Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment
Abstract
1. Introduction
2. Materials and Methods
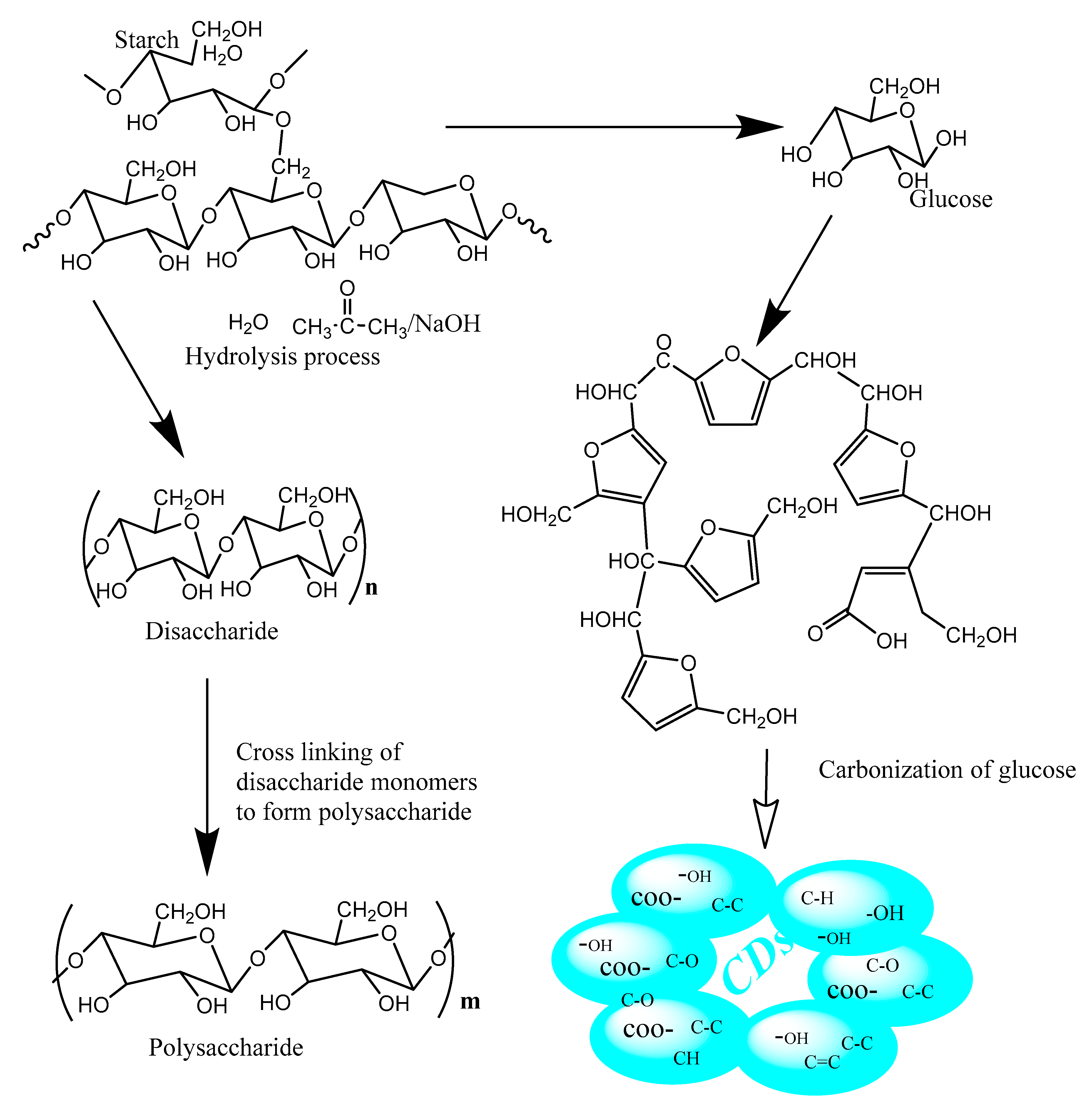
2.1. Synthesis of Carbon Dots
2.2. Carbon Dots Characterization
2.3. Adsorption Studies
2.4. Adsorption Isotherm
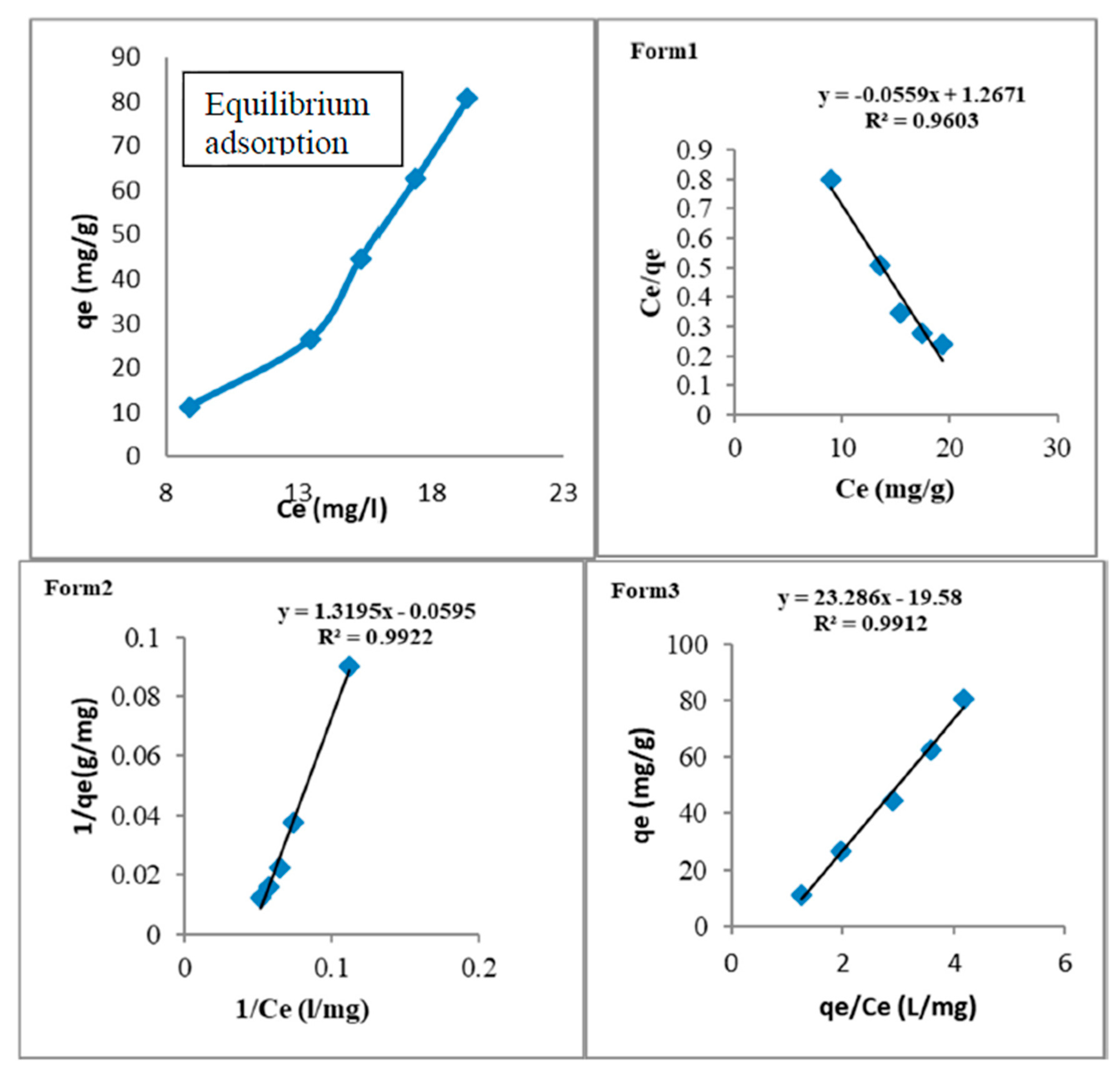
2.4.1. Langmuir Isotherm, Adsorption Equation, and Limitations
- qe = adsorption capacity (mg/g)
- qmax = maximum monolayer adsorption capacity of the adsorbent (mg/g)
- Ce = equilibrium concentration of the adsorbate (mg/L)
- KL = Langmuir adsorption constant related to free energy adsorption (L/mg)
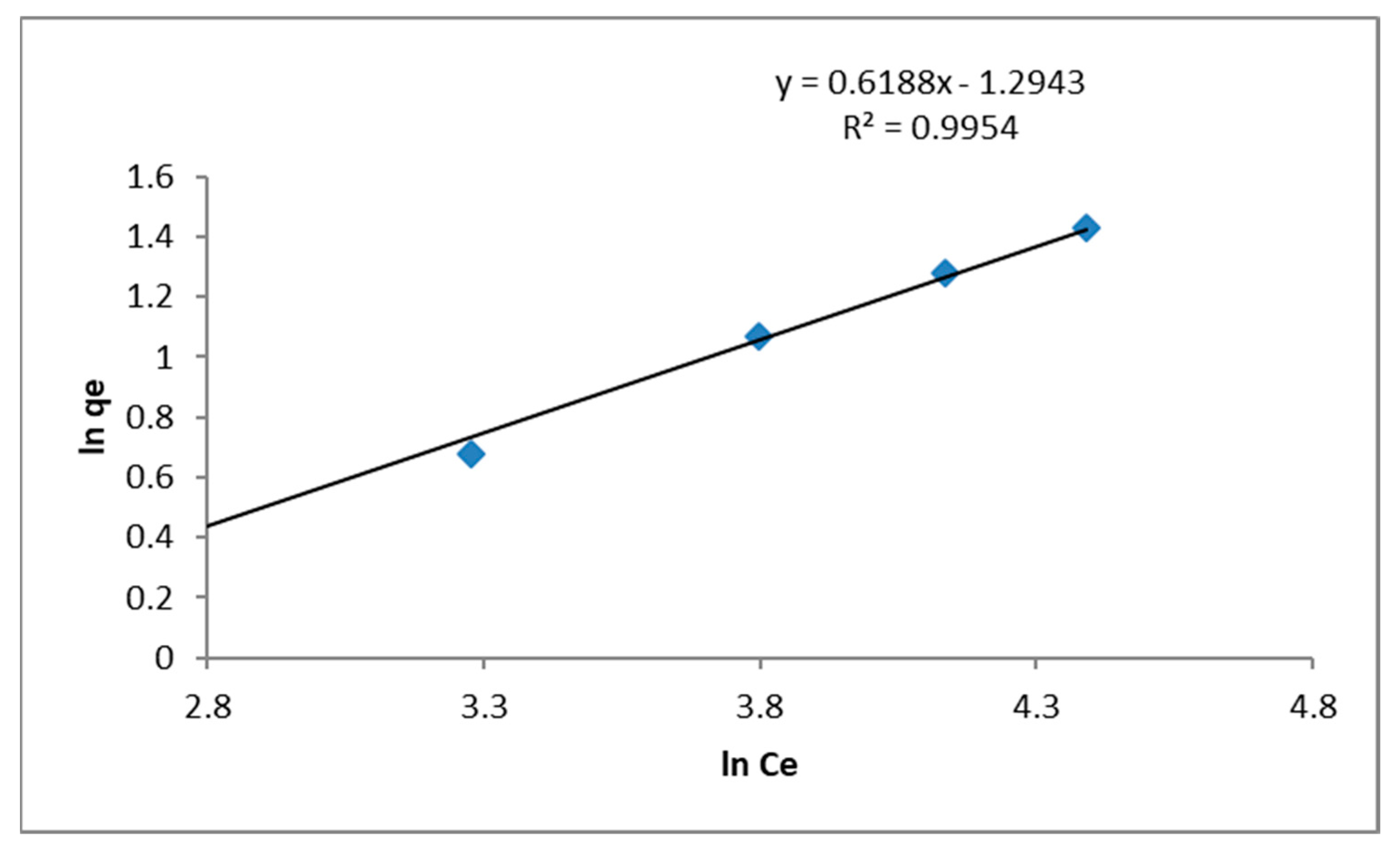
2.4.2. Freundlich Isotherm
- qe = adsorption capacity (mg/g)
- Kf = Freundlich isotherm constant (mg/g)
- Ce = equilibrium concentration of adsorbate (mg/L)
- 1/n = adsorption capacity (L/mg)
3. Results
3.1. Characterization of Carbon Dots (CDs)
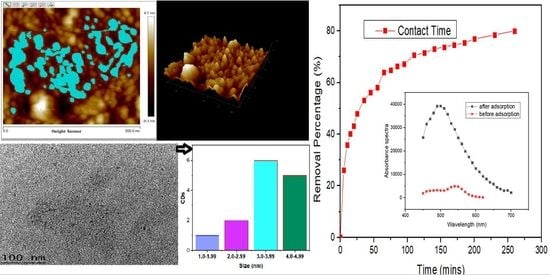
3.1.1. Atomic Force Microscopy (AFM) and High Resolution Transmission Electron Microscopy (HrTem) of Carbon Dots (CDs)
3.1.2. Zeta Potential of Carbon Dots
3.1.3. Energy Dispersive Spectroscopy (EDS)
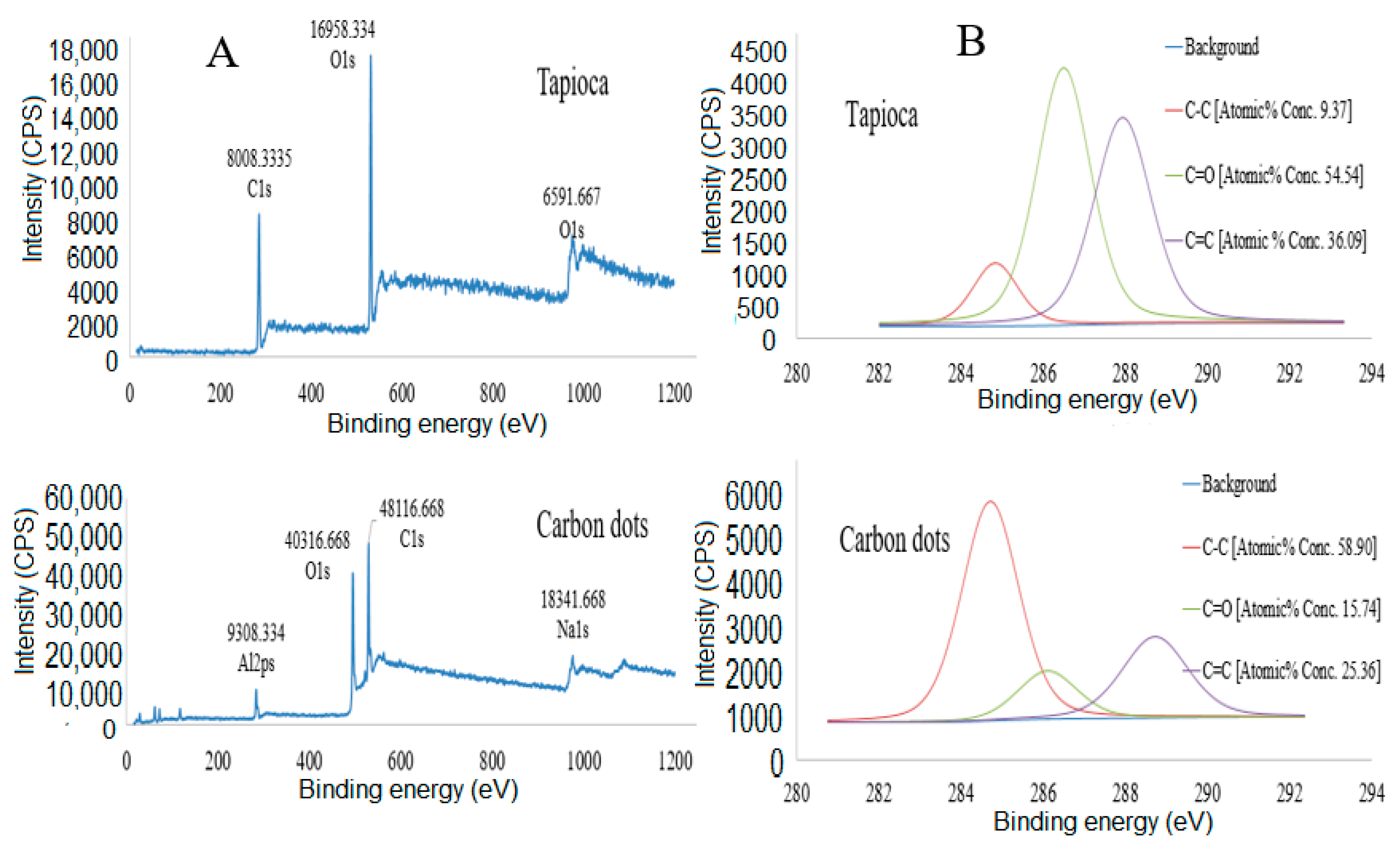
3.1.4. X-ray Photoelectron Spectroscopy (XPS)
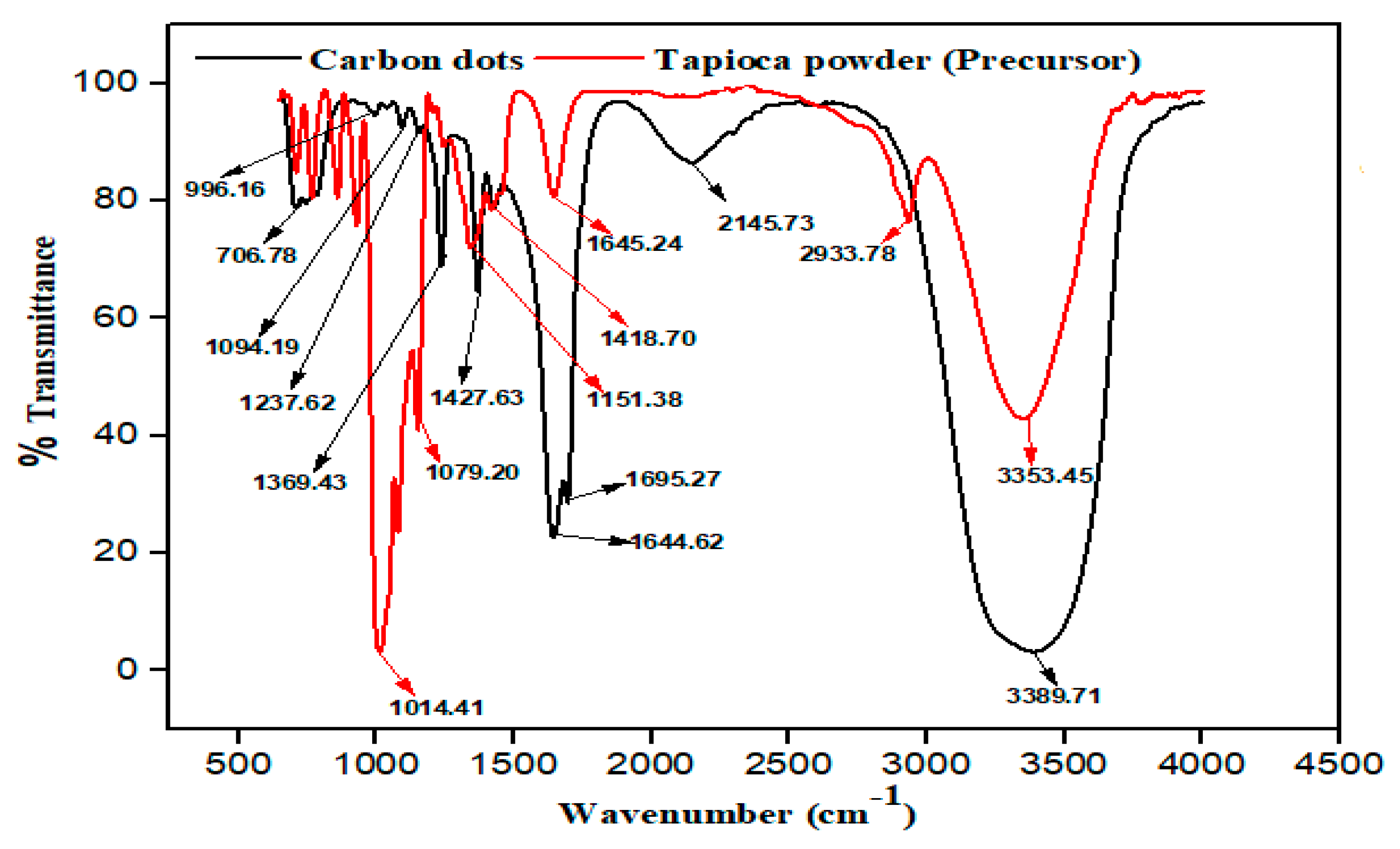
3.1.5. Fourier Transform Infrared (FTIR) Analysis
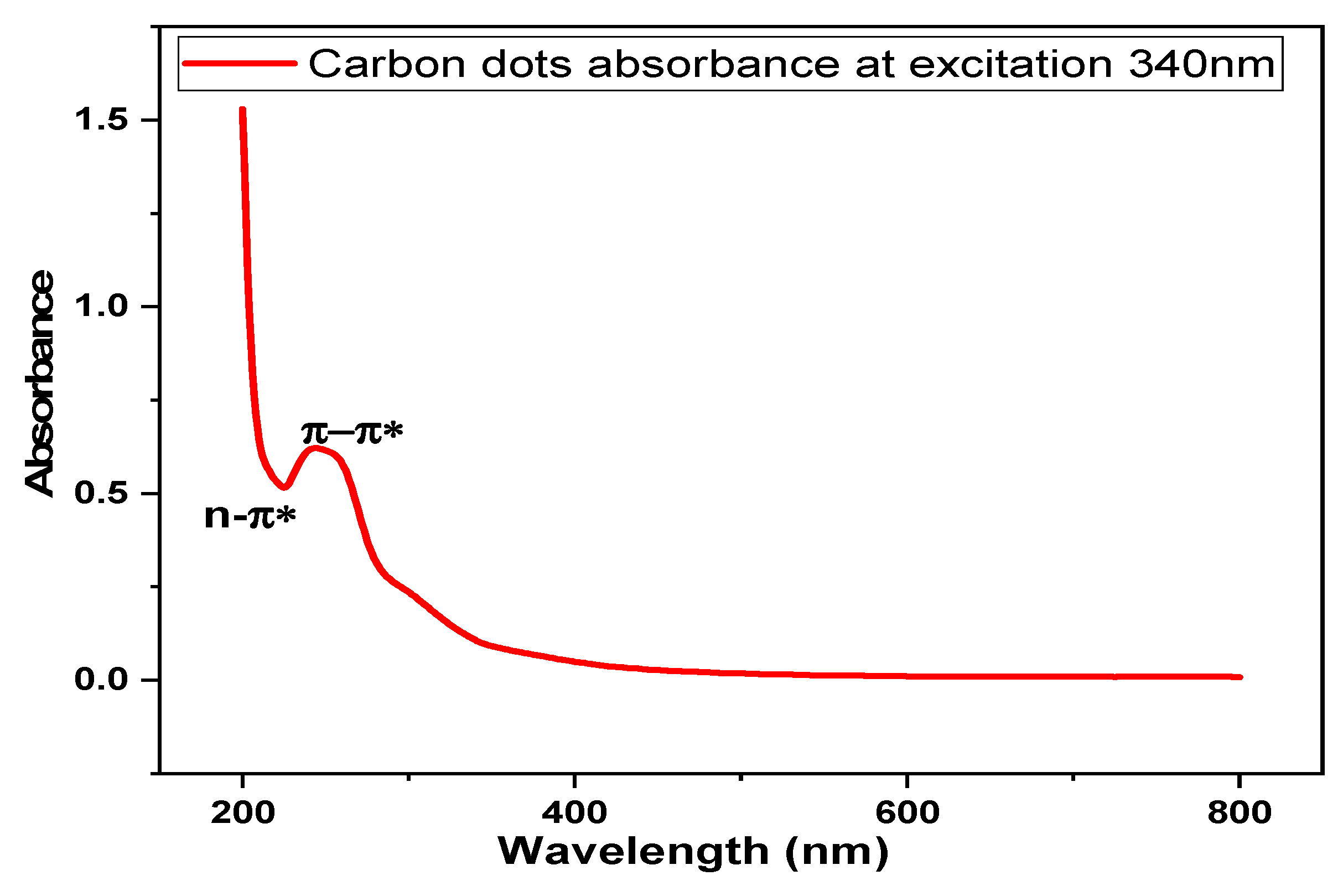
3.2. Optical Properties of Carbon Dots (CDs)
3.3. Adsorption of Lead Using Carbon Dots (CDs)
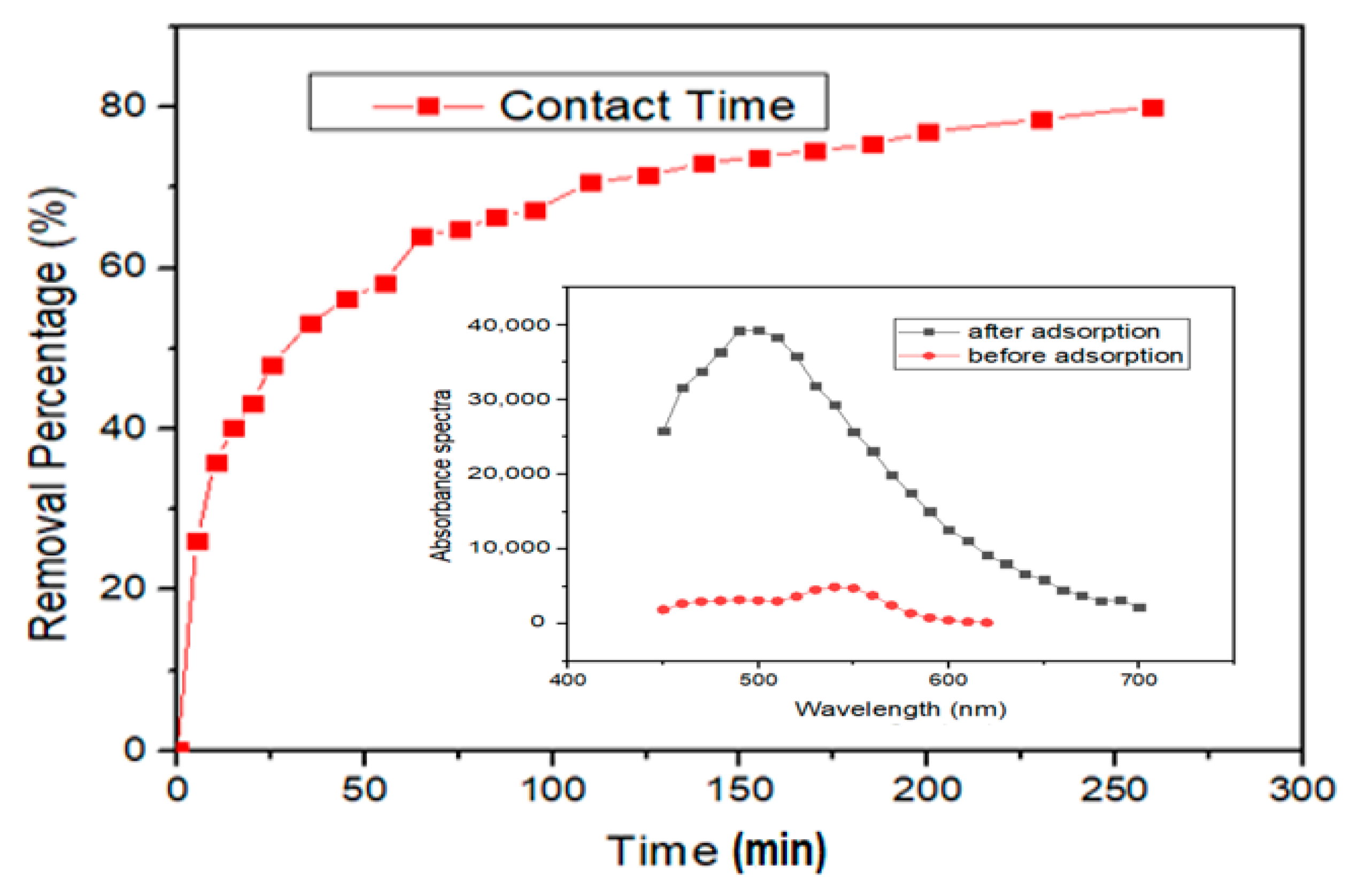
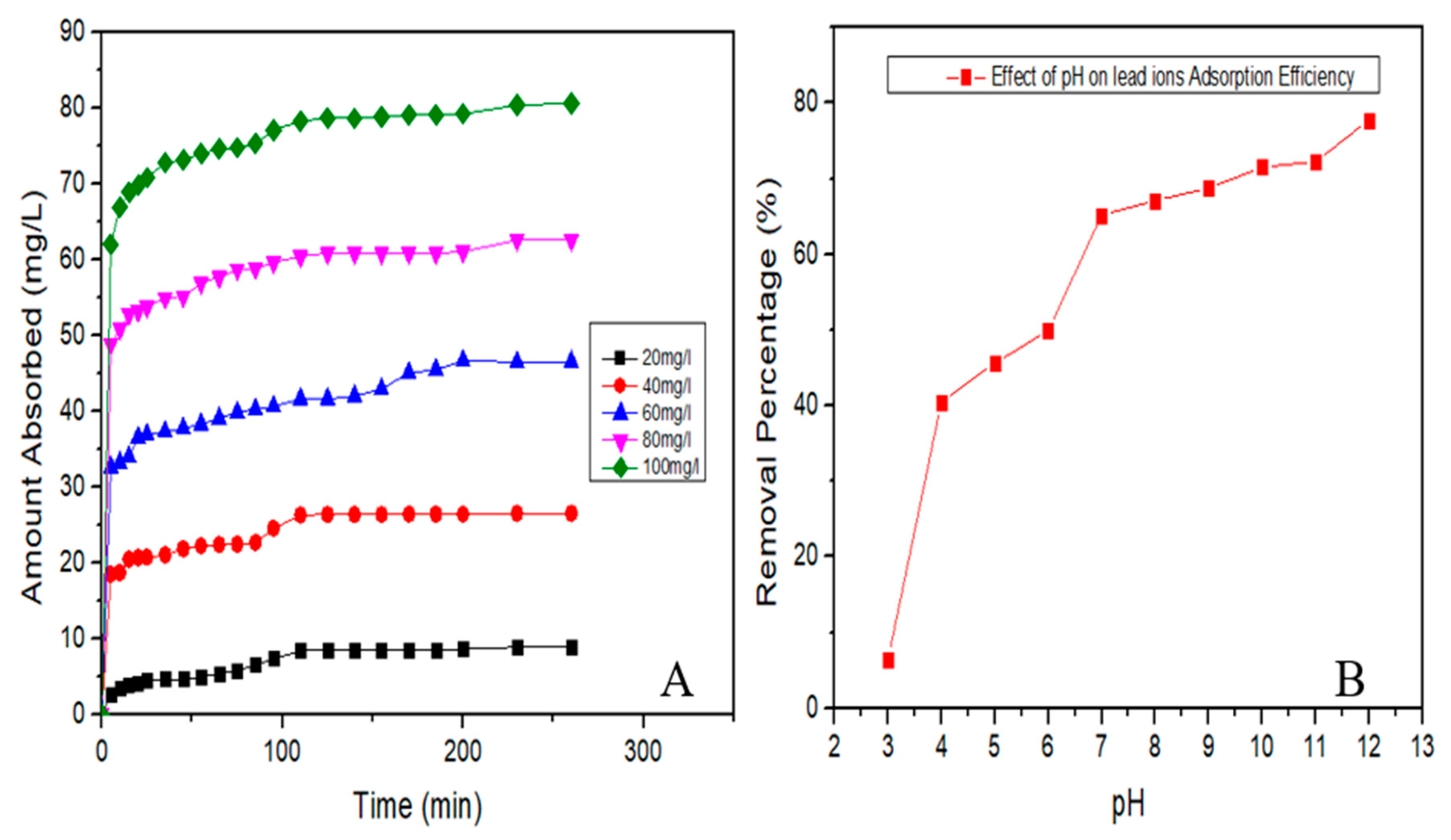
3.3.1. Adsorption Equilibrium
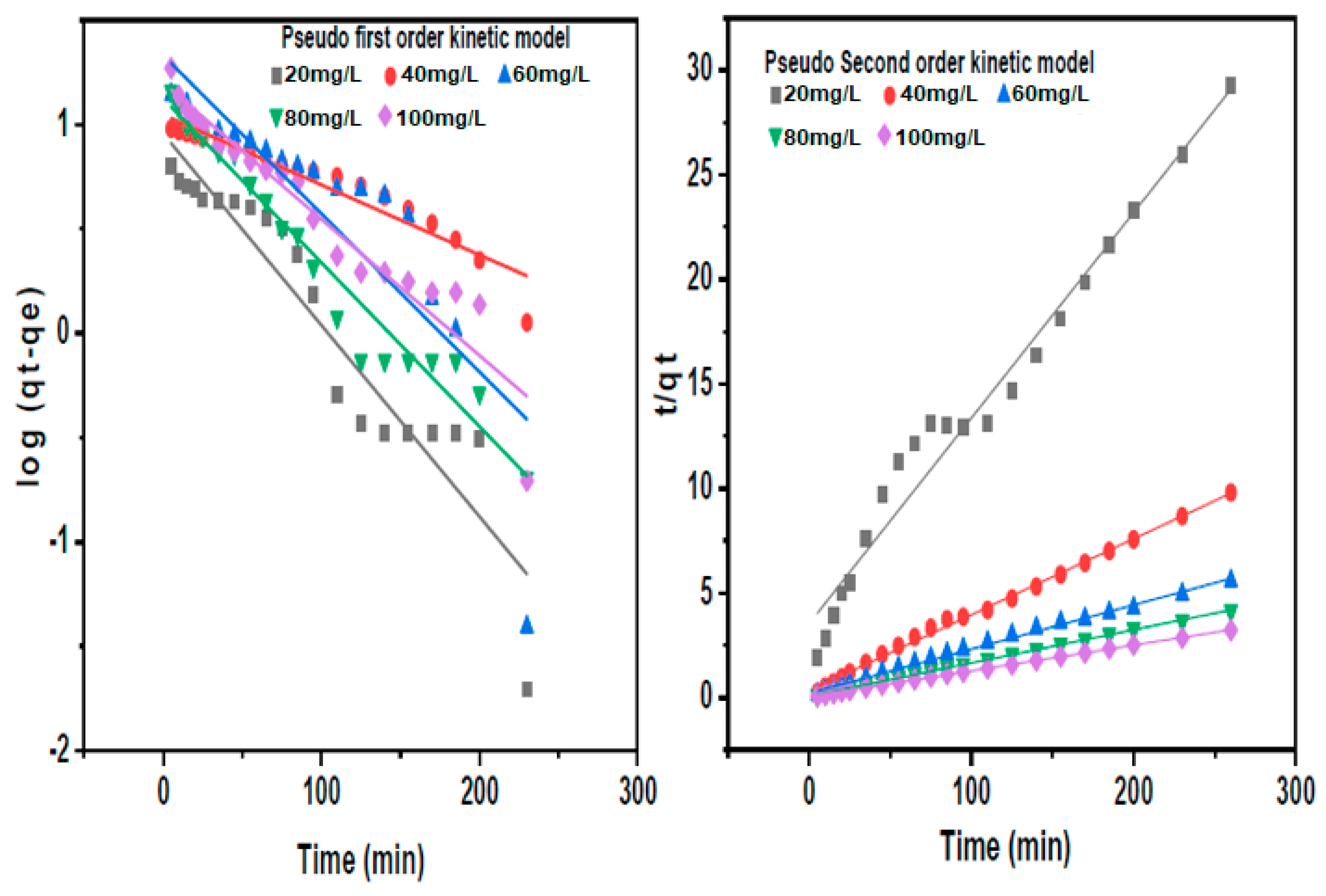
3.3.2. Adsorption Kinetics
Pseudo First and Second-Order Kinetic Models
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Shen, C.; Wang, J.; Lu, Y. Facile Synthesis of Biocompatible N, S-doped Carbon Dots for Cell Imaging and Ion Detecting. RSC Adv. 2015, 5, 16368–16375. [Google Scholar] [CrossRef]
