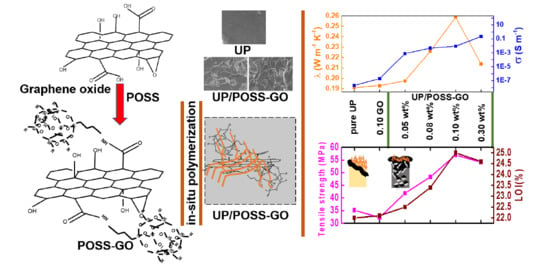
Novel Unsaturated Polyester Nanocomposites via Hybrid 3D POSS-Modified Graphene Oxide Reinforcement: Electro-Technical Application Perspective
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Graphene Oxide(GO)
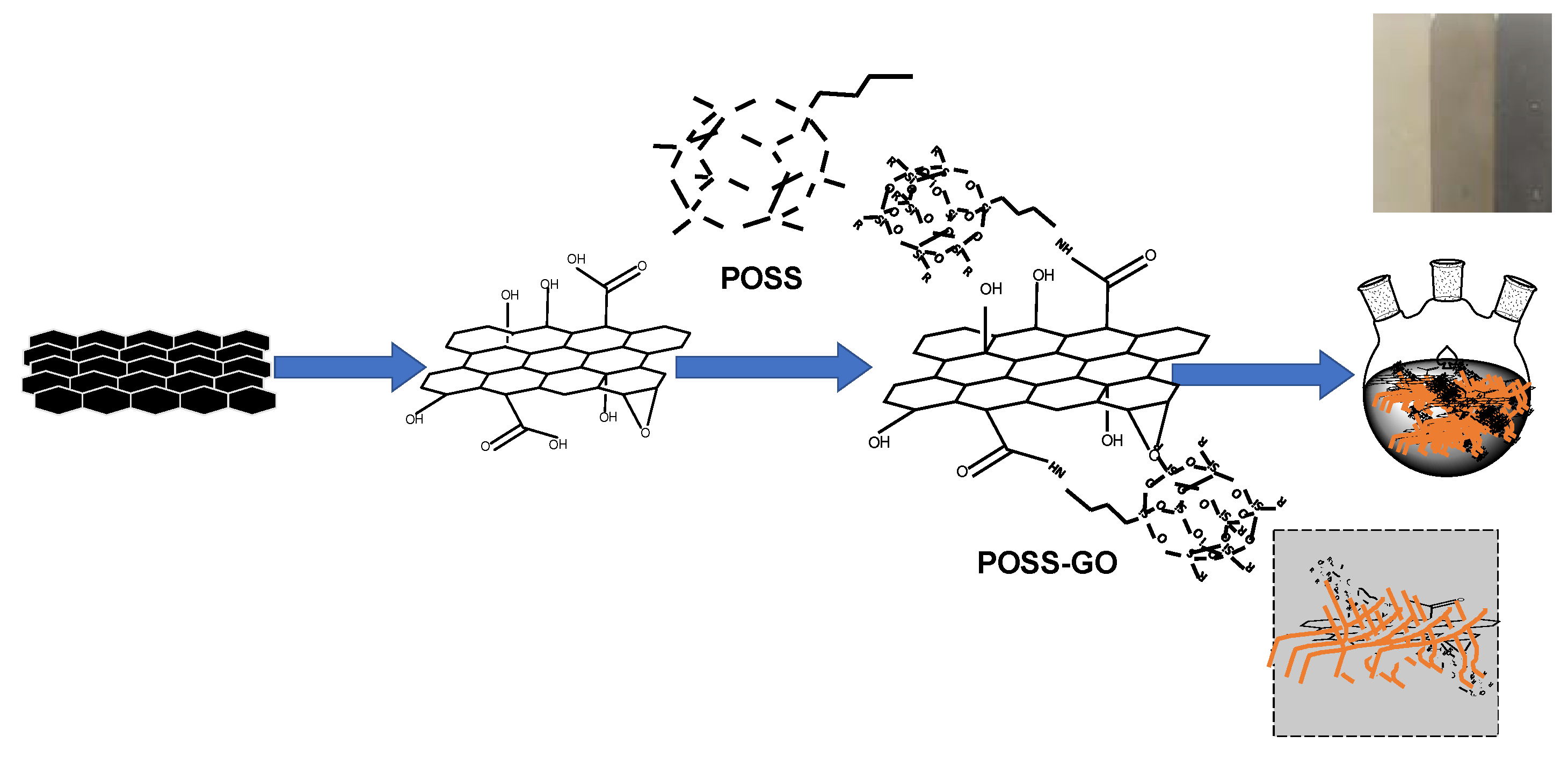
2.3. Synthesis of Polyhedral Oligomeric Silsesquioxane(POSS)-Functionalized GO (POSS-GO)
2.4. Synthesis of Pure Unsaturated Polyester (UP)
2.5. Synthesis of UP/POSS-GO Nanocomposites via In Situ Polymerization
2.6. Characterization
3. Results and Discussion
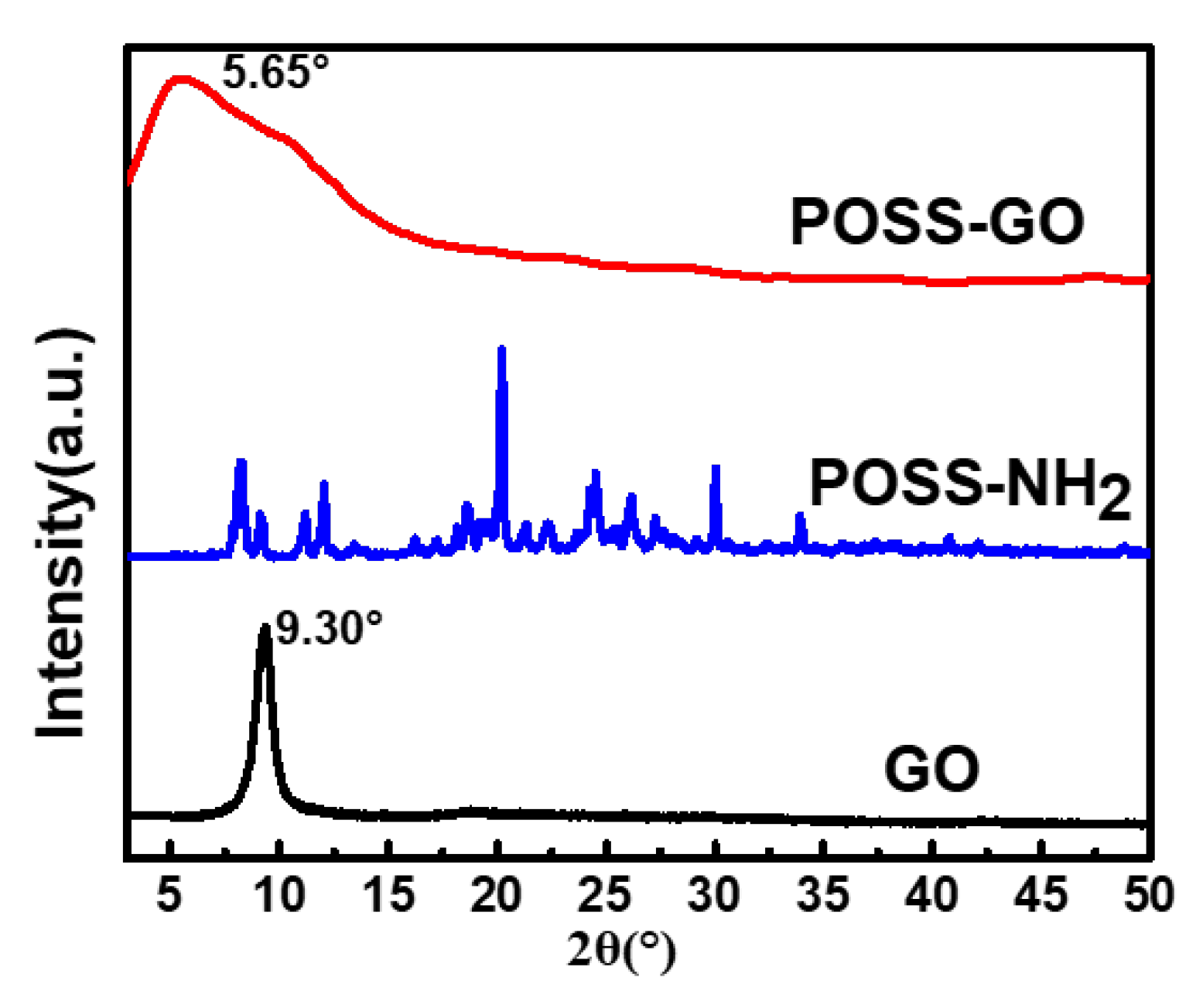
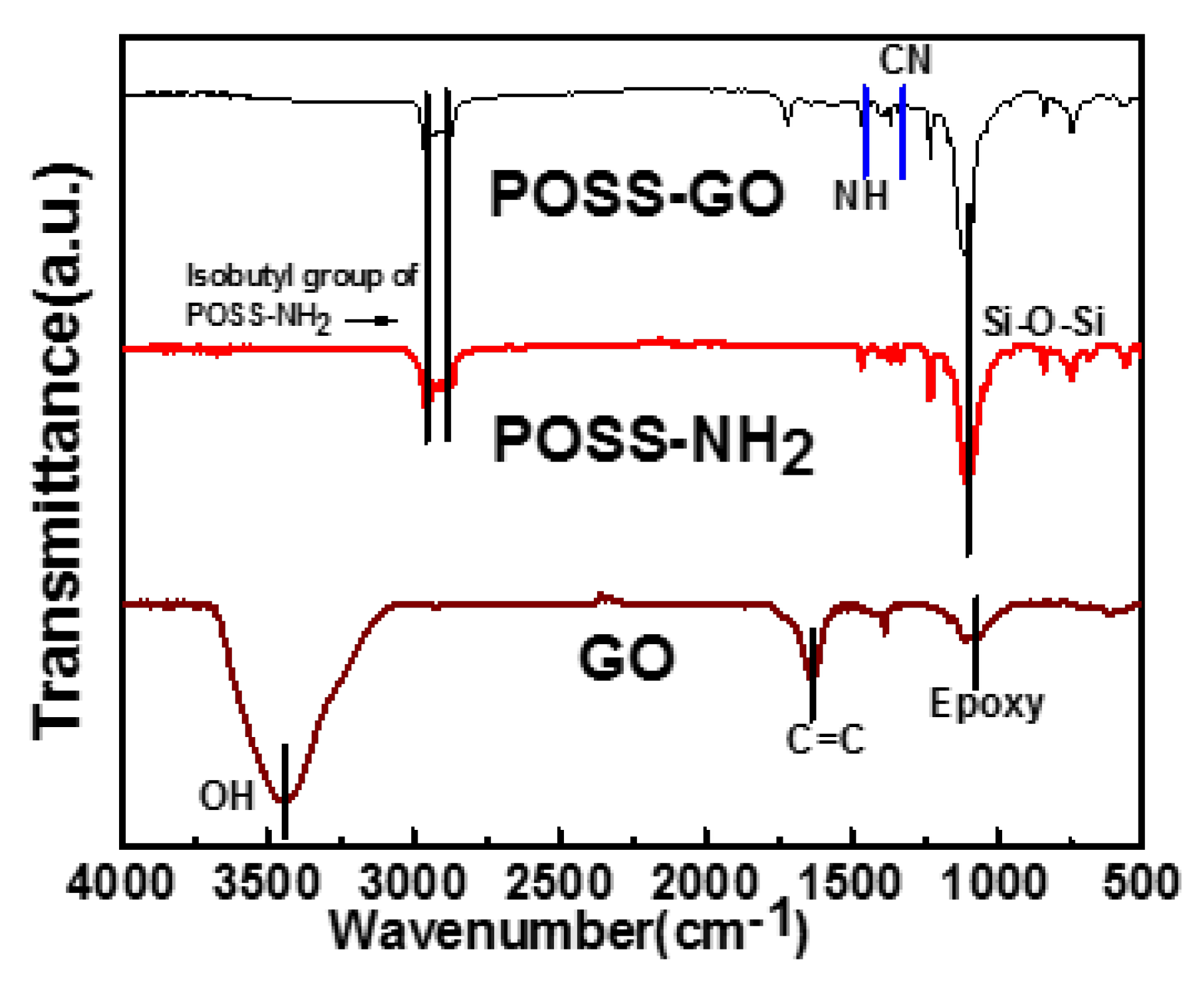
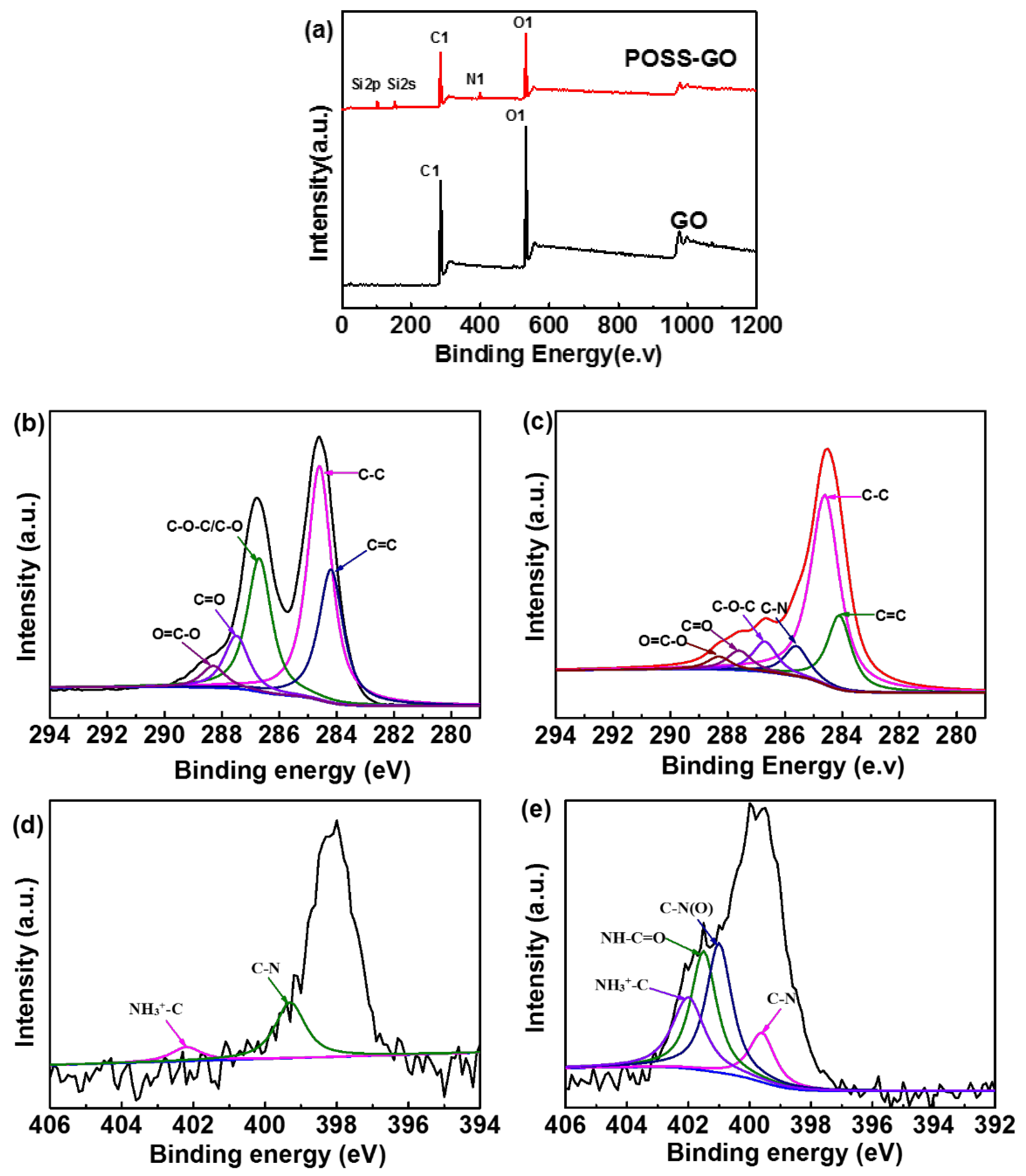
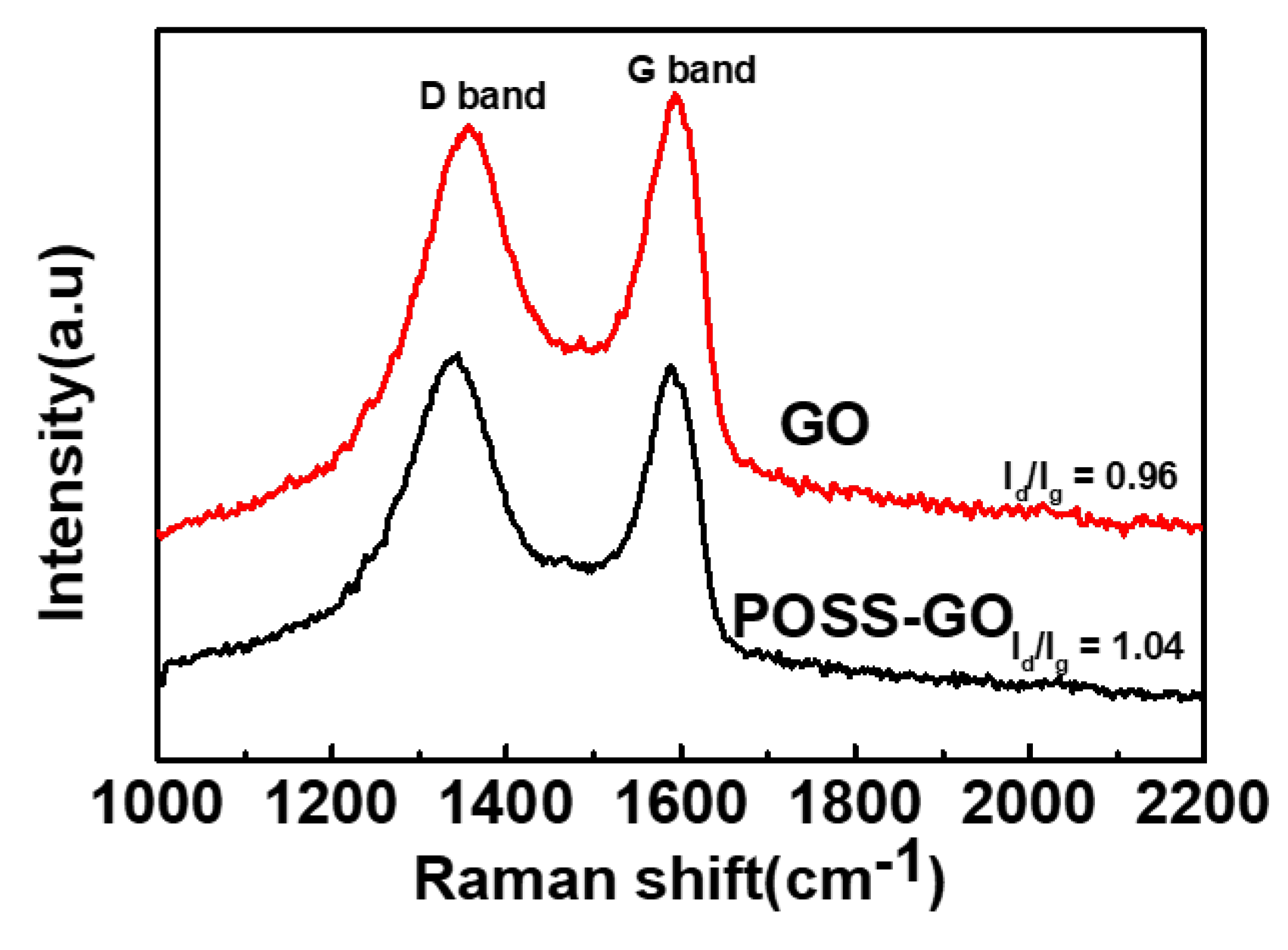
3.1. Characterization of GO and POSS-GO
3.2. Thermal Properties of UP/POSS-GO Nanocomposites
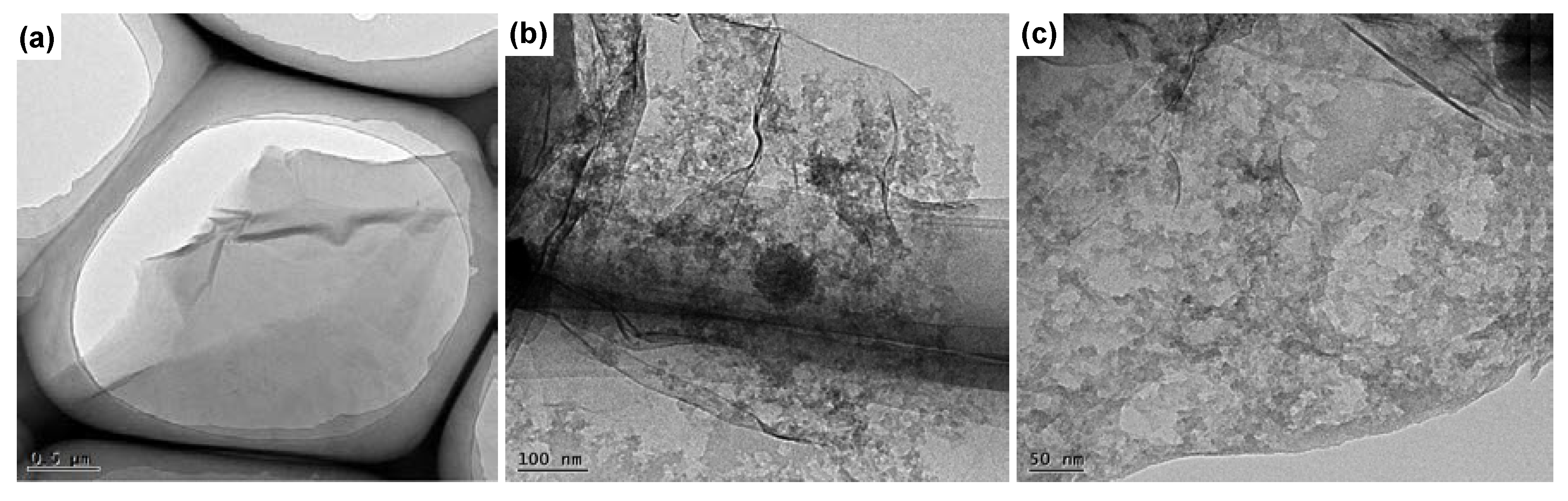
3.3. Morphological Properties of UP/POSS-GO Nanocomposites
3.4. Mechanical Properties of UP/POSS-GO Nanocomposites
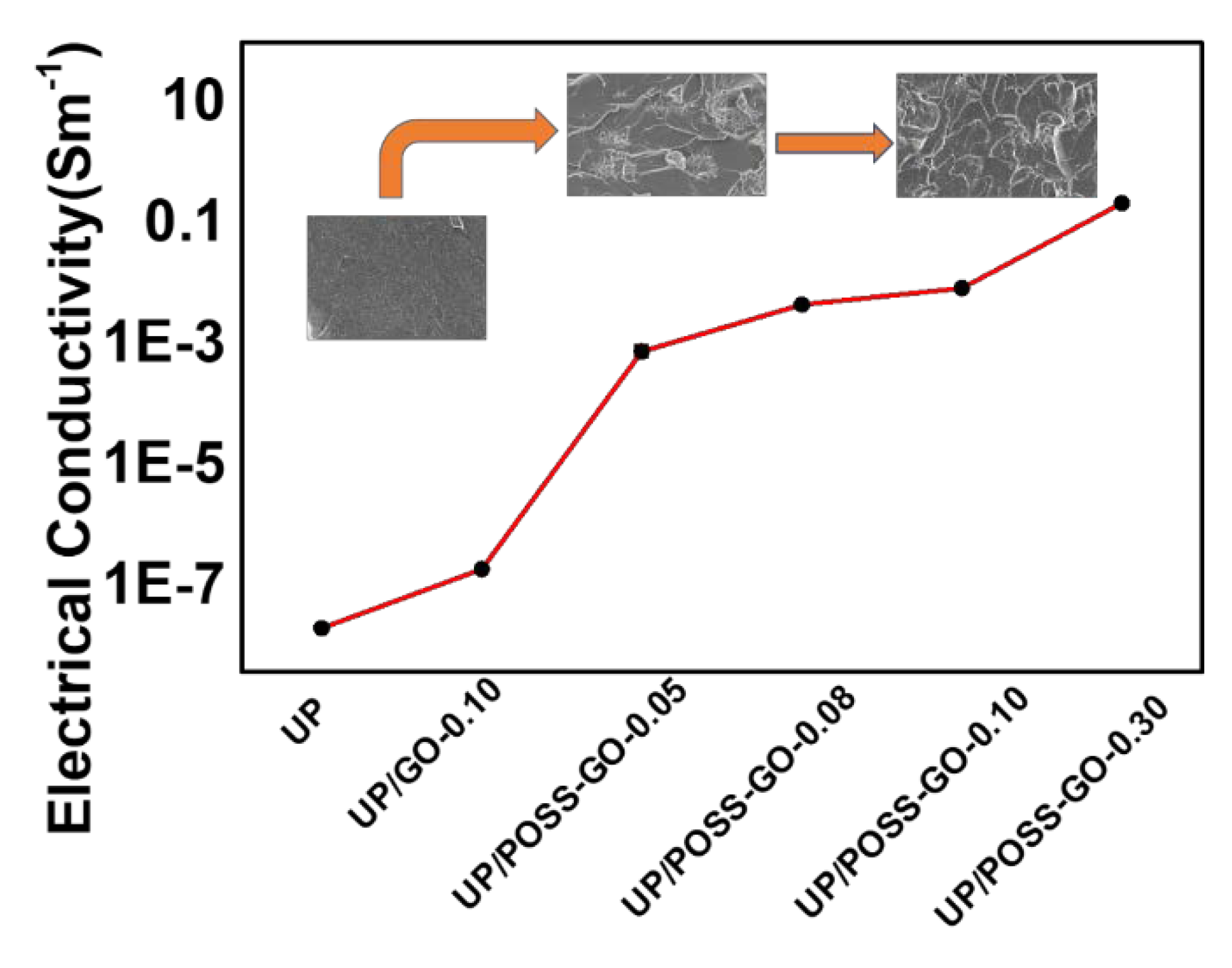
3.5. Electrical Properties of UP/POSS-GO Nanocomposites
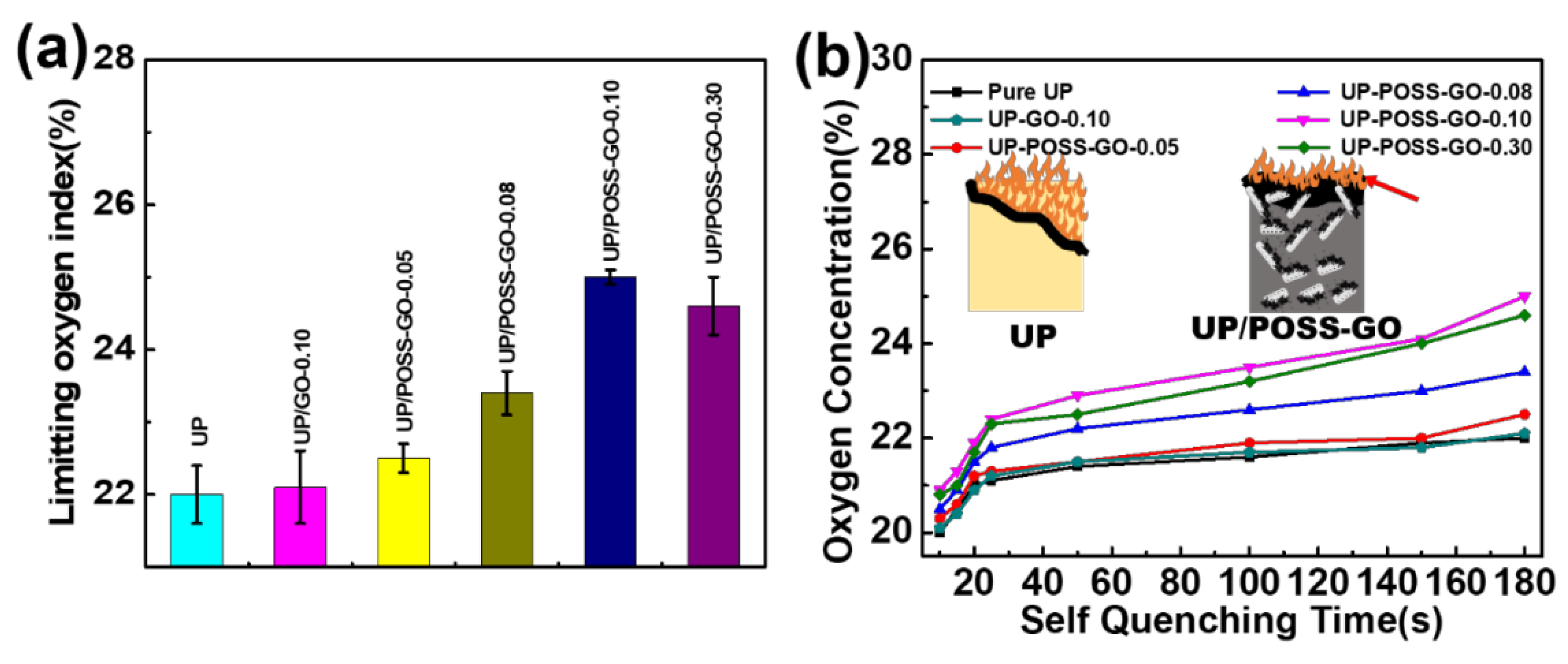
3.6. Limiting Oxygen Index of UP/POSS-GO Nanocomposites
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kuang, X.; Guo, E.; Chen, K.; Qi, H.J. Extraction of Biolubricant via Chemical Recycling of Thermosetting Polymers. ACS Sustain. Chem. Eng. 2019, 7, 6880–6888. [Google Scholar] [CrossRef]
- He, S.; Yu, Z.; Zhou, H.; Huang, Z.; Zhang, Y.; Li, Y.; Li, J.; Wang, Y.; Li, D. Polymer tubes as carrier boats of thermosetting and powder materials based on 3D printing for triboelectric nanogenerator with microstructure. Nano Energy 2018, 52, 134–141. [Google Scholar] [CrossRef]
- Ma, S.; Wei, J.; Jia, Z.; Yu, T.; Yuan, W.; Li, Q.; Wang, S.; You, S.; Liu, R.; Zhu, J. Readily recyclable, high-performance thermosetting materials based on a lignin-derived spiro diacetal trigger. J. Mater. Chem. A 2019, 7, 1233–1243. [Google Scholar] [CrossRef]
- Kumar, V.; Yokozeki, T.; Okada, T.; Hirano, Y.; Goto, T.; Takahashi, T.; Hassen, A.A.; Ogasawara, T. Polyaniline-based all-polymeric adhesive layer: An effective lightning strike protection technology for high residual mechanical strength of CFRPs. Compos. Sci. Technol. 2019, 172, 49–57. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, G.; Xu, N.; Lu, C.; Qiao, Y.; Zhang, D.; Wang, X. Preparation and properties of highly electroconductive and heat-resistant CMC/buckypaper/epoxy nanocomposites. Nanomaterials 2018, 8, 969. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, D.; Yuan, B.; Song, L.; Hu, Y. The effect of carbon nanotubes/NiFe2O4 on the thermal stability, combustion behavior and mechanical properties of unsaturated polyester resin. RSC Adv. 2016, 6, 96974–96983. [Google Scholar] [CrossRef]
