Facile Synthesis and Reuse of Magnetic Black Carbon Magnetite (BC-Mag) for Fast Carbamazepine Removal from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis and Characterization
2.2. Sorption Experiments
2.3. Desorption and Regeneration
2.4. Quantification of Carbamazepine
3. Results and Discussion
3.1. Characterization of Black Carbon Magnetite
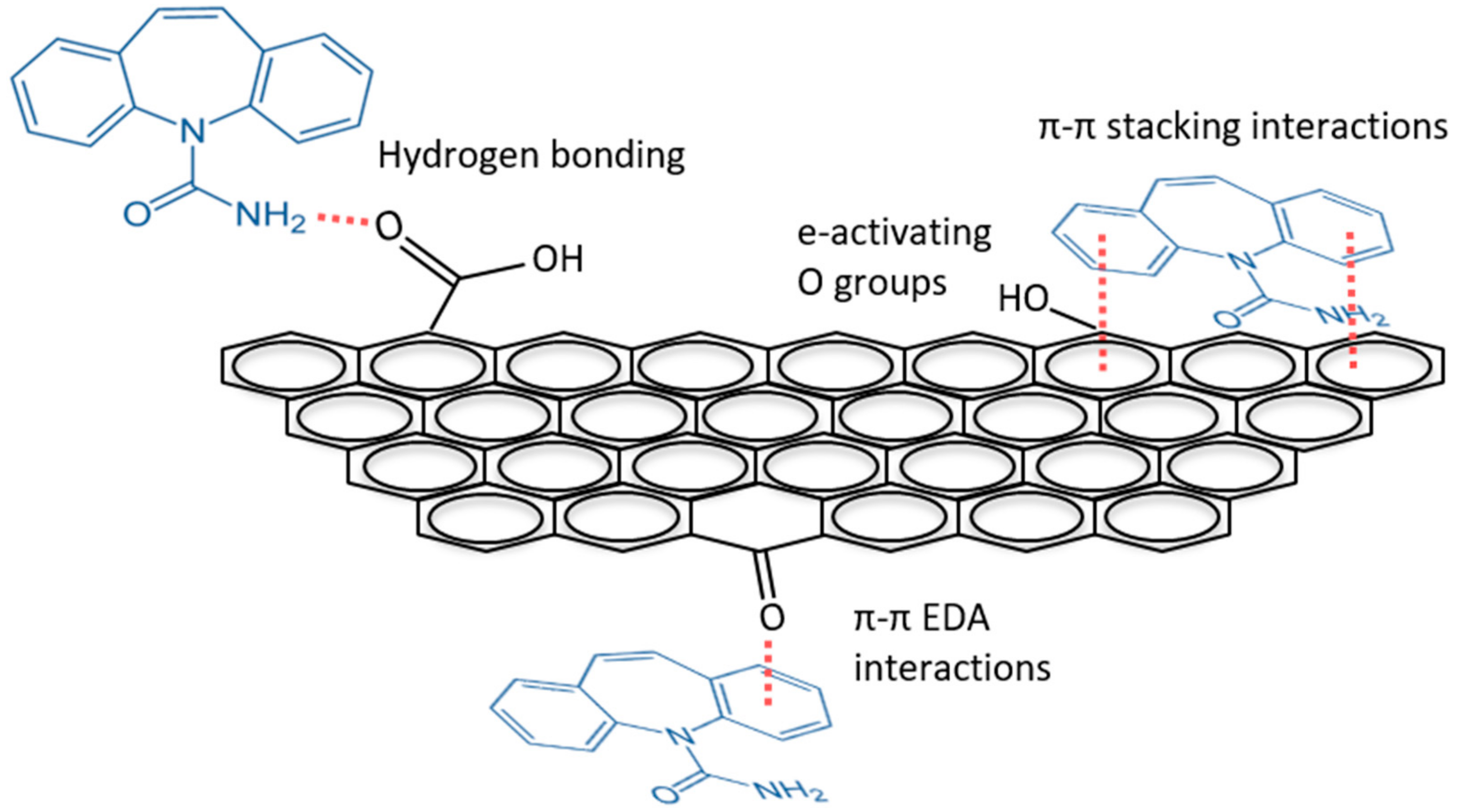
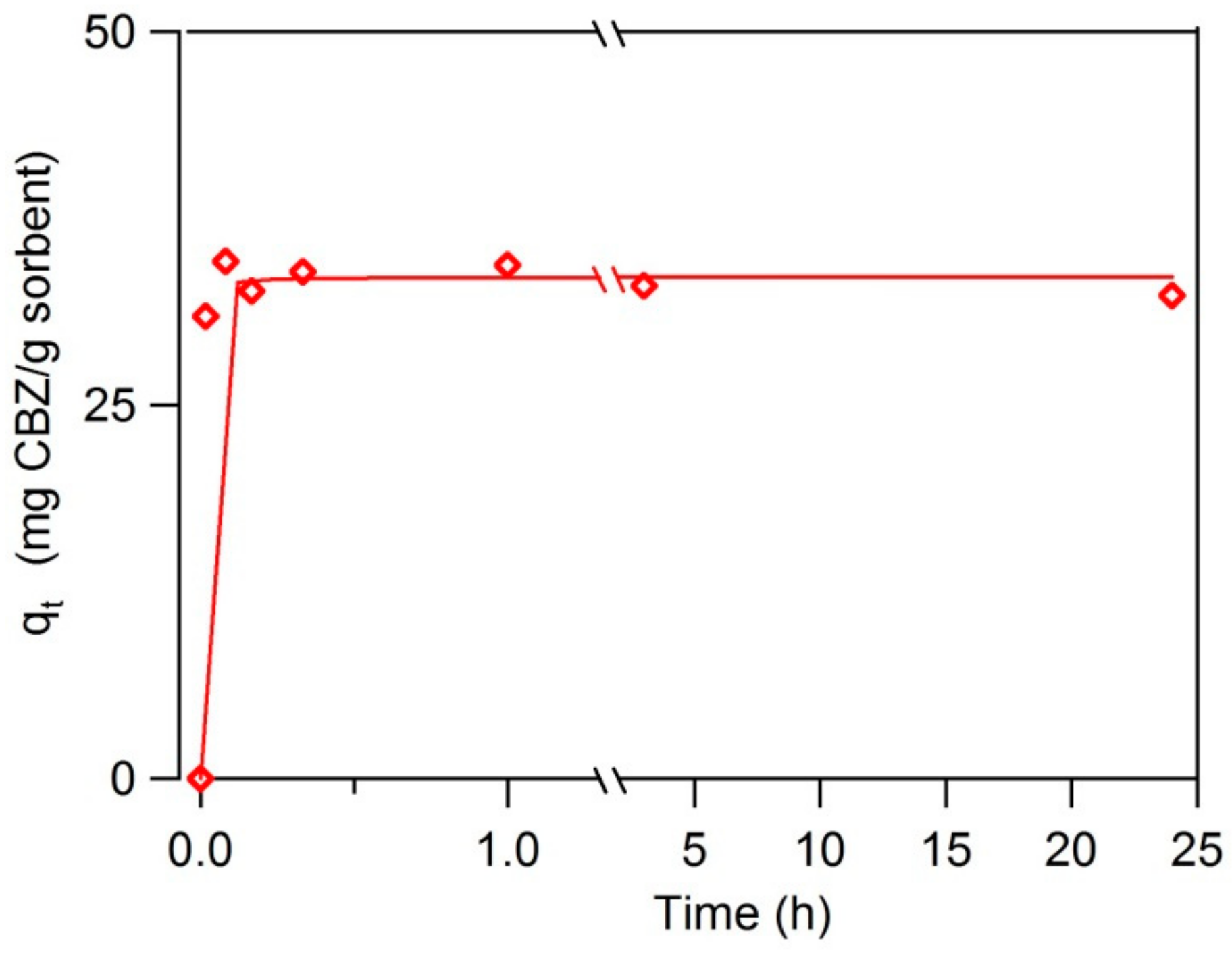
3.2. Sorption Rates and Capacity
3.3. Effect of Solution pH
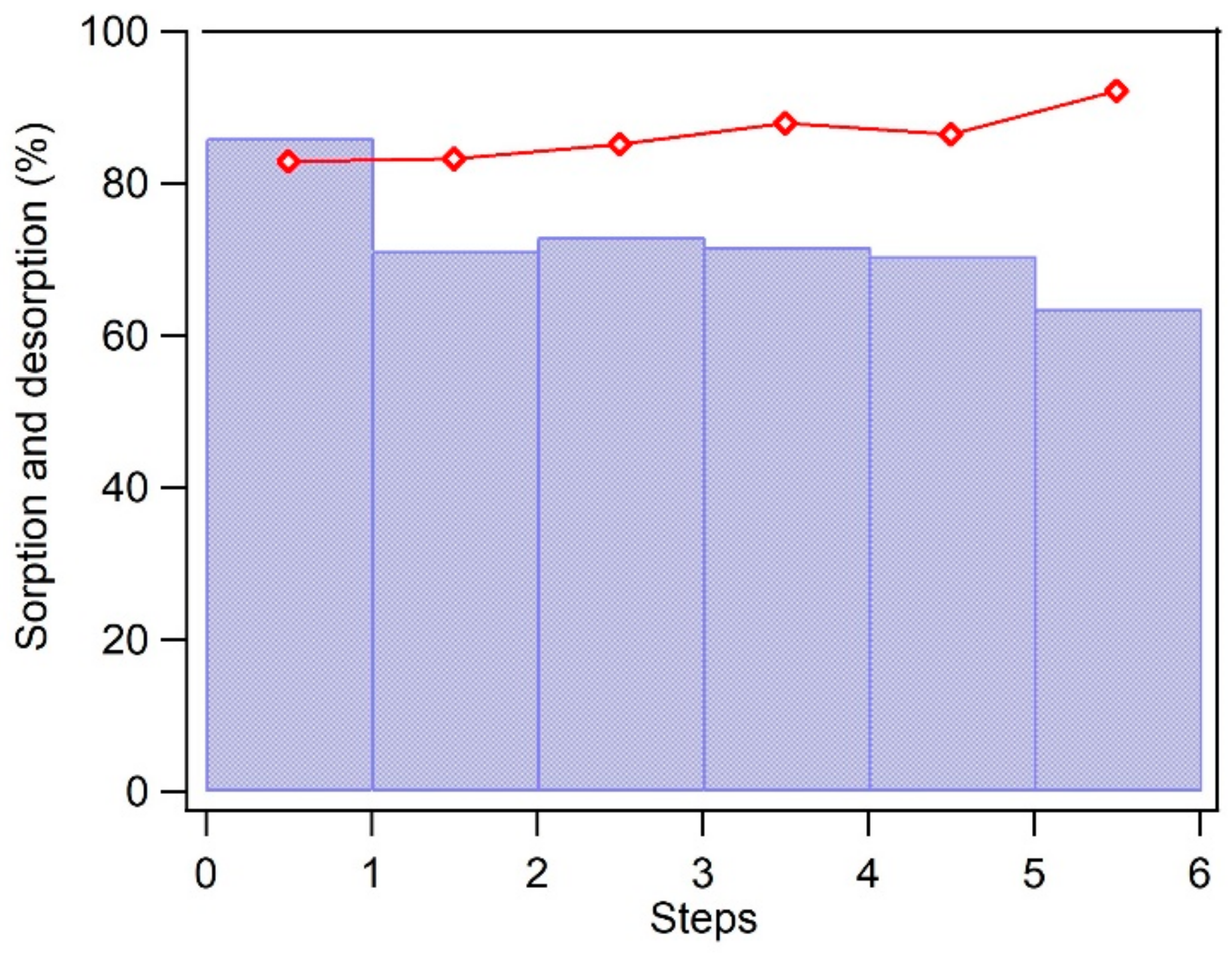
3.4. Recovery of Carbamazepine and Regeneration of BC-Mag
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Baig, U.; Uddin, M.K.; Gondal, M.A. Removal of hazardous azo dye from water using synthetic nano adsorbent: Facile synthesis, characterization, adsorption, regeneration and design of experiments. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124031. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zou, S.; Lu, C.; Bai, H.; Mu, H.; Duan, J. Removal and adsorption mechanism of tetracycline and cefotaxime contaminants in water by NiFe2O4-COF-chitosan-terephthalaldehyde nanocomposites film. Chem. Eng. J. 2019, 123008. [Google Scholar] [CrossRef]
- Luján-Facundo, M.J.; Iborra-Clar, M.I.; Mendoza-Roca, J.A.; Alcaina-Miranda, M.I. Pharmaceutical compounds removal by adsorption with commercial and reused carbon coming from a drinking water treatment plant. J. Clean Prod. 2019, 238, 117866. