Sonochemical Synthesis of Copper-doped BiVO4/g-C3N4 Nanocomposite Materials for Photocatalytic Degradation of Bisphenol A under Simulated Sunlight Irradiation
Abstract
1. Introduction
2. Methods
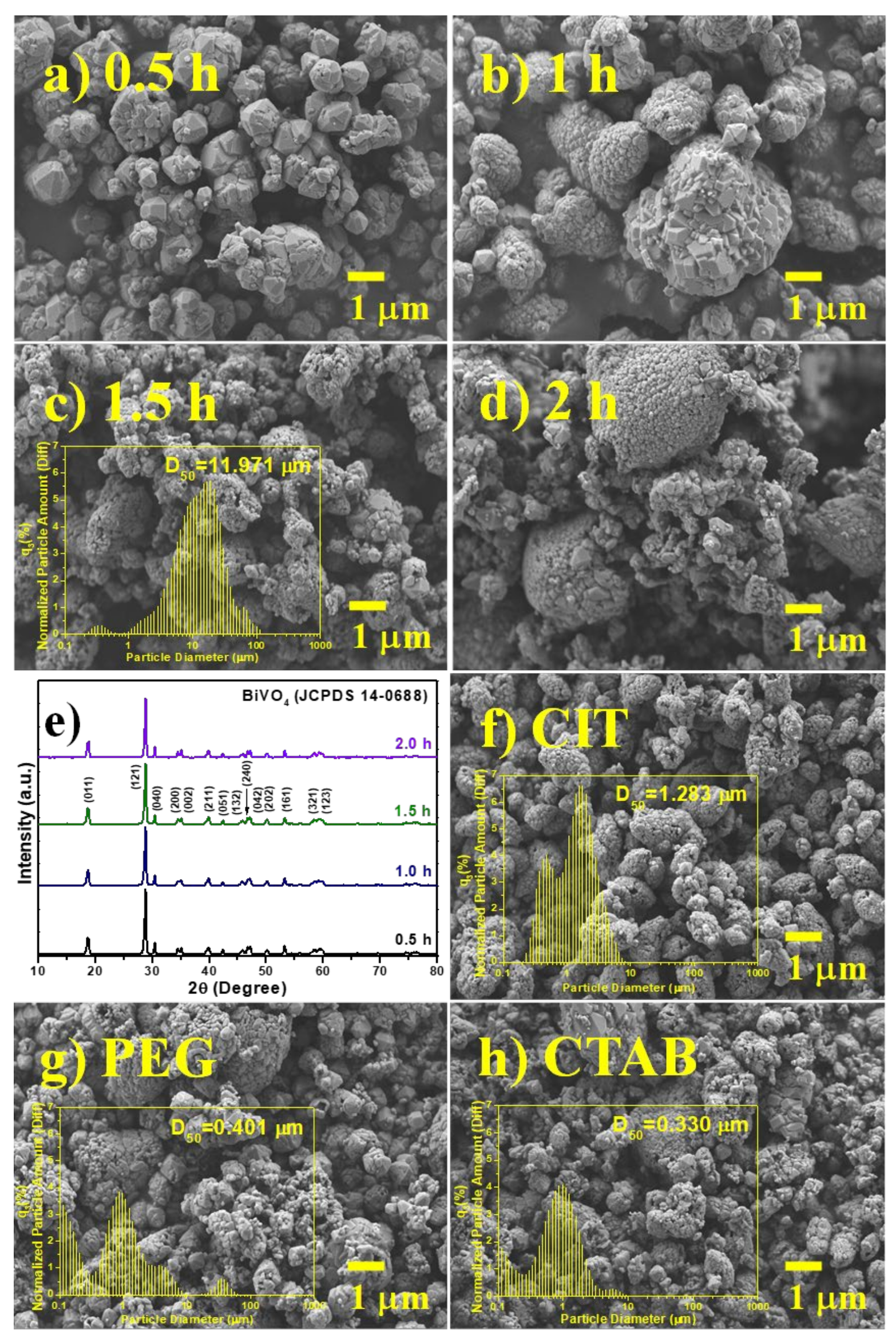
2.1. Sonochemical Synthesis of BiVO4 Nanocomposite Photocatalysts
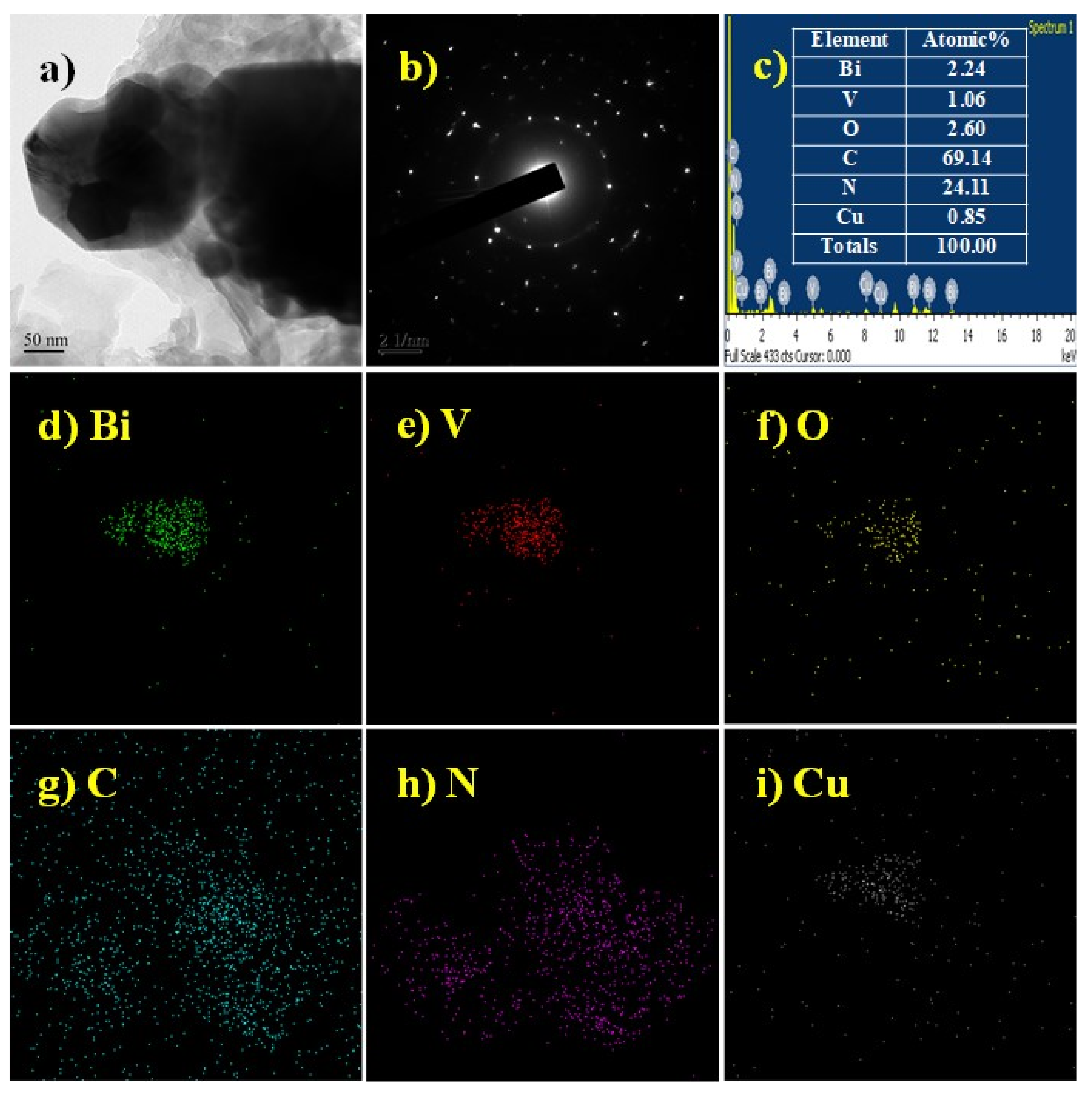
2.2. Characterization of BiVO4 Nanocomposite Photocatalysts
2.3. Photocatalytic Reaction
3. Results
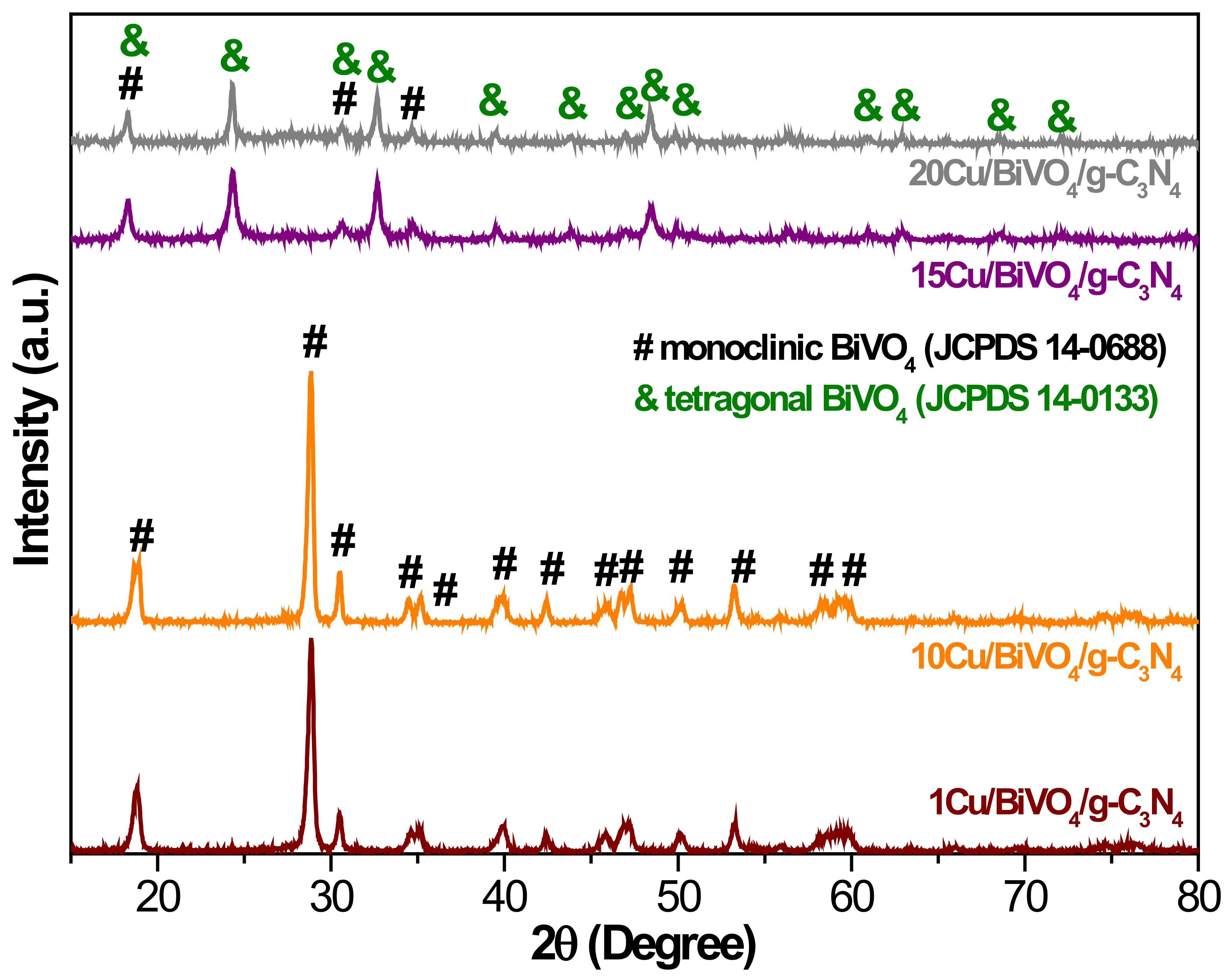
3.1. Characterization of BiVO4 Nanocomposite Photocatalysts
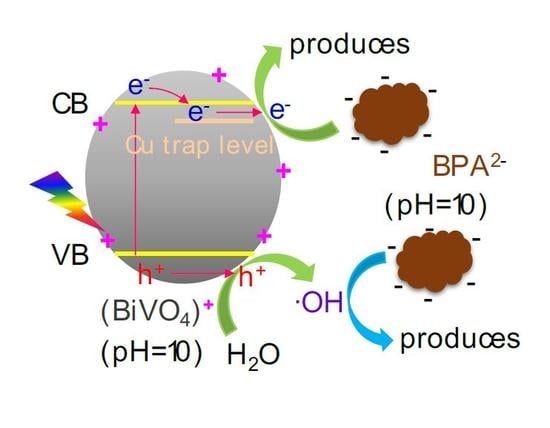
3.2. Photocatalytic Degradation Activity
3.2.1. Initial pH of the BPA Solution
3.2.2. Photocatalyst Dosage
3.2.3. Cu Doping Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Selective synthesis and visible-light photocatalytic activities of BiVO4 with different crystalline phases. Mater. Chem. Phys. 2007, 103, 162–167. [Google Scholar] [CrossRef]