- Fu, G.; Huo, D.; Shyha, I.; Pancholi, K.; Saharudin, M.S. Experimental investigation on micro milling of polyester/halloysite nano-clay nanocomposites. Nanomaterials 2019, 9, 917. [Google Scholar] [CrossRef]
- Lima, M.S.; Costa, C.S.M.F.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. A simple strategy toward the substitution of styrene by sobrerol-based monomers in unsaturated polyester resins. Green Chem. 2018, 20, 4880–4890. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, H.; Wei, Q.; Guo, B.; Zhang, L.; Jia, D. Incorporation of graphene into polyester/carbon nanofibers composites for better multi-stimuli responsive shape memory performances. Carbon 2013, 64, 487–498. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Antibacterial activities of aliphatic polyester nanocomposites with silver nanoparticles and/or graphene oxide sheets. Nanomaterials 2019, 9, 1102. [Google Scholar] [CrossRef]
- Wang, D.; Wen, P.; Wang, J.; Song, L.; Hu, Y. The effect of defect-rich molybdenum disulfide nanosheets with phosphorus, nitrogen and silicon elements on mechanical, thermal, and fire behaviors of unsaturated polyester composites. Chem. Eng. J. 2017, 313, 238–249. [Google Scholar] [CrossRef]
- Zhou, S.; Hrymak, A.N.; Kamal, M.R. Effect of hybrid carbon fillers on the electrical and morphological properties of polystyrene nanocomposites in microinjection molding. Nanomaterials 2018, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yang, D.; Fan, Z.; Li, X.; Liu, Y.; Guo, C.; Zhang, M.; Yu, Z.Z. Reduced graphene oxide/carbon nanotube hybrid fibers with narrowly distributed mesopores for flexible supercapacitors with high volumetric capacitances and satisfactory durability. Carbon 2019, 152, 134–143. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, M.; Li, J.; Yu, J.; Sun, S.; Ge, S.; Guo, X.; Xie, F.; Jiang, B.; Wujcik, E.K.; et al. Silver nanoparticles/graphene oxide decorated carbon fiber synergistic reinforcement in epoxy-based composites. Polymer 2017, 131, 263–271. [Google Scholar] [CrossRef]
- Kale, M.B.; Luo, Z.; Zhang, X.; Dhamodharan, D.; Divakaran, N.; Mubarak, S.; Wu, L.; Xu, Y. Waterborne polyurethane/graphene oxide-silica nanocomposites with improved mechanical and thermal properties for leather coatings using screen printing. Polymer 2019, 170, 43–53. [Google Scholar] [CrossRef]
