Real-Time In Vivo Imaging of Mouse Left Ventricle Reveals Fluctuating Movements of the Intercalated Discs
Abstract
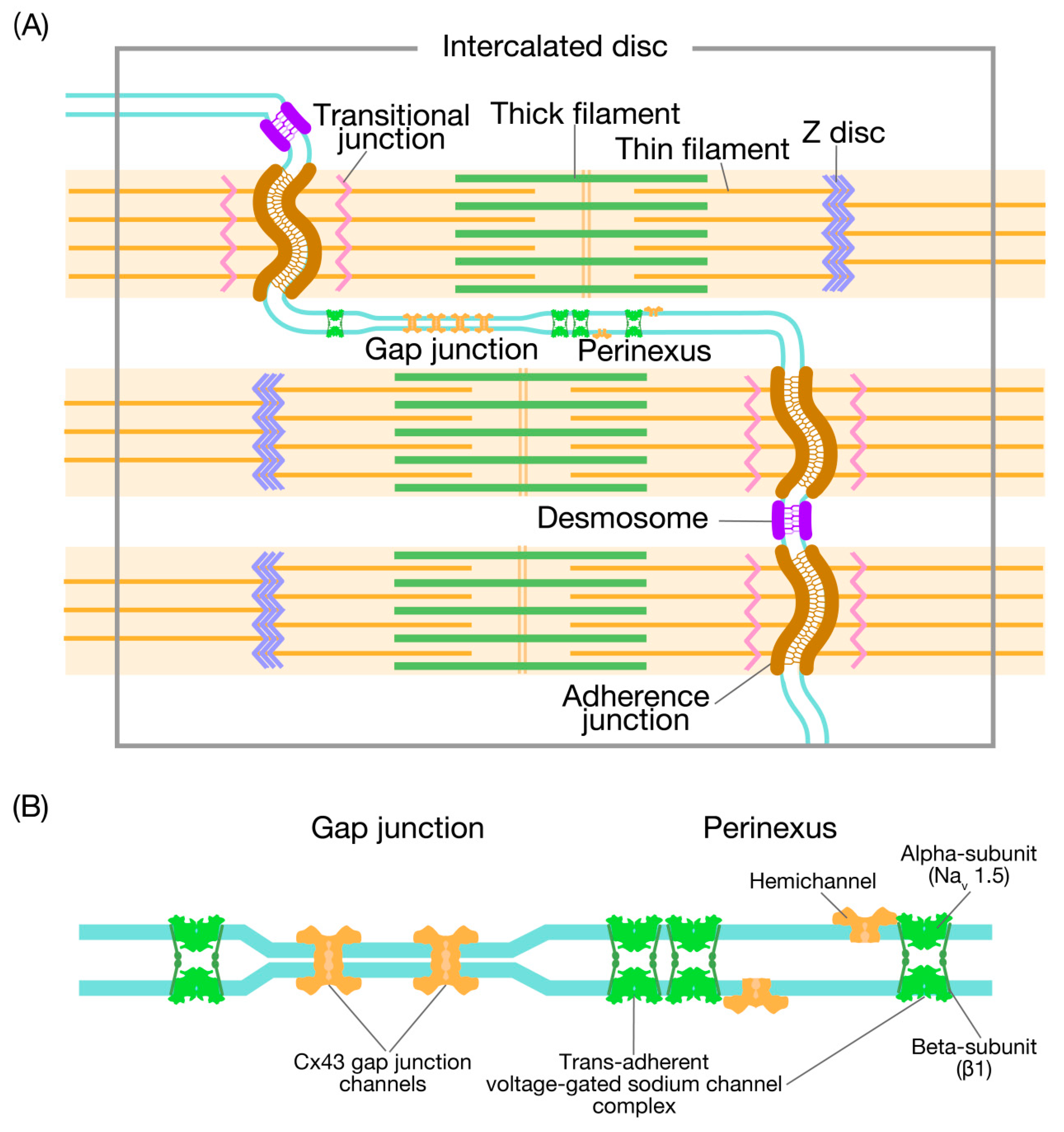
1. Introduction
2. Materials and Methods
2.1. CellMask Treatment in the Heart of Living Mice In Vivo
2.2. α-Actinin-AcGFP Expression in the Heart of Living Mice In Vivo
2.3. Microscopic System
2.4. In Vivo Nano-Imaging
3. Analyses
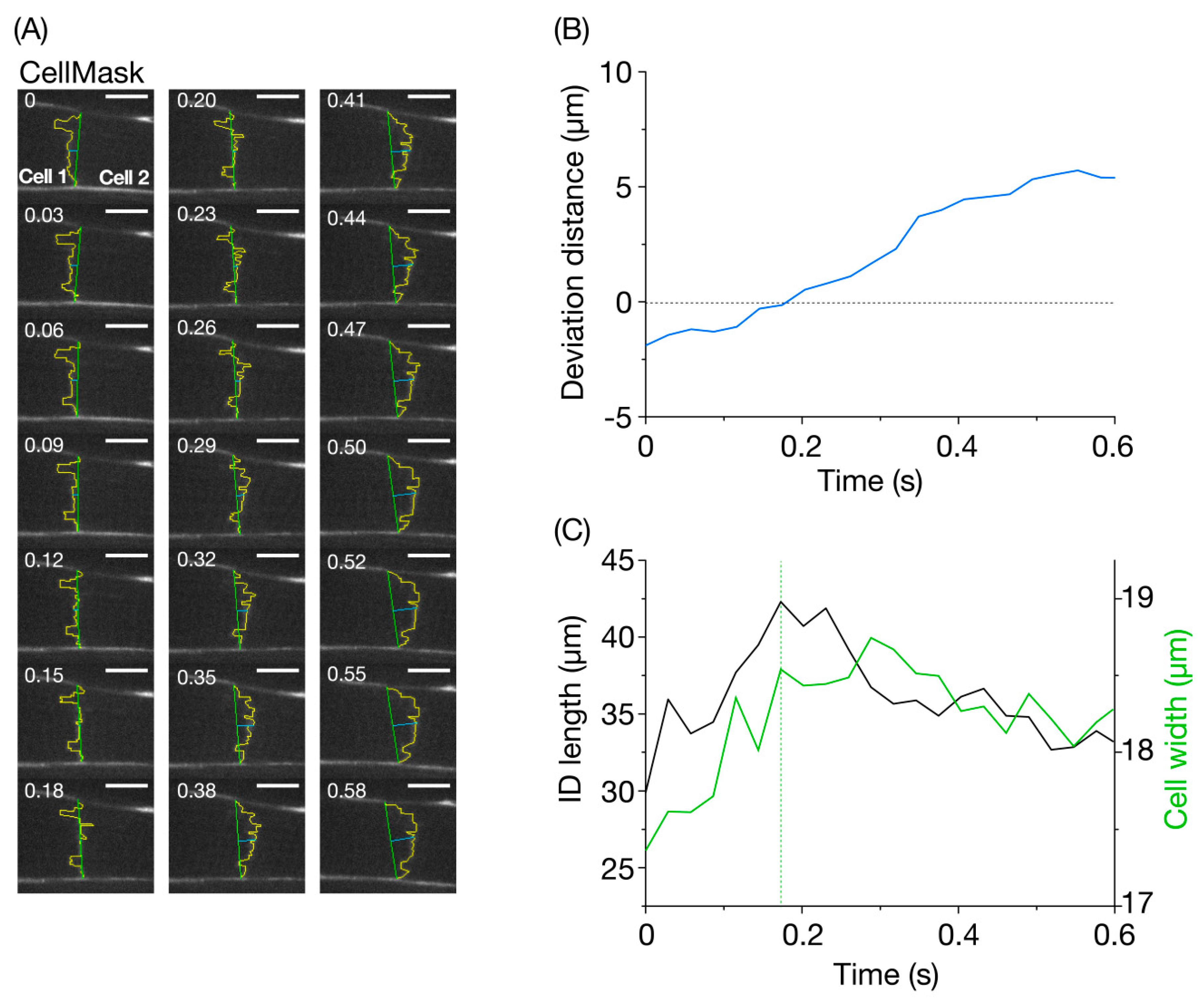
4. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bers, D.M. Excitation-Contraction Coupling and Cardiac Contractile Force. Vasc. Med. 2001, 63–100. [Google Scholar]
- Kobirumaki-Shimozawa, F.; Inoue, T.; Shintani, S.A.; Oyama, K.; Terui, T.; Minamisawa, S.; Ishiwata, S.; Fukuda, N. Cardiac thin filament regulation and the Frank-Starling mechanism. J. Physiol. Sci. 2014, 64, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Forbes, M.; Sperelakis, N. Intercalated discs of mammalian heart: A review of structure and function. Tissue Cell 1985, 17, 605–648. [Google Scholar] [CrossRef]
- Severs, N.J.; Slade, A.M.; Powell, T.; Twist, V.W. Ultrastructure of the sarcolemma and intercalated disc in isolated rat myocytes. Basic Res. Cardiol. 1985, 80, 35–40. [Google Scholar] [PubMed]
- Wilson, A.; Schoenauer, R.; Ehler, E.; Agarkova, I.; Bennett, P. Cardiomyocyte growth and sarcomerogenesis at the intercalated disc. Cell. Mol. Life Sci. 2013, 71, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P. Riding the waves of the intercalated disc of the heart. Biophys. Rev. 2018, 10, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Stroemlund, L.W.; Jensen, C.F.; Qvortrup, K.; Delmar, M.; Nielsen, M.S. Gap junctions–guards of excitability. Biochem. Soc. Trans. 2015, 43, 508–512. [Google Scholar] [CrossRef]
- Gourdie, R.G. The Cardiac Gap Junction has Discrete Functions in Electrotonic and Ephaptic Coupling. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2018, 302, 93–100. [Google Scholar] [CrossRef]
- Becker, D.; Cook, J.E.; Davies, C.S.; Evans, W.H.; Gourdie, R.G. Expression of major gap junction connexin types in the working myocardium of eight chordates. Cell Boil. Int. 1998, 22, 527–543. [Google Scholar] [CrossRef]
