Evaluation of the Oxidative Stress Response of Aging Yeast Cells in Response to Internalization of Fluorescent Nanodiamond Biosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanodiamonds
2.2. Yeast Strain and Cell Handling
2.3. Sample Conditions
2.4. Metabolic Activity
2.5. qPCR of Oxidative Stress Genes
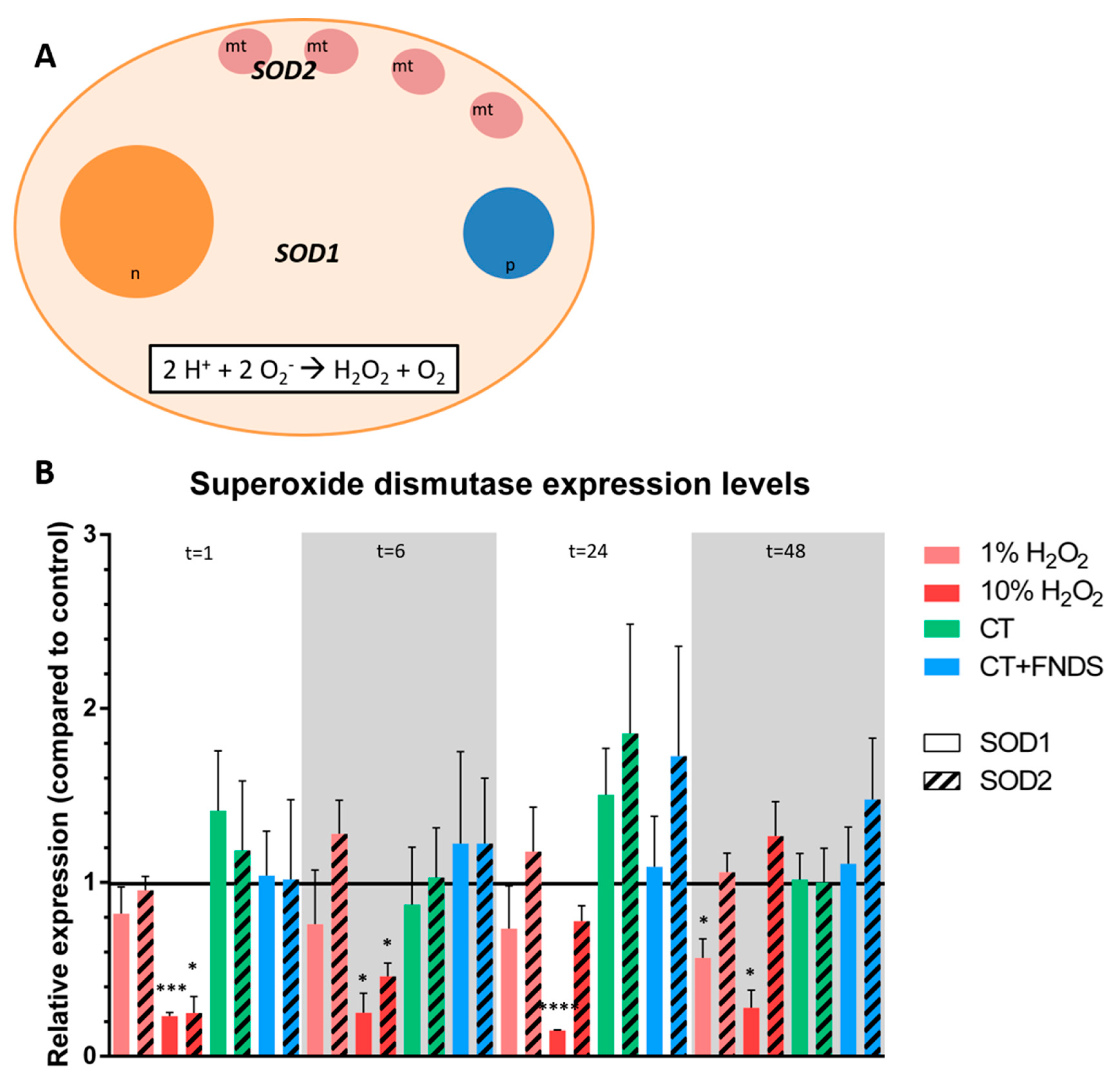
- Enzymatic response: catalase (CTT1, CTA1), superoxide dismutase (SOD1, SOD2) and thioredoxin.
