3D Nanoparticle Tracking Inside the Silver Nanofluid
Abstract
1. Introduction
2. Experimental Setup
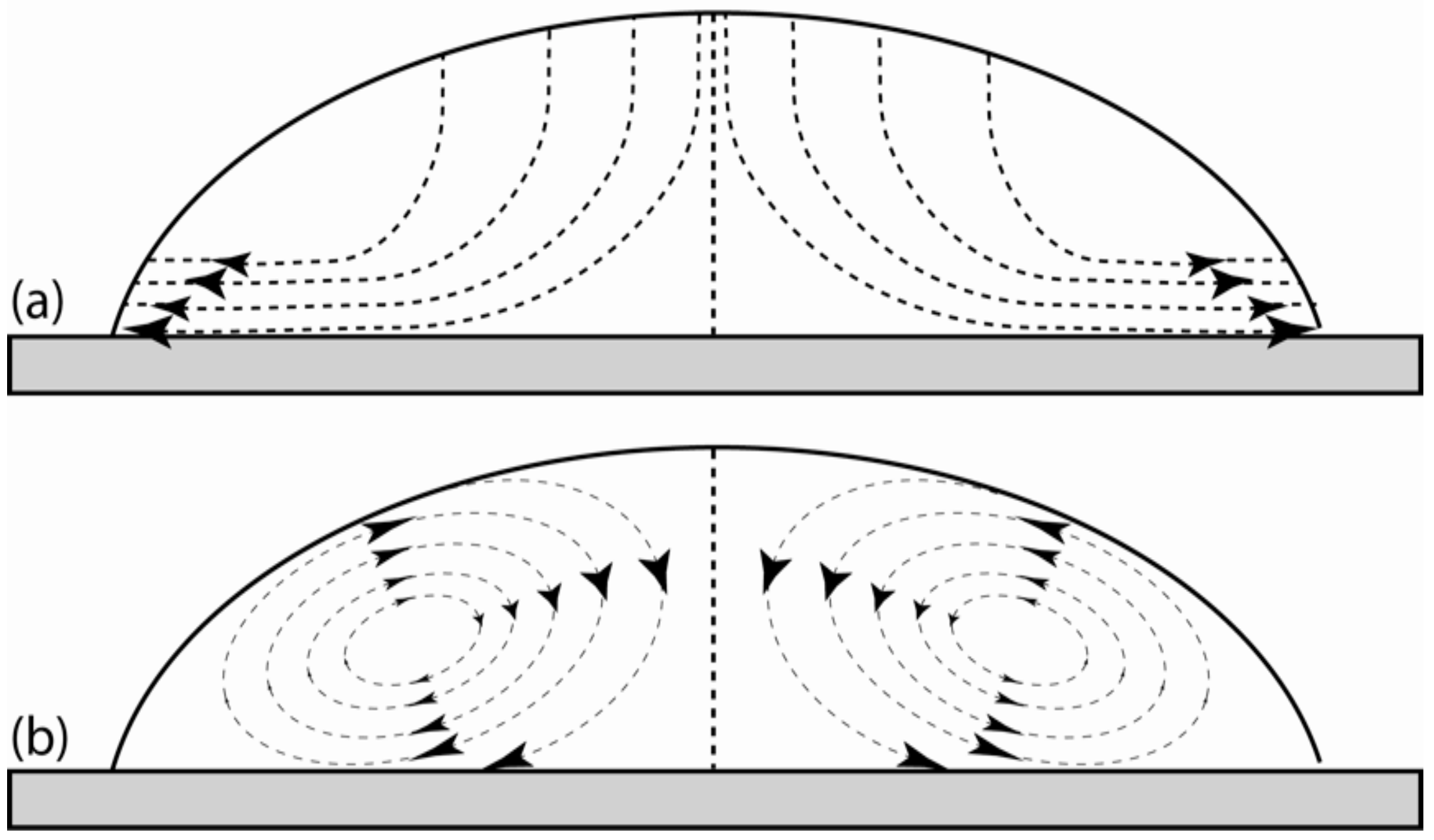
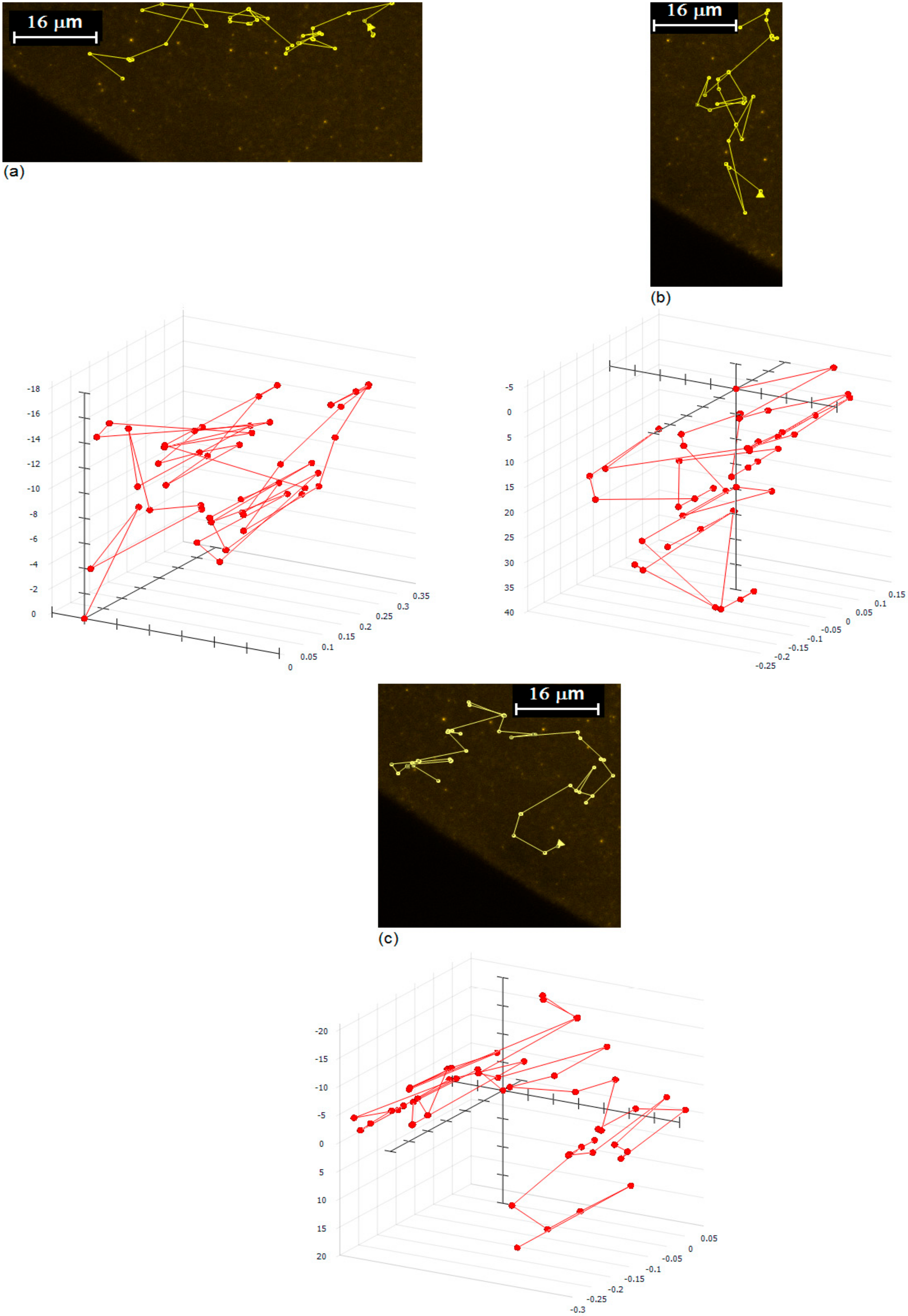
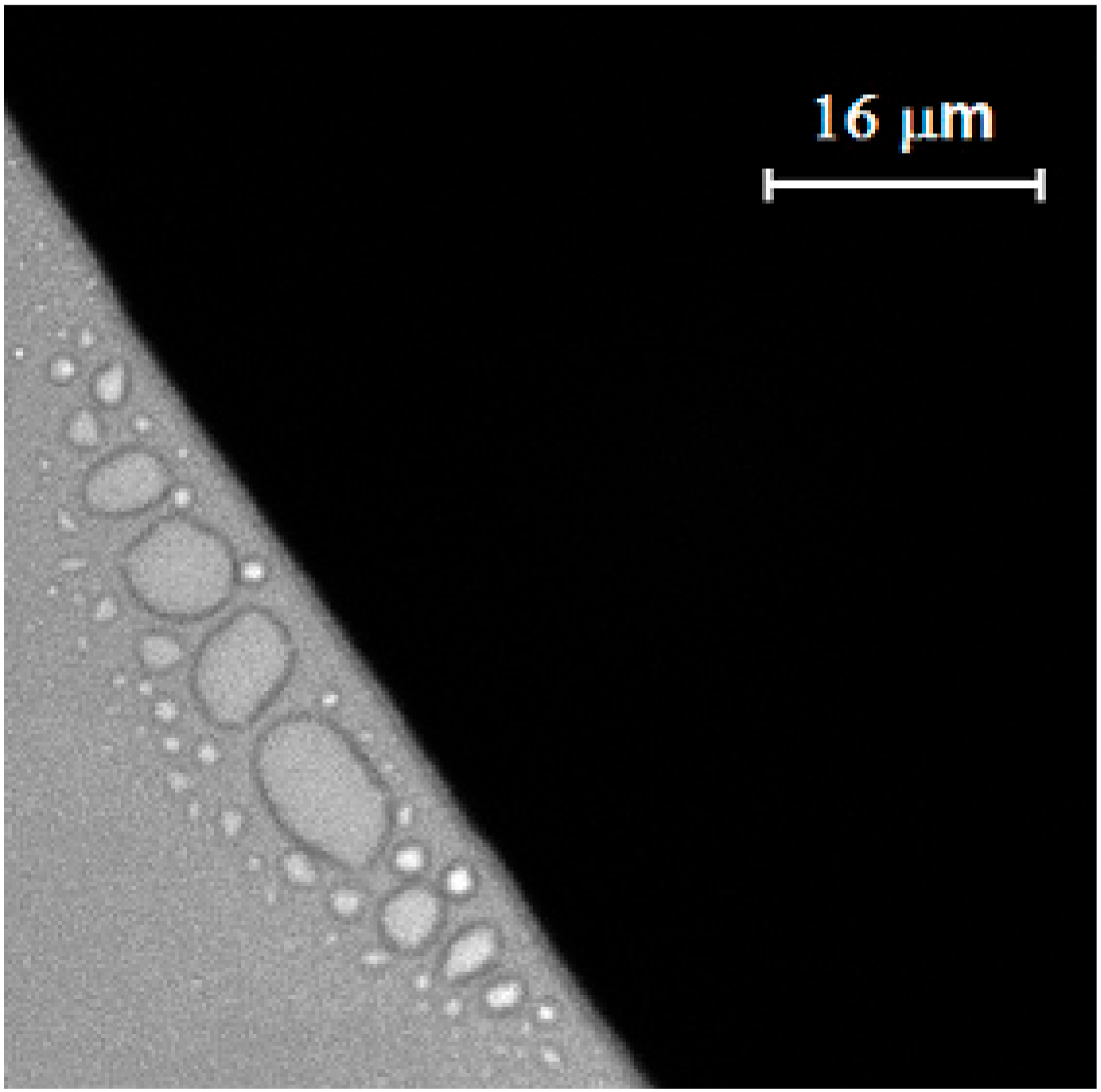
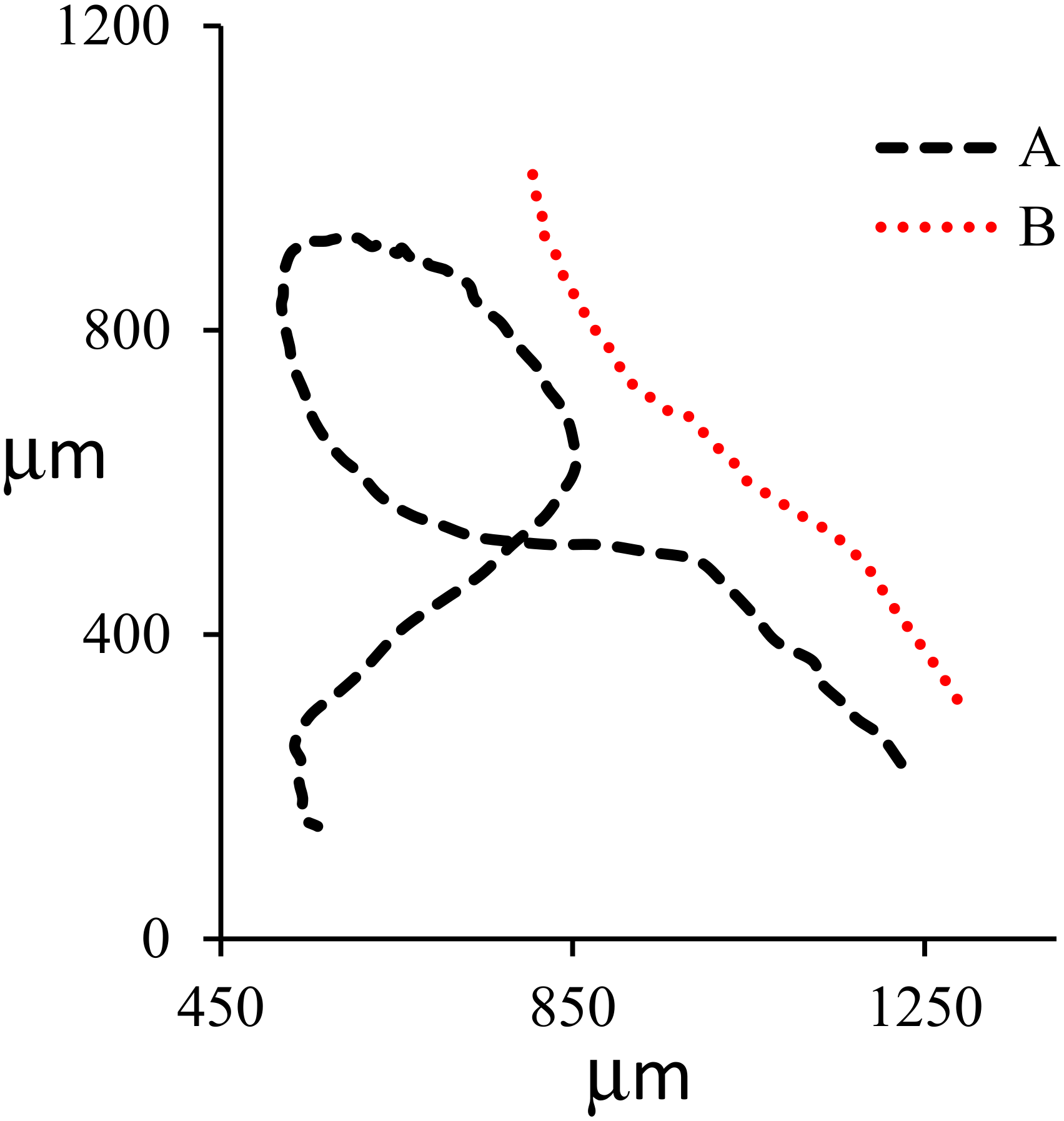
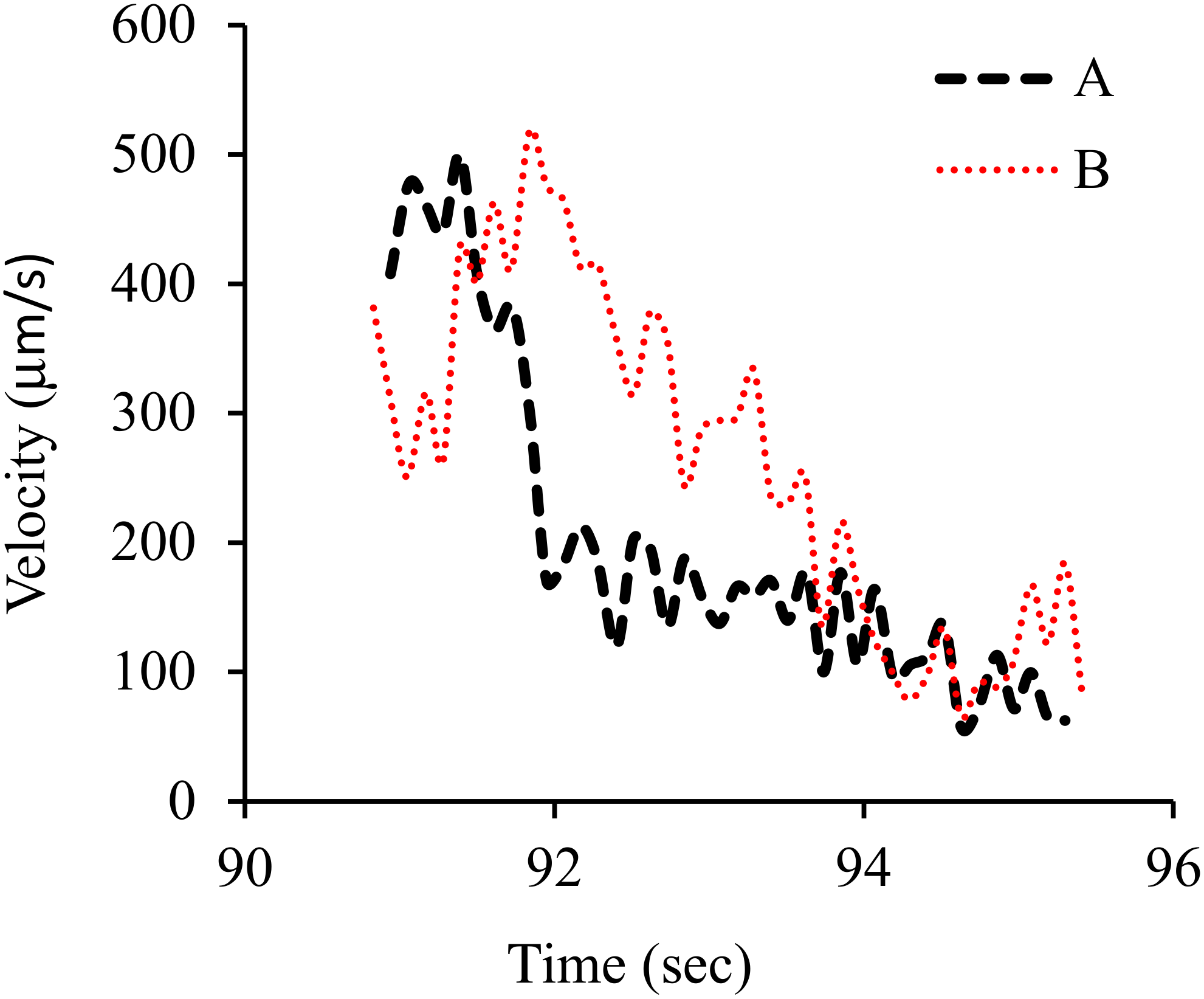
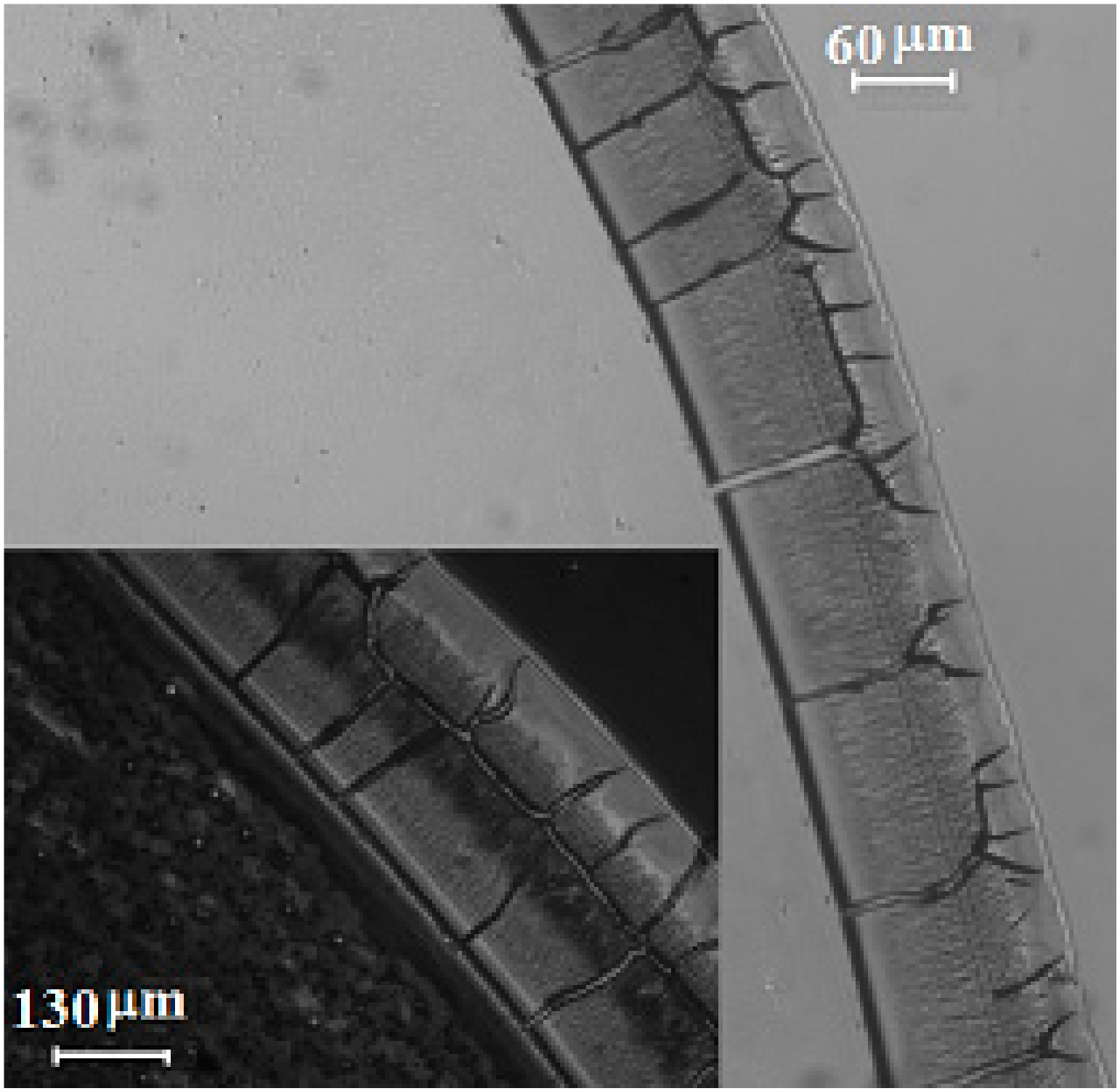
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| Greek Symbols | |
| σlg | liquid–gas surface tension (N/m) |
| σsg | solid–gas surface tension (N/m) |
| σsl | solid–liquid surface tension (N/m) |
References
- Xu, X.; Luo, J. Marangoni flow in an evaporating water droplet. Appl. Phys. Lett. 2007, 91, 124102. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Marangoni effect reverses coffee ring depositions. J. Phys. Chem. B 2006, 110, 7090–7094. [Google Scholar] [CrossRef] [PubMed]