- Puvvada, N.; Prashanth, K.; Suraj, K.; Himani, K.; Mahitosh, M.; Amita, P. Synthesis of biocompatible multicolor luminescent carbon dots for bioimaging applications. Sci. Technol. Adv. Mater 2012, 13, 045008. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Mouhamed, D.; Julien, L.; Gilles, L.; Christophe, D. Origin of the nano-carbon allotropes in pulsed laser ablation in liquids synthesis. J. Colloid Interface Sci. 2017, 489, 114–125. [Google Scholar]
- Marks, R.S. Handbook of Biosensors and Biochips, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2007. [Google Scholar]
- Zhu, X.; Zhao, T.; Nie, Z.; Miao, Z.; Liu, Y.; Yao, S. Nitrogen-doped carbon nanoparticle modulated turn-on fluorescent probes for histidine detection and its imaging in living cells. Nanoscale 2016, 8, 2205–2211. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, M.; Liu, Y.; Feng, X.Z.; Yin, X.B.; He, X.W.; Zhang, Y.K. Nitrogen-doped carbon dots: A facile and general preparation method, photoluminescence investigation, and imaging applications. Chem. Eur. J. 2013, 19, 2276–2283. [Google Scholar] [CrossRef]
- Shi, Q.-Q.; Yu-Hao, L.; Yang, X.; Kui, Y.-Z. High-yield and high-solubility nitrogen-doped carbon dots: Formation, fluorescence mechanism and imaging application. RSC Adv. 2013, 4, 1563. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Xiauyou, X.; Ray, R.; Yunlong, J.G.H.; Gearheart, L.; Kyle, A.R.; Scrivens, W.A. Electrophoretric analysis and purification of fluorescent single-walled carbon nanotube fragments. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar]
- Wang, X.; Cao, L.; Meziani, J.; Qi, G.; Zhou, B.; Harruff, A.B.; Sun, P.Y. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 25, 3774–3776. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Liu, G. Fabrication of carbon quantum dots and their application for efficient detecting Ru in the solution. Sens. Actuators 2013, 181, 209–214. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Robin, J.W.; Nicolas, B.; Vitaliy, L.B.; Dang, S.S.; Francisco, D.M.; James, H.C.; Mark, J.M. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- Cao, L.; Sheng-Tao, Y.; Xin, W.; Pengju, G.L.; Jia-Hui, L.; Sushant, S.; Yamin, L.; Ya-Ping, S. Competitive Performance of Carbon “Quantum” Dots in Optical Bioimaging. Theranostics 2012, 2, 295–301. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, R.; Lin, G.; Roy, I.; Yong, K.T. Functionalized quantum dots for biosensing and bioimaging and concerns on toxicity. ACS Appl. Mater. Interfaces 2013, 5, 2786–2799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921. [Google Scholar] [CrossRef]
- Zhou, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar]
- Tothill, I.E. Biosensors developments and potential applications in the agricultural diagnosis sector. Comput. Electron. Agric. 2001, 30, 205–218. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Vasudevan, D.; Narayan, R.; Raju, K.V. Controllable synthesis of biosourced blue-green fluorescent carbon dots from camphor for the detection of heavy metal ions in water. RSC Adv. 2014, 4, 57137–57143. [Google Scholar] [CrossRef]
- Ali, A.E.; Hghighat, S.; Kazemifard, N.; Rezaei, B.; Moradi, F. A novel one-step and green synthesis of highly fluorescent carbon dots from saffron for cell imaging and sensing of prilocaine. Sens. Actuators B Chem. 2017, 253, 451–460. [Google Scholar]
- Yang, Y.; Zhao, B.; Gao, Y.; Liu, H.; Tian, Y.; Qin, D.; Wu, H.; Huang, W.; Hou, L. Novel hybrid ligands for passivating PbS colloidal quantum dots to enhance the performance of solar cells. Nano-Micro Lett. 2015, 7, 325–331. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Shih, Z.; Lee, C.; Chang, H. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012, 14, 917–920. [Google Scholar] [CrossRef]
- Zhu, G.M.; Zeng, Y.; Zhang, L.; Tang, J.; Chen, M.; Cheng, L.H.; Zhang, L.; He, Y.; Guo, X.X.; He, M.Y. Highly sensitive electrochemical sensor using a MWCNTs/GNPs-modified electrode for lead (II) detection based on lead(II)-induced G-rich DNA conformation. Analyst 2014, 139, 5014–5020. [Google Scholar] [CrossRef] [PubMed]
- Saud, P.S.; Bishweshwar, P.; Al-Mahmnur, A.; Zafar, K.G.; Mira, P.; Hak, Y.K. Carbon quantum dots anchored TiO2 nanofibers: Effective photocatalyst for waste water treatment. Ceram. Int. 2015, 41, 11953–11959. [Google Scholar] [CrossRef]