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Coupling granular activated carbon adsorption with membrane bioreactor treatment for trace organic contaminant removal: Breakthrough behaviour of persistent and hydrophilic compounds. J. Environ. Manag. 2013, 119, 173–181. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Huang, Q.; Nie, Y.; Wang, B.; Huang, J.; Yu, G. Regenerable granular carbon nanotubes/alumina hybrid adsorbents for diclofenac sodium and carbamazepine removal from aqueous solution. Water Res. 2013, 47, 4139–4147. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, Q.; Nie, Y.; Wei, H.; Wang, B.; Huang, J.; Yu, G.; Xing, B. Sorption mechanisms of perfluorinated compounds on carbon nanotubes. Environ. Pollut. 2012, 168, 138–144. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, Z.; Gao, B.; Wang, Z.; Xu, D.; Jin, J.; Liu, X. Adsorption of diuron, fluridone and norflurazon on single-walled and multi-walled carbon nanotubes. Sci. Total Environ. 2012, 439, 1–7. [Google Scholar] [CrossRef]
- Sharma, V.K.; McDonald, T.J.; Kim, H.; Garg, V.K. Magnetic graphene–carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification. Adv. Colloid Interface 2015, 225, 229–240. [Google Scholar] [CrossRef]
- Park, C.M.; Chu, K.H.; Heo, J.; Her, N.; Jang, M.; Son, A.; Yoon, Y. Environmental behavior of engineered nanomaterials in porous media: A review. J. Hazard. Mater. 2016, 309, 133–150. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Functionalization, pH, and ionic strength influenced sorption of sulfamethoxazole on graphene. J. Environ. Chem. Eng. 2014, 2, 310–315. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, H.; Wu, C.; Feng, S.; Alsaedi, A.; Hayat, T.; Chen, C. Facile synthesis of magnetic Fe3O4/graphene composites for enhanced U(VI) sorption. Appl. Surf. Sci. 2018, 444, 691–698. [Google Scholar] [CrossRef]