- Steinchen, A.; Sefiane, K.J. Self-organised Marangoni motion at evaporating drops or in capillary menisci-Thermohydrodynamical model. J. Non-Equilib. Thermodyn. 2005, 30, 39–51. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.; Witten, T.A. Contact line deposits in an evaporating drop. Phys. Rev. E 2000, 62, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Larson, R.G. Analysis of the effects of Marangoni stresses on the microflow in an evaporating sessile droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Clint, S.; Rendall, C.S.; Eukel, J.A.; Wang, J.Y.L.; Behabtu, N.; Cary, L.; Pint, C.L.; Liu, T.Y.; Orbaek, A.W.; et al. Overcoming the coffee-stain effect by compositional Marangoni-flow-assisted drop-drying. J. Phys. Chem. B 2012, 116, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Trantum, J.R.; Eagleton, Z.E.; Patil, C.A.; Tucker-Schwartz, J.M.; Baglia, M.L.; Skala, M.C.; Haselton, F.R. Cross-sectional tracking of particle motion in evaporating drops: Flow fields and interfacial accumulation. Langmuir 2013, 29, 6221–6231. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Fang, X.; Attinger, D. Pattern formation during the evaporation of a colloidal nanoliter drop: A numerical and experimental study. New J. Phys. 2009, 11, 1–33. [Google Scholar] [CrossRef]
- Weon, B.M.; Je, J.H. Fingering inside the coffee ring. Phys. Rev. E 2013, 87, 013003. [Google Scholar]
- Li, H.; Fowler, N.; Struck, C.; Sivasankar, S. Flow triggered by instabilities at the contact line of a drop containing nanoparticles. Soft Matter 2011, 7, 5116–5119. [Google Scholar] [CrossRef]
- Shuja, S.Z. Laser heating of tungsten carbide-coated steel surface: Influence of coating thickness on temperature field and melt depth. Heat Transfer Eng. 2014, 35, 110–121. [Google Scholar] [CrossRef]