- Etacheria, V.; Cristiana, D.V.; Jenny, S.; Detlef, B.; Suresh, C.P. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Li, X.; Hongqiang, W.; Yoshiki, S.; Alexander, P.; Kenji, K.; Naoto, K. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2011, 47, 932–934. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.; Zeng, X.; Lu, Y. Preparation of carbon dots by non-focusing pulsed laser irradiation in toluene. Chem. Commun. 2016, 52, 819–822. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, S.; Li, D.; Zhu, C.; Ren, W.; Dong, S.; Wang, E. Easy synthesis and imaging applications of cross-linked green fluorescent hollow carbon nanoparticles. ACS Nano 2012, 6, 400–409. [Google Scholar] [CrossRef]
- Sun, D.; Ban, R.; Zhang, P.; Wu, G.; Zhang, J.; Zhu, J. Hair fiber as a precursor for synthesizing of sulfur—And nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Sudip, M.; Neelambaram, P.; Vasudevan, D.; Raju, K. Facile synthesis of carbon dot and residual carbon nanobeads: Implications for ion sensing, medicinal and biological applications. Mater. Sci. Eng. C 2017, 73, 643–652. [Google Scholar] [CrossRef]
- Zhou, J.; Christina, B.; Ruying, L.; Xingtai, Z.; Tsun-Kong, S.; Xueliang, S.; Zhifeng, D. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Hu, Y.; Li, G. A one-step sonoelectrochemical preparation method of pure blue fluorescent carbon nanoparticles under a high intensity electric field. Carbon 2014, 66, 77–83. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, Z.; Luo, D.; Yu, W.; Guo, Z.; Wang, T. Dual-peak electrogenerated chemiluminescence of carbon dots for iron ions detection. Anal. Chem. 2014, 86, 5620–5623. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical synthesis of carbon nanodots directly from alcohols. Chem. Eur. J. 2014, 20, 4993–4999. [Google Scholar] [CrossRef]
- Mahardika, P.A.; Pradita, A.A.; Jotti, K.; Annisa, L.W. Removal of Heavy Metal Nickel-Ions from Wastewaters Using Carbon Nanodots from Frying Oil. Procedia Eng. 2017, 170, 36–40. [Google Scholar]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835. [Google Scholar] [CrossRef]