- Mandeep; Gulati, A.; Kakkar, R. Graphene-based adsorbents for water remediation by removal of organic pollutants: Theoretical and experimental insights. Chem. Eng. Res. Des. 2020, 153, 21–36. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Zheng, M.; Zhou, J.; Quan, X. Enhanced permeability, contaminants removal and antifouling ability of CNTs-based hollow fiber membranes under electrochemical assistance. J. Membr. Sci. 2019, 582, 335–341. [Google Scholar] [CrossRef]
- Chu, G.; Zhao, J.; Liu, Y.; Lang, D.; Wu, M.; Pan, B.; Steinberg, C.E.W. The relative importance of different carbon structures in biochars to carbamazepine and bisphenol A sorption. J. Hazard. Mater. 2019, 373, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, A.P.K.; Koelmans, A.A.; Jonker, M.T.O. Modeling polychlorinated biphenyl sorption isotherms for soot and coal. Env. Pollut. 2010, 158, 2672–2678. [Google Scholar] [CrossRef]
- Hao, L.; Meng, X.; Wang, C.; Wu, Q.; Wang, Z. Preparation of nickel-doped nanoporous carbon microspheres from metal-organic framework as a recyclable magnetic adsorbent for phthalate esters. J. Chromatogr. A 2019, 460364. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xu, B.; Wu, J.; Brookes, P.C.; Xu, J. Adsorption and desorption of phenanthrene by magnetic graphene nanomaterials from water: Roles of pH, heavy metal ions and natural organic matter. Chem. Eng. J. 2019, 368, 390–399. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C.; Chen, K. Magnetic carbon bubble for pollutants removal. Sep. Purif. Technol. 2019, 225, 74–79. [Google Scholar] [CrossRef]
