Globular Flower-Like Reduced Graphene Oxide Design for Enhancing Thermally Conductive Properties of Silicone-Based Spherical Alumina Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
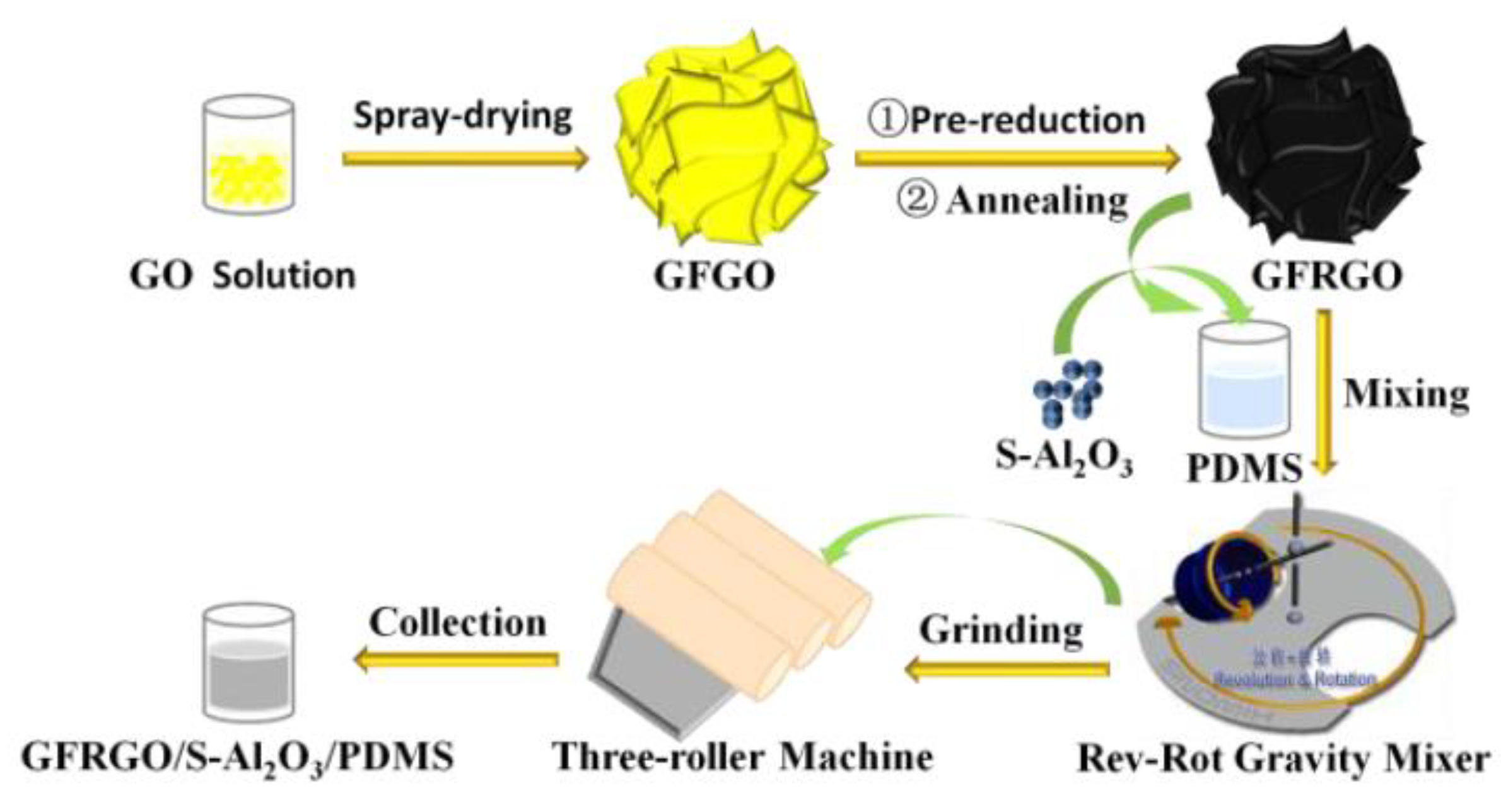
2.2. Preparation of GFRGO
2.3. Preparation of GFRGO/S-Al2O3/PDMS Composites
2.4. Characterization
3. Results and Discussion
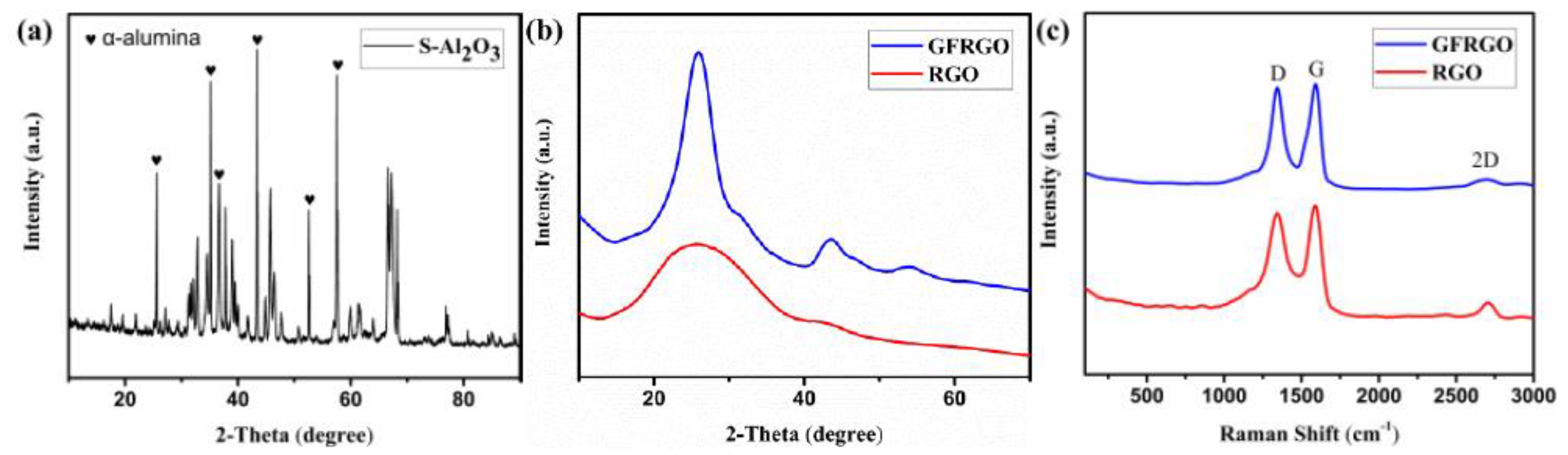
3.1. Morphology and Structure of S-Al2O3 and GFRGO
3.2. Density of GFRGO/S-Al2O3/PDMS Composites
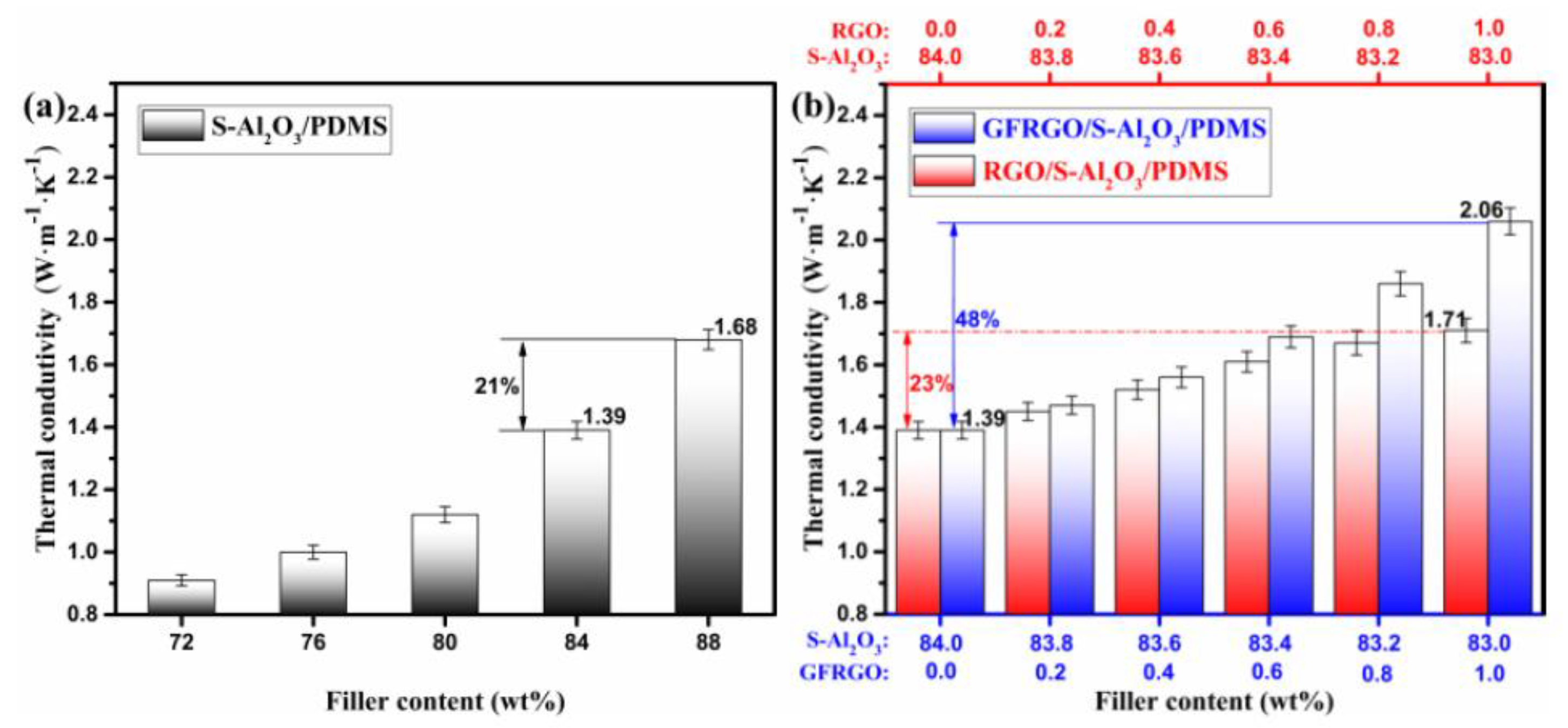
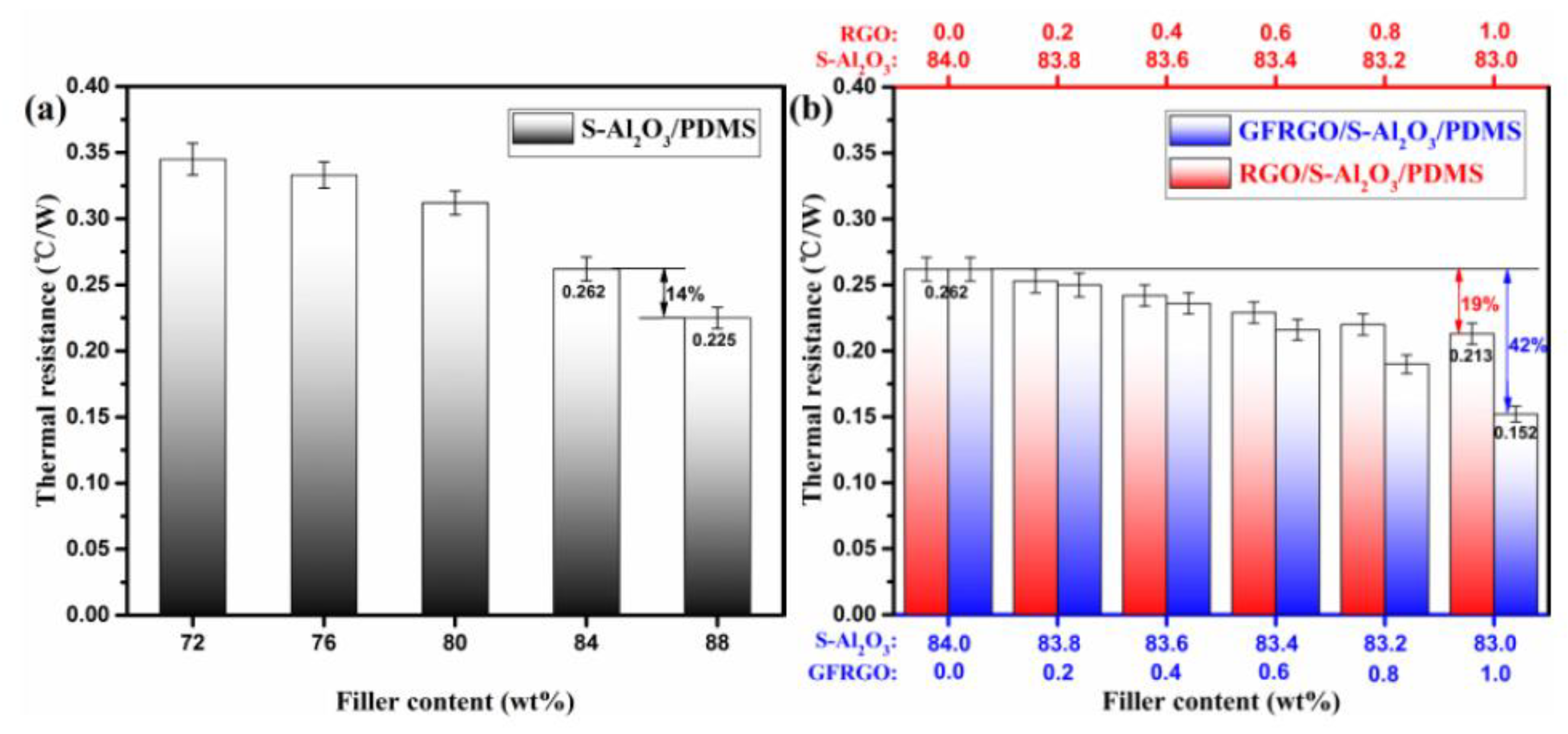
3.3. Thermally Conductive Properties of GFRGO/S-Al2O3/PDMS Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hansson, J.; Nilsson, T.M.J.; Ye, L.; Liu, J. Novel nanostructured thermal interface materials: A review. Int. Mater. Rev. 2018, 63, 22–45. [Google Scholar] [CrossRef]
- Leung, S.N. Thermally conductive polymer composites and nanocomposites: Processing-structure-property relationships. Compos. Part B Eng. 2018, 150, 78–92. [Google Scholar] [CrossRef]
- Chung, D.D.L. Thermal interface materials. J. Electron. Mater. 2020, 49, 268–270. [Google Scholar] [CrossRef]
- Razeeb, K.M.; Dalton, E.; Cross, G.L.W.; Robinson, A.J. Present and future thermal interface materials for electronic devices. Int. Mater. Rev. 2018, 63, 1–21. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal conductivity of polymers and their nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, M.; Hu, Y. Emerging interface materials for electronics thermal management: Experiments, modeling, and new opportunities. J. Mater. Chem. C 2020. [Google Scholar] [CrossRef]
- Mai, V.-D.; Lee, D.-I.; Park, J.-H.; Lee, D.-S. Rheological properties and thermal conductivity of epoxy resins filled with a mixture of alumina and boron nitride. Polymers 2019, 11, 597. [Google Scholar] [CrossRef]