- Rhett, J.M.; Gourdie, R.G. The perinexus: A new feature of Cx43 gap junction organization. Hear. Rhythm. 2011, 9, 619–623. [Google Scholar] [CrossRef]
- Rhett, J.M.; Ongstad, E.L.; Jourdan, J.; Gourdie, R.G. Cx43 associates with Nav1.5 in the cardiomyocyte perinexus. J. Membr. Boil. 2012, 245, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Rhett, J.M.; Veeraraghavan, R.; Poelzing, S.; Gourdie, R.G. The perinexus: Sign-post on the path to a new model of cardiac conduction? Trends Cardiovasc. Med. 2013, 23, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, R.; Lin, J.; Hoeker, G.S.; Keener, J.P.; Gourdie, R.G.; Poelzing, S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: An experimental and modeling study. Pflügers Arch. Eur. J. Physiol. 2015, 467, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Raisch, T.B.; Yanoff, M.S.; Larsen, T.; Farooqui, M.A.; King, D.R.; Veeraraghavan, R.; Gourdie, R.G.; Baker, J.W.; Arnold, W.S.; Almahameed, S.; et al. Intercalated Disk Extracellular Nanodomain Expansion in Patients With Atrial Fibrillation. Front. Physiol. 2018, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Leo-Macias, A.; Agullo-Pascual, E.; Sanchez-Alonso, J.L.; Keegan, S.; Lin, X.; Rothenberg, E.; Delmar, M.; Arcos, T.; Korchev, Y.E.; Gorelik, J.; et al. Nanoscale visualization of functional adhesion / excitability nodes at the intercalated disc. Nat. Commun. 2016, 7, 10342. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, R.; Hoeker, G.S.; Alvarez-Laviada, A.; Hoagland, D.; Wan, X.; King, D.R.; Sanchez-Alonso, J.; Chen, C.; Jourdan, J.; Isom, L.L.; et al. The adhesion function of the sodium channel beta subunit (β1) contributes to cardiac action potential propagation. eLife 2018, 7, e37610. [Google Scholar] [CrossRef]
- Veeraraghavan, R.; Poelzing, S.; Gourdie, R.G. Old cogs, new tricks: A scaffolding role for connexin43 and a junctional role for sodium channels? FEBS Lett. 2014, 588, 1244–1248. [Google Scholar] [CrossRef]
- Veeraraghavan, R.; Gourdie, R.G.; Poelzing, S. Mechanisms of cardiac conduction: A history of revisions. Am. J. Physiol. Circ. Physiol. 2014, 306, H619–H627. [Google Scholar] [CrossRef]