- De Castro Alves, L.; Yáñez-Vilar, S.; Piñeiro-Redondo, Y.; Rivas, J. Novel Magnetic Nanostructured Beads for Cadmium(II) Removal. Nanomaterials 2019, 9, 356. [Google Scholar] [CrossRef]
- Fernandes, T.; Soares, S.; Trindade, T.; Daniel-da-Silva, A. Magnetic Hybrid Nanosorbents for the Uptake of Paraquat from Water. Nanomaterials 2017, 7, 68. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, H.; Hu, Z.; Cai, Y.; Shi, Y. Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2010, 1217, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.H.; Qawariq, R.F.; Abdelghani, J.I. Adsorption and magnetic solid-phase extraction of NSAIDs from pharmaceutical wastewater using magnetic carbon nanotubes: Effect of sorbent dimensions, magnetite loading and competitive adsorption study. Env. Technol. Inno. 2019, 16, 100496. [Google Scholar] [CrossRef]
- Nas, M.S.; Kuyuldar, E.; Demirkan, B.; Calimli, M.H.; Demirbaş, O.; Sen, F. Magnetic nanocomposites decorated on multiwalled carbon nanotube for removal of Maxilon Blue 5G using the sono-Fenton method. Sci. Rep. 2019, 9, 10850. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Ramaprabhu, S. Magnetite Decorated Multiwalled Carbon Nanotube Based Supercapacitor for Arsenic Removal and Desalination of Seawater. J. Phys. Chem. C 2010, 114, 2583–2590. [Google Scholar] [CrossRef]