- Zhang, P.; Ding, X.; Wang, Y.; Shu, M.; Gong, Y.; Zheng, K.; Tian, X.; Zhang, X. Low-melting-point alloy continuous network construction in a polymer matrix for thermal conductivity and electromagnetic shielding enhancement. ACS Appl. Polym. Mater. 2019, 1, 2006–2014. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.J.; Son, Y.R.; In, I.; An, K.H.; Kim, B.J.; Park, S.J. Recent advanced thermal interfacial materials: A review of conducting mechanisms and parameters of carbon materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- An, D.; Cheng, S.; Xi, S.; Zhang, Z.; Duan, X.; Ren, Y.; Li, J.; Sun, Z.; Liu, Y.; Wong, C.-P. Flexible thermal interfacial materials with covalent bond connections for improving high thermal conductivity. Chem. Eng. J. 2020, 383, 123151. [Google Scholar] [CrossRef]
- Mehra, N.; Mu, L.; Ji, T.; Yang, X.; Kong, J.; Gu, J.; Zhu, J. Thermal transport in polymeric materials and across composite interfaces. Appl. Mater. Today 2018, 12, 92–130. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, C.; Feng, Y.; Xue, Z.; Zhou, X.; Xie, X.; Mai, Y.-W. Effects of selective distribution of alumina micro-particles on rheological, mechanical and thermal conductive properties of asphalt/SBS/alumina composites. Compos. Sci. Technol. 2020, 186, 107917. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, M.; Cheng, Y.; Liu, J.; Xiao, B.; Chen, S.; Huang, J.; Xie, Q.; Wu, H. Dielectric properties and thermal conductivity of epoxy composites using quantum-sized silver decorated core/shell structured alumina/polydopamine. Compos. Part A Appl. Sci. Manuf. 2019, 118, 302–311. [Google Scholar] [CrossRef]
- Feng, Y.Z.; He, C.G.; Wen, Y.F.; Zhou, X.P.; Xie, X.L.; Ye, Y.S.; Mai, Y.W. Multi-functional interface tailoring for enhancing thermal conductivity, flame retardancy and dynamic mechanical property of epoxy/Al2O3 composites. Compos. Sci. Technol. 2018, 160, 42–49. [Google Scholar] [CrossRef]
