The Nanosized Dye Adsorbents for Water Treatment
Abstract
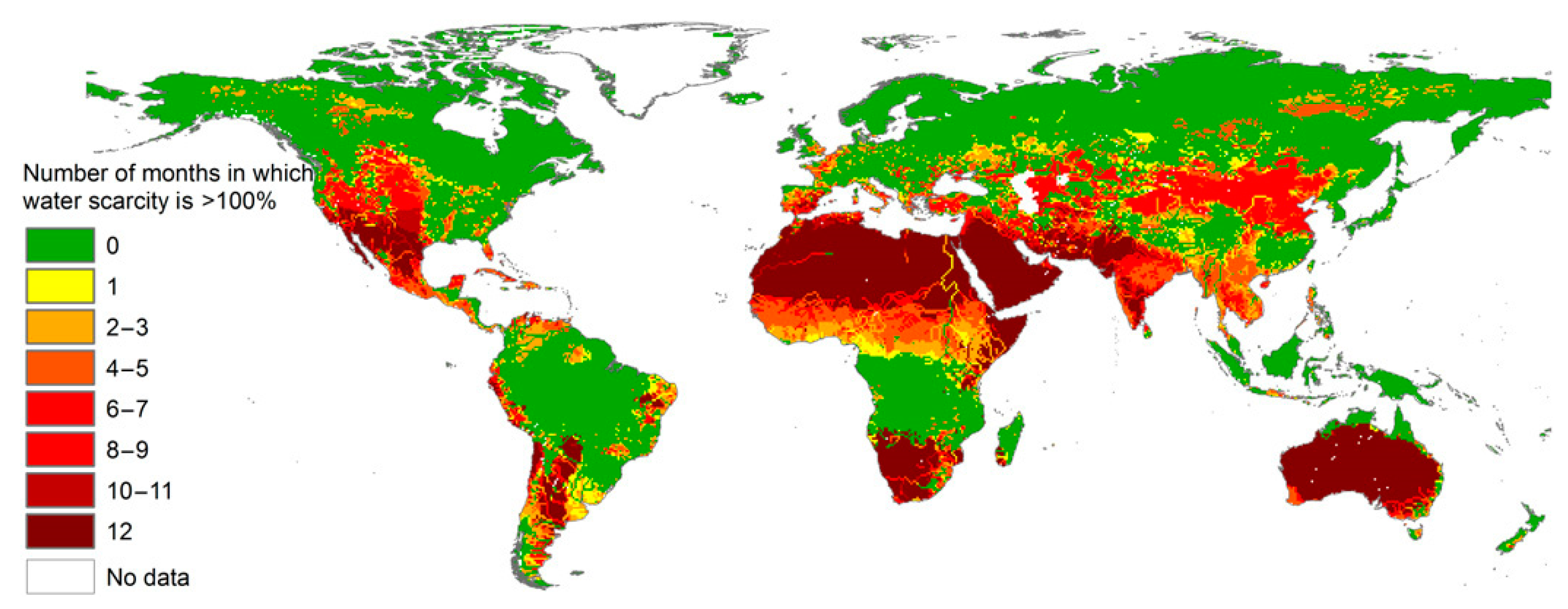
1. Introduction
2. Adsorption-Based Water Treatment
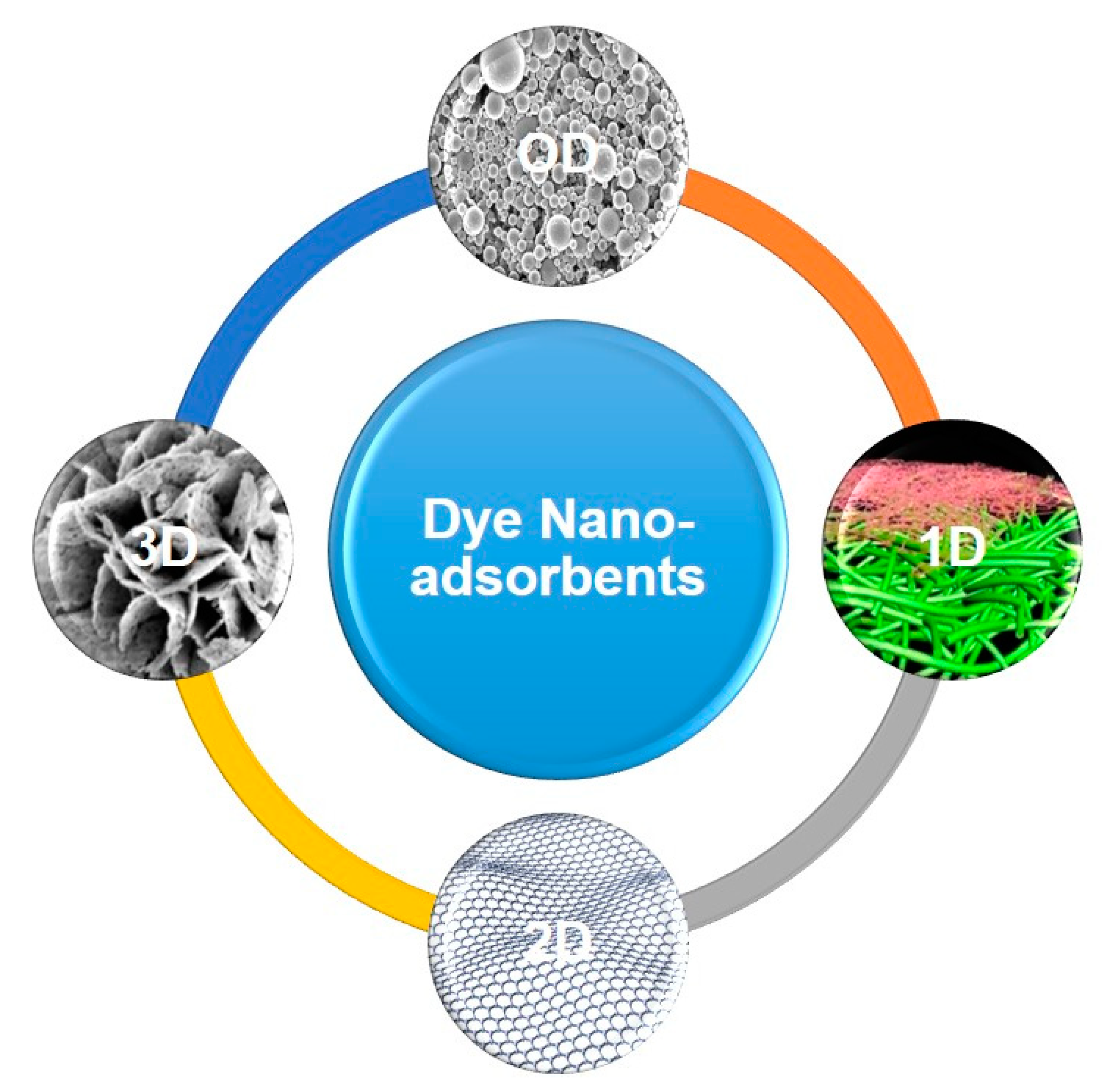
3. Different Classes of Nanostructured Dye Adsorbents
3.1. 0D Nano-Adsorbents
3.1.1. Passive 0D Nano-Adsorbents
Biopolymer Derived 0D Nano-Adsorbents
Synthetic Polymer Derived 0D Nano-Adsorbents
Inorganic 0D Nano-Adsorbents
Nanocomposite 0D Nano-Adsorbents
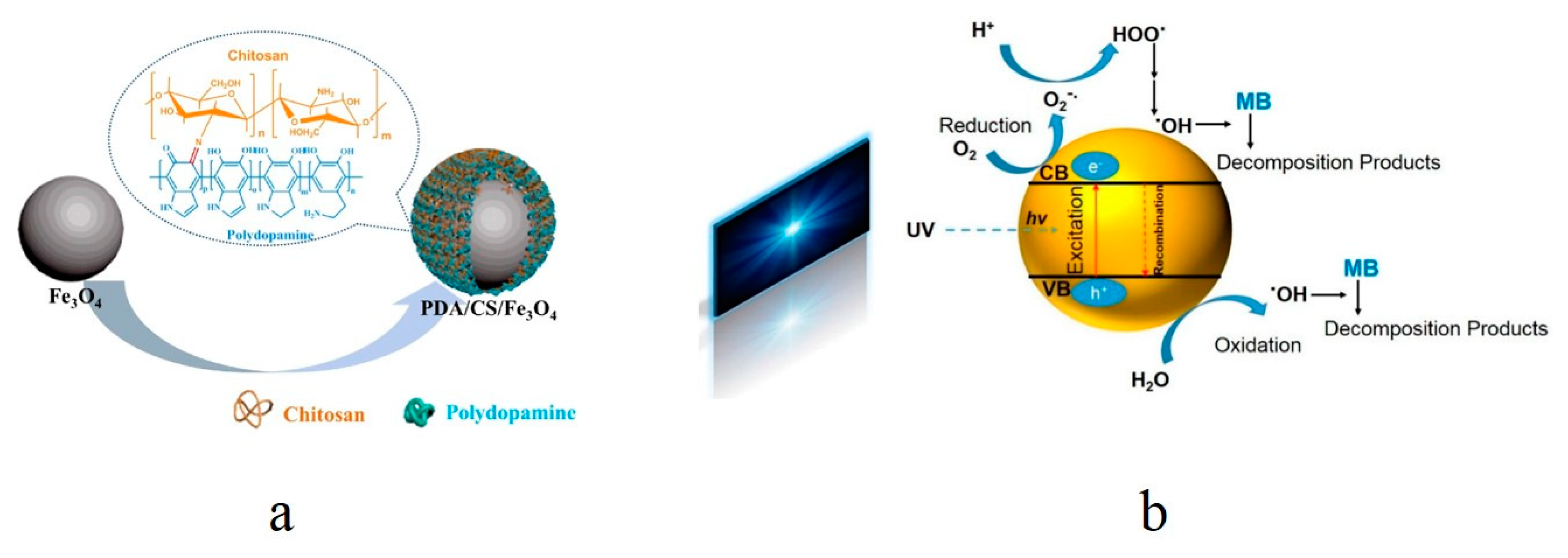
3.1.2. Active 0D Nano-Adsorbents
3.2. 1D Nano-Adsorbents
3.2.1. 1D Carbonaceous Nano-Adsorbents
3.2.2. 1D Biopolymer Nano-Adsorbents
3.2.3. 1D Inorganic Nano-Adsorbents
3.2.4. 1D Nanocomposite Nano-Adsorbents
3.3. 2D Nano-Adsorbents
3.3.1. 2D Carbonaceous Nano-Adsorbents
3.3.2. 2D Inorganic Nano-Adsorbents
3.4. 3D Nano-Adsorbents
4. Conclusions and Future Perspectives
Abbreviation
| a-COx | Oxygenated Amorphous Carbon |
| AFM | Atomic Force Microscopy |
| AO | Acid Orange |
| AOP | Advanced Oxidation Process |
| BB | Basic Blue |
| BET | Brunauer–Emmett–Teller |
| BN | Boron Nitride |
| BPB | Bromophenol Blue |
| BR | Basic Red |
| C-PAM | Cationic Polyacrylamide |
| CB | Conduction Band |
| CBB | Coomassie Brilliant Blue |
| CNC | Cellulose Nanocrystal |
| CNT | Carbon Nanotube |
| COD | Chemical Oxygen Demand |
| CQD | Carbon Quantum Dot |
| CR | Congo Red |
| CS | Chitosan |
| CSB | Chicago Sky Blue |
| CTAB | Cetyltrimethylammonium Bromide |
| CV | Crystal Violet |
| D | Dimension |
| DFT | Density Functional Theory |
| DR | Disperse Red |
| ER | Eosin Red |
| FA | Fuchsin Acid |
| FESEM | Field Emission Scanning Electron Microscopy |
| FFT | Fast Fourier Transform |
| G | Graphite |
| GH | Graphene Hydrogel |
| GO | Graphene Oxide |
| HCP | Hyper-crosslinked-polymer |
| HDA | Hexanediamine |
| HRTEM | High Resolution Transmission Electron Microscopy |
| IC | Indigo Carmine |
| MB | Methylene Blue |
| MG | Malachite Green |
| MO | Methyl Orange |
| MOF | Metal-Organic Framework |
| MV | Methyl Violet |
| MWCNT | Multi-walled Carbon Nanotube |
| nZVI | Nanoscale Zerovalent Iron |
| NBB | Napthol Blue Black |
| NIR | Near Infrared |
| NR | Neutral Red |
| PAN | Polyacrylonitrile |
| PDA | Polydopamine |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PES | Polyethersulfone |
| PVDF | Polyvinylidene fluoride |
| RAPOP | Rich Amine Porous Organic Polymer |
| RBB | Remazol Brilliant Blue |
| rGO | Reduced Graphene Oxide |
| RhB | Rhodamine B |
| ROS | Reactive Oxygen Species |
| SEM | Scanning Electron Microscopy |
| SET-LRP | Single-Electron Transfer Living Radical Polymerization |
| TEMPO | (2,2,6,6-tetramethylpiperidin-1-yl)oxyl |
| TMD | Transition Metal Dichalcogenide |
| TNT | Titanate Nanotubes |
| TOC | Total Organic Carbon |
| UV | Ultraviolet |
| VB | Valence Band |
| ZIF | Zeolitic Imidazolate Framework |
Funding
Acknowledgments
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Elbahri, M. Nanocomposite Electrospun Nanofiber Membranes for Environmental Remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A Novel Nanohybrid Nanofibrous Adsorbent for Water Purification from Dye Pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, N.; Gupta, V.K.; Thirumal, E.; Thangadurai, P.; Narayanan, V.; Stephen, A. ZnO/Ag nanocomposite: An efficient catalyst for degradation studies of textile effluents under visible light. Mater. Sci. Eng. C 2013, 33, 2235–2244. [Google Scholar] [CrossRef]
- Janaki, V.; Oh, B.-T.; Shanthi, K.; Lee, K.-J.; Ramasamy, A.K.; Kamala-Kannan, S. Polyaniline/chitosan composite: An eco-friendly polymer for enhanced removal of dyes from aqueous solution. Synth. Met. 2012, 162, 974–980. [Google Scholar] [CrossRef]
- Raghuvanshi, S.; Singh, R.; Kaushik, C.; Raghav, A. Kinetics study of methylene blue dye bioadsorption on baggase. Appl. Ecol. Environ. Res. 2004, 2, 35–43. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Lahtinen, M.; Sillanpää, M. Preparation and characterization of a novel chitosan/Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of Methyl Orange adsorption. Chem. Eng. J. 2015, 259, 1–10. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Ezechi, E.H.; bin Mohamed Kutty, S.R.; Malakahmad, A.; Isa, M.H. Characterization and optimization of effluent dye removal using a new low cost adsorbent: Equilibrium, kinetics and thermodynamic study. Process Saf. Environ. Prot. 2015, 98, 16–32. [Google Scholar] [CrossRef]
- Almasian, A.; Olya, M.E.; Mahmoodi, N.M. Synthesis of polyacrylonitrile/polyamidoamine composite nanofibers using electrospinning technique and their dye removal capacity. J. Taiwan Inst. Chem. Eng. 2015, 49, 119–128. [Google Scholar] [CrossRef]
- Clarke, E.A.; Anliker, R. Organic Dyes and Pigments. In Anthropogenic Compounds; Springer: Berlin/Heidelberg, Germany, 1980; pp. 181–215. [Google Scholar]
- Ali, I.; Khan, T.A.; Asim, M. Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep. Purif. Rev. 2011, 40, 25–42. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Column with CNT/magnesium oxide composite for lead (II) removal from water. Environ. Sci. Pollut. Res. 2012, 19, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interface Sci. 2011, 362, 337–344. [Google Scholar] [CrossRef]
- Saleh, T.A.; Agarwal, S.; Gupta, V.K. Synthesis of MWCNT/MnO2 and their application for simultaneous oxidation of arsenite and sorption of arsenate. Appl. Catal. B Environ. 2011, 106, 46–53. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Kim, I.; Han, J.-I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.R. Effect of particle size and surface area on the adsorption of albumin-bonded bilirubin on activated carbon. Carbon 2010, 48, 3607–3615. [Google Scholar] [CrossRef]
- Dolez, P.I. Chapter 1.1—Nanomaterials Definitions, Classifications, and Applications. In Nanoengineering; Dolez, P.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–40. [Google Scholar]
- Homaeigohar, S.S.; Buhr, K.; Ebert, K. Polyethersulfone electrospun nanofibrous composite membrane for liquid filtration. J. Membr. Sci. 2010, 365, 68–77. [Google Scholar] [CrossRef]
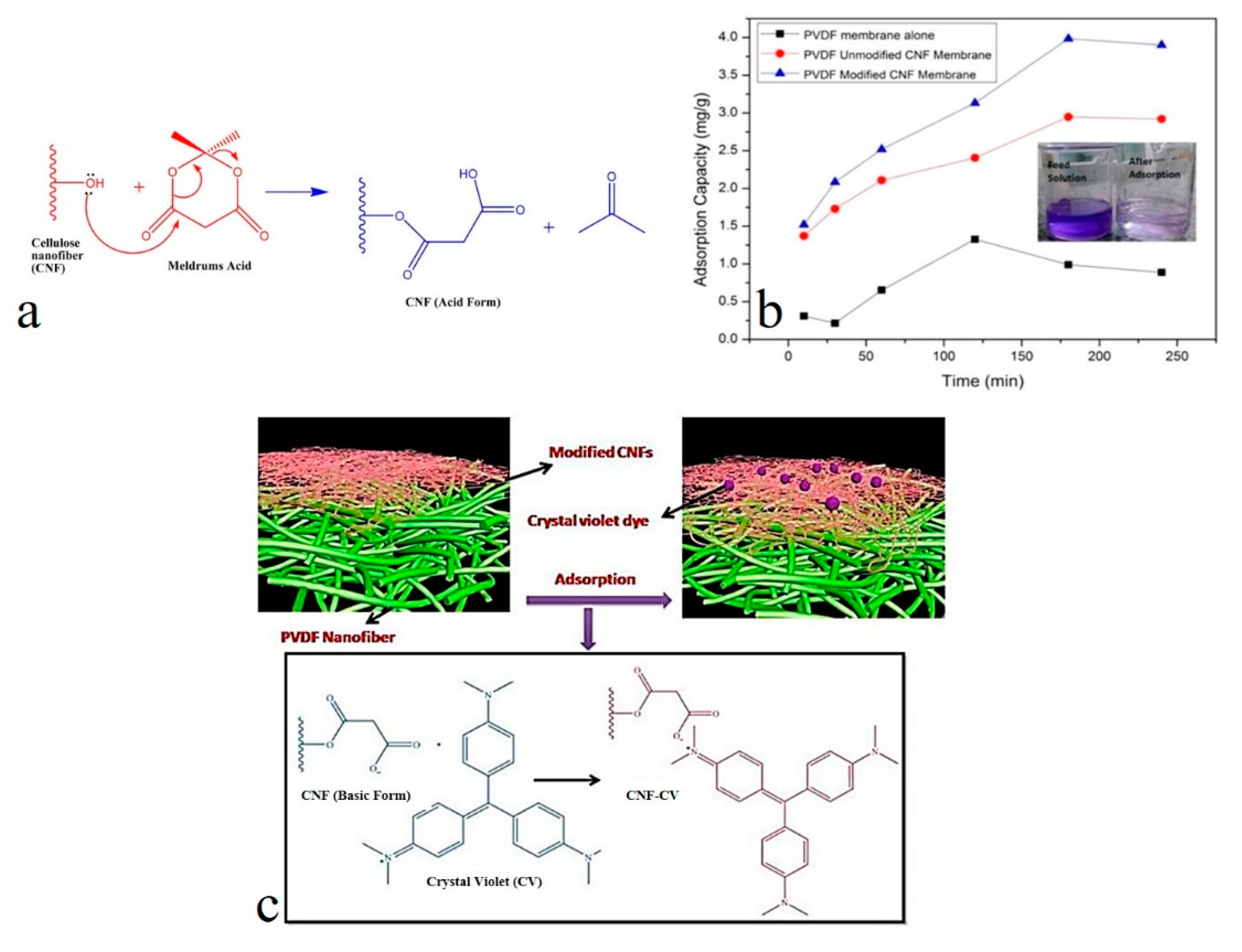
- Gopakumar, D.A.; Pasquini, D.; Henrique, M.A.; de Morais, L.C.; Grohens, Y.; Thomas, S. Meldrum’s Acid Modified Cellulose Nanofiber-Based Polyvinylidene Fluoride Microfiltration Membrane for Dye Water Treatment and Nanoparticle Removal. ACS Sustain. Chem. Eng. 2017, 5, 2026–2033. [Google Scholar] [CrossRef]
- Gnayem, H.; Dandapat, A.; Sasson, Y. Development of Hybrid BiOClxBr1−x-Embedded Alumina Films and Their Application as Highly Efficient Visible-Light-Driven Photocatalytic Reactors. Chem. A Eur. J. 2016, 22, 370–375. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Batool, S.; Akib, S.; Ahmad, M.; Balkhair, K.S.; Ashraf, M.A. Study of modern nano enhanced techniques for removal of dyes and metals. J. Nanomater. 2014. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Hosseini-Bandegharaei, A.; Fu, J.; Mitropoulos, A.C.; Kyzas, G.Z. Use of nanoparticles for dye adsorption. J. Dispers. Sci. Technol. 2018, 39, 836–847. [Google Scholar] [CrossRef]