- Radulescu, C.; Robinson, A.J. Mixed convective heat transfer due to forced and thermocapillary flow around bubbles in a miniature channel: A 2D numerical study. Heat Transfer Eng. 2010, 31, 335–343. [Google Scholar] [CrossRef]
- Radulescu, C. Mixed thermocapillary and forced convection heat transfer around a hemispherical bubble in a miniature channel: A 3D numerical study. Heat Transfer Eng. 2012, 33, 596–608. [Google Scholar] [CrossRef]
- O’Shaughnessy, S.M.; Robinson, A.J. The influence of the magnitude of gravitational acceleration on Marangoni convection about an isolated bubble under a heated wall. Heat Transfer Eng. 2009, 30, 1096–1107. [Google Scholar] [CrossRef][Green Version]
- Qu, X.; Qiu, H. Thermal bubble dynamics under the effects of an acoustic field. Heat Transfer Eng. 2011, 32, 636–647. [Google Scholar] [CrossRef]
- Takeuchi, H.; Motosuke, M.; Honami, S. Noncontact bubble manipulation in microchannel by using photothermal Marangoni effect. Heat Transfer Eng. 2012, 33, 234–244. [Google Scholar] [CrossRef]
- Vafaei, S.; Wen, D.; Borca-Tasciuc, T. Nanofluids surface wettability through asymptotic contact angle. Langmuir 2011, 27, 2211–2218. [Google Scholar] [CrossRef]
- Vafaei, S.; Purkayastha, A.; Jain, A.; Ramanath, G.; Borca-Tasciuc, T. The effect of nanoparticles on the liquid-gas surface tension of Bi2Te3 nanofluids. Nanotechnology 2009, 20, 185702–185708. [Google Scholar] [CrossRef]
- Vafaei, S.; Podowski, M.Z. Analysis of the relationship between liquid droplet size and contact angle. Adv. Colloid Interface Sci. 2005, 113, 133–146. [Google Scholar] [CrossRef]