- Wei, L.; Sichun, W.; Ying, L.; Chunhui, M.; Shouxin, L. One-step hydrothermal synthesis of fluorescent nanocrystalline cellulose/carbon dot hydrogels. Carbohydr. Polym. 2017, 175, 7–17. [Google Scholar]
- Luyao, L.; Xingxian, W.; Zheng, F.; Fengling, C. One-step hydrothermal synthesis of nitrogen- and sulfur-co-doped carbon dots from ginkgo leaves and application in biology. Mater. Lett. 2017, 196, 300–303. [Google Scholar]
- Wang, J.; Wang, C.F.; Chen, S. Amphiphilic Egg-Derived Carbon Dots: Rapid Plasma Fabrication, Pyrolysis Process, and Multicolor Printing Patterns. Angew. Chem. Int. Ed. 2012, 51, 9297–9301. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, N.; Gong, N.; Wang, H.; Shi, X.; Gu, W.; Ye, L. One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon 2014, 68, 258–264. [Google Scholar] [CrossRef]
- Choi, Y.; Nichaphat, T.; Ari, C.; Seongho, J.; Insik, I. Microwave-assisted synthesis of luminescent and biocompatible lysine-based carbon quantum dots. J. Ind. Eng. Chem. 2017, 47, 329–335. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Prashant, K.S.; Michael, L.F. Quantum dots and carbon dots based fluorescent sensors for TB biomarkers detection. Vacuum 2017, 146, 606–613. [Google Scholar] [CrossRef]
- Ke, Y.; Garg, B.; Ling, Y.C. Waste chicken eggshell as low-cost precursor for efficient synthesis of nitrogen-doped fluorescent carbon nanodots and their multi-functional applications. RSC Adv. 2014, 4, 58329–58336. [Google Scholar] [CrossRef]
- Lu, W.; Xiaoyun, Q.; Sen, L.; Guohui, C.; Yingwei, Z.; Yonglan, L.; Abdullah, M.A.; Abdulrahman, O.; Xuping, S. Economical, Green Synthesis of Fluorescent Carbon Nanoparticles and Their Use as Probes for Sensitive and Selective Detection of Mercury(II) Ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Khan, M.R.; Alothman, Z.A. Adsorptive removal of toxic dye using Fe3O4–TSC nanocomposite: Equilibrium, kinetic, and thermodynamic studies. J. Chem. Eng. Data 2016, 61, 3806–3813. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ahamad, T. Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: Kinetics, isotherm and mechanism. J. Environ. Manag. 2018, 223, 29–36. [Google Scholar] [CrossRef]