- Ma, J.; Sun, S.; Chen, K. Facile and scalable synthesis of magnetite/carbon adsorbents by recycling discarded fruit peels and their potential usage in water treatment. Bioresour. Technol. 2017, 233, 110–115. [Google Scholar] [CrossRef]
- Alfe, M.; Ammendola, P.; Gargiulo, V.; Raganati, F.; Chirone, R. Magnetite loaded carbon fine particles as low-cost CO2 adsorbent in a sound assisted fluidized bed. Proc. Combust. Inst. 2015, 35, 2801–2809. [Google Scholar] [CrossRef]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Isotherms and thermodynamics of CO2 adsorption on a novel carbon-magnetite composite sorbent. Chem. Eng. Res. Des. 2018, 134, 540–552. [Google Scholar] [CrossRef]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Kinetic study and breakthrough analysis of the hybrid physical/chemical CO2 adsorption/desorption behavior of a magnetite-based sorbent. Chem. Eng. J. 2019, 372, 526–535. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Al-Hartomy, O.A.; Al-Solamy, F.R.; Dishovsky, N.; Malinova, P.; Atanasova, G.; Atanasov, N. Conductive carbon black/magnetite hybrid fillers in microwave absorbing composites based on natural rubber. Compos. B Eng. 2016, 96, 231–241. [Google Scholar] [CrossRef]
- Tokoro, H.; Fujii, S.; Oku, T. Iron nanoparticles coated with graphite nanolayers and carbon nanotubes. Diam. Relat. Mater. 2004, 13, 1270–1273. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.-C.; Kim, K.S. Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wei, S.; Gu, H.; Rapole, S.B.; Wang, Q.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal. Environ. Sci. Technol. 