- Ouyang, Y.G.; Hou, G.L.; Bai, L.Y.; Li, B.; Yuan, F. Constructing continuous networks by branched alumina for enhanced thermal conductivity of polymer composites. Compos. Sci. Technol. 2018, 165, 307–313. [Google Scholar] [CrossRef]
- Zhang, S.; Ke, Y.; Cao, X.; Ma, Y.; Wang, F. Effect of Al2O3 fibers on the thermal conductivity and mechanical properties of high density polyethylene with the absence and presence of compatibilizer. J. Appl. Polym. Sci. 2012, 124, 4874–4881. [Google Scholar] [CrossRef]
- Permal, A.; Devarajan, M.; Hung, H.L.; Zahner, T.; Lacey, D.; Ibrahim, K. Improved thermal and mechanical properties of aluminium oxide filled epoxy composites by reinforcing milled carbon fiber by partial replacement method. J. Mater. Sci. Mater. Electron. 2017, 28, 13487–13495. [Google Scholar] [CrossRef]
- Yeo, H.; Islam, A.M.; You, N.H.; Ahn, S.; Goh, M.; Hahn, J.R.; Jang, S.G. Characteristic correlation between liquid crystalline epoxy and alumina filler on thermal conducting properties. Compos. Sci. Technol. 2017, 141, 99–105. [Google Scholar] [CrossRef]
- Chena, C.; Xueb, Y.; Lib, X.; Wenb, Y.; Liub, J.; Xueb, Z.; Shia, D.; Zhoub, X.; Xieb, X.; Maib, Y.-W. High-performance epoxy/binary spherical alumina composite as underfill material for electronic packaging. Compos. Part A Appl. Sci. Manuf. 2019, 118, 67–74. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Shen, Y.; Zhou, Y.; Wang, D.; Lei, Z.; Feng, W.; Min, Z. Silicone-based alumina composites synthesized through in situ polymerization for high thermal conductivity and thermal stability. Mater. Lett. 2020, 261, 127002. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Zhang, L.; Yin, J.; Wang, J.; Xie, H. Thermal conductivity and mechanical properties of low-density silicone rubber filled with Al2O3 and graphene nanoplatelets. J. Therm. Sci. Eng. Appl. 2018, 10, 011014. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Nika, D.L.; Balandin, A.A. Phonons and thermal transport in graphene and graphene-based materials. Rep. Phys. 2017, 80, 036502. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020. [Google Scholar] [CrossRef]
- Wu, J.; Feng, S.; Wei, X.; Shen, J.; Lu, W.; Shi, H.; Tao, K.; Lu, S.; Sun, T.; Yu, L.; et al. Facile synthesis of 3D graphene flowers for ultrasensitive and highly reversible gas sensing. Adv. Funct. Mater. 2016, 26, 7462–7469. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Liu, Y.; Zhao, X.; Ananth, N.; Zheng, B.; Peng, Li.; Huang, T.; Gao, W.; Gao, C. High-quality graphene microflower design for high-performance Li-S and Al-Ion batteries. Adv. Energy Mater. 2017, 7, 1700051. [Google Scholar] [CrossRef]
- Chen, C.; Xi, J.B.; Zhou, E.Z.; Peng, L.; Chen, Z.C.; Gao, C. Porous graphene microflowers for high-performance microwave absorption. Nano Micro Lett. 2018, 10, 26. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, X.; Sun, R.; Xu, J.-B.; Wong, C.-P. Spray-assisted assembled spherical boron nitride as fillers for polymers with enhanced thermally conductivity. Chem. Eng. J. 2019, 370, 166–175. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Song, Y.; Cui, Y.; Khan, M.; Ji, J.; et al. Reduced graphene oxide embedded with MQ silicone resin nano-aggregates for silicone rubber composites with enhanced thermal conductivity and mechanical performance. Polymers 2018, 10, 1254. [Google Scholar] [CrossRef]