- Kucera, J.; Rohr, S.; Rudy, Y. Localization of Sodium Channels in Intercalated Disks Modulates Cardiac Conduction. Circ. Res. 2002, 91, 1176–1182. [Google Scholar] [CrossRef]
- Fedele, F.; Severino, P.; Bruno, N.; Stio, R.; Caira, C.; D’Ambrosi, A.; Brasolin, B.; Ohanyan, V.; Mancone, M. Role of ion channels in coronary microcirculation: A review of the literature. Future Cardiol. 2013, 9, 897–905. [Google Scholar] [CrossRef]
- Brugada, R.; Campuzano, O.; Sarquella-Brugada, G.; Brugada, J.; Brugada, P. Brugada Syndrome. Methodist DeBakey Cardiovasc. J. 2014, 10, 25–28. [Google Scholar] [CrossRef]
- Fedele, F.; Mancone, M.; Chilian, W.M.; Severino, P.; Canali, E.; Logan, S.; De Marchis, M.L.; Volterrani, M.; Palmirotta, R.; Guadagni, F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res. Cardiol. 2013, 108, 387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yoon, J.-Y.; Morley, M.; McLendon, J.; Mapuskar, K.A.; Gutmann, R.; Mehdi, H.; Bloom, H.L.; Dudley, S.C.; Ellinor, P.T.; et al. A common variant alters SCN5A–miR-24 interaction and associates with heart failure mortality. J. Clin. Investig. 2018, 128, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kobirumaki-Shimozawa, F.; Kagemoto, T.; Fujii, T.; Terui, T.; Kusakari, Y.; Hongo, K.; Morimoto, S.; Ohtsuki, I.; Hashimoto, K.; et al. Depressed Frank–Starling mechanism in the left ventricular muscle of the knock-in mouse model of dilated cardiomyopathy with troponin T deletion mutation ΔK210. J. Mol. Cell. Cardiol. 2013, 63, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kobirumaki-Shimozawa, F.; Shimozawa, T.; Oyama, K.; Kushida, Y.; Terui, T.; Ishiwata, S.; Fukuda, N. Optimization of fluorescent labeling for in vivo nanoimaging of sarcomeres in the mouse heart. BioMed Res. Int. 2018, 2018, 4349170. [Google Scholar] [CrossRef]
- Kobirumaki-Shimozawa, F.; Oyama, K.; Shimozawa, T.; Mizuno, A.; Ohki, T.; Terui, T.; Minamisawa, S.; Ishiwata, S.; Fukuda, N. Nano-imaging of the beating mouse heart in vivo: Importance of sarcomere dynamics, as opposed to sarcomere length per se, in the regulation of cardiac function. J. Gen. Physiol. 2015, 147, 53–62. [Google Scholar] [CrossRef]