- Ally, J.; Kappl, M.; Butt, H.J.; Amirfazli, A. Detachment force of particles from air-liquid interfaces of films and bubbles. Langmuir 2010, 26, 18135–18143. [Google Scholar] [CrossRef] [PubMed]
- Sefiane, K.; Skilling, J.; MacGillivray, J. Contact line motion and dynamic wetting of nanofluid solutions. Adv. Colloid Interface Sci. 2008, 138, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Bang, I.C.; Buongiorno, J.; Hu, L.H. Surface wettability change during pool boiling of nanofluids and its effect on critical heat flux. Int. J. Heat Mass Transfer 2007, 50, 4105–4116. [Google Scholar] [CrossRef]
- Vafaei, S.; Tuck, C.; Wildman, R.; Ashcroft, I. Spreading of the nanofluid triple line in ink jet printed electronics tracks. Addit. Manuf. 2016, 11, 77–84. [Google Scholar] [CrossRef]
- Ogino, K.; Tsubaki, N.; Abe, M. Solution properties of mixed surfactant system: VI. The effect of oxyethylene groups in nonionic surfactant on surface tension of anionic-nonionic surfactant systems. J. Colloid Interface Sci. 1985, 107, 509–513. [Google Scholar] [CrossRef]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafaei, S. 3D Nanoparticle Tracking Inside the Silver Nanofluid. Nanomaterials 2020, 10, 397. https://doi.org/10.3390/nano10020397
Vafaei S. 3D Nanoparticle Tracking Inside the Silver Nanofluid. Nanomaterials. 2020; 10(2):397. https://doi.org/10.3390/nano10020397
Chicago/Turabian StyleVafaei, Saeid. 2020. "3D Nanoparticle Tracking Inside the Silver Nanofluid" Nanomaterials 10, no. 2: 397. https://doi.org/10.3390/nano10020397
APA StyleVafaei, S. (2020). 3D Nanoparticle Tracking Inside the Silver Nanofluid. Nanomaterials, 10(2), 397. https://doi.org/10.3390/nano10020397