- Cobos, M.; Fernández, M.J.; Fernández, M.D. Graphene based poly(vinyl alcohol) nanocomposites prepared by in situ green reduction of graphene oxide by ascorbic acid: Influence of graphene content and glycerol plasticizer on properties. Nanomaterials 2018, 8, 1013. [Google Scholar] [CrossRef]
- Pan, W.; He, M.; Zhang, L.; Hou, Y.; Chen, C. Interfacial engineering of graphene nanosheets at mgo particles for thermal conductivity enhancement of polymer composites. Nanomaterials 2019, 9, 798. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral oligosilsesquioxane-modified boron nitride nanotube based epoxy nanocomposites: An ideal dielectric material with high thermal conductivity. Adv. Funct. Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral oligomeric silsesquioxane (POSS)—Containing polymer nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef]
- Constantin, F.; Gârea, S.A.; Iovu, H. The influence of organic substituents of polyhedral oligomeric silsesquioxane on the properties of epoxy-based hybrid nanomaterials. Compos. B Eng. 2013, 44, 558–564. [Google Scholar] [CrossRef]
- Jeong, H.G.; Han, Y.S.; Jung, K.H.; Kim, Y.J. Poly(vinylidene fluoride) composite nanofibers containing polyhedral oligomeric silsesquioxane-epigallocatechin gallate conjugate for bone tissue regeneration. Nanomaterials 2019, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, G.; Wu, G.; Cai, C. Thermal and dynamic mechanical properties of epoxy resin/poly (urethane-imide)/polyhedral oligomeric silsesquioxane nanocomposites. Polym. Adv. Technol. 2011, 22, 2069–2074. [Google Scholar] [CrossRef]
- Chen, X.; Dumée, L.F. Polyhedral oligomeric silsesquioxane (POSS) nano-composite separation membranes—A review. Adv. Eng. Mater. 2018, 21, 1800667. [Google Scholar] [CrossRef]
- Li, J.G.; Chu, W.C.; Kuo, S.W. Hybrid mesoporous silicas and microporous POSS-based frameworks incorporating evaporation-induced self-assembly. Nanomaterials 2015, 5, 1087–1101. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, R. Flame retardancy mechanisms of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS) in polycarbonate/acryonitrile-butadiene-styrene blends. Polym. Adv. Technol. 2012, 23, 588–595. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, H.; Yuan, L.; Liu, C. Effect of functionalized graphene oxide with hyperbranched POSS polymer on mechanical and dielectric properties of cyanate ester composites. Rsc Adv. 2016, 6, 38887–38896. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Song, H.; Han, Y.; Dong, Y.; Wang, Y.; Wang, Q. Improving the thermal conductivity and property of epoxy composites by introducing polyhedral oligomeric silsesquioxanes—Grafted graphene oxide. Polym. Compos. 2018, 39, E1890–E1899. [Google Scholar] [CrossRef]