- Shimozawa, T.; Hirokawa, E.; Kobirumaki-Shimozawa, F.; Oyama, K.; Shintani, S.A.; Terui, T.; Kushida, Y.; Tsukamoto, S.; Fujii, T.; Ishiwata, S.; et al. In vivo cardiac nano-imaging: A new technology for high-precision analyses of sarcomere dynamics in the heart. Prog. Biophys. Mol. Boil. 2017, 124, 31–40. [Google Scholar] [CrossRef]
- Boyett, M.; Frampton, J.; Kirby, M. The length, width and volume of isolated rat and ferret ventricular myocytes during twitch contractions and changes in osmotic strength. Exp. Physiol. 1991, 76, 259–270. [Google Scholar] [CrossRef]
- Petitprez, S.; Zmoos, A.-F.; Ogrodnik, J.; Balse, E.; Raad, N.; El-Haou, S.; Albesa, M.; Bittihn, P.; Luther, S.; Lehnart, S.E.; et al. SAP97 and Dystrophin Macromolecular Complexes Determine Two Pools of Cardiac Sodium Channels Nav1.5 in Cardiomyocytes. Circ. Res. 2011, 108, 294–304. [Google Scholar] [CrossRef]
- Morris, C.; Juranka, P.F. Nav Channel Mechanosensitivity: Activation and Inactivation Accelerate Reversibly with Stretch. Biophys. J. 2007, 93, 822–833. [Google Scholar] [CrossRef]
- Banderali, U.; Juranka, P.F.; Clark, R.B.; Giles, W.; Morris, C. Impaired stretch modulation in potentially lethal cardiac sodium channel mutants. Channels 2010, 4, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Apel, D.; Gonzalez Casanova, J.; von Salisch, S.; Mohr, F.W.; Daehnert, I.; Dhein, S. On the different roles of AT1 and AT2 receptors in stretch-induced changes of connexin 43 expression and localization. Pflügers Arch. Eur. J. Physiol. 2012, 464, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.; Maggs, A.M.; Baines, A.; Pinder, J.C. The Transitional Junction: A New Functional Subcellular Domain at the Intercalated Disc. Mol. Boil. Cell 2006, 17, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.H.; Ruska, H. Electron microscope study of mammalian cardiac muscle cells. J. Cell Boil. 1957, 3, 261–268. [Google Scholar] [CrossRef]
- Lockard, V. Trans-cellular Desmin-Lamin B Intermediate Filament Network in Cardiac Myocytes. J. Mol. Cell. Cardiol. 1993, 25, 303–309. [Google Scholar] [CrossRef]
- Epstein, N.D.; Davis, J.S. Sensing stretch is fundamental. Cell 2003, 112, 147–150. [Google Scholar] [CrossRef][Green Version]