- Tara, N.; Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A.; Asiri, A.M. Nano-engineered Adsorbent for the removal of dyes from water: A review. Curr. Anal. Chem. 2020, 16, 14–40. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Garud, H.B.; Patil, A.H.; Patil, G.D.; Patil, C.R.; Dongale, T.D.; Patil, P.S. Recent advancements in silica nanoparticles based technologies for removal of dyes from water. Colloid Interface Sci. Commun. 2019, 30, 100181. [Google Scholar] [CrossRef]
- Cai, Z.; Sun, Y.; Liu, W.; Pan, F.; Sun, P.; Fu, J. An overview of nanomaterials applied for removing dyes from wastewater. Environ. Sci. Pollut. Res. 2017, 24, 15882–15904. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, H.; Ali, G.A.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostructure Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Kavithayeni, V.; Geetha, K.; Akash Prabhu, S. A Review on Dye Reduction Mechanism using Nano Adsorbents in Waste Water. Adsorption 2019, 43, 44. [Google Scholar]
- Yang, K.; Xing, B. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ. Pollut. 2007, 145, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpää, M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013, 47, 4812–4832. [Google Scholar] [CrossRef]
- Alves, N.; Mano, J. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Fluoride removal from water by chitosan derivatives and composites: A review. J. Fluor. Chem. 2011, 132, 231–240. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin-and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Shajahan, A.; Shankar, S.; Sathiyaseelan, A.; Narayan, K.S.; Narayanan, V.; Kaviyarasan, V.; Ignacimuthu, S. Comparative studies of chitosan and its nanoparticles for the adsorption efficiency of various dyes. Int. J. Biol. Macromol. 2017, 104, 1449–1458. [Google Scholar] [CrossRef]
- Dhananasekaran, S.; Palanivel, R.; Pappu, S. Adsorption of Methylene Blue, Bromophenol Blue, and Coomassie Brilliant Blue by α-chitin nanoparticles. J. Adv. Res. 2016, 7, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Tahir, H.; Khan, J.; Hameed, U.; Saud, A. Synthesis of polyaniline nanoparticles and their application for the removal of Crystal Violet dye by ultrasonicated adsorption process based on Response Surface Methodology. Ultrason. Sonochemistry 2017, 34, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, D.; Zheng, J.; Zeng, L.; Jiang, J.; Jiang, R.; Zhu, F.; Shen, Y.; Wu, D.; Ouyang, G. The sensitive and selective adsorption of aromatic compounds with highly crosslinked polymer nanoparticles. Nanoscale 2015, 7, 16943–16951. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; You, Q.; Li, J.; Liao, G.; Xia, H.; Wang, D. A rich-amine porous organic polymer: An efficient and recyclable adsorbent for removal of azo dye and chlorophenol. RSC Adv. 2016, 6, 98487–98497. [Google Scholar] [CrossRef]
- Li, L.H.; Xiao, J.; Liu, P.; Yang, G.W. Super adsorption capability from amorphousization of metal oxide nanoparticles for dye removal. Sci. Rep. 2015, 5, 9028. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Xing, J.; Li, Z.; Li, Q. Cr-Doped ZnO Nanoparticles: Synthesis, Characterization, Adsorption Property, and Recyclability. ACS Appl. Mater. Interfaces 2015, 7, 27449–27457. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A. Ternary dye adsorption onto MnO 2 nanoparticle-loaded activated carbon: Derivative spectrophotometry and modeling. RSC Adv. 2015, 5, 72300–72320. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A.; Bazrafshan, A.A. Simultaneous ultrasound-assisted ternary adsorption of dyes onto copper-doped zinc sulfide nanoparticles loaded on activated carbon: Optimization by response surface methodology. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 145, 203–212. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Hajati, S.; Bazrafshan, A.A. Rapid adsorption of ternary dye pollutants onto copper (I) oxide nanoparticle loaded on activated carbon: Experimental optimization via response surface methodology. J. Environ. Chem. Eng. 2016, 4, 1769–1779. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Bazrafshan, A.A. Sonochemical assisted hydrothermal synthesis of ZnO: Cr nanoparticles loaded activated carbon for simultaneous ultrasound-assisted adsorption of ternary toxic organic dye: Derivative spectrophotometric, optimization, kinetic and isotherm study. Ultrason. Sonochemistry 2016, 32, 119–131. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.-M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid Interface Sci. 2013, 201, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Donia, A.M.; Atia, A.A.; Elwakeel, K.Z. Selective separation of mercury (II) using magnetic chitosan resin modified with Schiff’s base derived from thiourea and glutaraldehyde. J. Hazard. Mater. 2008, 151, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Dalvand, A.; Nabizadeh, R.; Reza Ganjali, M.; Khoobi, M.; Nazmara, S.; Hossein Mahvi, A. Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J. Magn. Magn. Mater. 2016, 404, 179–189. [Google Scholar] [CrossRef]
- Dai, R.; Zhang, Y.; Shi, Z.-Q.; Yang, F.; Zhao, C.-S. A facile approach towards amino-coated ferroferric oxide nanoparticles for environmental pollutant removal. J. Colloid Interface Sci. 2018, 513, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, G.; Tang, L.; Cai, Y.; Pang, Y.; Zhang, Y.; Yang, G.; Zhou, Y.; He, X.; He, Y. Highly effective adsorption of cationic and anionic dyes on magnetic Fe/Ni nanoparticles doped bimodal mesoporous carbon. J. Colloid Interface Sci. 2015, 448, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, G.-D.; Zeng, G.-M.; Cai, Y.; Li, S.-S.; Zhou, Y.-Y.; Pang, Y.; Liu, Y.-Y.; Zhang, Y.; Luna, B. Synergistic effect of iron doped ordered mesoporous carbon on adsorption-coupled reduction of hexavalent chromium and the relative mechanism study. Chem. Eng. J. 2014, 239, 114–122. [Google Scholar] [CrossRef]
- Tang, L.; Cai, Y.; Yang, G.; Liu, Y.; Zeng, G.; Zhou, Y.; Li, S.; Wang, J.; Zhang, S.; Fang, Y.; et al. Cobalt nanoparticles-embedded magnetic ordered mesoporous carbon for highly effective adsorption of rhodamine B. Appl. Surf. Sci. 2014, 314, 746–753. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Z.; Zeng, G.; Tang, L.; Pang, Y.; Li, Z.; Liu, C.; Lei, X.; Wu, M.; Ren, P.; et al. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour. Technol. 2012, 115, 21–26. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Z.; Liu, J.; Jiang, G. Effective heavy metal removal from aqueous systems by thiol functionalized magnetic mesoporous silica. J. Hazard. Mater. 2011, 192, 277–283. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Switchable Plasmonic Nanocomposites. Adv. Opt. Mater. 2019, 7, 1801101. [Google Scholar] [CrossRef]