- Macedo, M.I.F.; Bertran, C.A.; Osawa, C.C. Kinetics of the γ→α-alumina phase transformation by quantitative X-ray diffraction. J. Mater. Sci. 2007, 42, 2830–2836. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Soliman, F.S.; El Maghraby, H.; Moustfa, Y.M. Thermal conductivity enhancement of treated petroleum waxes, as phase change material, by α nano alumina: Energy storage. Renew. Sustain. Energy Rev. 2017, 70, 1052–1058. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Liao, M.; Dai, W.; Wang, Z.; Yan, C.; Li, He.; Lin, C.-T.; Jiang, N.; Yu, J. Constructing a “pea-pod-like” alumina-graphene binary architecture for enhancing thermal conductivity of epoxy composite. Chem. Eng. J. 2020, 381, 122690. [Google Scholar] [CrossRef]
- Idumah, C.I.; Hassan, A. Recently emerging trends in thermal conductivity of polymer nanocomposites. Rev. Chem. Eng. 2016, 32, 413–457. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Zhang, M.; Ji, J.; Pang, X.; Liu, R. Three-dimensional heterostructured reduced graphene oxide-hexagonal boron nitride-stacking material for silicone thermal grease with enhanced thermally conductive properties. Nanomaterials 2019, 9, 938. [Google Scholar] [CrossRef] [PubMed]
- Barani, Z.; Mohammadzadeh, A.; Geremew, A.; Huang, C.Y.; Coleman, D.; Mangolini, L.; Kargar, F.; Balandin, A.A. Thermal Properties of the Binary-Filler Hybrid Composites with Graphene and Copper Nanoparticles. Adv. Funct. Mater. 2019, 30, 1904008. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Balinskiy, M.; Magana, A.S.; Lewis, J.S.; Balandin, A.A. Dual-Functional Graphene Composites for Electromagnetic Shielding and Thermal Management. Adv. Electron. Mater. 2019, 5, 1800558. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Salgado, R.; Debnath, B.; Lewis, J.S.; Aytan, E.; Lake, R.K.; Balandin, A.A. Thermal percolation threshold and thermal properties of composites with high loading of graphene and boron nitride fillers. ACS Appl. Mater. Interfaces 2018, 10, 37555–37565. [Google Scholar] [CrossRef]