2012, 46, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and Endocrine Disrupting Compounds in U.S. Drinking Water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J. Carbamazepine degradation by gamma irradiation coupled to biological treatment. J. Hazard. Mater. 2017, 321, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Gurung, K.; Ncibi, M.C.; Shestakova, M.; Sillanpää, M. Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode. Appl. Catal. B Environ. 2018, 221, 329–338. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, J.; Luo, Z.; Zeng, W.; Wei, Z.; Spinney, R.; Hu, W.P.; Dionysiou, D.D. Experimental and theoretical insight into hydroxyl and sulfate radicals-mediated degradation of carbamazepine. Environ. Pollut. 2019, 113498. [Google Scholar] [CrossRef]
- Lu, G.; Hu, J. Effect of alpha-hydroxy acids on transformation products formation and degradation mechanisms of carbamazepine by UV/H2O2 process. Sci. Total Environ. 2019, 689, 70–78. [Google Scholar] [CrossRef]
- Shayanfar, A.; Velaga, S.; Jouyban, A. Solubility of carbamazepine, nicotinamide and carbamazepine–nicotinamide cocrystal in ethanol–water mixtures. Fluid Phase Equilibr. 2014, 363, 97–105. [Google Scholar] [CrossRef]
- Cai, N.; Larese-Casanova, P. Sorption of carbamazepine by commercial graphene oxides: A comparative study with granular activated carbon and multiwalled carbon nanotubes. J. Colloid Interf. Sci. 2014, 426, 152–161. [Google Scholar] [CrossRef]
- Goetz, S.A.; Nguyen, D.T.; Esser-Kahn, A.P. Surface modification of carbon black nanoparticles enhances photothermal separation and release of CO2. Carbon 2016, 105, 126–135. [Google Scholar] [CrossRef]