- Hu, L.; Jiang, P.; Bian, G.; Huang, M.; Haryono, A.; Zhang, P.; Bao, Y.; Xia, J. Effect of ocata(aminopropyl) polyhedral oligomeric silsesquioxane (OapPOSS) functionalized graphene oxide on the mechanical, thermal and hydrophobic properties of waterborne polyurethane composites. J. Appl. Polym. Sci. 2016, 134, 44440. [Google Scholar]
- Raimondo, M.; Guadagno, L.; Speranza, V.; Bonnaud, L.; Dubois, P.; Lafdi, K. Multifunctional graphene/POSS epoxy resin tailored for aircraft lightning strike protection. Compos. B Eng. 2018, 140, 44–56. [Google Scholar] [CrossRef]
- Lin, J.; Zhong, B.; Jia, Z.; Hu, D.; Ding, Y.; Luo, Y.; Jia, D. In-situ fabrication of halloysite nanotubes/silica nanohybrid and its application in unsaturated polyester resin. Appl. Surf. Sci. 2017, 407, 130–136. [Google Scholar] [CrossRef]
- Jiang, D.; Xing, L.; Liu, L.; Yan, X.; Guo, J.; Zhang, X.; Zhang, Q.; Wu, Z.; Zhao, F.; Huang, Y.; et al. Interfacially reinforced unsaturated polyester composites by chemically grafting diiferent functional POSS onto carbon fibres. J. Mater. Chem. A 2014, 2, 18293–18303. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, H.; Chen, L.; Jiang, D.; Weng, L.; Ma, Y.; Li, X.; Zhang, X.; Liu, H.; Wang, N.; et al. Interfacially reinforced unsaturated polyester carbon fiber composites with a vinyl ester- carbon nanotubes sizing agent. Compos. Sci. Technol. 2018, 164, 195–203. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Tang, J.; Zhang, J.; Shang, Q.; Hu, Y.; Wang, H.; Wu, Q.; Zhou, Y.; Lei, W.; et al. High-performance biobased unsaturated polyester nanocomposites with very low loadings of Graphene. Polymers 2018, 10, 1288. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, L.; Jiang, S.; Zhou, K.; Shi, X.; Mou, W. Establishment of highly effective flame-retardant unsaturated polyester resin system based on multiple strategies. Adv. Polym. Technol. 2018, 37, 2674–2686. [Google Scholar] [CrossRef]