- Lewis, J.S.; Barani, Z.; Magana, A.S.; Kargar, F.; Balandin, A.A. Thermal and Electrical Conductivity Control in Hybrid Composites with Graphene and Boron Nitride Fillers. Mater. Res. Express 2019, 6, 085325. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, X.; Zhang, X.; Sun, R.; Tian, X.; Zeng, Y.; Xu, J.-B.; Wong, C.-P. Silver nanoparticle-modified alumina microsphere hybrid composites for enhanced energy density and thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2019, 119, 299–309. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Liang, Y.; Li, B.; Ma, H.; Zhang, L. Improved thermal conductivity and electromechanical properties of natural rubber by constructing Al2O3-PDA-Ag hybrid nanoparticles. Compos. Sci. Technol. 2019, 180, 86–93. [Google Scholar] [CrossRef]
- Ren, L.; Li, Q.; Lu, J.; Zeng, X.; Sun, R.; Wu, J.; Xu, J.-B.; Wong, C.-P. Enhanced thermal conductivity for Ag-deposited alumina sphere/epoxy resin composites through manipulating interfacial thermal resistance. Compos. Part A Appl. Sci. Manuf. 2018, 107, 561–569. [Google Scholar] [CrossRef]
- Bian, W.; Yao, T.; Chen, M.; Zhang, C.; Shao, T.; Yang, Y. The synergistic effects of the micro-BN and nano-Al2O3 in micro-nano composites on enhancing the thermal conductivity for insulating epoxy resin. Compos. Sci. Technol. 2018, 168, 420–428. [Google Scholar] [CrossRef]
- Guan, F.L.; Gui, C.X.; Zhang, H.B.; Jiang, Z.G.; Jiang, Y.; Yu, Z.Z. Enhanced thermal conductivity and satisfactory flame retardancy of epoxy/alumina composites by combination with graphene nanoplatelets and magnesium hydroxide. Compos. Part B Eng. 2016, 98, 134–140. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, L. Effect of nano-fillers on the thermal conductivity of epoxy composites with micro-Al2O3 particles. Mater. Des. 2015, 66, 176–182. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.Q.; Yin, L.Q.; Zhao, J.C.; Xia, L.G.; Chen, L.F. Exceptionally high thermal conductivity of thermal grease: Synergistic effects of graphene and alumina. Int. J. Therm. Sci. 2015, 91, 76–82. [Google Scholar] [CrossRef]