- Liu, W.; Cao, L.; Su, G.; Liu, H.; Wang, X.; Zhang, L. Ultrasound assisted synthesis of monoclinic structured spindle BiVO4 particles with hollow structure and its photocatalytic property. Ultrason. Sonochem. 2010, 17, 669–674. [Google Scholar] [CrossRef]
- Martinez Suarez, C.; Hernández, S.; Russo, N. BiVO4 as photocatalyst for solar fuels production through water splitting: A short review. Appl. Catal. A Gen. 2015, 504, 158–170. [Google Scholar] [CrossRef]
- Dong, P.; Xi, X.; Zhang, X.; Hou, G.; Guan, R. Template-free synthesis of monoclinic BiVO4 with porous structure and its high photocatalytic activity. Materials 2016, 9, 685. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Shen, T.; Song, H.; Li, D.; Zhao, R.; Wang, X. Engineering the dimensional interface of BiVO4-2D reduced graphene oxide (RGO) nanocomposite for enhanced visible light photocatalytic performance. Nanomaterials 2019, 9, 907. [Google Scholar] [CrossRef]
- Xie, T.; Li, H.; Liu, C.; Yang, J.; Xiao, T.; Xu, L. Magnetic photocatalyst BiVO4/Mn-Zn ferrite/reduced graphene oxide: Synthesis strategy and its highly photocatalytic activity. Nanomaterials 2018, 8, 380. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Peng, X.; Wang, Z.; Zhou, L.; Yin, Q. A novel route to manufacture 2D layer MoS2 and g-C3N4 by atmospheric plasma with enhanced visible-light-driven photocatalysis. Nanomaterials 2019, 9, 1139. [Google Scholar] [CrossRef]
- Smýkalová, A.; Sokolová, B.; Foniok, K.; Matejka, V.; Praus, P. Photocatalytic degradation of selected pharmaceuticals using g-C3N4 and TiO2 nanomaterials. Nanomaterials 2019, 9, 1194. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Cui, W.; Sui, H. Synthesis and characterization of a core–shell BiVO4@g-C3N4 photo-catalyst with enhanced photocatalytic activity under visible light irradiation. RSC Adv. 2017, 7, 8167–8177. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Fu, X.; Lu, C.; Kim, S.H. Mesoporous sulfur-modified iron oxide as an effective Fenton-like catalyst for degradation of bisphenol A. Appl. Catal. B Environ. 