- Toupin, M.; Bélanger, D. Spontaneous Functionalization of Carbon Black by Reaction with 4-Nitrophenyldiazonium Cations. Langmuir 2008, 24, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiong, Z.; Zheng, J.; Luo, Z.; Zhu, G.; Xiao, C.; Meng, Z.; Li, Y.; Luo, K. Nitrogen-doped graphite encapsulated Fe/Fe3C nanoparticles and carbon black for enhanced performance towards oxygen reduction. J. Mater. Sci. Technol. 2019, 35, 2543–2551. [Google Scholar] [CrossRef]
- Ratajczak, K.; Stobiecka, M. Ternary Interactions and Energy Transfer between Fluorescein Isothiocyanate, Adenosine Triphosphate, and Graphene Oxide Nanocarriers. J. Phys. Chem. B 2017, 121, 6822–6830. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Ezra, F.S. Role of surface acidity in the adsorption of organic pollutants on the surface of carbon. Environ. Sci. Technol. 1968, 2, 291–297. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory. Preparation and Characterization., 2nd ed.; WILEY-VCH Verlag GmbH: Weinheim, Germany, 1991; pp. 138–140. [Google Scholar]
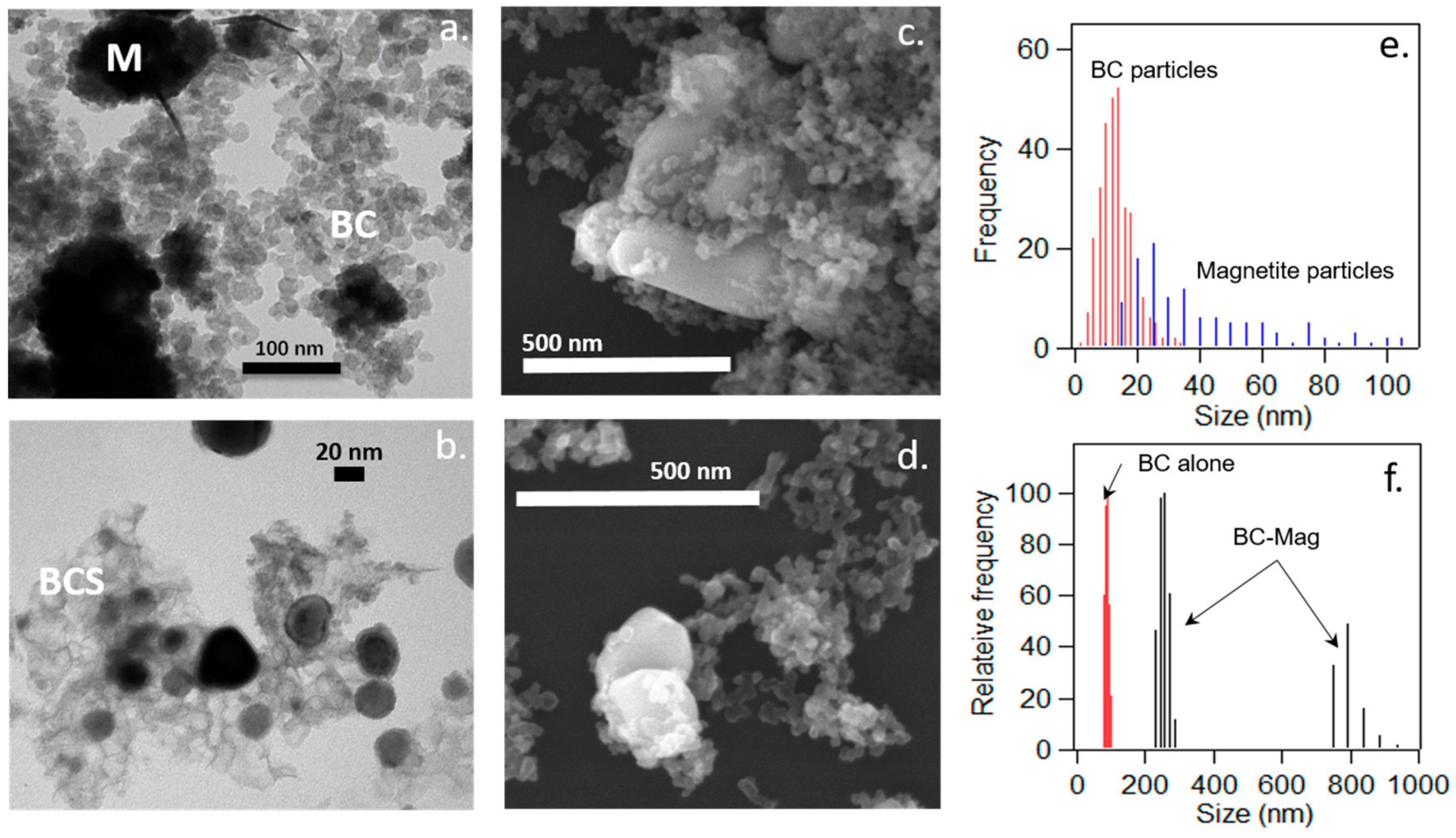
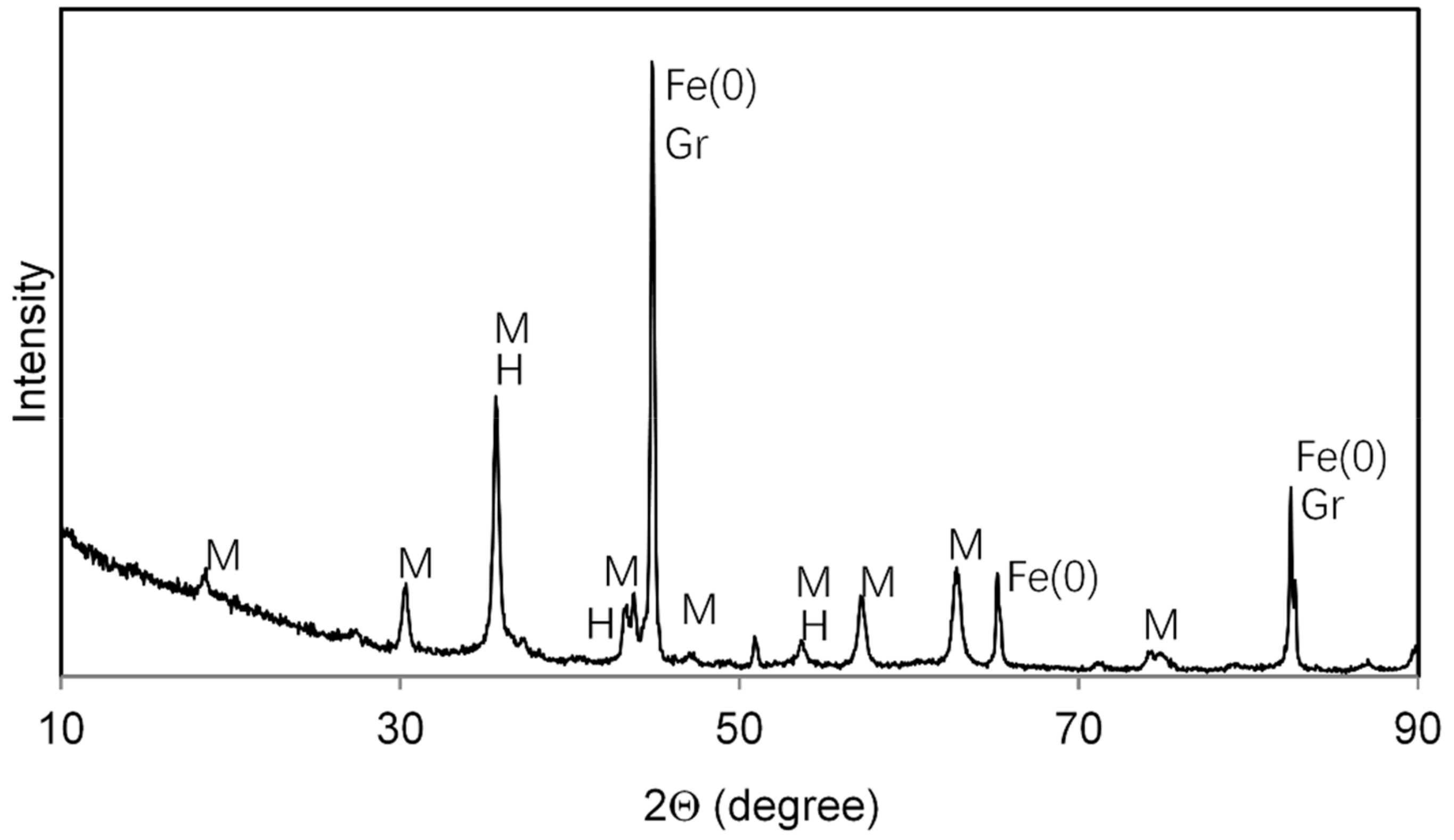
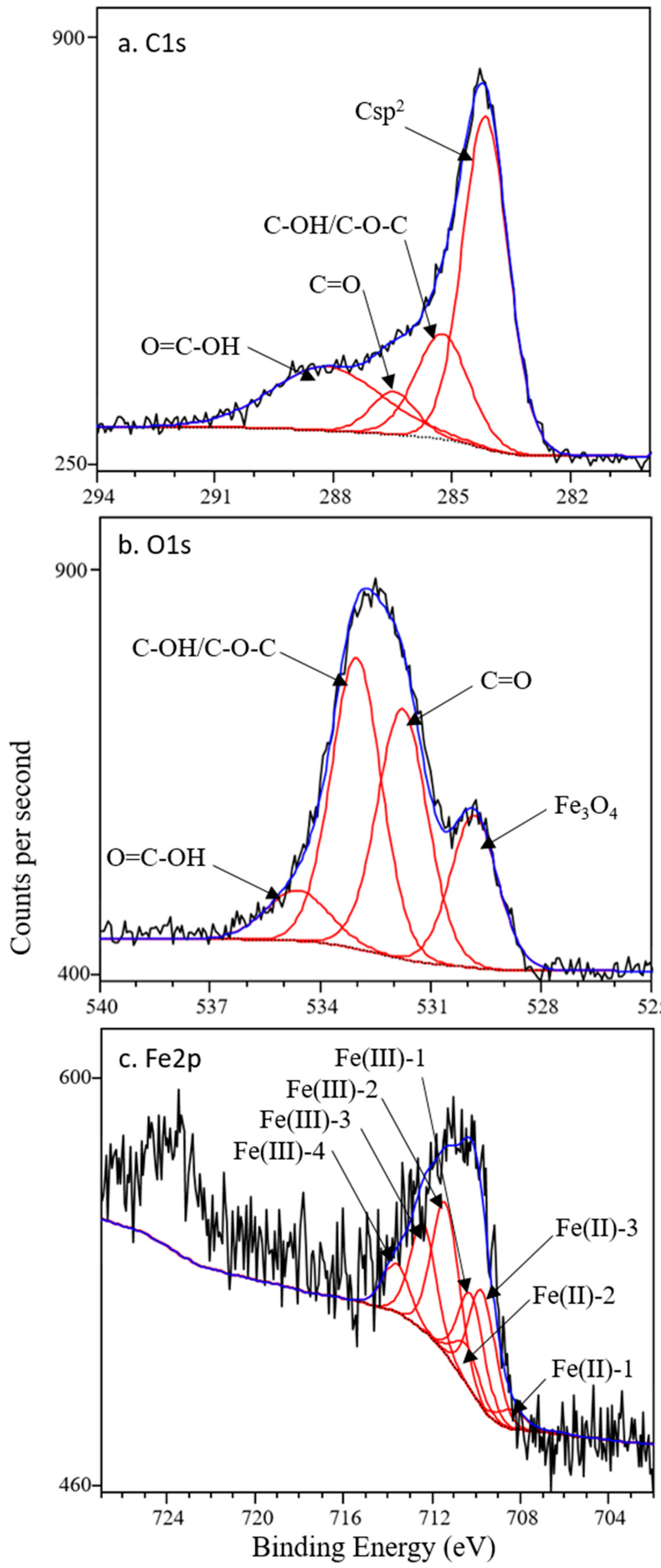



| Binding Energy | Rel. Area | FWHM | |
|---|---|---|---|
| eV | % | ||
| Cls | |||
| C=C sp2 | 284.1 | 51.2 | 1.4 |
| C–OH/C–O–C | 285.2 | 18.8 | 1.5 |
| C=O | 286.5 | 6.5 | 1.3 |
| O=C–OH | 288.2 | 23.5 | 3.2 |
| O1s | |||
| Fe3O4 | 529.8 | 19.1 | 1.5 |
| C=O | 531.7 | 33.5 | 1.6 |
| C–OH/C–O–C | 533.0 | 39.0 | 1.6 |