- Leung, S.N.; Khan, M.O.; Chan, E.; Naguib, H.; Dawson, F.; Adinkrah, V.; Lakatos-Hayward, L. Analytical modeling and characterization of heat transfer in thermally conductive polymer composites filled with spherical particulates. Compos. Part B Eng. 2013, 45, 43–49. [Google Scholar] [CrossRef]
- Wu, H.; Lawrence, T.D. High thermally conductive graphite nanoplatelet/polyetherimide composite by precoating: Effect of percolation and particle size. Polym. Compos. 2013, 34, 2148–2153. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, C.; Songfeng, E.; Xie, L.; Geng, R.; Lin, C.-T.; Li, L.; Yao, Y. Enhanced thermal conductivity of polyurethane composites via engineering small/large sizes interconnected boron nitride nanosheets. Compos. Sci. Technol. 2019, 170, 93–100. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.B.; Wong, C.P. Construction of 3D skeleton for polymer composites achieving a high thermal conductivity. Small 2018, 14, 1704044. [Google Scholar] [CrossRef]
- Ji, C.; Yan, C.; Wang, Y.; Xiong, S.; Zhou, F.; Li, Y.; Sun, R.; Wong, C.-P. Thermal conductivity enhancement of CNT/MoS2/graphene−epoxy nanocomposites based on structural synergistic effects and interpenetrating network. Compos. Part B Eng. 2019, 163, 363–370. [Google Scholar] [CrossRef]
- Oh, H.; Kim, J. Fabrication of polymethyl methacrylate composites with silanized boron nitride by in-situ polymerization for high thermal conductivity. Compos. Sci. Technol. 2019, 172, 153–162. [Google Scholar] [CrossRef]



| Matrix | Filler | TCE (%) | References |
|---|---|---|---|
| Epoxy | 22.90% Ag decorated S-Al2O3 hybrid | 6 | [13] |