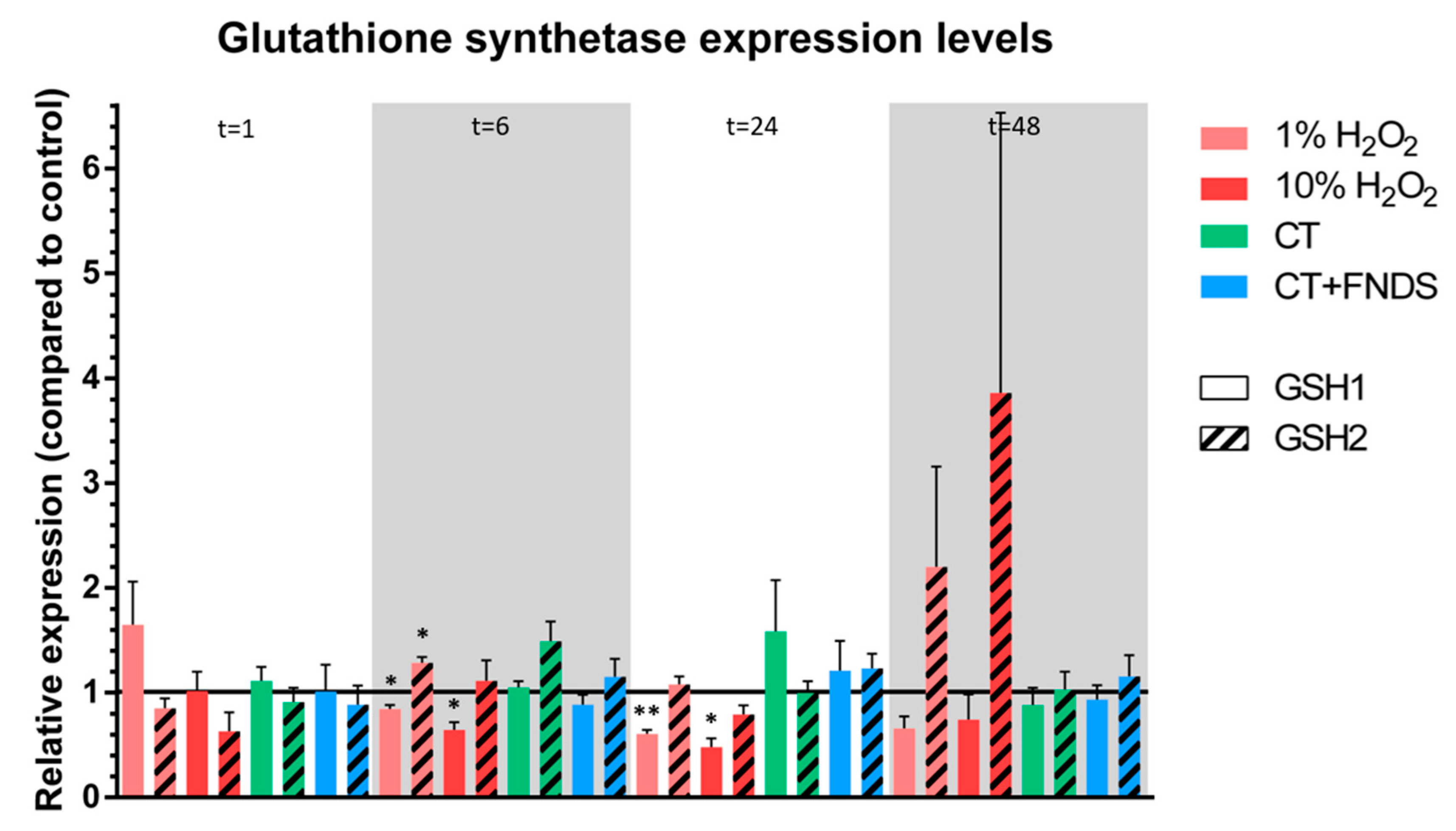
- Non-enzymatic antioxidant scavenger that was tested: glutathione (GSH1, GSH2).
- Three reference genes were selected: ALG9, TAF10 and TFC1 [21].