- Xiangtao, W.; Yifei, G.; Li, Y.; Meihua, H.; Jing, Z.; Xiaoliang, C. Nanomaterials as Sorbents to Remove Heavy Metal Ions in Wastewater Treatment. J. Environ. Anal. Toxicol 2012, 2, 145. [Google Scholar]
- Nejib, A.; Joelle, D.; Amane, J.; Emna, E.; Malika, G.; Khadija, S.; Malika, T.A. Removal of anionic dye from textile industries’ effluents by using Tunisian clays as adsorbents. Ζeta potential and streaming-induced potential measurements. C. R. Chim. 2019, 22, 113–125. [Google Scholar]
- Adeyi, A.A.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y.; Lau, K.L.; Abdullah, M. Adsorptive Removal of Methylene Blue from Aquatic Environments Using Thiourea-Modified Poly(Acrylonitrile-co-Acrylic Acid). Materials 2019, 12, 1734. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Manique, M.C.; Silva, A.P.; Alves, A.K.; Bergmann, C.P. Titanate nanotubes produced from microwave-assisted hydrothermal synthesis: Characterization, adsorption and photocatalytic activity. Braz. J. Chem. Eng. 2017, 34, 331–339. [Google Scholar] [CrossRef]
- Jhonsi, M.A.; Thulasi, S. A novel fluorescent carbon dots derived from tamarind. Chem. Phys. Lett. 2016, 661, 179–184. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar]
- Barhoum, A.; García-Betancourt, M.L.; Rahier, H.; Van-Assche, G. Physicochemical characterization of nanomaterials: Polymorph, composition, wettability, and thermal stability. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 255–278. [Google Scholar]
- Yongli, L.; Qingxiang, Z.; Yongyong, Y.; Yalin, W. Hydrothermal synthesis of fluorescent carbon dots from sodium citrate and polyacrylamide and their highly selective detection of lead and pyrophosphate. Carbon 2017, 115, 550–560. [Google Scholar]
- Jie, W.; Yuhao, C.; Fengxian, Q.; Xin, L.; Dongya, Y. One-pot simple green synthesis of water-soluble cleaner fluorescent carbon dots from cellulose and its sensitive detection of iron ion. J. Clean. Prod. 2017, 23–30. [Google Scholar] [CrossRef]