- Elbahri, M.; Abdelaziz, M.; Homaeigohar, S.; Elsharawy, A.; Keshavarz Hedayati, M.; Röder, C.; El Haj Assad, M.; Abdelaziz, R. Plasmonic Metaparticles on a Blackbody Create Vivid Reflective Colors for Naked-Eye Environmental and Clinical Biodetection. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Hou, C.; Liu, M. Mussel-inspired synthesis of magnetic polydopamine-chitosan nanoparticles as biosorbent for dyes and metals removal. J. Taiwan Inst. Chem. Eng. 2016, 61, 292–298. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, J.; Ma, J.; Shao, L. Highly regenerable alkali-resistant magnetic nanoparticles inspired by mussels for rapid selective dye removal offer high-efficiency environmental remediation. J. Mater. Chem. A 2015, 3, 19960–19968. [Google Scholar] [CrossRef]
- He, X.; Yang, D.-P.; Zhang, X.; Liu, M.; Kang, Z.; Lin, C.; Jia, N.; Luque, R. Waste eggshell membrane-templated CuO-ZnO nanocomposites with enhanced adsorption, catalysis and antibacterial properties for water purification. Chem. Eng. J. 2019, 369, 621–633. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Botcha, N.K.; Zarie, E.S.; Elbahri, M. Ups and Downs of Water Photodecolorization by Nanocomposite Polymer Nanofibers. Nanomaterials 2019, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Chaudhary, G.R.; Mehta, S.K. Comparative study of catalytic activity of ZrO2 nanoparticles for sonocatalytic and photocatalytic degradation of cationic and anionic dyes. Chem. Eng. J. 2015, 280, 475–485. [Google Scholar] [CrossRef]
- Hisaindee, S.; Meetani, M.; Rauf, M. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. TrAC Trends Anal. Chem. 2013, 49, 31–44. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Hu, Y.; Guo, C.; Qian, H.; Zhang, F.; Lou, X.W.D. Magnetic-field induced formation of 1D Fe3O4/C/CdS coaxial nanochains as highly efficient and reusable photocatalysts for water treatment. J. Mater. Chem. 2011, 21, 18359–18364. [Google Scholar] [CrossRef]
- Seema, H.; Kemp, K.C.; Chandra, V.; Kim, K.S. Graphene-SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology 2012, 23, 355705. [Google Scholar] [CrossRef]
- Jorfi, S.; Barzegar, G.; Ahmadi, M.; Darvishi Cheshmeh Soltani, R.; alah Jafarzadeh Haghighifard, N.; Takdastan, A.; Saeedi, R.; Abtahi, M. Enhanced coagulation-photocatalytic treatment of Acid red 73 dye and real textile wastewater using UVA/synthesized MgO nanoparticles. J. Environ. Manag. 2016, 177, 111–118. [Google Scholar] [CrossRef]
- Nishimoto, S.; Mano, T.; Kameshima, Y.; Miyake, M. Photocatalytic water treatment over WO3 under visible light irradiation combined with ozonation. Chem. Phys. Lett. 2010, 500, 86–89. [Google Scholar] [CrossRef]
- Nakano, K.; Obuchi, E.; Takagi, S.; Yamamoto, R.; Tanizaki, T.; Taketomi, M.; Eguchi, M.; Ichida, K.; Suzuki, M.; Hashimoto, A. Photocatalytic treatment of water containing dinitrophenol and city water over TiO2/SiO2. Sep. Purif. Technol. 2004, 34, 67–72. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Salem, I.A.; Salem, M.A.; El-Ghobashy, M.A. The dual role of ZnO nanoparticles for efficient capture of heavy metals and Acid blue 92 from water. J. Mol. Liq. 2017, 248, 527–538. [Google Scholar] [CrossRef]
- Cao, S.-W.; Zhu, Y.-J. Hierarchically nanostructured α-Fe2O3 hollow spheres: Preparation, growth mechanism, photocatalytic property, and application in water treatment. J. Phys. Chem. C 2008, 112, 6253–6257. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Davoudpour, Y.; Habibi, Y.; Elbahri, M. The Electrospun Ceramic Hollow Nanofibers. Nanomaterials 2017, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Csanady, M.; Straub, I. Health damage due to water pollution in Hungary. Iahs Publ. Ser. Proc. Rep. Intern Assoc Hydrol. Sci. 1995, 233, 147–152. [Google Scholar]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Muuronen, M.; Parker, S.M.; Berardo, E.; Le, A.; Zwijnenburg, M.A.; Furche, F. Mechanism of photocatalytic water oxidation on small TiO2 nanoparticles. Chem. Sci. 2017, 8, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. Photocatalytic water oxidation on ZnO: A review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Deng, X.; Tüysüz, H. Cobalt-Oxide-Based Materials as Water Oxidation Catalyst: Recent Progress and Challenges. ACS Catal. 2014, 4, 3701–3714. [Google Scholar] [CrossRef]
- Roy, C.; Sebok, B.; Scott, S.B.; Fiordaliso, E.M.; Sørensen, J.E.; Bodin, A.; Trimarco, D.B.; Damsgaard, C.D.; Vesborg, P.C.K.; Hansen, O.; et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 2018, 1, 820–829. [Google Scholar] [CrossRef]
- Senasu, T.; Nanan, S. Photocatalytic performance of CdS nanomaterials for photodegradation of organic azo dyes under artificial visible light and natural solar light irradiation. J. Mater. Sci. Mater. Electron. 2017, 28, 17421–17441. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Zhou, Y.; Liu, J.; Wang, Z. Facile synthesis of cerium oxide nanoparticles decorated flower-like bismuth molybdate for enhanced photocatalytic activity toward organic pollutant degradation. J. Colloid Interface Sci. 2018, 530, 171–178. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Zhou, Y.; Liu, Y.; Mo, L. Hierarchical architectures of bismuth molybdate nanosheets onto nickel titanate nanofibers: Facile synthesis and efficient photocatalytic removal of tetracycline hydrochloride. J. Colloid Interface Sci. 2018, 521, 42–49. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Liu, J.; Wang, Z. Facile synthesis of flower-like Ag3VO4/Bi2WO6 heterojunction with enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2017, 501, 156–163. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Liu, Y.; Zhou, Y.; Mo, L.; Liu, J. Ag3VO4 nanoparticles decorated Bi2O2CO3 micro-flowers: An efficient visible-light-driven photocatalyst for the removal of toxic contaminants. Front. Chem. 2018, 6, 255. [Google Scholar] [CrossRef]
- Li, S.; Shen, X.; Liu, J.; Zhang, L. Synthesis of Ta3N5/Bi2MoO6 core–shell fiber-shaped heterojunctions as efficient and easily recyclable photocatalysts. Environ. Sci. Nano 2017, 4, 1155–1167. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Roshan, R.; Kumar, V. Mg-doped ZnO nanoparticles for efficient sunlight-driven photocatalysis. ACS Appl. Mater. Interfaces 2012, 4, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Woodley, S.B.; Sokol, A.A.; Catlow, C.R.A.; Al-Sunaidi, A.A.; Woodley, S.M. Structural and optical properties of Mg and Cd doped ZnO nanoclusters. J. Phys. Chem. C 2013, 117, 27127–27145. [Google Scholar] [CrossRef]