2.6. Fluorescent Marker: DCDFA
2.7. Statistical Data Analysis
3. Results
3.1. No FND-Induced Reduction in Metabolic Activity
3.2. Transient FND-Induced Changes in Oxidative Stress Transcriptome
3.3. Unaltered Total Free Radical Activity after Diamond Internalization
4. Discussion
4.1. Diamond Internalization Inaging Yeast Cells Does Not Provoke a Prolonged Oxidative Stress Response
4.2. The Many Faces of Cell Viability and the Challenges in Measuring Oxidative Stress Levels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef]
- Harman, D. The Free Radical Theory of Aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef]
- Gruber, A.; Dräbenstedt, A.; Tietz, C.; Fleury, L.; Wrachtrup, J.; Von Borczyskowski, C. Scanning confocal optical microscopy and magnetic resonance on single defect centers. Science 1997, 276, 2012–2014. [Google Scholar] [CrossRef]
- Grinolds, M.S.; Hong, S.; Maletinsky, P.; Luan, L.; Lukin, M.D.; Walsworth, R.L.; Yacoby, A. Nanoscale magnetic imaging of a single electron spin under ambient conditions. Nat. Phys. 2013, 9, 215–219. [Google Scholar] [CrossRef]
- Mamin, H.J.; Kim, M.; Sherwood, M.H.; Rettner, C.T.; Ohno, K.; Awschalom, D.D.; Rugar, D. Nanoscale nuclear magnetic resonance with a nitrogen-vacancy spin sensor. Science 2013, 339, 557–560. [Google Scholar] [CrossRef]
- Rondin, L.; Tetienne, J.P.; Rohart, S.; Thiaville, A.; Hingant, T.; Spinicelli, P.; Roch, J.F.; Jacques, V. Stray-field imaging of magnetic vortices with a single diamond spin. Nat. Commun. 2013, 4, 1–5. [Google Scholar] [CrossRef]
- Pelliccione, M.; Jenkins, A.; Ovartchaiyapong, P.; Reetz, C.; Emmanouilidou, E.; Ni, N.; Bleszynski Jayich, A.C. Scanned probe imaging of nanoscale magnetism at cryogenic temperatures with a single-spin quantum sensor. Nat. Nanotechnol. 2016, 11, 700–705. [Google Scholar] [CrossRef]
- Rondin, L.; Tetienne, J.P.; Spinicelli, P.; Dal Savio, C.; Karrai, K.; Dantelle, G.; Thiaville, A.; Rohart, S.; Roch, J.F.; Jacques, V. Nanoscale magnetic field mapping with a single spin scanning probe magnetometer. Appl. Phys. Lett. 2012, 100, 153118. [Google Scholar] [CrossRef]
- Tetienne, J.P.; Lombard, A.; Simpson, D.A.; Ritchie, C.; Lu, J.; Mulvaney, P.; Hollenberg, L.C.L. Scanning Nanospin Ensemble Microscope for Nanoscale Magnetic and Thermal Imaging. Nano Lett. 2016, 16, 326–333. [Google Scholar] [CrossRef]
- Grinolds, M.S.; Maletinsky, P.; Hong, S.; Lukin, M.D.; Walsworth, R.L.; Yacoby, A. Quantum control of proximal spins using nanoscale magnetic resonance imaging. Nat. Phys. 2011, 7, 687–692. [Google Scholar] [CrossRef]
- Jamnik, P.; Raspor, P. Methods for monitoring oxidative stress response in yeasts. J. Biochem. Mol. Toxicol. 2005, 19, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Drakulic, T.; Temple, M.D.; Guido, R.; Jarolim, S.; Breitenbach, M.; Attfield, P.V.; Dawes, I.W. Involvement of oxidative stress response genes in redox homeostasis, the level of reactive oxygen species, and ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 2005, 5, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Van Der Laan, K.J.; Naulleau, J.; Damle, V.G.; Sigaeva, A.; Jamot, N.; Perona-Martinez, F.P.; Chipaux, M.; Schirhagl, R. Toward Using Fluorescent Nanodiamonds to Study Chronological Aging in Saccharomyces cerevisiae. Anal. Chem. 2018, 90, 13506–13513. [Google Scholar] [CrossRef] [PubMed]
- Hemelaar, S.R.; Saspaanithy, B.; L’Hommelet, S.R.M.; Perona Martinez, F.P.; van der Laan, K.J.; Schirhagl, R. The response of HeLa cells to fluorescent nanodiamond uptake. Sensors 2018, 18, 355. [Google Scholar] [CrossRef] [PubMed]
- Shenderova, O.A.; Shames, A.I.; Nunn, N.A.; Torelli, M.D.; Vlasov, I.; Zaitsev, A. Review Article: Synthesis, properties, and applications of fluorescent diamond particles. J. Vac. Sci. Technol. B 2019, 37, 030802. [Google Scholar] [CrossRef] [PubMed]
- Hemelaar, S.R.; van der Laan, K.J.; Hinterding, S.R.; Koot, M.V.; Ellermann, E.; Perona-Martinez, F.P.; Roig, D.; Hommelet, S.; Novarina, D.; Takahashi, H.; et al. Generally Applicable Transformation Protocols for Fluorescent Nanodiamond Internalization into Cells. Sci. Rep. 2017, 7, 5862. [Google Scholar] [CrossRef]