- Lingling, L.; Luyao, L.; Chang-Po, C.; Fengling, C. Green synthesis of nitrogen-doped carbon dots from ginkgo fruits and the application in cell imaging. Inorg. Chem. Commun. 2017, 86, 227–231. [Google Scholar]
- Bin, W.; Feng, L.; Yuanya, W.; Yanfen, C.; Chang, M.L. Synthesis of catalytically active multielement-doped carbon dots and application for colorimetric detection of glucose. Sens. Actuators B Chem. 2018, 225, 2601–2607. [Google Scholar]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.W.; Jeong, S.W.; Kim, C.H.; Lee, Y.C.; Huh, Y.S.; Lee, J. Photoluminescent green carbon nanodots from food-waste-derived sources: Large-scale synthesis, properties, and biomedical applications. ACS Appl. Mater. Interfaces 2014, 6, 3365–3370. [Google Scholar] [CrossRef]
- Han, S.; He, Z.; Jian, Z.; Yujie, X.; Liangliang, L.; Hangxing, W.; Xiangkai, L.; Weisheng, L.; Yu, T. Fabrication, gradient extraction and surface polarity-dependent photoluminescence of cow milk-derived carbon dots. RSC Adv. 2014, 4, 58084–58089. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Sayan, G.; Poushali, D.; Madhuparna, B.; Subhadip, M.; Amit, K.D.; Das, N.C. Strongly blue-luminescent N-doped carbogenic dots as a tracer metalsensing probe in aqueous medium and its potential activity towardsin situ Ag-nanoparticle synthesis. Sens. Actuators B 2017, 252, 735–746. [Google Scholar]
- Yuqian, P.; Hui, G.; Shaohui, W.; Xiaolong, L. Facile synthesis the nitrogen and sulfur co-doped carbon dots for selective fluorescence detection of heavy metal ions. Mater. Lett. 2017, 193, 236–239. [Google Scholar]
- Nisar, U.; Muhammad, M.; Ibrahim, K.; Ahsanulhaq, Q. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar]
- Laxman, W.; Tanushree, D.; Pawan, K.; Yong, S.O.; Samadhan, P.; Akash, D.; Ki-Hyun, K. Functionalized fluorescent nanomaterials for sensing pollutants in the environment: A critical review. Trends Anal. Chem. 2017, 97, 458–467. [Google Scholar]
- Xu, L.; Chen, C.; Huo, J.B.; Chen, X.; Yang, J.C.; Fu, M.L. Iron hydroxyphosphate composites derived from waste lithium-ion batteries for lead adsorption and Fenton-like catalytic degradation of methylene blue. Environ. Technol. Innov. 2019, 16, 100504–100515. [Google Scholar] [CrossRef]