- Bora, C.; Bharali, P.; Baglari, S.; Dolui, S.K.; Konwar, B.K. Strong and conductive reduced graphene oxide/polyester resin composite films with improved mechanical strength, thermal stability and its antibacterial activity. Compos. Sci. Technol. 2013, 87, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.J.; Son, Y.R.; In, I.; An, K.H.; Kim, B.J.; Park, S.J. Recent advanced thermal interfacial materials: A review of conducting mechanisms and parameters of carbon materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Wu, L.; Weng, Z.; Zhou, Y. Unsaturated polyester/montmorillonite nanocomposites with improved mechanical and thermal properties fabricated by in situ polymerization. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Valentini, L.; Cardinali, M.; Kenny, J.M.; Prato, M.; Monticelli, O. A photoresponsive hybrid nanomaterial based on graphene and polyhedral oligomeric silsesquioxane. Eur. J. Inorg. Chem. 2012, 2012, 5282–5287. [Google Scholar] [CrossRef]
- Yuan, G.; Yang, B.; Chen, Y.; Jia, Y. Synthesis of a novel multi-structure synergistic POSS-GO-DOPO ternary graft flame retardant and its application in polypropylene. Compos. A Appl. Sci. Manuf. 2019, 117, 345–356. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Y.; Lu, F.; Qu, J.; Chen, H.; Dai, L. Functionalization of graphene oxide with polyhedral oligomeric silsesquioxane (POSS) for multifunctional applications. J. Phys. Chem. Lett. 2012, 3, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Kandola, B.; Hu, Y. Simultaneous reduction and surface functionalization of graphene oxide with POSS for reducing fire hazards in epoxy composites. J. Mater. Chem. 2012, 22, 22037–22043. [Google Scholar] [CrossRef]
- Yuan, R.; Ji, L.; Wu, Y.; Li, H.; Ju, P.; Chen, L.; Zhou, H.; Chen, J. “Plate-anchor” shaped POSS- functionalized graphene oxide with self fixing effect in polyimide matrix: Molecular dynamic simulations and experimental analysis. Compos. Sci. Tehnol. 2019, 176, 103–110. [Google Scholar] [CrossRef]
- Liao, W.H.; Yang, S.Y.; Hsiao, S.T.; Wang, Y.S.; Li, S.M.; Ma, C.C.M.; Tien, H.W.; Zeng, S.J. Effect of octa(aminophenyl) polyhedral oligomeric silsesquioxane functionalized graphene oxide on the mechanical and dielectric properties of polyimide composites. ACS Appl. Mater. Interfaces 2014, 6, 15802–15812. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, Y.Z.; Wang, M.Y.; Li, Q.; Ren, R. Using maleic anhydride functionalized graphene oxide for improving the interfacial properties of carbon fiber/BMI composites. Express Polym. Lett. 2016, 10, 874–882. [Google Scholar] [CrossRef]