| O=C–OH | 534.6 | 8.3 | 2 |
| Fe2p3/2 | |||
| Fe(II)-1 | 708.4 | 3.3 | 1.4 |
| Fe(II)-2 | 709.7 | 19.7 | 1.4 |
| Fe(II)-3 | 710.4 | 6.6 | 1.4 |
| Fe(III)-1 | 710.2 | 16.4 | 1.4 |
| Fe(III)-2 | 711.3 | 27.9 | 1.5 |
| Fe(III)-3 | 712.4 | 18.0 | 1.4 |
| Fe(III)-4 | 713.6 | 8.1 | 1.4 |
| Sorbent | Sorbent Concentration g L−1 | tr h | k2 g mg−1 h−1 | qe mg g−1 | qe/SSA mg m−2 |
|---|---|---|---|---|---|
| BC-Mag | 0.5 | 0.0012 | 24.8 | 33.6 | 0.27 |
| Freundlich Isotherm | Langmuir Isotherm | ||||
|---|---|---|---|---|---|
| KFa | n | R2 | KL (L mg−1) | qmax (mg g−1) | R2 |
| 5.8 | 0.33 | 0.95 | 0.05 | 31.8 | 0.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, N.; Larese-Casanova, P. Facile Synthesis and Reuse of Magnetic Black Carbon Magnetite (BC-Mag) for Fast Carbamazepine Removal from Water. Nanomaterials 2020, 10, 213. https://doi.org/10.3390/nano10020213
Cai N, Larese-Casanova P. Facile Synthesis and Reuse of Magnetic Black Carbon Magnetite (BC-Mag) for Fast Carbamazepine Removal from Water. Nanomaterials. 2020; 10(2):213. https://doi.org/10.3390/nano10020213
Chicago/Turabian StyleCai, Nan, and Philip Larese-Casanova. 2020. "Facile Synthesis and Reuse of Magnetic Black Carbon Magnetite (BC-Mag) for Fast Carbamazepine Removal from Water" Nanomaterials 10, no. 2: 213. https://doi.org/10.3390/nano10020213
APA StyleCai, N., & Larese-Casanova, P. (2020). Facile Synthesis and Reuse of Magnetic Black Carbon Magnetite (BC-Mag) for Fast Carbamazepine Removal from Water. Nanomaterials, 10(2), 213. https://doi.org/10.3390/nano10020213