- Mahar, F.K.; He, L.; Wei, K.; Mehdi, M.; Zhu, M.; Gu, J.; Kim, I. Rapid adsorption of lead ions using porous carbon nanofibers. Chemosphere 2019, 225, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Z.; Song, S.; Han, J.; Chen, H.; Wang, X.; Cheng, J. Adsorption of copper (II) and lead (II) from seawater using hydrothermal biochar derived from Enteromorpha. Mar. Pollut. Bull. 2019, 149, 110586–110592. [Google Scholar] [CrossRef]
- Silas, K.; Ab Karim Ghani, W.W.; Choong, T.S.; Rashid, U. Optimization of Activated Carbon Monolith Co3O4-Based Catalyst for Simultaneous SO2/NOx Removal from Flue Gas Using Response Surface Methodology. Combust. Sci. Technol. 2019, 2–19. [Google Scholar] [CrossRef]
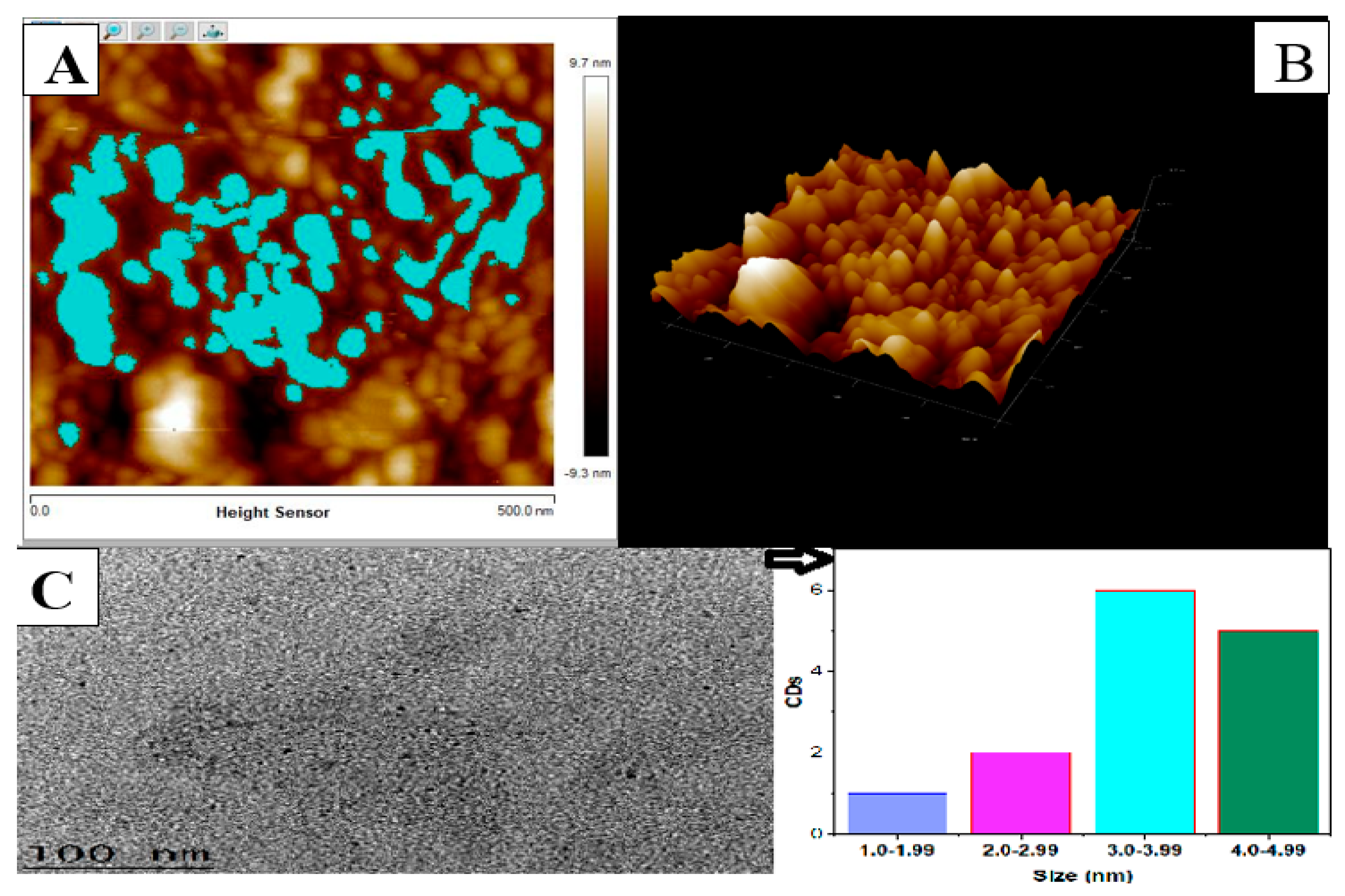

| Parameter | Mean | Minimum | Maximum | Sigma |
|---|---|---|---|---|
| Total Count | 32.000 | 32.000 | 32.000 | 0.000 |
| Height | 2.440 (nm) | 0.409 (nm) | 8.168 (nm) | 1.875 (nm) |
| Area | 1801.610 (nm2) | 95.367 (nm2) | 31,333.924 (nm2) | 5432.314 (nm2) |
| Diameter | 32.387 (nm) | 11.019 (nm) | 199.739 (nm) | 35.284 (nm) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahaya Pudza, M.; Zainal Abidin, Z.; Abdul Rashid, S.; Md Yasin, F.; Noor, A.S.M.; Issa, M.A. Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment. Nanomaterials 2020, 10, 315. https://doi.org/10.3390/nano10020315
Yahaya Pudza M, Zainal Abidin Z, Abdul Rashid S, Md Yasin F, Noor ASM, Issa MA. Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment. Nanomaterials. 2020; 10(2):315. https://doi.org/10.3390/nano10020315
Chicago/Turabian StyleYahaya Pudza, Musa, Zurina Zainal Abidin, Suraya Abdul Rashid, Faizah Md Yasin, A. S. M. Noor, and Mohammed A. Issa. 2020. "Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment" Nanomaterials 10, no. 2: 315. https://doi.org/10.3390/nano10020315
APA StyleYahaya Pudza, M., Zainal Abidin, Z., Abdul Rashid, S., Md Yasin, F., Noor, A. S. M., & Issa, M. A. (2020). Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment. Nanomaterials, 10(2), 315. https://doi.org/10.3390/nano10020315