- Srivastava, V.C. Photocatalytic oxidation of dye bearing wastewater by iron doped zinc oxide. Ind. Eng. Chem. Res. 2013, 52, 17790–17799. [Google Scholar]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Modwi, A.; Abbo, M.A.; Hassan, E.A.; Al-Duaij, O.K.; Houas, A. Adsorption kinetics and photocatalytic degradation of malachite green (MG) via Cu/ZnO nanocomposites. J. Environ. Chem. Eng. 2017, 5, 5954–5960. [Google Scholar] [CrossRef]
- Sun, J.-H.; Dong, S.-Y.; Feng, J.-L.; Yin, X.-J.; Zhao, X.-C. Enhanced sunlight photocatalytic performance of Sn-doped ZnO for Methylene Blue degradation. J. Mol. Catal. A Chem. 2011, 335, 145–150. [Google Scholar] [CrossRef]
- Jena, M.; Manjunatha, C.; Shivaraj, B.W.; Nagaraju, G.; Ashoka, S.; Sham Aan, M.P. Optimization of parameters for maximizing photocatalytic behaviour of Zn1-xFexO nanoparticles for methyl orange degradation using Taguchi and Grey relational analysis Approach. Mater. Today Chem. 2019, 12, 187–199. [Google Scholar] [CrossRef]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef]
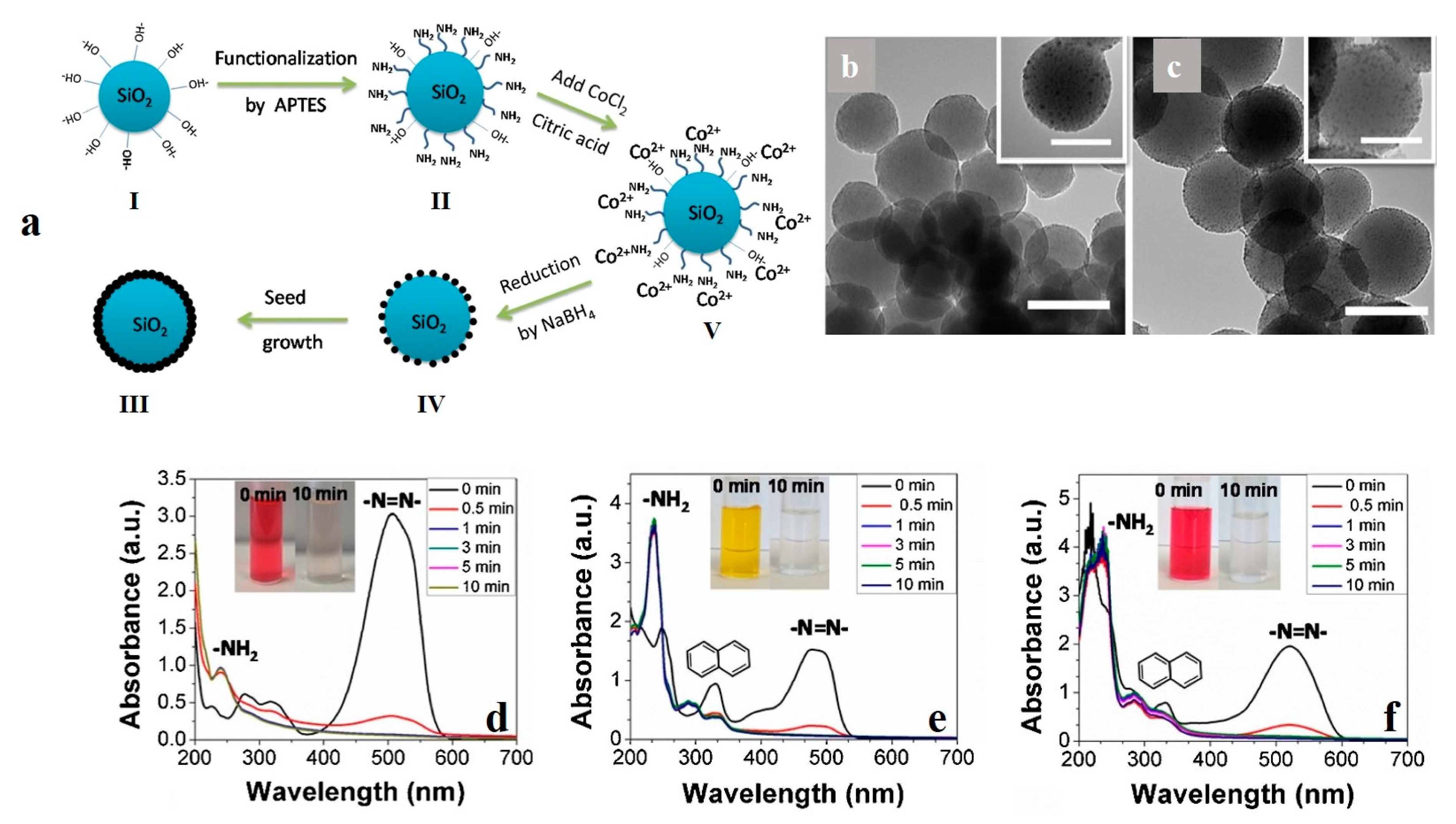
- Zhang, Y.; Gao, F.; Wanjala, B.; Li, Z.; Cernigliaro, G.; Gu, Z. High efficiency reductive degradation of a wide range of azo dyes by SiO2-Co core-shell nanoparticles. Appl. Catal. B Environ. 2016, 199, 504–513. [Google Scholar] [CrossRef]
- Seabra, A.B.; Duran, N. Nanotoxicology of metal oxide nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef]
- Seabra, A.B.; Haddad, P.S. Cytotoxicity and genotoxicity of iron oxides nanoparticles. In Nanotoxicology; Springer: New York, NY, USA, 2014; pp. 265–279. [Google Scholar]
- Sheng, L.; Wang, L.; Sang, X.; Zhao, X.; Hong, J.; Cheng, S.; Yu, X.; Liu, D.; Xu, B.; Hu, R. Nano-sized titanium dioxide-induced splenic toxicity: A biological pathway explored using microarray technology. J. Hazard. Mater. 2014, 278, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Takamura, K.; Hayashi, K.; Ishinishi, N.; Yamada, T.; Sugioka, Y. Evaluation of carcinogenicity and chronic toxicity associated with orthopedic implants in mice. J. Biomed. Mater. Res. 1994, 28, 583–589. [Google Scholar] [CrossRef]
- Ko, K.-S.; Kong, I.C. Toxic effects of nanoparticles on bioluminescence activity, seed germination, and gene mutation. Appl. Microbiol. Biotechnol. 2014, 98, 3295–3303. [Google Scholar] [CrossRef]
- Xie, Y.; He, C.; Liu, L.; Mao, L.; Wang, K.; Huang, Q.; Liu, M.; Wan, Q.; Deng, F.; Huang, H.; et al. Carbon nanotube based polymer nanocomposites: Biomimic preparation and organic dye adsorption applications. Rsc Adv. 2015, 5, 82503–82512. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef]
- Robati, D.; Mirza, B.; Ghazisaeidi, R.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Adsorption behavior of methylene blue dye on nanocomposite multi-walled carbon nanotube functionalized thiol (MWCNT-SH) as new adsorbent. J. Mol. Liq. 2016, 216, 830–835. [Google Scholar] [CrossRef]
- Mahmoodian, H.; Moradi, O.; Shariatzadeha, B.; Salehf, T.A.; Tyagi, I.; Maity, A.; Asif, M.; Gupta, V.K. Enhanced removal of methyl orange from aqueous solutions by poly HEMA-chitosan-MWCNT nano-composite. J. Mol. Liq. 2015, 202, 189–198. [Google Scholar] [CrossRef]
- Sun, X.; Ou, H.; Miao, C.; Chen, L. Removal of sudan dyes from aqueous solution by magnetic carbon nanotubes: Equilibrium, kinetic and thermodynamic studies. J. Ind. Eng. Chem. 2015, 22, 373–377. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.; Qian, X.; Dong, M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J. Colloid Interface Sci. 2018, 509, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety considerations for graphene: Lessons learnt from carbon nanotubes. Acc. Chem. Res. 2012, 46, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Strunskus, T.; Strobel, J.; Kienle, L.; Elbahri, M. A Flexible Oxygenated Carbographite Nanofilamentous Buckypaper as an Amphiphilic Membrane. Adv. Mater. Interfaces 2018, 5, 1800001. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.-Y.; Young, T.-H.; Yang, H.J.; Ji, Y.-R. An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohydr. Polym. 2019, 224, 115112. [Google Scholar] [CrossRef]
- Homaeigohar, S. Amphiphilic Oxygenated Amorphous Carbon-Graphite Buckypapers with Gas Sensitivity to Polar and Non-Polar VOCs. Nanomaterials 2019, 9, 1343. [Google Scholar] [CrossRef]
- Cao, X.; Huang, M.; Ding, B.; Yu, J.; Sun, G. Robust polyacrylonitrile nanofibrous membrane reinforced with jute cellulose nanowhiskers for water purification. Desalination 2013, 316, 120–126. [Google Scholar] [CrossRef]
- Qiao, H.; Zhou, Y.; Yu, F.; Wang, E.; Min, Y.; Huang, Q.; Pang, L.; Ma, T. Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 2015, 141, 297–303. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Jin, L.; Li, W.; Xu, Q.; Sun, Q. Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 2015, 22, 2443–2456. [Google Scholar] [CrossRef]
- Jin, L.; Sun, Q.; Xu, Q.; Xu, Y. Adsorptive removal of anionic dyes from aqueous solutions using microgel based on nanocellulose and polyvinylamine. Bioresour. Technol. 2015, 197, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultra-fine cellulose nanofibers: New nano-scale materials for water purification. J. Mater. Chem. 2011, 21, 7507–7510. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous Microfiltration Membrane Based on Cellulose Nanowhiskers. Biomacromolecules 2012, 13, 180–186. [Google Scholar] [CrossRef]
- Lee, J.; Ham, S.; Jang, D.-J. Facile fabrication of Cu-exchanged ZnS nanoadsorbents for highly efficient removal of contaminants. J. Environ. Chem. Eng. 2017, 5, 4431–4440. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Wu, Y.-n.; Zhang, B.; Li, F. Dendrimer-based preparation of mesoporous alumina nanofibers by electrospinning and their application in dye adsorption. Chem. Eng. J. 2015, 264, 48–55. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Ruan, Z.; Cui, G.; Yan, S. Synthesis of NiCo2O4 nanostructures with different morphologies for the removal of methyl orange. Appl. Surf. Sci. 2017, 393, 434–440. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, E.; Dai, W.; Li, Z.; Zhong, Y.; Hu, Y. A facile sacrificial template method to synthesize one-dimensional porous CdO/CdFe2O4 hybrid nanoneedles with superior adsorption performance. RSC Adv. 2017, 7, 5093–5100. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.; Wang, Q.; Han, X.; Li, X.; Liu, J.; Lin, H.; Qu, F. The synthesis of ZnO/SnO2 porous nanofibers for dye adsorption and degradation. Dalton Trans. 2015, 44, 3034–3042. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Dai, T.; Elbahri, M. Biofunctionalized nanofibrous membranes as super separators of protein and enzyme from water. J. Colloid Interface Sci. 2013, 406, 86–93. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Disci-Zayed, D.; Dai, T.; Elbahri, M. Biofunctionalized nanofibrous membranes mimicking carnivorous plants. BioinspiredBiomim. Nanobiomater. 2013, 2, 186–193. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. An Amphiphilic, Graphitic Buckypaper Capturing Enzyme Biomolecules from Water. Water 2019, 11, 2. [Google Scholar] [CrossRef]