| Epoxy | 70% Ag modified S-Al2O3 hybrid | 16 | [39] |
| Natural rubber | 10 vol% S-Al2O3-poly(dopamine)-Ag hybrid | 15 | [40] |
| Epoxy | 70% GO coating S-Al2O3 hybrid | 58 | [14] |
| Silicone rubber | 89% S-Al2O3 + 1% RGO | 47 | [21] |
| Epoxy | 68.63% S-Al2O3 + 1.37% Ag | 43 | [41] |
| Epoxy | 22.5% S-Al2O3 + 7.5% BN | 45 | [42] |
| Epoxy | 79% S-Al2O3 + 1% graphene | 20 | [43] |
| Epoxy | 45% S-Al2O3 + 5% AlN | 17 | [44] |
| 45% S-Al2O3 + 5% AlN/graphene hybrid | 24 | ||
| 45% S-Al2O3 + 5% AlN/CNT hybrid | 20 | ||
| PDMS | 83% S-Al2O3 + 1% RGO | 23 | This work |
| 83% S-Al2O3 + 1% GFRGO | 48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Li, T.; Zhou, X.; Ge, X.; Chen, X.; Lin, Z.; Pang, X.; Ge, J. Globular Flower-Like Reduced Graphene Oxide Design for Enhancing Thermally Conductive Properties of Silicone-Based Spherical Alumina Composites. Nanomaterials 2020, 10, 544. https://doi.org/10.3390/nano10030544
Liang W, Li T, Zhou X, Ge X, Chen X, Lin Z, Pang X, Ge J. Globular Flower-Like Reduced Graphene Oxide Design for Enhancing Thermally Conductive Properties of Silicone-Based Spherical Alumina Composites. Nanomaterials. 2020; 10(3):544. https://doi.org/10.3390/nano10030544
Chicago/Turabian StyleLiang, Weijie, Tiehu Li, Xiaocong Zhou, Xin Ge, Xunjun Chen, Zehua Lin, Xiaoyan Pang, and Jianfang Ge. 2020. "Globular Flower-Like Reduced Graphene Oxide Design for Enhancing Thermally Conductive Properties of Silicone-Based Spherical Alumina Composites" Nanomaterials 10, no. 3: 544. https://doi.org/10.3390/nano10030544
APA StyleLiang, W., Li, T., Zhou, X., Ge, X., Chen, X., Lin, Z., Pang, X., & Ge, J. (2020). Globular Flower-Like Reduced Graphene Oxide Design for Enhancing Thermally Conductive Properties of Silicone-Based Spherical Alumina Composites. Nanomaterials, 10(3), 544. https://doi.org/10.3390/nano10030544