- Tien, H.W.; Hsiao, S.T.; Liao, W.H.; Yu, Y.H.; Lin, F.C.; Wang, Y.S.; Li, S.M.; Ma, C.C.M. Using self-assembly to prepare a graphene-silver nanowire hybrid film that is transparent and electrically conductive. Carbon 2013, 58, 198–207. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Geng, Z.; Zhu, K.; Zhang, M.; Na, R.; Wang, G. Hybrid formation of graphene oxide-POSS and their effect on the dielectric properties of poly(aryl ether ketone) composites. New J. Chem. 2017, 41, 3089–3096. [Google Scholar] [CrossRef]
- Compton, O.C.; Dikin, D.A.; Putz, K.W.; Brinson, C.; Nguyen, S.T. Electrically conductive "alkylated" graphene paper via chemical reduction of amine-functionalized graphene oxide paper. Adv. Mater. 2010, 22, 892–896. [Google Scholar] [CrossRef]
- Tong, W.; Zhang, Y.; Zhang, Q.; Luan, X.; Duan, Y.; Pan, S.; Lv, F.; An, Q. Achieving significantly enhanced dielectric performance of reduced graphene oxide/polymer composite by covalent modification of graphene oxide surface. Carbon 2015, 84, 590–598. [Google Scholar] [CrossRef]
- Yoon, Y.; Seo, S.; Kim, G.; Lee, H. Atomic dopants involved in the structural evolution of thermally graphitized graphene. Chem. Eur. J. 2012, 18, 13466–13472. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Liu, L.; Wang, Y.; Xie, F.; Zhong, Z.; Zhao, M.; Jiang, B.; Huang, Y. Interfacially reinforced methylphenylsilicone resin composites by chemically grafting multiwall carbon nanotubes onto carbon fibers. Compos. Beng. 2015, 82, 50–58. [Google Scholar] [CrossRef]
- Liu, X.; Cao, L.; Song, W.; Ai, K.; Lu, L. Functionalizing metal nanostructured film with graphene oxide for ultrasensitive detection of aromatic molecules by surface-enhanced Raman spectroscopy. ACS Appl. Mater. Interfaces 2011, 3, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
- Kouloumpis, A.; Thomou, E.; Chalmpes, N.; Dimos, K.; Spyrou, K.; Bourlinos, A.B.; Koutselas, I.; Gournis, D.; Rudolf, P. Graphene/carbon dot hybrid thin films prepared by a dodified langmuir-schaefer method. ACS Omega 2017, 2, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Nimita Jebaranjitham, J.; Mageshwari, C.; Saravanan, R.; Mu, N. Fabrication of amine functionalized graphene oxide—AgNPs nanocomposite with improved dispersibility for reduction of 4-nitrophenol. Compos. B Eng. 2019, 171, 302–309. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymer 2013, 54, 5087–5103. [Google Scholar] [CrossRef]
- García, P.G.; Ramírez-Aguilar, R.; Torres, M.; Franco-Urquiza, E.A.; May-Crespo, J.; Camacho, N. Mechanical and thermal behavior dependence on graphite and oxidized graphite content in polyester composites. Polymer 2018, 153, 9–16. [Google Scholar] [CrossRef]