- Homaeigohar, S.S.; Elbahri, M. Novel compaction resistant and ductile nanocomposite nanofibrous microfiltration membranes. J. Colloid Interface Sci. 2012, 372, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Elbahri, M.; Homaeigohar, S.; Dai, T.; Abdelaziz, R.; Khalil, R.; Zillohu, A.U. Smart Metal-Polymer Bionanocomposites as Omnidirectional Plasmonic Black Absorbers Formed by Nanofluid Filtration. Adv. Funct. Mater. 2012, 22, 4771–4777. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Koll, J.; Lilleodden, E.T.; Elbahri, M. The solvent induced interfiber adhesion and its influence on the mechanical and filtration properties of polyethersulfone electrospun nanofibrous microfiltration membranes. Sep. Purif. Technol. 2012, 98, 456–463. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Sagadevan, V. Polyethylene glycol and iron oxide nanoparticles blended polyethersulfone ultrafiltration membrane for enhanced performance in dye removal studies. e-Polymers 2015, 15, 151–159. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Sun, C.; Ji, H.; Zhao, W.; Sun, S.; Zhao, C. Graphene oxide-based polymeric membranes for broad water pollutant removal. RSC Adv. 2015, 5, 100651–100662. [Google Scholar] [CrossRef]
- Lin, C.-H.; Gung, C.-H.; Sun, J.-J.; Suen, S.-Y. Preparation of polyethersulfone/plant-waste-particles mixed matrix membranes for adsorptive removal of cationic dyes from water. J. Membr. Sci. 2014, 471, 285–298. [Google Scholar] [CrossRef]
- Lala, N.L.; Jose, R.; Yusoff, M.M.; Ramakrishna, S. Continuous tubular nanofibers of vanadium pentoxide by electrospinning for energy storage devices. J. Nanoparticle Res. 2012, 14, 1–9. [Google Scholar] [CrossRef]
- Im, J.S.; Kwon, O.; Kim, Y.H.; Park, S.-J.; Lee, Y.-S. The effect of embedded vanadium catalyst on activated electrospun CFs for hydrogen storage. Microporous Mesoporous Mater. 2008, 115, 514–521. [Google Scholar] [CrossRef]
- Huang, J.-S.; Chou, C.-Y.; Liu, M.-Y.; Tsai, K.-H.; Lin, W.-H.; Lin, C.-F. Solution-processed vanadium oxide as an anode interlayer for inverted polymer solar cells hybridized with ZnO nanorods. Org. Electron. 2009, 10, 1060–1065. [Google Scholar] [CrossRef]
- González, G.; Saraiva, S.M.; Aliaga, W. Isoelectric points for niobium and vanadium pentoxides. J. Dispers. Sci. 1994, 15, 249. [Google Scholar] [CrossRef]
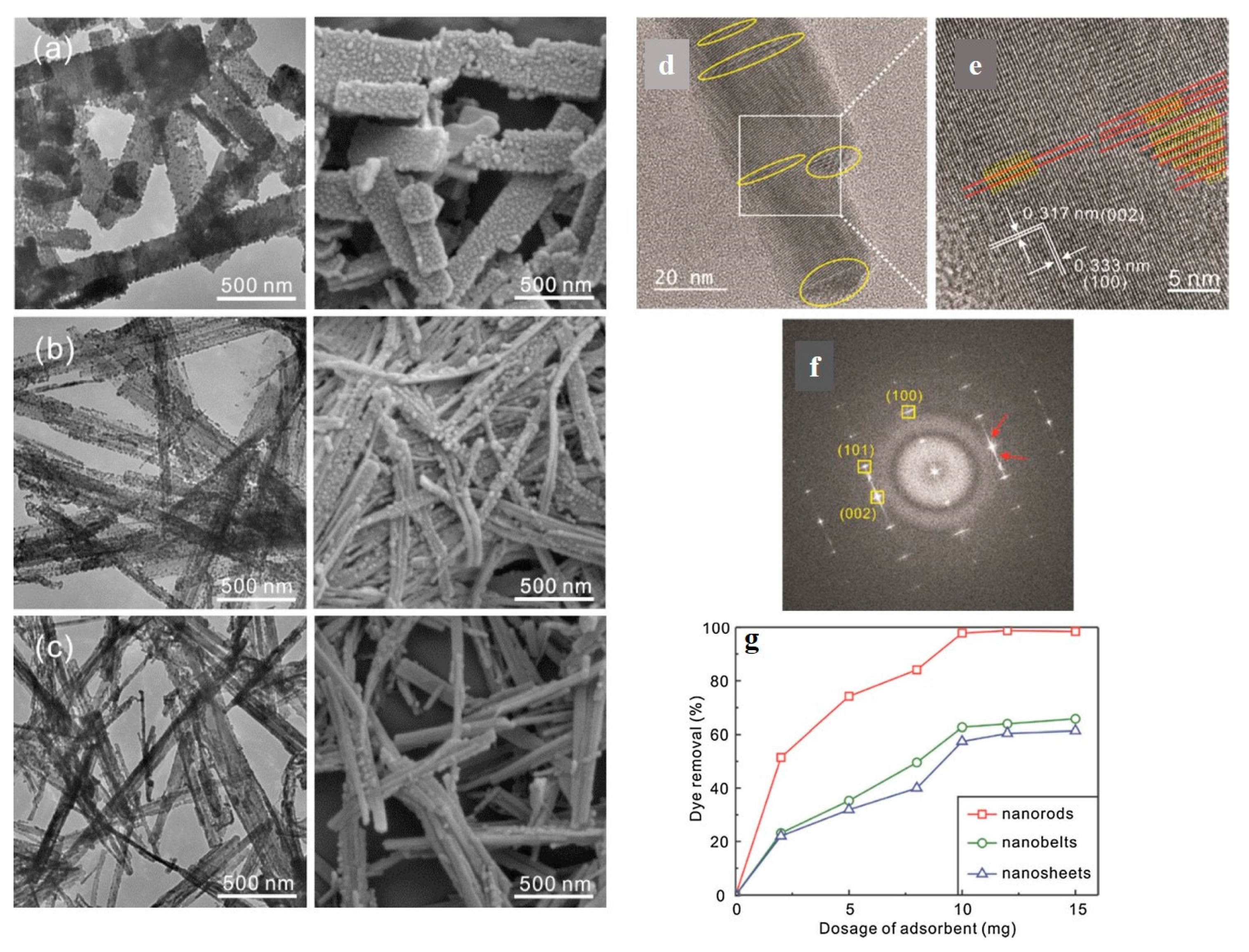
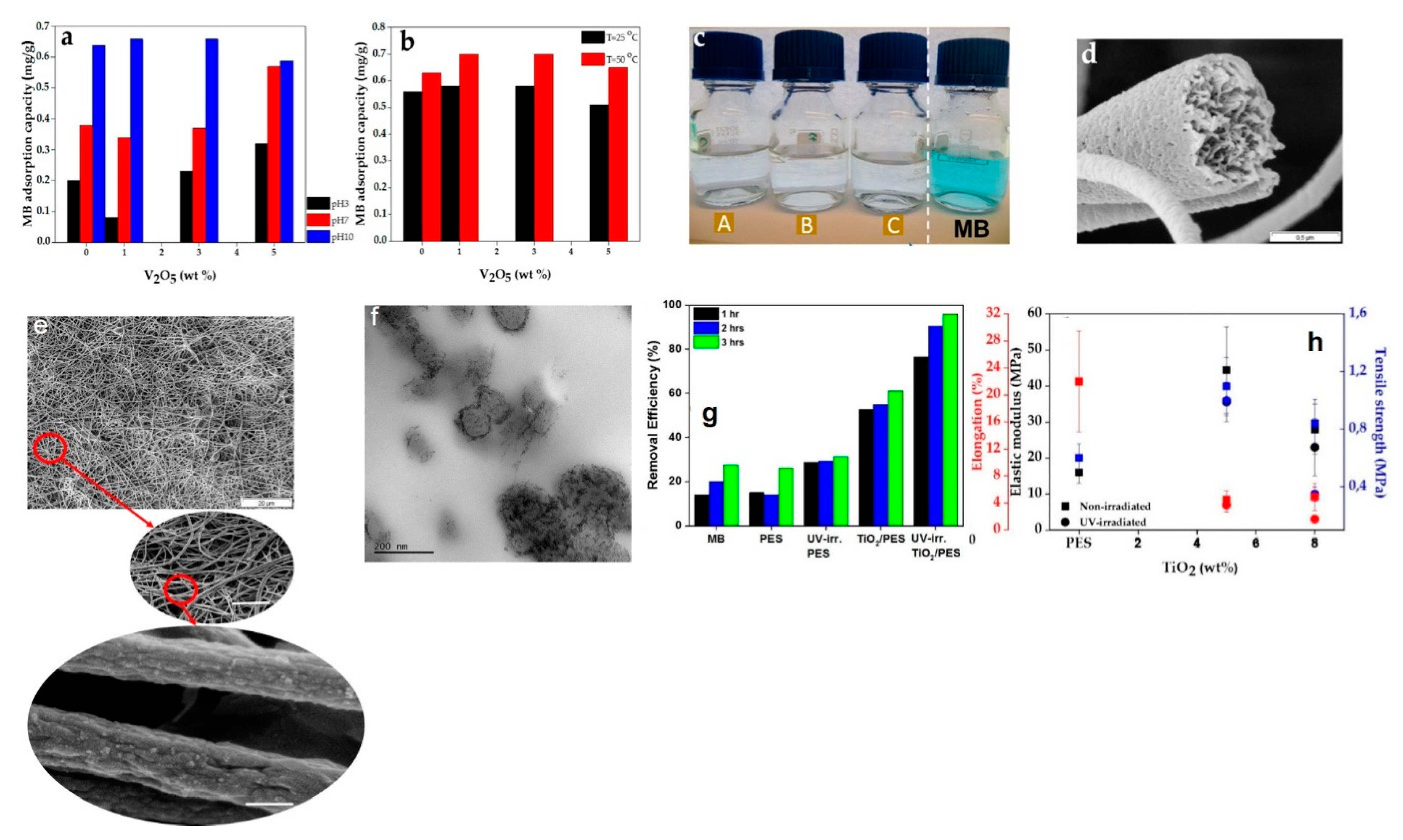
- Avansi, W.; de Mendonça, V.R.; Lopes, O.F.; Ribeiro, C. Vanadium pentoxide 1-D nanostructures applied to dye removal from aqueous systems by coupling adsorption and visible-light photodegradation. RSC Adv. 2015, 5, 12000–12006. [Google Scholar] [CrossRef]
- Homaeigohar, S.S.; Mahdavi, H.; Elbahri, M. Extraordinarily water permeable sol gel formed nanocomposite nanofibrous membranes. J. Colloid Interface Sci. 2012, 366, 51–56. [Google Scholar] [CrossRef]
- Cho, S.; Choi, W. Solid-phase photocatalytic degradation of PVC-TiO2 polymer composites. J. Photochem. Photobiol. A Chem. 2001, 143, 221–228. [Google Scholar] [CrossRef]
- Lei, X.; Li, X.; Ruan, Z.; Zhang, T.; Pan, F.; Li, Q.; Xia, D.; Fu, J. Adsorption-photocatalytic degradation of dye pollutant in water by graphite oxide grafted titanate nanotubes. J. Mol. Liq. 2018, 266, 122–131. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of anionic azo-dyes from aqueous solutions onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef]
- Chang, J.; Zhou, G.; Christensen, E.R.; Heideman, R.; Chen, J. Graphene-based sensors for detection of heavy metals in water: A review. Anal. Bioanal. Chem. 2014, 406, 3957–3975. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, W.; Xie, Z.; Tan, Y.; Song, Y.; Zheng, Q. Environmentally friendly reduced graphene oxide as a broad-spectrum adsorbent for anionic and cationic dyes via π–π interactions. J. Mater. Chem. A 2016, 4, 12126–12135. [Google Scholar] [CrossRef]
- Minitha, C.R.; Lalitha, M.; Jeyachandran, Y.L.; Senthilkumar, L.; Rajendra Kumar, R.T. Adsorption behaviour of reduced graphene oxide towards cationic and anionic dyes: Co-action of electrostatic and π—π interactions. Mater. Chem. Phys. 2017, 194, 243–252. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q. Health and ecosystem risks of graphene. Chem. Rev. 2013, 113, 3815–3835. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A. Graphene: Safe or toxic? The two faces of the medal. Angew. Chem. Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Sharma, S.K.; An, N.; Lee, H.; Kim, D.Y.; Im, Y.B.; Cho, Y.D.; Ganesh, R.S.; Ponnusamy, S.; Raji, P.; et al. Photocatalytic properties of Mn-doped NiO spherical nanoparticles synthesized from sol-gel method. Optik 2016, 127, 10727–10734. [Google Scholar] [CrossRef]
- Roffi, T.M.; Nozaki, S.; Uchida, K. Growth mechanism of single-crystalline NiO thin films grown by metal organic chemical vapor deposition. J. Cryst. Growth 2016, 451, 57–64. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Wang, C. Synthesis of β-cyclodextrin-based electrospun nanofiber membranes for highly efficient adsorption and separation of methylene blue. ACS Appl. Mater. Interfaces 2015, 7, 26649–26657. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Haixia, L.; Huali, L.; Yu, L.; Huayong, Z.; Tianduo, L. Solvothermal synthesis and photocatalytic properties of NiO ultrathin nanosheets with porous structure. Appl. Surf. Sci. 2015, 328, 525–530. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kaur, Y.; Jayee, B.; Chaudhary, G.R.; Umar, A. NiO nanodisks: Highly efficient visible-light driven photocatalyst, potential scaffold for seed germination of Vigna Radiata and antibacterial properties. J. Clean. Prod. 2018, 190, 563–576. [Google Scholar] [CrossRef]
- Ye, L.; Xu, H.; Zhang, D.; Chen, S. Synthesis of bilayer MoS2 nanosheets by a facile hydrothermal method and their methyl orange adsorption capacity. Mater. Res. Bull. 2014, 55, 221–228. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.; Lee, Y.-H.; Shi, Y.; Hsu, A.; Chin, M.L.; Li, L.-J.; Dubey, M.; Kong, J.; Palacios, T. Integrated Circuits Based on Bilayer MoS2 Transistors. Nano Lett. 2012, 12, 4674–4680. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Min, S.-W.; Chang, Y.-G.; Park, M.K.; Nam, T.; Kim, H.; Kim, J.H.; Ryu, S.; Im, S. MoS2 Nanosheet Phototransistors with Thickness-Modulated Optical Energy Gap. Nano Lett. 2012, 12, 3695–3700. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; You, S.; Jia, X.H.; Yang, J. MoS2 nanosheets decorated with magnetic Fe3O4 nanoparticles and their ultrafast adsorption for wastewater treatment. Ceram. Int. 2015, 41, 13896–13902. [Google Scholar] [CrossRef]