- Nikko, E.; Pelham, H.R.B. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic 2009, 10, 1856–1867. [Google Scholar] [CrossRef]
- Teparić, R.; Stuparević, I.; Mrša, V. Increased mortality of Saccharomyces cerevisiae cell wall protein mutants. Microbiology 2004, 150, 3145–3150. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.; Hiltunen, K.; Chen, Z. Controllable Drug Release System in Living Cells Triggered by Enzyme-Substrate Recognition. ACS Appl. Mater. Interfaces 2015, 7, 26811–26818. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Balkis, M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 2003, 41, 506–508. [Google Scholar] [CrossRef]
- Teste, M.A.; Duquenne, M.; François, J.M.; Parrou, J.L. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol. Biol. 2009, 10, 99. [Google Scholar] [CrossRef]
- Pérez-Gallardo, R.V.; Briones, L.S.; Díaz-Pérez, A.L.; Gutiérrez, S.; Rodríguez-Zavala, J.S.; Campos-García, J. Reactive oxygen species production induced by ethanol in Saccharomyces cerevisiae increases because of a dysfunctional mitochondrial iron-sulfur cluster assembly system. FEMS Yeast Res. 2013, 13, 804–819. [Google Scholar] [CrossRef]
- Herker, E.; Jungwirth, H.; Lehmann, K.A.; Maldener, C.; Fröhlich, K.U.; Wissing, S.; Büttner, S.; Fehr, M.; Sigrist, S.; Madeo, F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004, 164, 501–507. [Google Scholar] [CrossRef]
- Fabrizio, P.; Longo, V.D. Chronological aging-induced apoptosis in yeast. Biochim. Biophys. - Acta Mol. Cell Res. 2008, 1783, 1280–1285. [Google Scholar] [CrossRef]
- Fabrizio, P.; Longo, V.D. The chronological life span of Saccharomyces cerevisiae. Methods Mol. Biol. 2007, 371, 89–95. [Google Scholar]
- Chipaux, M.; van der Laan, K.J.; Hemelaar, S.R.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds and their applications in cells. Small 2018, 1704263, 1–25. [Google Scholar] [CrossRef]
- Dworak, N.; Wnuk, M.; Zebrowski, J.; Bartosz, G.; Lewinska, A. Genotoxic and mutagenic activity of diamond nanoparticles in human peripheral lymphocytes in vitro. Carbon 2014, 68, 763–776. [Google Scholar] [CrossRef]
- Schrand, A.M.; Huang, H.; Carlson, C.; Schlager, J.J.; Osawa, E.; Hussain, S.M.; Dai, L. Are diamond nanoparticles cytotoxic? J. Phys. Chem. B 2007, 111, 2–7. [Google Scholar] [CrossRef]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J.; Vina, J.; et al. Even free radicals should follow some rules: A Guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; II, J.R.; Ischiropoulos, H. measuring reactive oxygen and nitrogen species with fluorescent probes challenges limitations 2012 Kalyanaraman Darley Davies Dennery Forman Grisham Mann Moore Roberts Ischiroinoulos. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]
- Sigaeva, A.; Ong, Y.; Damle, V.G.; Morita, A.; van der Laan, K.J.; Schirhagl, R. Optical Detection of Intracellular Quantities Using Nanoscale Technologies. Acc. Chem. Res. 2019, 52, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Laan, K.J.; Morita, A.; Perona-Martinez, F.P.; Schirhagl, R. Evaluation of the Oxidative Stress Response of Aging Yeast Cells in Response to Internalization of Fluorescent Nanodiamond Biosensors. Nanomaterials 2020, 10, 372. https://doi.org/10.3390/nano10020372
van der Laan KJ, Morita A, Perona-Martinez FP, Schirhagl R. Evaluation of the Oxidative Stress Response of Aging Yeast Cells in Response to Internalization of Fluorescent Nanodiamond Biosensors. Nanomaterials. 2020; 10(2):372. https://doi.org/10.3390/nano10020372
Chicago/Turabian Stylevan der Laan, Kiran J., Aryan Morita, Felipe P. Perona-Martinez, and Romana Schirhagl. 2020. "Evaluation of the Oxidative Stress Response of Aging Yeast Cells in Response to Internalization of Fluorescent Nanodiamond Biosensors" Nanomaterials 10, no. 2: 372. https://doi.org/10.3390/nano10020372
APA Stylevan der Laan, K. J., Morita, A., Perona-Martinez, F. P., & Schirhagl, R. (2020). Evaluation of the Oxidative Stress Response of Aging Yeast Cells in Response to Internalization of Fluorescent Nanodiamond Biosensors. Nanomaterials, 10(2), 372. https://doi.org/10.3390/nano10020372