- Guo, M.; David, É.; Fréchette, M.; Demarquette, N.R. Polyethylene/polyhedral oligomeric silsesquioxanes composites: Dielectric, thermal and rheological properties. Polymer 2017, 115, 60–69. [Google Scholar] [CrossRef]
- Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Thermal conductivity of polymeric composites: A review. In Proceedings of the 2013 IEEE International Conferenceon Solid Dielectrics(ICSD), Bologna, Italy, 30 June–4 July 2013. [Google Scholar]
- Istrate, O.M.; Paton, K.R.; Khan, U.; O’Neill, A.; Bell, A.P.; Coleman, J.N. Reinforcement in melt-processed polymer-graphene composites at extremely low graphene loading level. Carbon 2014, 78, 243–249. [Google Scholar] [CrossRef]
- Ajorloo, M.; Fasihi, M.; Ohshima, M.; Taki, K. How are the thermal properties of polypropylene/graphene nanoplatelet composites affected by polymer chain configuration and size of nanofiller. Mater. Des. 2019, 181, 108068. [Google Scholar]
- Divakaran, N.; Zhang, X.; Kale, M.B.; Senthil, T.; Mubarak, S.; Dhamodharan, D.; Wu, L.; Wang, J. Fabrication of surface modified graphene oxide/unsaturated polyester nanocomposites via in-situ polymerization: Comprehensive property enhancement. Appl. Surf. Sci. 2019, 502, 144164. [Google Scholar] [CrossRef]
- Nezakati, T.; Tan, A.; Seifalian, A.M. Enhancing the electrical conductivity of a hybrid POSS-PCL/graphene nanocomposite polymer. J. Colloid Interface Sci. 2014, 435, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Sang, B.; Li, Z.; Li, X.; Yu, L.; Zhang, Z. Graphene-based flame retardants: A review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Bao, C.; Song, L.; Xing, W.; Yuan, B.; Wilkie, C.A.; Huang, J.; Guo, Y.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, L.; García Molleja, J.; Wang, D.Y. Bimetallic metal-organic frameworks and graphene oxide nano-hybrids for enhanced fire retardant epoxy composites: A novel carbonization mechanism. Carbon 2019, 153, 407–416. [Google Scholar] [CrossRef]
- Ni, M.; Chen, G.; Wang, Y.; Peng, H.; Liao, Y.; Xie, X. Holographic polymer nanocomposites with ordered structures and improved electro-optical performance by doping POSS. Compos. B Eng. 2019, 174, 107045. [Google Scholar] [CrossRef]
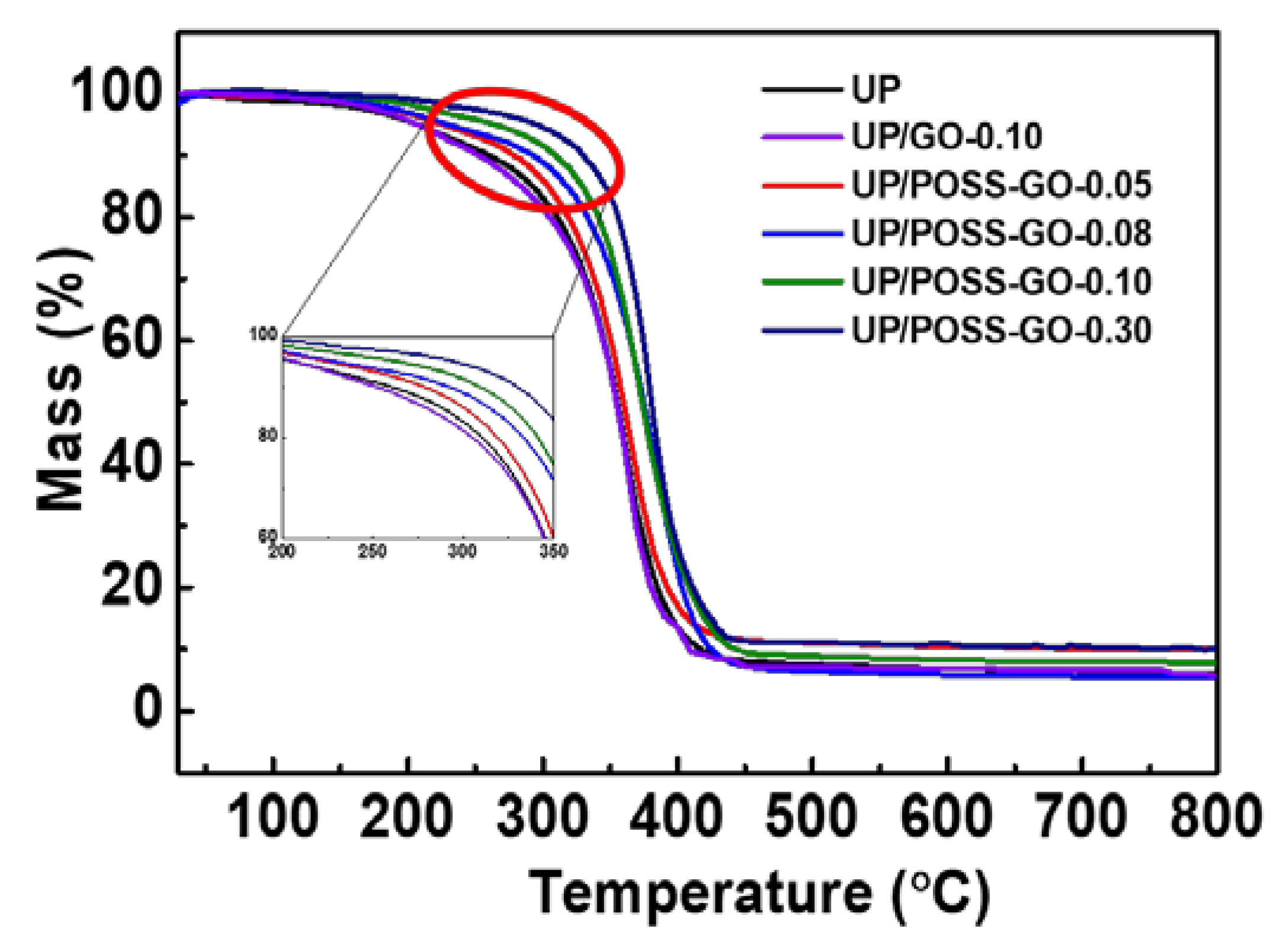
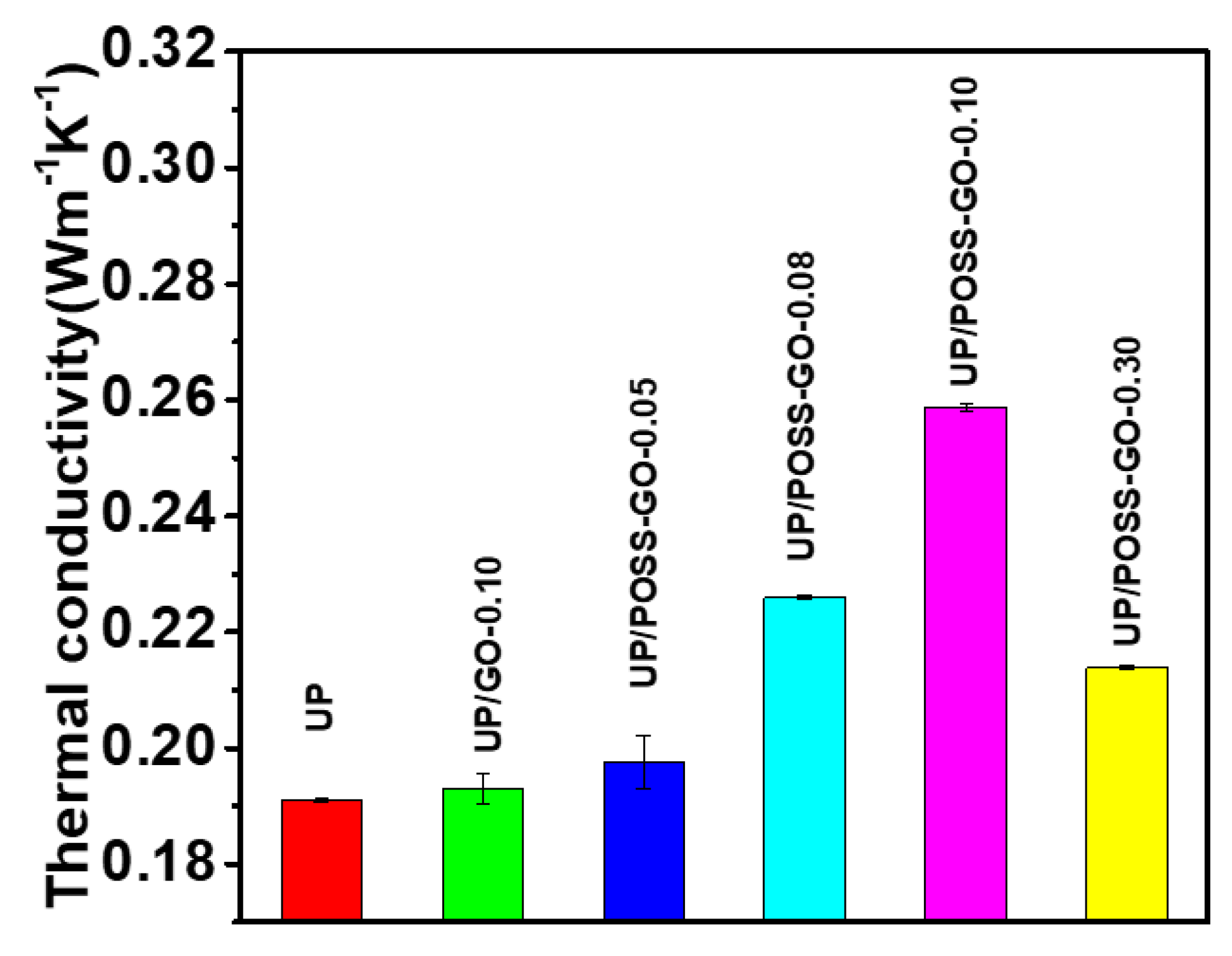
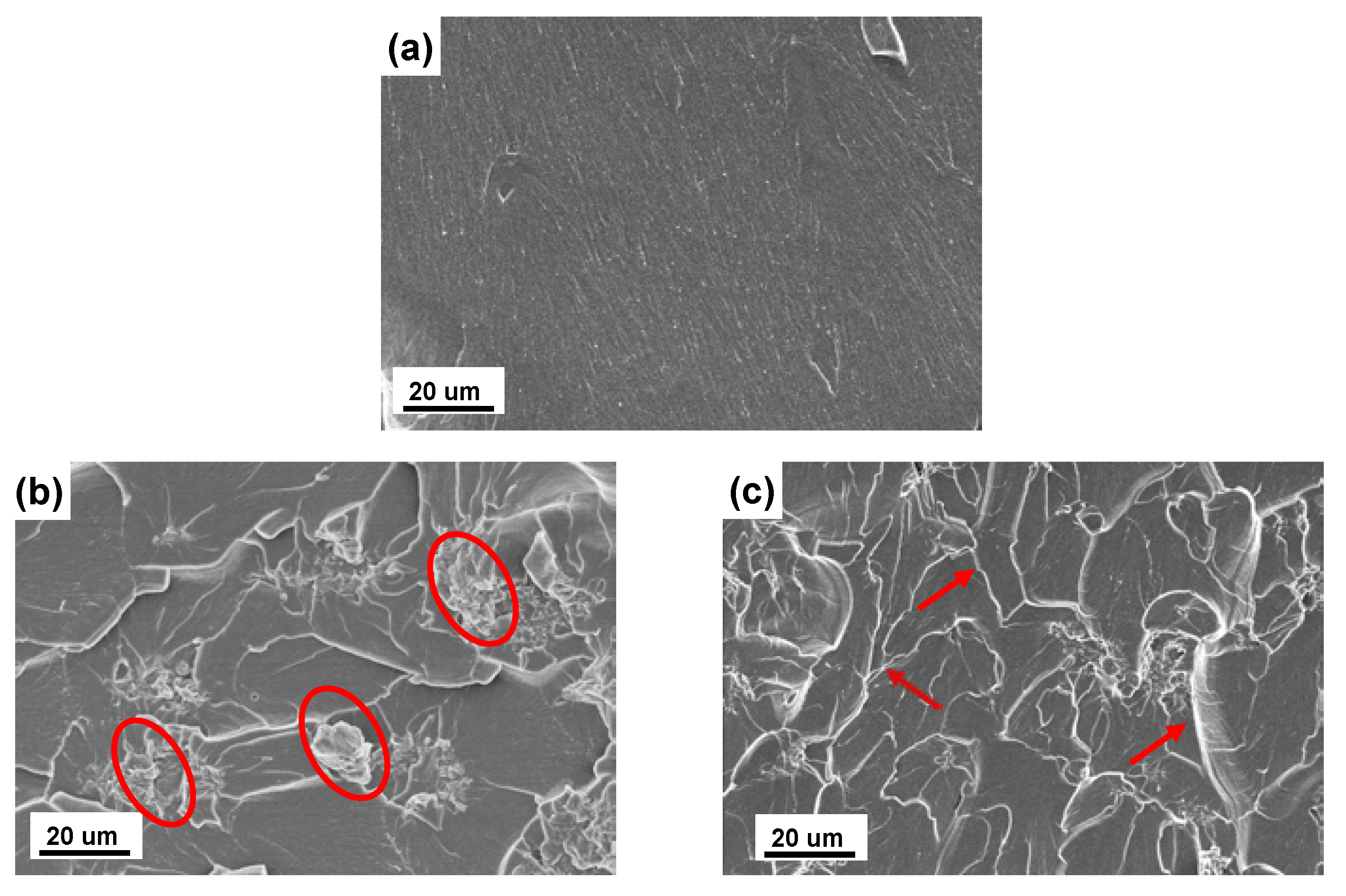
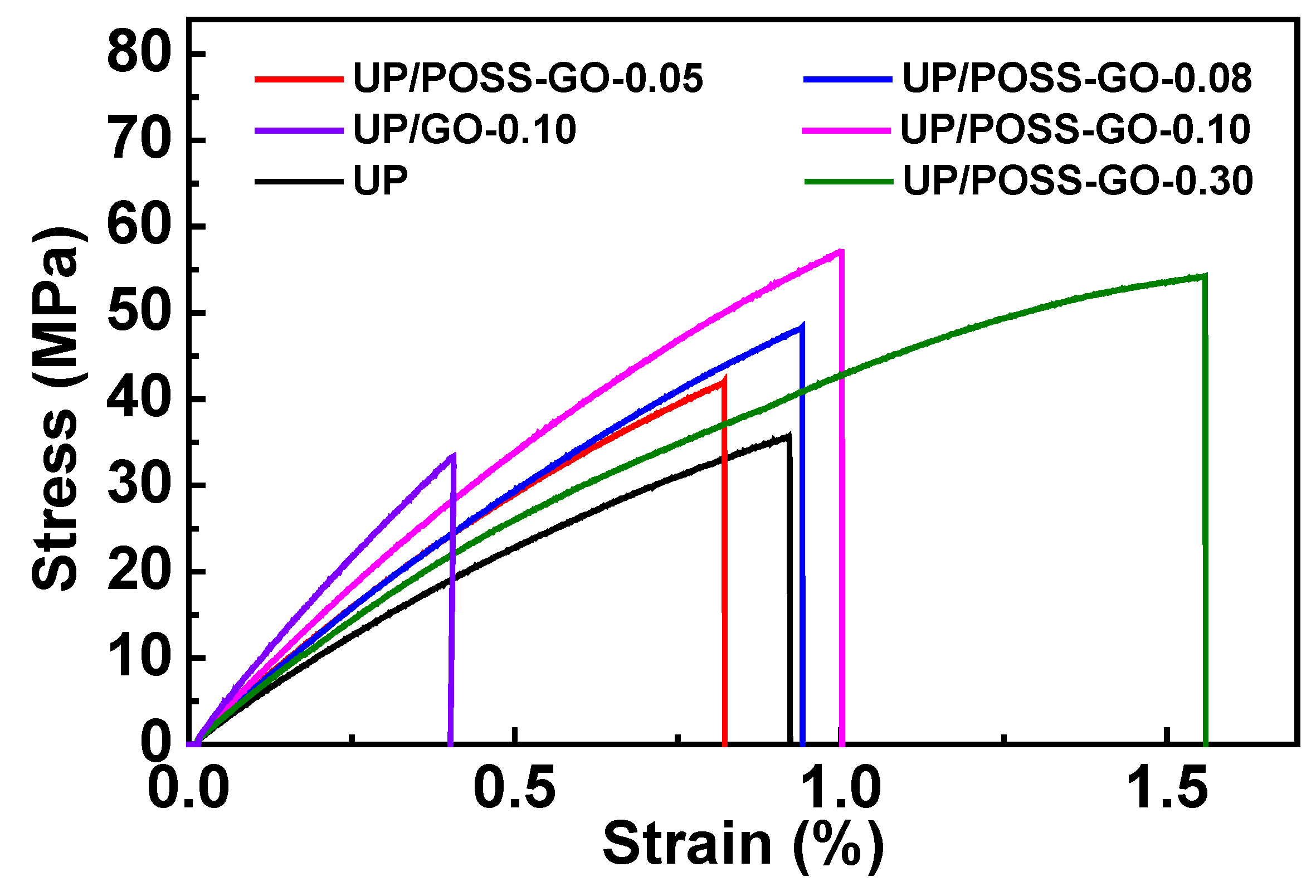
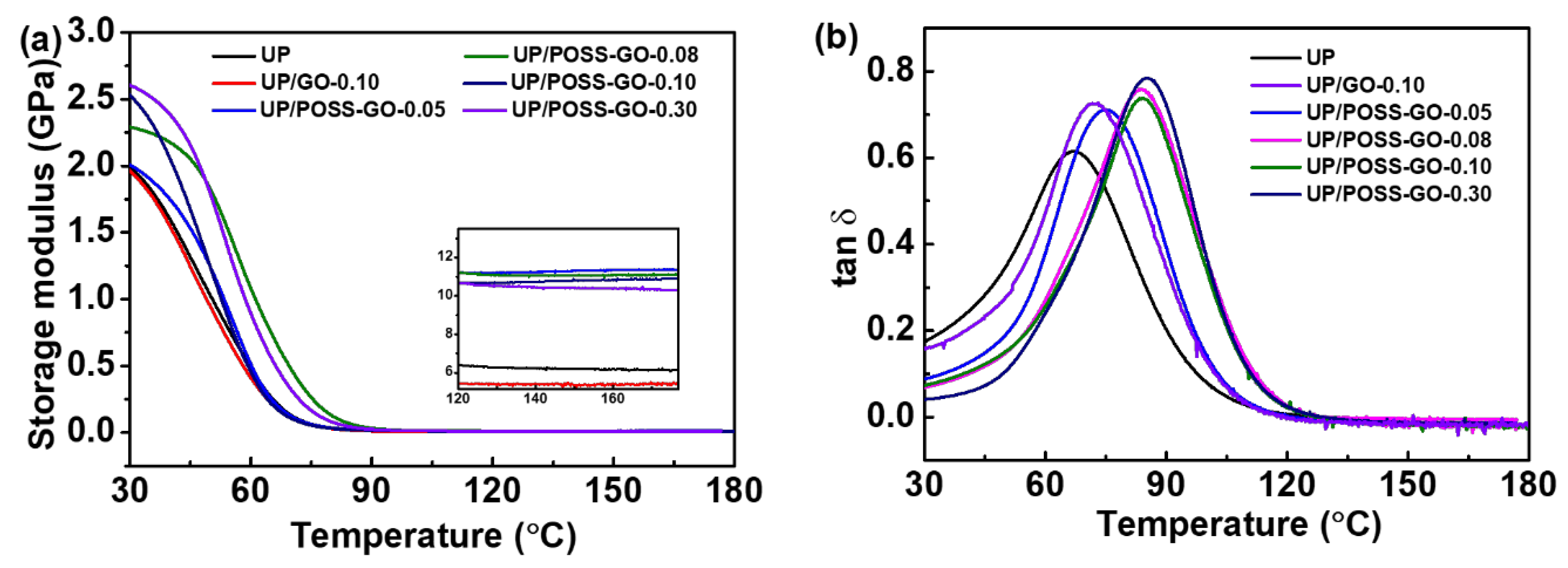