- Roobakhsh, S.; Rostami, Z.; Azizian, S. Can MoS2 nanosheets be used as adsorbent for water treatment? Sep. Purif. Technol. 2018, 200, 23–28. [Google Scholar] [CrossRef]
- Song, H.; You, S.; Jia, X. Synthesis of fungus-like MoS2 nanosheets with ultrafast adsorption capacities toward organic dyes. Appl. Phys. A 2015, 121, 541–548. [Google Scholar] [CrossRef]
- Xie, H.; Xiong, X. A porous molybdenum disulfide and reduced graphene oxide nanocomposite (MoS2- rGO) with high adsorption capacity for fast and preferential adsorption towards Congo red. J. Environ. Chem. Eng. 2017, 5, 1150–1158. [Google Scholar] [CrossRef]
- Tian, C.; Xiang, X.; Wu, J.; Li, B.; Cai, C.; Khan, B.; Chen, H.; Yuan, Y.; Zu, X. Facile Synthesis of MoS2/CuS Nanosheet Composites as an Efficient and Ultrafast Adsorbent for Water-Soluble Dyes. J. Chem. Eng. Data 2018, 63, 3966–3974. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental Applications of 2D Molybdenum Disulfide (MoS2) Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Lei, W.; Portehault, D.; Liu, D.; Qin, S.; Chen, Y. Porous boron nitride nanosheets for effective water cleaning. Nat. Commun. 2013, 4, 1777. [Google Scholar] [CrossRef]
- Pang, J.; Chao, Y.; Chang, H.; Li, H.; Xiong, J.; Zhang, Q.; Chen, G.; Qian, J.; Zhu, W.; Li, H. Silver Nanoparticle-Decorated Boron Nitride with Tunable Electronic Properties for Enhancement of Adsorption Performance. ACS Sustain. Chem. Eng. 2018, 6, 4948–4957. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Li, P.; Ye, J.; Lin, J. Synthesis and photocatalytic properties of metastable β-Bi2O3 stabilized by surface-coordination effects. J. Mater. Chem. A 2015, 3, 5119–5125. [Google Scholar] [CrossRef]
- Li, K.; Liang, Y.; Yang, J.; Gao, Q.; Zhu, Y.; Liu, S.; Xu, R.; Wu, X. Controllable synthesis of {001} facet dependent foursquare BiOCl nanosheets: A high efficiency photocatalyst for degradation of methyl orange. J. Alloy. Compd. 2017, 695, 238–249. [Google Scholar] [CrossRef]
- Ye, L.; Jin, X.; Liu, C.; Ding, C.; Xie, H.; Chu, K.H.; Wong, P.K. Thickness-ultrathin and bismuth-rich strategies for BiOBr to enhance photoreduction of CO2 into solar fuels. Appl. Catal. B Environ. 2016, 187, 281–290. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, K.; Liu, J.; Luo, H.; Huang, Y.; Yu, X. Controllable synthesis of hollow/flower-like BiOI microspheres and highly efficient adsorption and photocatalytic activity. CrystEngComm 2012, 14, 4384–4390. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, G.; Liu, J.; Zhang, Y. Hierarchically structured α-Fe2O3/Bi2WO6 composite for photocatalytic degradation of organic contaminants under visible light irradiation. RSC Adv. 2013, 3, 2963–2970. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Sun, Z.; Zhang, Q.; Wang, L.; Chen, Z. In-situ synthesis of heterostructured BiVO4/BiOBr core-shell hierarchical mesoporous spindles with highly enhanced visible-light photocatalytic performance. J. Alloy. Compd. 2017, 713, 78–86. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B Environ. 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.; Li, K.; Zhu, Y.; Liu, S.; Xu, R.; Zhou, W. Design of 3D flowerlike BiOClxBr1-x nanostructure with high surface area for visible light photocatalytic activities. J. Alloy. Compd. 2017, 725, 1144–1157. [Google Scholar] [CrossRef]
- Wang, G.; Luo, X.; Huang, Y.; Kuang, A.; Yuan, H.; Chen, H. BiOX/BiOY (X, Y = F, Cl, Br, I) superlattices for visible light photocatalysis applications. RSC Adv. 2016, 6, 91508–91516. [Google Scholar] [CrossRef]
- Pare, B.; Sarwan, B.; Jonnalagadda, S. Photocatalytic mineralization study of malachite green on the surface of Mn-doped BiOCl activated by visible light under ambient condition. Appl. Surf. Sci. 2011, 258, 247–253. [Google Scholar] [CrossRef]
- Fu, J.; Tian, Y.; Chang, B.; Xi, F.; Dong, X. BiOBr-carbon nitride heterojunctions: Synthesis, enhanced activity and photocatalytic mechanism. J. Mater. Chem. 2012, 22, 21159–21166. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.-D. Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity. J. Mater. Chem. 2010, 20, 5866–5870. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Hu, J.; Lv, J.; Wang, J.; Wu, Y. Fabrication and photocatalytic performances of BiOCl nanosheets modified with ultrafine Bi2O3 nanocrystals. RSC Adv. 2016, 6, 63241–63249. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Q.; Ji, M.; Zhu, H.; Hou, H.; Yang, Q.; Jiang, C.; Wang, J.; Tian, L.; Chen, J. Constructing Z-scheme charge separation in 2D layered porous BiOBr/graphitic C3N4 nanosheets nanojunction with enhanced photocatalytic activity. J. Alloy. Compd. 2017, 723, 1121–1131. [Google Scholar] [CrossRef]
- Song, C.; Feng, Y.; Shi, W.; Liu, C. Fabrication and mechanism of a novel direct solid-state Z-scheme photocatalyst CdS/BiOI under visible light. CrystEngComm 2016, 18, 7796–7804. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Two different roles of metallic Ag on Ag/AgX/BiOX (X= Cl, Br) visible light photocatalysts: Surface plasmon resonance and Z-scheme bridge. Acs Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, X.; Wei, Z.; Li, X.; Xi, X.; Hu, R.; Tang, B.; Wang, R.; Wang, S.; Wang, T. Photocatalytic properties of hierarchical structures based on Fe-doped BiOBr hollow microspheres. J. Mater. Chem. A 2013, 1, 2406–2410. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, G.; Wang, X.; Hu, R.; Xi, X.; Bao, S.; Zhou, Y.; Tong, T.; Wang, S.; Wang, T.; et al. Efficient visible-light-induced photocatalytic activity over the novel Ti-doped BiOBr microspheres. Powder Technol. 2012, 228, 258–263. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Yin, S.; Zhang, Q.; Chen, Z.; Li, H. Carbon Quantum Dots Modified BiOCl Ultrathin Nanosheets with Enhanced Molecular Oxygen Activation Ability for Broad Spectrum Photocatalytic Properties and Mechanism Insight. ACS Appl. Mater. Interfaces 2015, 7, 20111–20123. [Google Scholar] [CrossRef]
- Ren, K.; Liu, J.; Liang, J.; Zhang, K.; Zheng, X.; Luo, H.; Huang, Y.; Liu, P.; Yu, X. Synthesis of the bismuth oxyhalide solid solutions with tunable band gap and photocatalytic activities. Dalton Trans. 2013, 42, 9706–9712. [Google Scholar] [CrossRef]
- Ouyang, S.; Ye, J. β-AgAl1-xGaxO2 Solid-Solution Photocatalysts: Continuous Modulation of Electronic Structure toward High-Performance Visible-Light Photoactivity. J. Am. Chem. Soc. 2011, 133, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Gnayem, H.; Sasson, Y. Hierarchical Nanostructured 3D Flowerlike BiOClxBr1–x Semiconductors with Exceptional Visible Light Photocatalytic Activity. ACS Catal. 2013, 3, 186–191. [Google Scholar] [CrossRef]