2016, 184, 132–141. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Jiang, H.; Jiang, W.; Su, R.; Luo, S.; Luo, Y. Photoelectrocatalytic oxidation of bisphenol A over mesh of TiO2/graphene/Cu2O. Appl. Catal. B Environ. 2016, 183, 75–85. [Google Scholar] [CrossRef]
- Lee, G.J.; Anandan, S.; Masten, S.J.; Wu, J.J. Sonochemical synthesis of hollow copper doped zinc sulfide nanostructures: Optical and catalytic properties for visible light assisted photosplitting of water. Ind. Eng. Chem. Res. 2014, 53, 8766–8772. [Google Scholar] [CrossRef]
- Lee, G.J.; Chen, H.C.; Wu, J.J. Enhancing the photocatalytic hydrogen evolution of copper doped zinc sulfide nanoballs through surfactants modification. Int. J. Hydrog. Energy 2019, 44, 30563–30573. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Meng, D.; Xing, Y.; Tian, X.; Yu, X.; Xu, K.; Wu, X. Effects of citric acid and urea on the structural and morphological characteristics of BiVO4 synthesized by the sol–gel combustion method. J. Sol-Gel Sci. Technol. 2015, 76, 562–571. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Suzuki, M.; Ichikuni, N. Ni/SiO2 prepared by sol–gel process using citric acid. Microporous Mesoporous Mater. 2003, 66, 197–208. [Google Scholar] [CrossRef]
- Shahi, A.K.; Pandey, B.K.; Gopal, R. PEG mediated solvothermal synthesis of fine ZnS sub-micro and microspheres and their optical properties. Mater. Lett. 2014, 116, 112–115. [Google Scholar] [CrossRef]
- Li, H.; Hong, W.; Cui, Y.; Hu, X.; Fan, S.; Zhu, L. Enhancement of the visible light photocatalytic activity of Cu2O/BiVO4 catalysts synthesized by ultrasonic dispersion method at room temperature. Mater. Sci. Eng. B 2014, 181, 1–8. [Google Scholar] [CrossRef]
- Safaei, J.; Ullah, H.; Mohamed, N.A.; Noh, M.F.M.; Soh, M.F.; Tahir, A.A.; Ludin, N.A.; Ibrahim, M.A.; Isahak, W.N.R.W.; Teridi, M.A.M. Enhanced photoelectrochemical performance of Z-scheme g-C3N4/BiVO4 photocatalyst. Appl. Catal. B Environ. 2018, 234, 296–310. [Google Scholar] [CrossRef]