| Sample Name | T10 * (°C) | T50 * (°C) | Residual (%) | Thermal Conductivity (W·m−1 K−1) |
|---|---|---|---|---|
| UP | 259.7 | 354.6 | 5.68 | 0.1911 ± 0.00038 |
| UP/GO-0.10 | 255.4 | 352.2 | 5.89 | 0.1930 ± 0.0024 |
| UP/POSS-GO-0.05 | 273.4 | 357.8 | 10.10 | 0.1976 ± 0.0045 |
| UP/POSS-GO-0.08 | 293.3 | 376.4 | 5.99 | 0.2260 ± 0.0004 |
| UP/POSS-GO-0.10 | 309.1 | 379.6 | 7.88 | 0.2587 ± 0.0005 |
| UP/POSS-GO-0.30 | 329.5 | 380.6 | 10 | 0.2139 ± 0.00042 |
| Sample Name | Tensile Strength (MPa) | Elongation at Break (%) | Storage Modulus at 30 °C (MPa) | Tg * (°C) |
|---|---|---|---|---|
| UP | 35.2 ± 0.5 | 0.92 ± 0.008 | 1963 | 69.5 |
| UP/GO-0.10 | 32.3 ± 1.5 | 0.4 ± 0.100 | 1913 | 71.2 |
| UP/POSS-GO-0.05 | 41.9 ± 3.2 | 0.82 ± 0.009 | 1982 | 74.9 |
| UP/POSS-GO-0.08 | 48.3 ± 2 | 0.94 ± 0.012 | 2287 | 83.6 |
| UP/POSS-GO-0.10 | 57 ± 1.4 | 1 ± 0.033 | 2516 | 84.7 |
| UP/POSS-GO-0.30 | 54.1 ± 1.5 | 1.56 ± 0.044 | 2600 | 85.2 |
| Sample Name | Electrical Conductivity (S m−1) | LOI (%) |
|---|---|---|
| UP | 2.12 × 10−8 | 22 ± 0.4 |
| UP/GO-0.10 | 2 × 10−7 | 22.1 ± 0.5 |
| UP/POSS-GO-0.05 | 7.87 × 10−4 | 22.5 ± 0.2 |
| UP/POSS-GO-0.08 | 0.0047 | 23.4 ± 0.3 |
| UP/POSS-GO-0.10 | 0.00878 | 25 ± 0.1 |
| UP/POSS-GO-0.30 | 0.223 | 24.6 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divakaran, N.; Kale, M.B.; Senthil, T.; Mubarak, S.; Dhamodharan, D.; Wu, L.; Wang, J. Novel Unsaturated Polyester Nanocomposites via Hybrid 3D POSS-Modified Graphene Oxide Reinforcement: Electro-Technical Application Perspective. Nanomaterials 2020, 10, 260. https://doi.org/10.3390/nano10020260
Divakaran N, Kale MB, Senthil T, Mubarak S, Dhamodharan D, Wu L, Wang J. Novel Unsaturated Polyester Nanocomposites via Hybrid 3D POSS-Modified Graphene Oxide Reinforcement: Electro-Technical Application Perspective. Nanomaterials. 2020; 10(2):260. https://doi.org/10.3390/nano10020260
Chicago/Turabian StyleDivakaran, Nidhin, Manoj B. Kale, T. Senthil, Suhail Mubarak, Duraisami Dhamodharan, Lixin Wu, and Jianlei Wang. 2020. "Novel Unsaturated Polyester Nanocomposites via Hybrid 3D POSS-Modified Graphene Oxide Reinforcement: Electro-Technical Application Perspective" Nanomaterials 10, no. 2: 260. https://doi.org/10.3390/nano10020260
APA StyleDivakaran, N., Kale, M. B., Senthil, T., Mubarak, S., Dhamodharan, D., Wu, L., & Wang, J. (2020). Novel Unsaturated Polyester Nanocomposites via Hybrid 3D POSS-Modified Graphene Oxide Reinforcement: Electro-Technical Application Perspective. Nanomaterials, 10(2), 260. https://doi.org/10.3390/nano10020260