- Gnayem, H.; Sasson, Y. Nanostructured 3D Sunflower-like Bismuth Doped BiOClxBr1–x Solid Solutions with Enhanced Visible Light Photocatalytic Activity as a Remarkably Efficient Technology for Water Purification. J. Phys. Chem. C 2015, 119, 19201–19209. [Google Scholar] [CrossRef]
- Qin, Q.; Guo, Y.; Zhou, D.; Yang, Y.; Guo, Y. Facile growth and composition-dependent photocatalytic activity of flowerlike BiOCl1−xBrxhierarchical microspheres. Appl. Surf. Sci. 2016, 390, 765–777. [Google Scholar] [CrossRef]
- Kim, W.J.; Pradhan, D.; Min, B.-K.; Sohn, Y. Adsorption/photocatalytic activity and fundamental natures of BiOCl and BiOClxI1−x prepared in water and ethylene glycol environments, and Ag and Au-doping effects. Appl. Catal. B Environ. 2014, 147, 711–725. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Yang, G.; Zhu, Y.; Si, H.; Zhang, J.; Li, Y. Preparation and characterization of bifunctional BiOClxIy solid solutions with excellent adsorption and photocatalytic abilities for removal of organic dyes. Mater. Sci. Semicond. Process. 2016, 41, 193–199. [Google Scholar] [CrossRef]
- Xie, J.; Cao, Y.; Jia, D.; Li, Y. Dahlia-shaped BiOClxI1−x structures prepared by a facile solid-state method: Evidence and mechanism of improved photocatalytic degradation of rhodamine B dye. J. Colloid Interface Sci. 2017, 503, 115–123. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, J.; Hng, H.H.; Ma, J.; Chen, X. A Leavening Strategy to Prepare Reduced Graphene Oxide Foams. Adv. Mater. 2012, 24, 4144–4150. [Google Scholar] [CrossRef]
- Yang, S.J.; Kang, J.H.; Jung, H.; Kim, T.; Park, C.R. Preparation of a freestanding, macroporous reduced graphene oxide film as an efficient and recyclable sorbent for oils and organic solvents. J. Mater. Chem. A 2013, 1, 9427–9432. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Hu, Y.; Cheng, H.; Shi, G.; Qu, L. A Versatile, Ultralight, Nitrogen-Doped Graphene Framework. Angew. Chem. Int. Ed. 2012, 51, 11371–11375. [Google Scholar] [CrossRef]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy Graphene as a Highly Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Pham, H.D.; Pham, V.H.; Cuong, T.V.; Nguyen-Phan, T.-D.; Chung, J.S.; Shin, E.W.; Kim, S. Synthesis of the chemically converted graphene xerogel with superior electrical conductivity. Chem. Commun. 2011, 47, 9672–9674. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.-O.; Park, S.; Park, H.S. Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J. Ind. Eng. Chem. 2015, 21, 1191–1196. [Google Scholar] [CrossRef]
- Perazzolo, V.; Grądzka, E.; Durante, C.; Pilot, R.; Vicentini, N.; Rizzi, G.A.; Granozzi, G.; Gennaro, A. Chemical and Electrochemical Stability of Nitrogen and Sulphur Doped Mesoporous Carbons. Electrochim. Acta 2016, 197, 251–262. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Wang, A.-J.; Wu, X.-L.; Chen, J.-R.; Feng, J.-J. Green-assembly of three-dimensional porous graphene hydrogels for efficient removal of organic dyes. J. Colloid Interface Sci. 2016, 484, 254–262. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Xu, A.; Tang, K.; Huang, Y.; Lu, C. In situ reduced and assembled three-dimensional graphene aerogel for efficient dye removal. J. Alloy. Compd. 2017, 714, 522–529. [Google Scholar] [CrossRef]
- Massey, A.T.; Gusain, R.; Kumari, S.; Khatri, O.P. Hierarchical Microspheres of MoS2 Nanosheets: Efficient and Regenerative Adsorbent for Removal of Water-Soluble Dyes. Ind. Eng. Chem. Res. 2016, 55, 7124–7131. [Google Scholar] [CrossRef]
- Ren, B.; Shen, W.; Li, L.; Wu, S.; Wang, W. 3D CoFe2O4 nanorod/flower-like MoS2 nanosheet heterojunctions as recyclable visible light-driven photocatalysts for the degradation of organic dyes. Appl. Surf. Sci. 2018, 447, 711–723. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Xu, C.; Yang, P.; Ng, D.H.L.; Song, P.; Zuo, M. Hierarchically porous MoS2/CoAl-LDH/HCF with synergistic adsorption-photocatalytic performance under visible light irradiation. J. Alloy. Compd. 2017, 698, 852–862. [Google Scholar] [CrossRef]
- Wu, M.-H.; Li, L.; Xue, Y.-C.; Xu, G.; Tang, L.; Liu, N.; Huang, W.-Y. Fabrication of ternary GO/g-C3N4/MoS2 flower-like heterojunctions with enhanced photocatalytic activity for water remediation. Appl. Catal. B Environ. 2018, 228, 103–112. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Tan, S.; Yu, X.; Zhu, Q.; Zhou, W. Fabrication of 3D flower-like black N-TiO2-x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal. B Environ. 2017, 201, 119–127. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of Few-Layer MoS2 Nanosheet-Coated TiO2 Nanobelt Heterostructures for Enhanced Photocatalytic Activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef]
- Lai, H.; Ma, G.; Shang, W.; Chen, D.; Yun, Y.; Peng, X.; Xu, F. Multifunctional magnetic sphere-MoS2@Au hybrid for surface-enhanced Raman scattering detection and visible light photo-Fenton degradation of aromatic dyes. Chemosphere 2019, 223, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Bruno, R.; Tiburcio, E.; Casteran, P.-E.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Efficient Capture of Organic Dyes and Crystallographic Snapshots by a Highly Crystalline Amino-Acid-Derived Metal-Organic Framework. Chem. A Eur. J. 2018, 24, 17712–17718. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Li, J.-R.; Lv, X.-L.; Zhang, Y.-Q.; Guo, G. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Nickerl, G.; Notzon, A.; Heitbaum, M.; Senkovska, I.; Glorius, F.; Kaskel, S. Selective Adsorption Properties of Cationic Metal-Organic Frameworks Based on Imidazolic Linker. Cryst. Growth Des. 2013, 13, 198–203. [Google Scholar] [CrossRef]
- Cordova, K.E.; Yaghi, O.M. The ‘folklore’and reality of reticular chemistry. Mater. Chem. Front. 2017, 1, 1304–1309. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- He, Y.-C.; Yang, J.; Kan, W.-Q.; Zhang, H.-M.; Liu, Y.-Y.; Ma, J.-F. A new microporous anionic metal-organic framework as a platform for highly selective adsorption and separation of organic dyes. J. Mater. Chem. A 2015, 3, 1675–1681. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Metal organic frameworks as adsorbents for dye adsorption: Overview, prospects and future challenges. Toxicol. Environ. Chem. 2012, 94, 1846–1863. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the use of metal-organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Grey, D.; Garrick, D.; Blackmore, D.; Kelman, J.; Muller, M.; Sadoff, C. Water security in one blue planet: Twenty-first century policy challenges for science. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120406. [Google Scholar] [CrossRef]
- Levin, R.B.; Epstein, P.R.; Ford, T.E.; Harrington, W.; Olson, E.; Reichard, E.G. US drinking water challenges in the twenty-first century. Environ. Health Perspect. 2002, 110, 43–52. [Google Scholar] [CrossRef]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Acc. Chem. Res. 2012, 46, 834–843. [Google Scholar] [CrossRef]

