Surface-Bound Humic Acid Increased Propranolol Sorption on Fe3O4/Attapulgite Magnetic Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
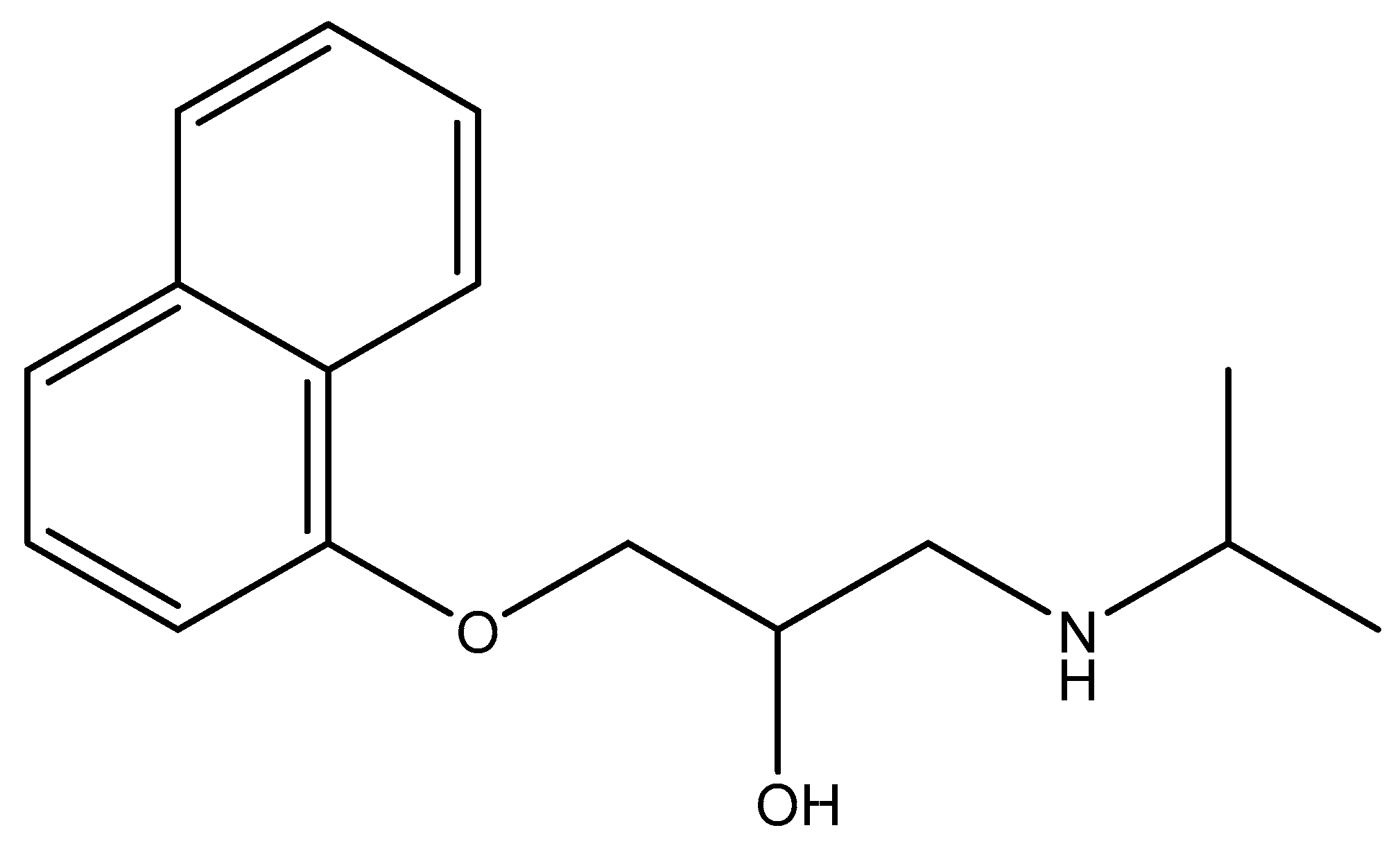