- Conover, G.M.; Gregorio, C.C. The desmin coil 1B mutation K190A impairs nebulin Z-disc assembly and destabilizes actin thin filaments. J. Cell Sci. 2011, 124, 3464–3476. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Elzey, B.; Williams, G.; Lu, S.; Law, D.J.; Horowits, R. Ultrastructural and biochemical localization of N-RAP at the interface between myofibrils and intercalated disks in the mouse heart. Biochemistry 2001, 40, 14898–14906. [Google Scholar] [CrossRef]
- Granzier, H.L.; Hutchinson, K.R.; Tonino, P.; Methawasin, M.; Li, F.W.; Slater, R.E.; Bull, M.M.; Saripalli, C.; Pappas, C.T.; Gregorio, C.C.; et al. Deleting titin’s I-band/A-band junction reveals critical roles for titin in biomechanical sensing and cardiac function. Proc. Natl. Acad. Sci. USA 2014, 111, 14589–14594. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobirumaki-Shimozawa, F.; Nakanishi, T.; Shimozawa, T.; Terui, T.; Oyama, K.; Li, J.; Louch, W.E.; Ishiwata, S.; Fukuda, N. Real-Time In Vivo Imaging of Mouse Left Ventricle Reveals Fluctuating Movements of the Intercalated Discs. Nanomaterials 2020, 10, 532. https://doi.org/10.3390/nano10030532
Kobirumaki-Shimozawa F, Nakanishi T, Shimozawa T, Terui T, Oyama K, Li J, Louch WE, Ishiwata S, Fukuda N. Real-Time In Vivo Imaging of Mouse Left Ventricle Reveals Fluctuating Movements of the Intercalated Discs. Nanomaterials. 2020; 10(3):532. https://doi.org/10.3390/nano10030532
Chicago/Turabian StyleKobirumaki-Shimozawa, Fuyu, Tomohiro Nakanishi, Togo Shimozawa, Takako Terui, Kotaro Oyama, Jia Li, William E. Louch, Shin’ichi Ishiwata, and Norio Fukuda. 2020. "Real-Time In Vivo Imaging of Mouse Left Ventricle Reveals Fluctuating Movements of the Intercalated Discs" Nanomaterials 10, no. 3: 532. https://doi.org/10.3390/nano10030532
APA StyleKobirumaki-Shimozawa, F., Nakanishi, T., Shimozawa, T., Terui, T., Oyama, K., Li, J., Louch, W. E., Ishiwata, S., & Fukuda, N. (2020). Real-Time In Vivo Imaging of Mouse Left Ventricle Reveals Fluctuating Movements of the Intercalated Discs. Nanomaterials, 10(3), 532. https://doi.org/10.3390/nano10030532