- Bautista-Toledo, I.; Ferro-Garcia, M.A.; Rivera-Utrilla, J.; Moreno-Castilla, C.; Vegas Fernandez, F.J. Bisphenol A removal from water by activated carbon effects of carbon characteristics and solution chemistry. Environ. Sci. Technol. 2005, 39, 6246–6250. [Google Scholar] [CrossRef] [PubMed]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Degradation of bisphenol A by ozonation: Rate constants, influence of inorganic anions, and by-products. Maejo Int. J. Sci. Technol. 2012, 6, 77–94. [Google Scholar]
- Kuo, C.Y.; Wu, C.H.; Lin, H.Y. Photocatalytic degradation of bisphenol A in a visible light/TiO2 system. Desalination 2010, 256, 37–42. [Google Scholar] [CrossRef]
- Lee, G.J.; Wang, J.C.; Wu, J.J. Sonochemical synthesis of Ga-doped ZnS nanoballs with enhanced photocatalytic activity for Orange II dye degradation in wastewater. Int. J. Nanotech. 2018, 15, 804–815. [Google Scholar] [CrossRef]
- Lee, G.J.; Chen, H.C.; Wu, J.J. (In, Cu) Co-doped ZnS nanoparticles for photoelectrochemical hydrogen production. Int. J. Hydrog. Energy 2019, 44, 110–117. [Google Scholar] [CrossRef]
- Lee, G.J.; Hou, Y.H.; Liu, N.; Wu, J.J. Enhanced photocatalytic hydrogen and methane evolution using chalcogenide with metal ion modification via a microwave-assisted solvothermal method. Catal. Today 2019, in press. [Google Scholar] [CrossRef]

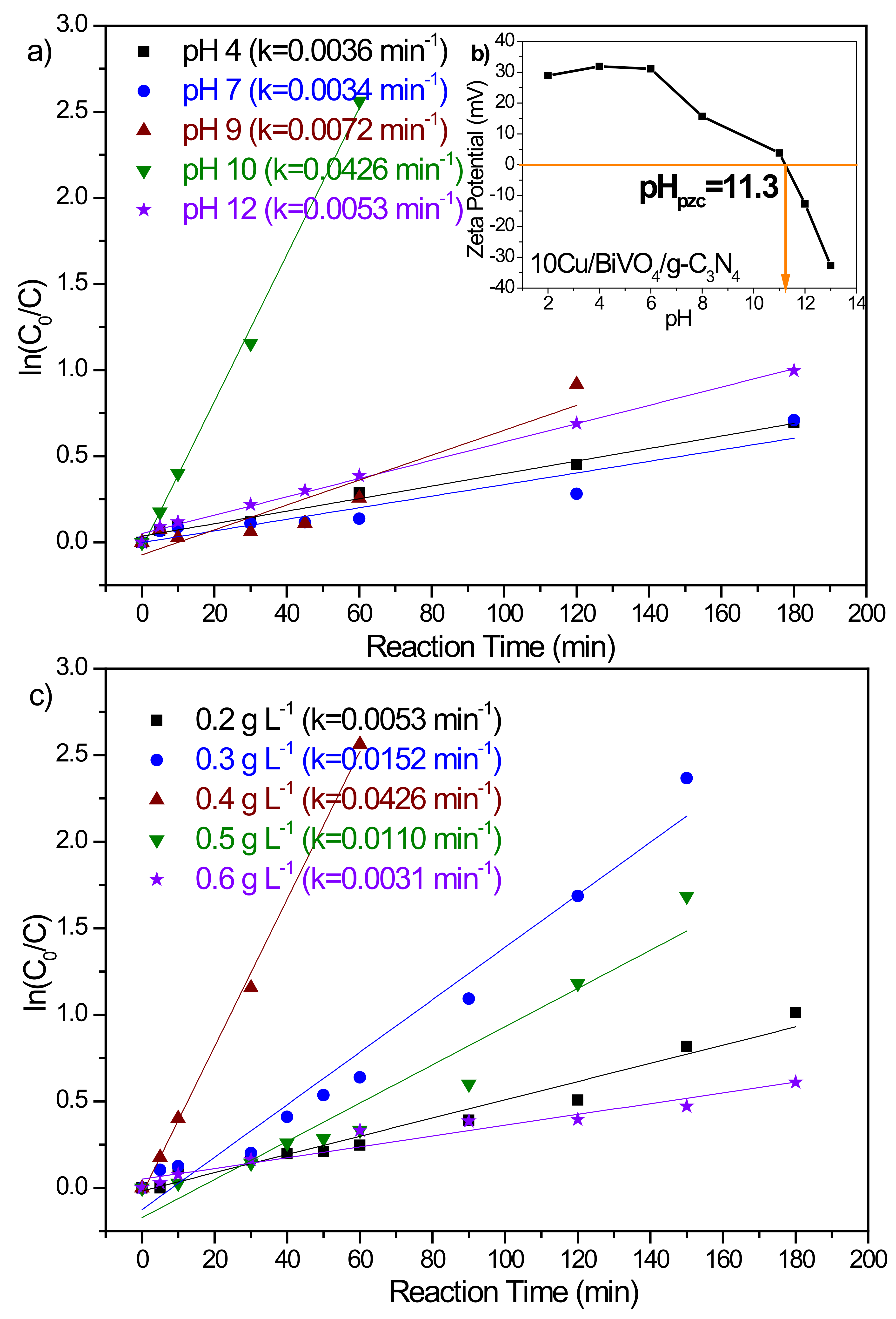
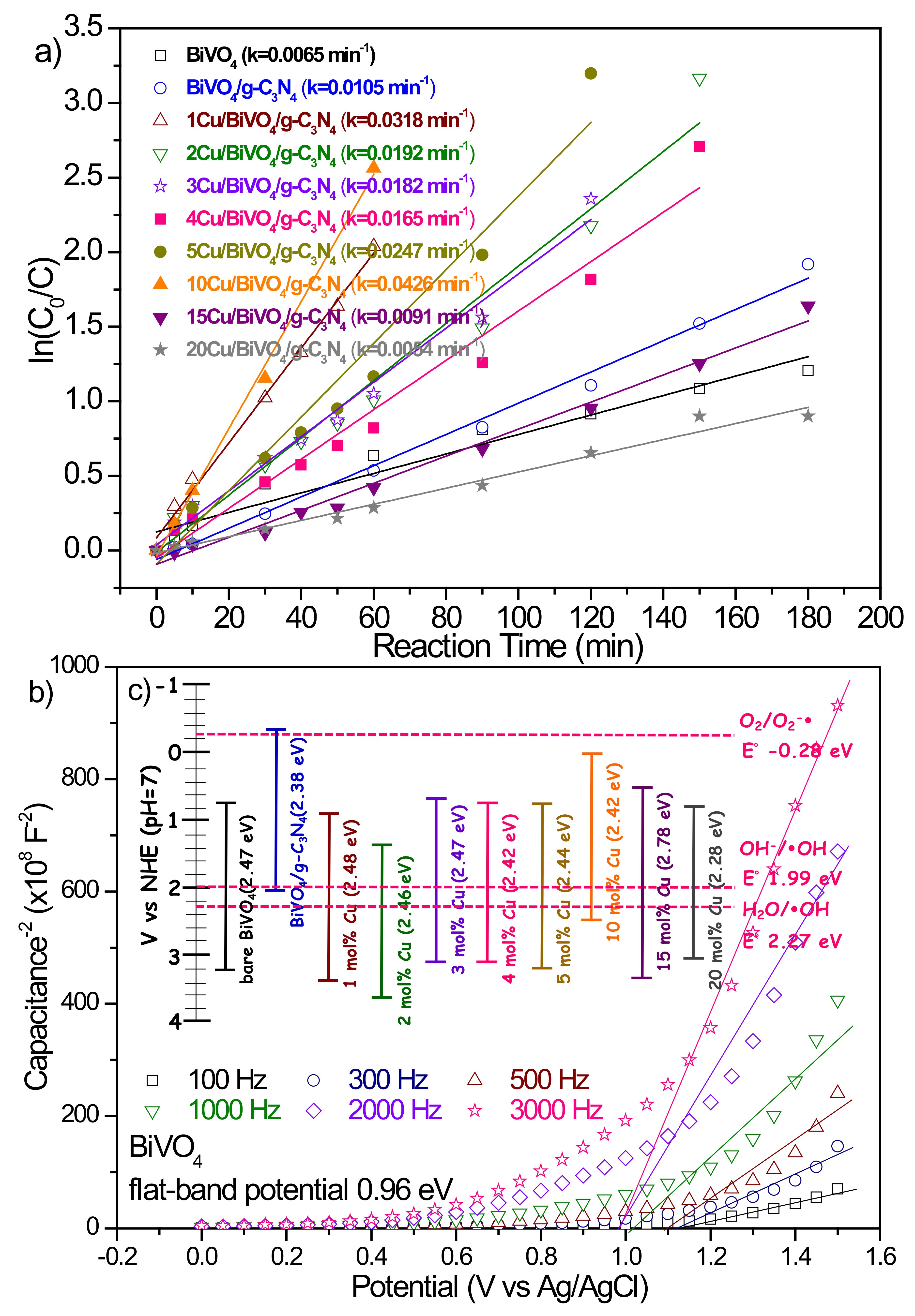
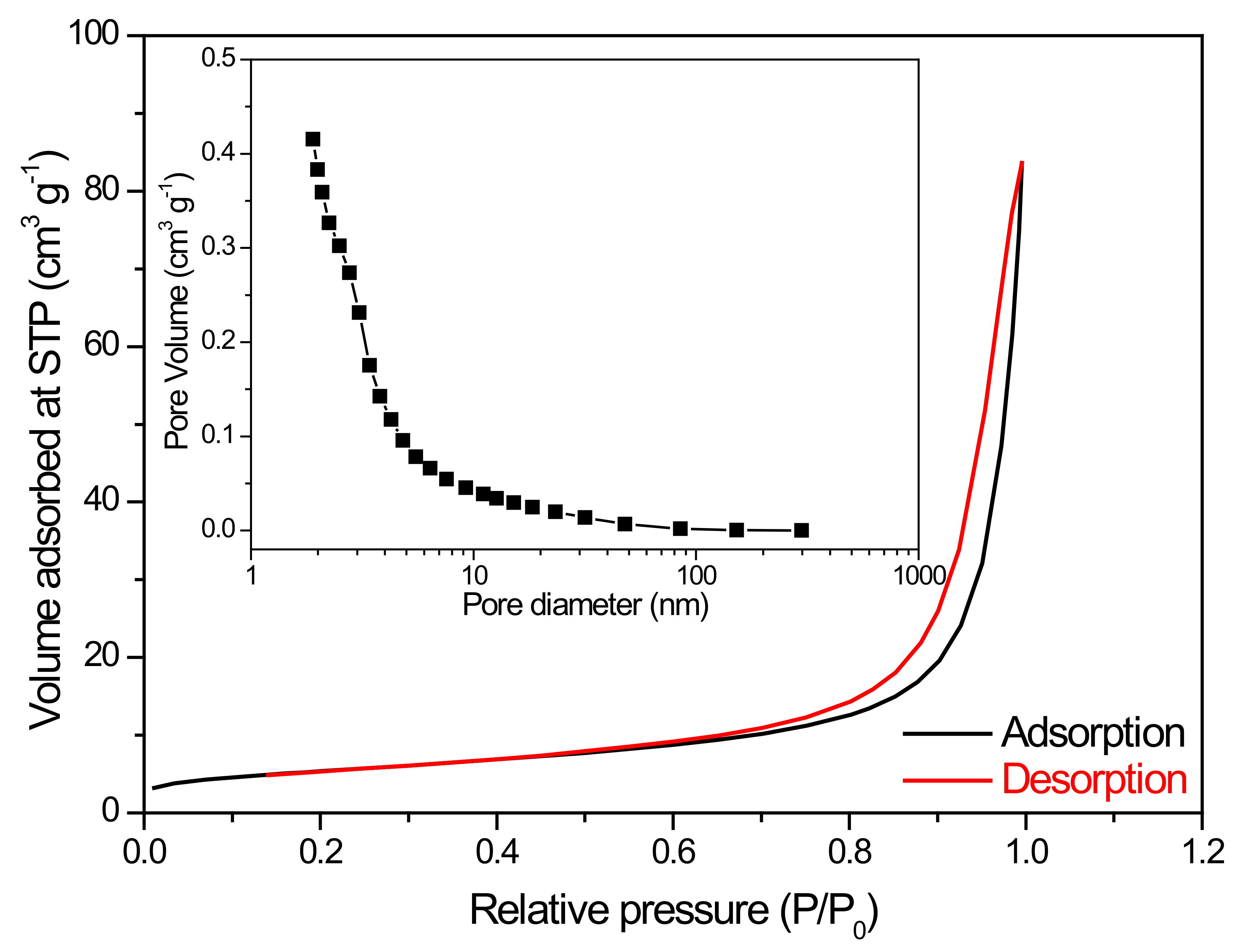
| Cu (mol%) | 0 | 1 | 2 | 3 | 4 | 5 | 10 | 15 | 20 |
|---|---|---|---|---|---|---|---|---|---|
| Surface area (m2/g) | 23.1 | 22.6 | 22.1 | 21.5 | 20.6 | 20.0 | 19.4 | 17.0 | 15.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-J.; Lee, X.-Y.; Lyu, C.; Liu, N.; Andandan, S.; Wu, J.J. Sonochemical Synthesis of Copper-doped BiVO4/g-C3N4 Nanocomposite Materials for Photocatalytic Degradation of Bisphenol A under Simulated Sunlight Irradiation. Nanomaterials 2020, 10, 498. https://doi.org/10.3390/nano10030498
Lee G-J, Lee X-Y, Lyu C, Liu N, Andandan S, Wu JJ. Sonochemical Synthesis of Copper-doped BiVO4/g-C3N4 Nanocomposite Materials for Photocatalytic Degradation of Bisphenol A under Simulated Sunlight Irradiation. Nanomaterials. 2020; 10(3):498. https://doi.org/10.3390/nano10030498
Chicago/Turabian StyleLee, Gang-Juan, Xin-Yu Lee, Cong Lyu, Na Liu, Sambandam Andandan, and Jerry J. Wu. 2020. "Sonochemical Synthesis of Copper-doped BiVO4/g-C3N4 Nanocomposite Materials for Photocatalytic Degradation of Bisphenol A under Simulated Sunlight Irradiation" Nanomaterials 10, no. 3: 498. https://doi.org/10.3390/nano10030498
APA StyleLee, G.-J., Lee, X.-Y., Lyu, C., Liu, N., Andandan, S., & Wu, J. J. (2020). Sonochemical Synthesis of Copper-doped BiVO4/g-C3N4 Nanocomposite Materials for Photocatalytic Degradation of Bisphenol A under Simulated Sunlight Irradiation. Nanomaterials, 10(3), 498. https://doi.org/10.3390/nano10030498