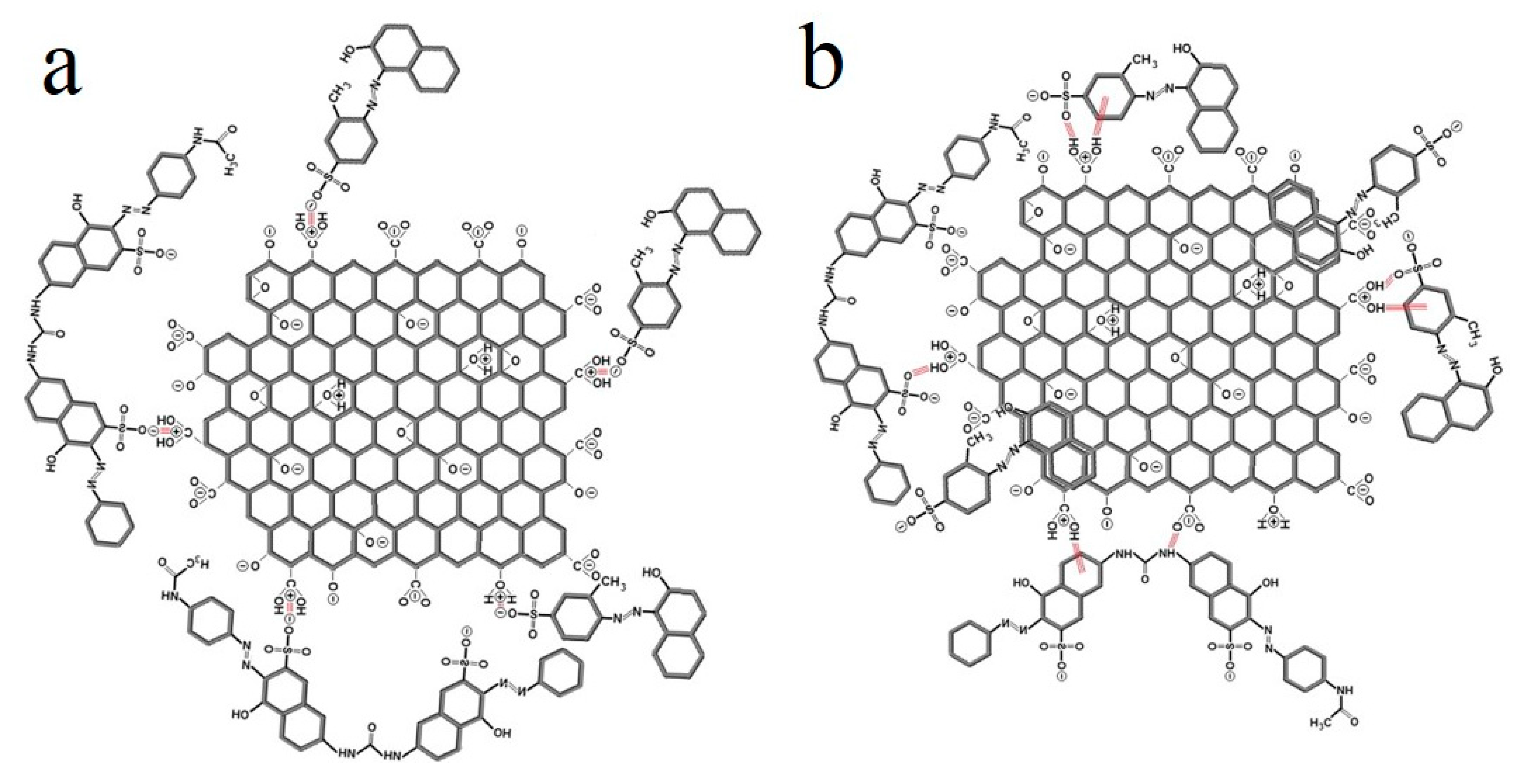
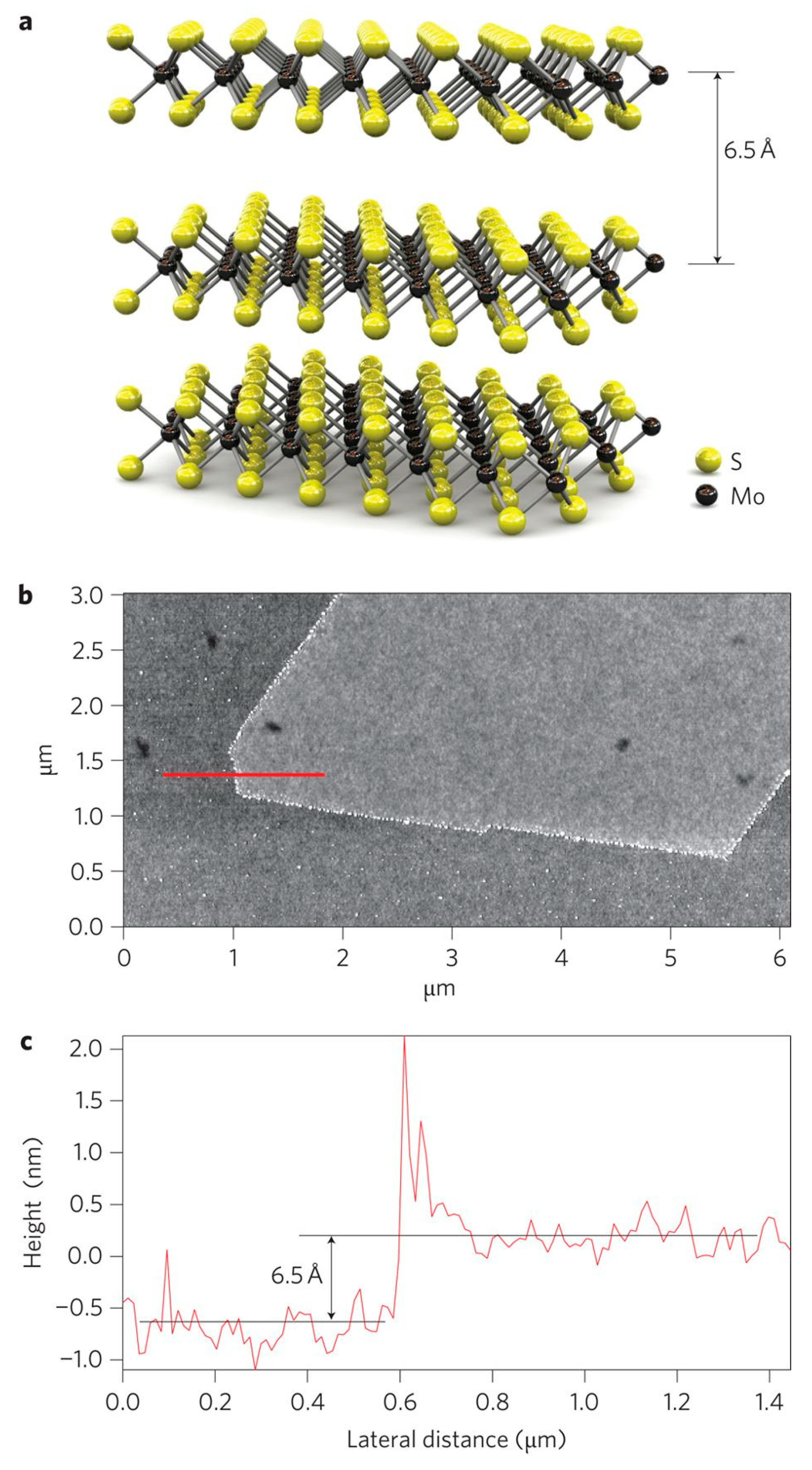
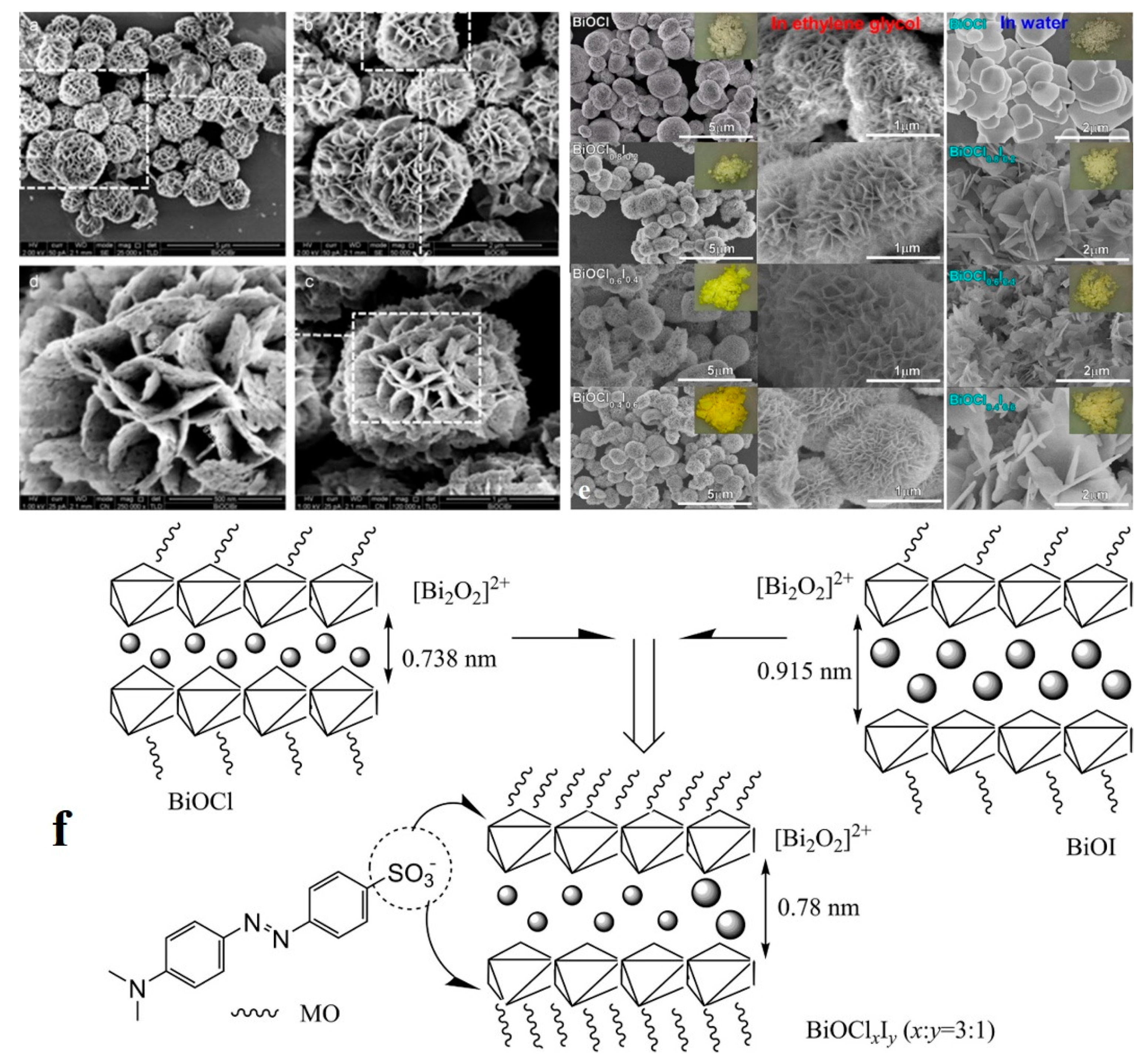
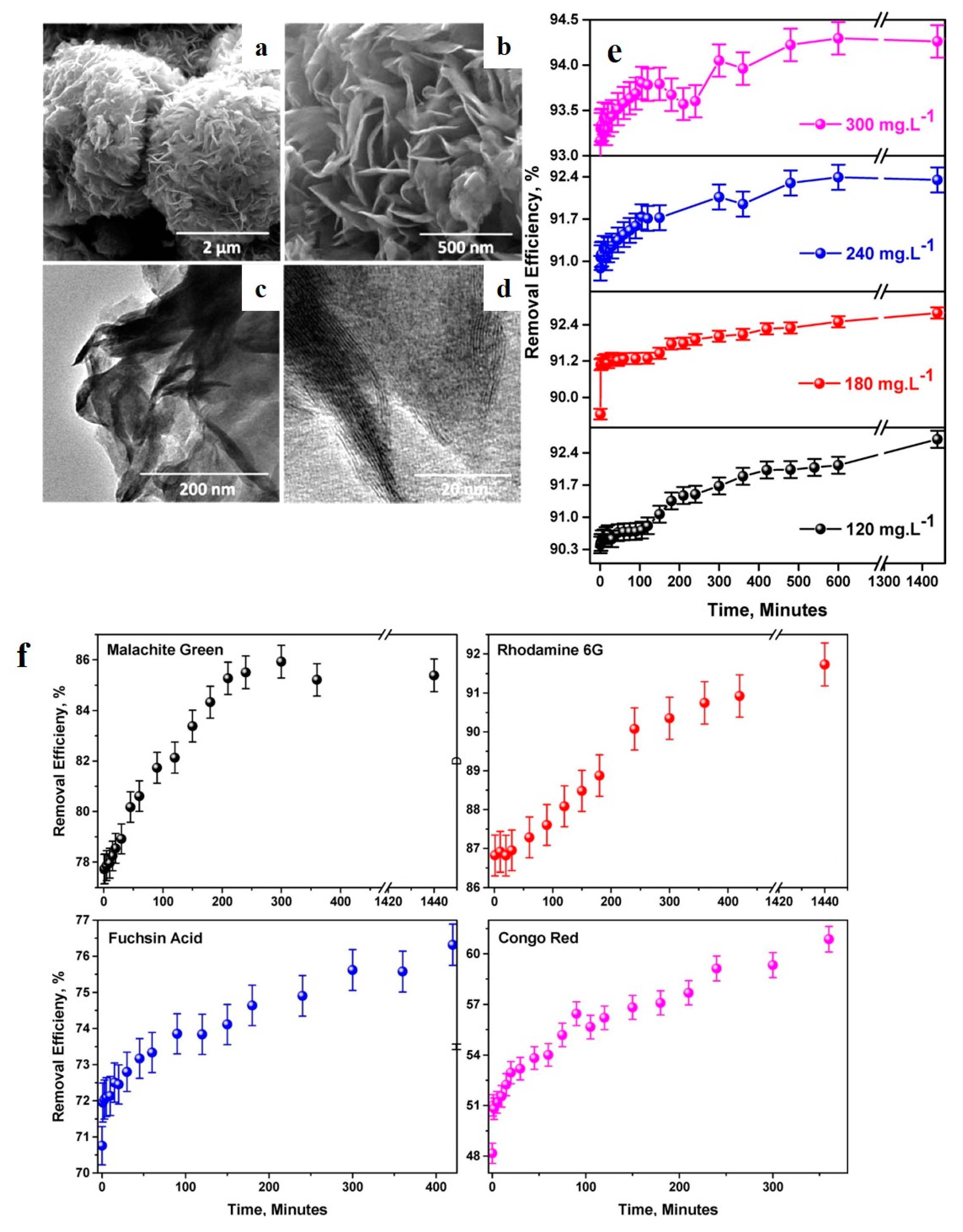
| Dyes | Properties | Applications | Toxicity | Examples |
|---|---|---|---|---|
| Acidic | Soluble in water, anionic | Nylon, wool, silk, paper, leather, ink-jet printing | Carcinogenic | Acid red 183, acid orange 10, acid orange 12, acid orange 8, acid red 73, acid red 18, sunset yellow, acid green 27, methyl orange, amido black 10B, indigo carmine |
| Cationic | Soluble in water, and liberates colored cations | Paper, PAN, treated nylons, treated polyesters, as antiseptic for biomedicine | Carcinogenic | MB, janus green, basic green 5, basic violet 10, rhodamine 6G |
| Disperse | Insoluble in water, non-ionic, for the aqueous/hydrophobic dispersions | Polyester, nylon, cellulose, cellulose acetate, acrylic fibers | Allergenic (skin), carcinogenic | Disperse orange 3, disperse red, disperse red 1, disperse yellow 1 |
| Direct | Soluble in water, anionic, promotes wash fastness in case chelated with metal salts | Cotton, regenerated cellulose, paper, leather | Bladder cancer | CR, direct red 23, direct orange 39, direct blue 86 |
| Reactive | Very high wash fastness thanks to its covalent bond with fiber, generates brighter colors compared to the direct dyes | Cotton, wool, nylon, ink-jet printing of textiles | Dermatitis, allergic conjunctivitis, rhinitis, occupational asthma | Reactive black 5, reactive green 19, reactive blue 4, reactive red 195, reactive red 198, reactive blue 19, reactive red 120 |
| Vat | employs soluble leuco salts following reduction in an alkaline bath (NaOH) | Cellulosic fibers | - | Vat blue 4, vat green 11, vat orange 15, vat orange 28, vat yellow 20 |
| Adsorbent System | Dimension-ality | Dye Models Studied | Adsorption Mechanism | Production Method | Reference |
|---|---|---|---|---|---|
| fungal chitosan nanoparticles | 0D | RBB, MO, DR, NBB, CSB | electrostatic interaction | ionic gelation method | [42] |
| α-chitin nanoparticles | 0D | MB, BPB, CBB | physical adsorption | chemical treatment of Penaeus monodon shell waste | [43] |
| cellulose nanoparticles in chitosan | 0D | Rh | hydrogen binding and electrostatic interaction | freeze drying and compacting | [122] |
| Davankov-type hyper-crosslinked-polymer (HCP) nanoparticles | 0D | MB, nigrosine, and AO | π–π stacking | emulsion polymerization then the Friedel–crafts crosslinking reaction using FeCl3 as the catalyst | [45] |
| Fe2O3, CoO, and NiO nanoparticles | 0D | MB | ionic bonding | laser irradiation in the liquid for amorphization | [47] |
| Cr-doped ZnO nanoparticles | 0D | MO | ionic bonding | solvothermal treatment | [48] |
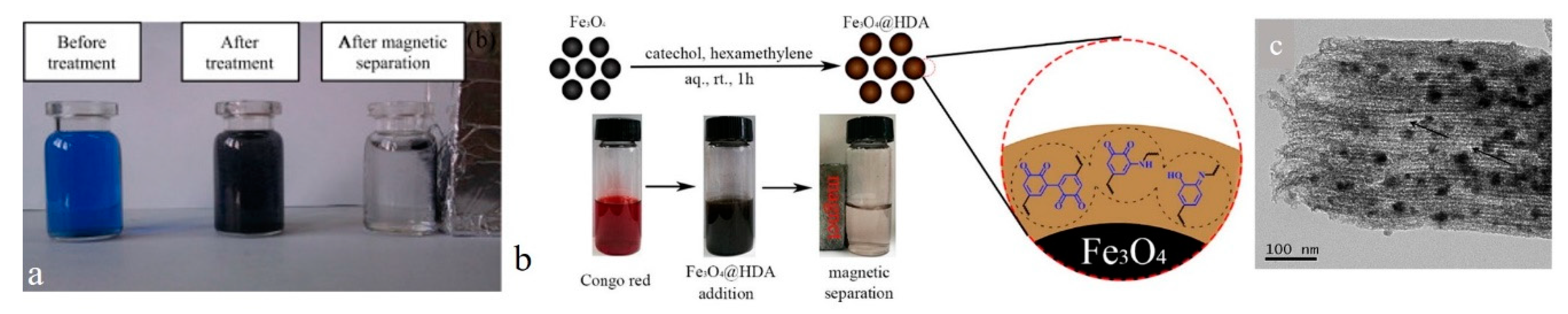
| amino-coated Fe3O4 nanoparticles | 0D | CR | π–π stacking, hydrogen binding, and electrostatic interaction | mussel-inspired polymerization | [56] |
| chitosan/Al2O3/magnetic iron oxide nanoparticle | 0D | MO | electrostatic interaction | dispersion of iron oxide nanoparticles in aluminium isopropoxide/ethanol solution. The as-prepared core-shell nanoparticles were then dispersed in chitosan solution. | [10] |
| hollow cobalt nanoparticles | 0D | MO | reductive degradation | A galvanic replacement reaction using aluminum nanoparticle templates | [102] |
| poly HEMA-CS-f-MWCNT | 1D | MO | electrostatic interaction | functionalization of the nanotube with chitosan and polyHEMA | [112] |
| Fe3O4/CNTs | 1D | sudan I, sudan II, sudan III, and sudan IV dye | electrostatic interaction | hydrothermal synthesis of Fe3O4 nanoparticles onto carbon nanotube | [113] |
| OMWCNT-κ-carrageenan-Fe3O4 nanocomposites | 1D | MB | π–π stacking, hydrogen binding, and electrostatic interaction | chemical oxidation of CNTs and their functionalization with κ-carrageenan | [110] |
| a-COx/G nanofilaments | 1D | MB | electrostatic interaction and π–π stacking | electrospinning and carbonization of PAN nanofibers | [117] |
| Functionalized cellulose nanofibers | 1D | CV | electrostatic interaction | functionalization of the cellulose nanofibers using Meldrum’s acid (2,2-dimethyl-1,3-dioxane-4,6-dione) | [26] |
| Cu(I)-exchanged ZnS 1D nanorods | 1D | Rh B | electrostatic interaction | cation exchange of ZnS with CuCl | [127] |
| ZnO/SnO2 hybrid electro-spun nanofibers | 1D | MB, CR, MO, and ER | photocatalysis | electrospinning, sol-gel process and pyrolysis | [131] |
| V2O5/PES nanofibers | 1D | MB | electrostatic interaction | sol-gel and electrospinning | [6] |
| TiO2/PES nanofibers | 1D | MB | Electrostatic interaction and photocatalysis | sol-gel and electrospinning | [67] |
| TNTs@GO | 1D | MB | electrostatic interaction and photocatalysis | hydrothermal treatment | [148] |
| cysteine-modified rGO | 2D | IC and NR | π–π stacking, and electrostatic interaction | hydrothermally (or hydrazine based) reduced GO | [152] |
| NiO nano-disks | 2D | MB | photocatalysis | hydrothermal treatment | [160] |
| MoS2/rGO | 2D | CR | π–π stacking | hydrothermal treatment | [168] |
| MoS2/CuS nanosheet | 2D | RhB, MB, MO and RhB 6G | molecular diffusion, the van der Waals, and the electrostatic interactions | hydrothermal treatment | [169] |
| Ag/BN nanosheets | 2D | RhB | lewis acid/base interactions | one-pot pyrolysis | [172] |
| BiOCl nanosheets doped with carbon quantum dots | 2D | RhB | photocatalysis | solvothermal treatment | [191] |
| BiOClxBr1-x (x = 0–1) | 3D | MO | photocatalysis | glycol-assisted hydrothermal treatment | [180] |
| dahlia-like BiOClxI1−x (x = 0.75) | 3D | RhB | photocatalysis | solid-state chemical approach | [199] |
| N/S-GHs | 3D | MG, MB, and CV | π–π stacking, hydrogen, and covalent bonding | using glutathione as the binding and reducing material | [208] |
| 3D MoS2 | 3D | MB, MG, rhodamine 6G, FA, and CR | physio-sorption induced by weak Van der Waals forces or dipole-based interactions, electrostatic interactions | synthesized based on a polyethylene glycol (PEG 200) template | [210] |
| MoS2 flowers onto CoFe2O4 nanorods | 3D | CR, MB, and MO | photocatalysis | electrospinning and then hydrothermal treatment | [211] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homaeigohar, S. The Nanosized Dye Adsorbents for Water Treatment. Nanomaterials 2020, 10, 295. https://doi.org/10.3390/nano10020295
Homaeigohar S. The Nanosized Dye Adsorbents for Water Treatment. Nanomaterials. 2020; 10(2):295. https://doi.org/10.3390/nano10020295
Chicago/Turabian StyleHomaeigohar, Shahin. 2020. "The Nanosized Dye Adsorbents for Water Treatment" Nanomaterials 10, no. 2: 295. https://doi.org/10.3390/nano10020295
APA StyleHomaeigohar, S. (2020). The Nanosized Dye Adsorbents for Water Treatment. Nanomaterials, 10(2), 295. https://doi.org/10.3390/nano10020295