2.1. Materials
2.2. Synthesis of HA Coated MATP Magnetic Nanoparticles
2.3. Characterization of the As-Made Magnetic Nanoparticles
2.4. Sorption Experiments
3. Results and Discussion
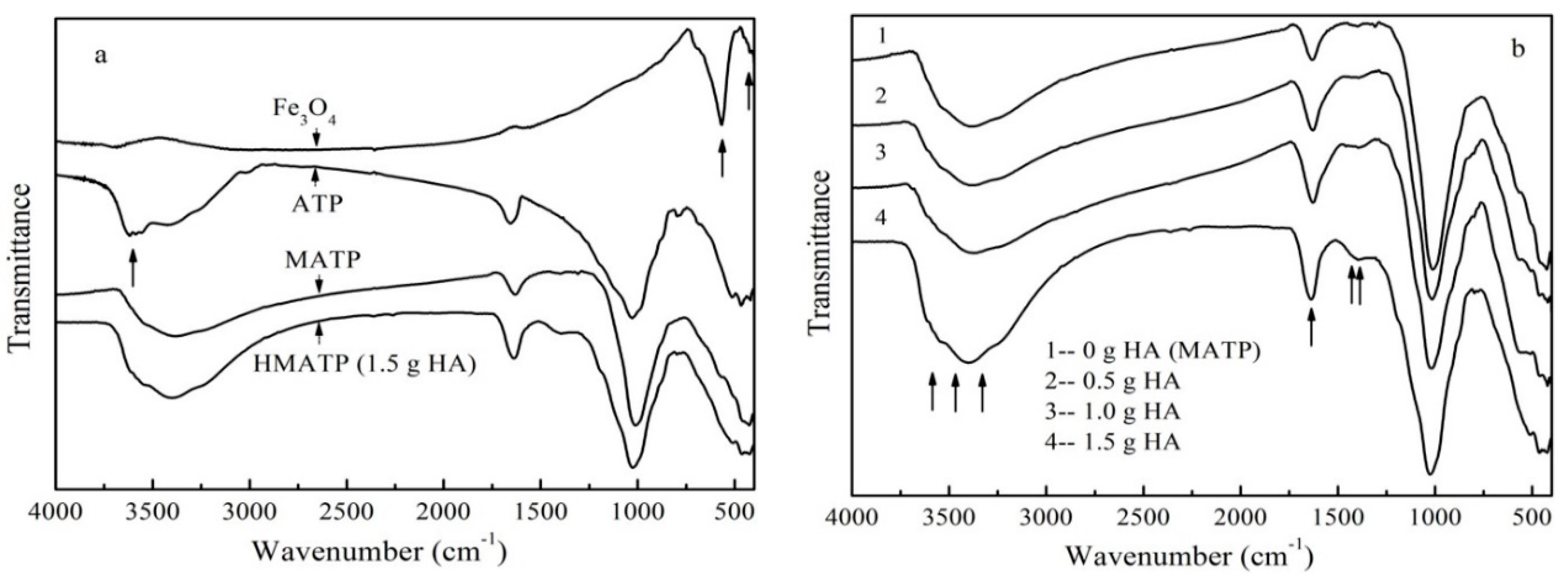
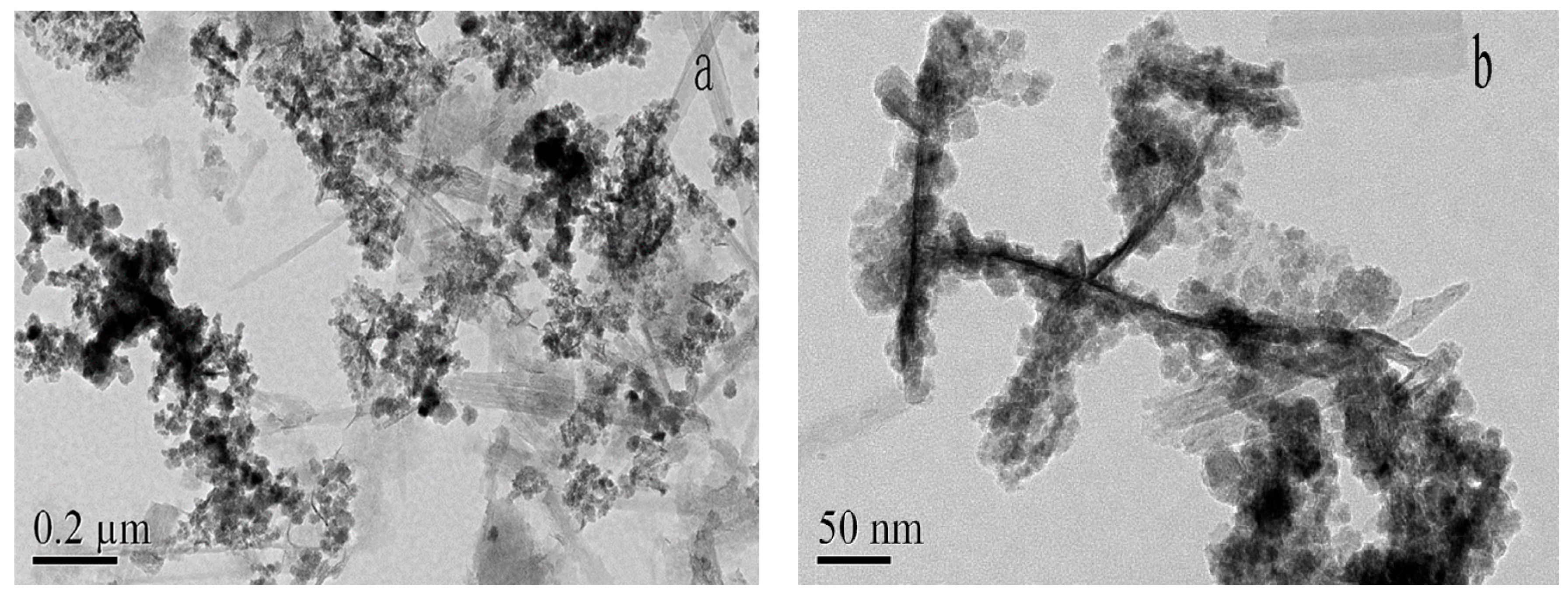
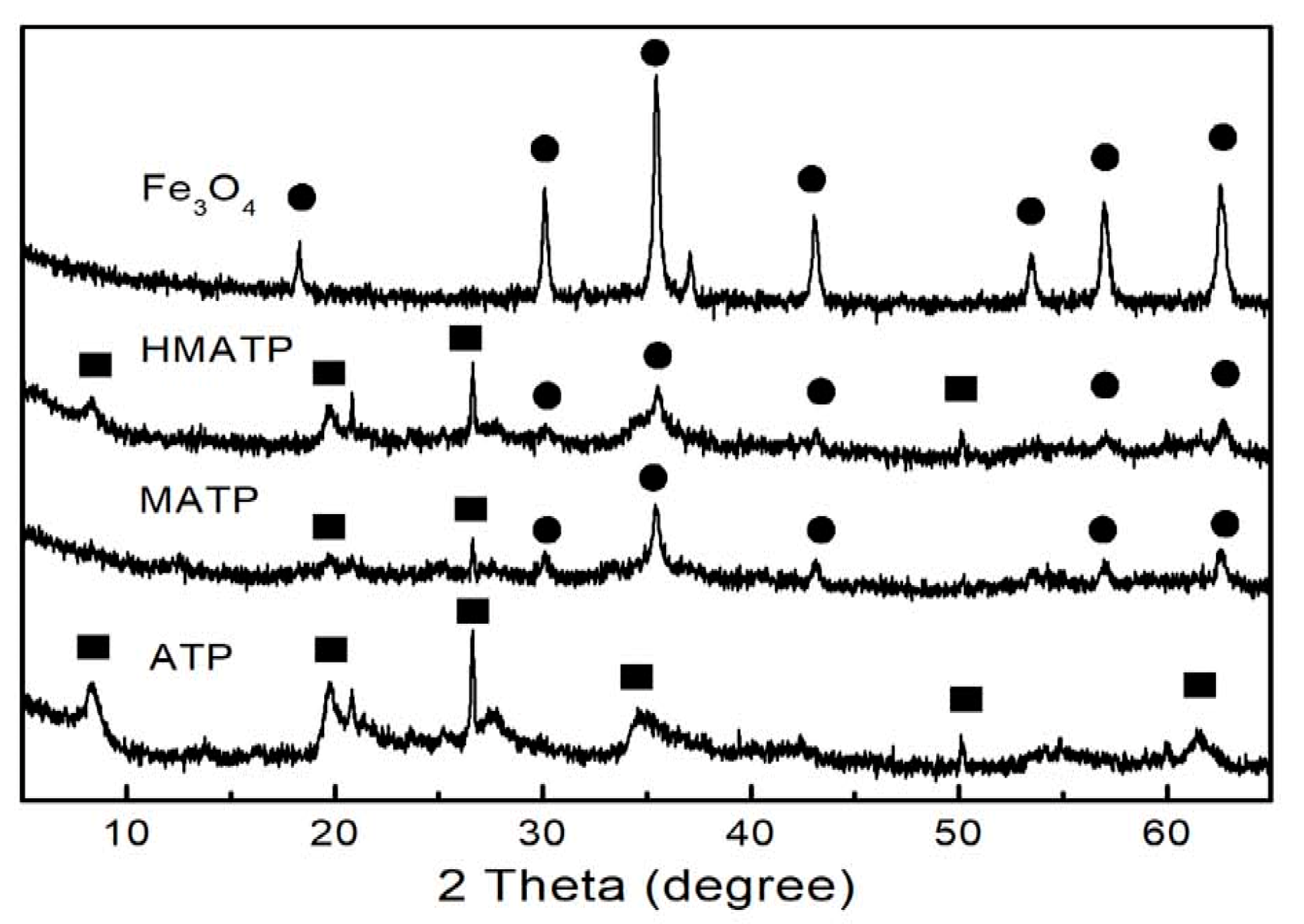
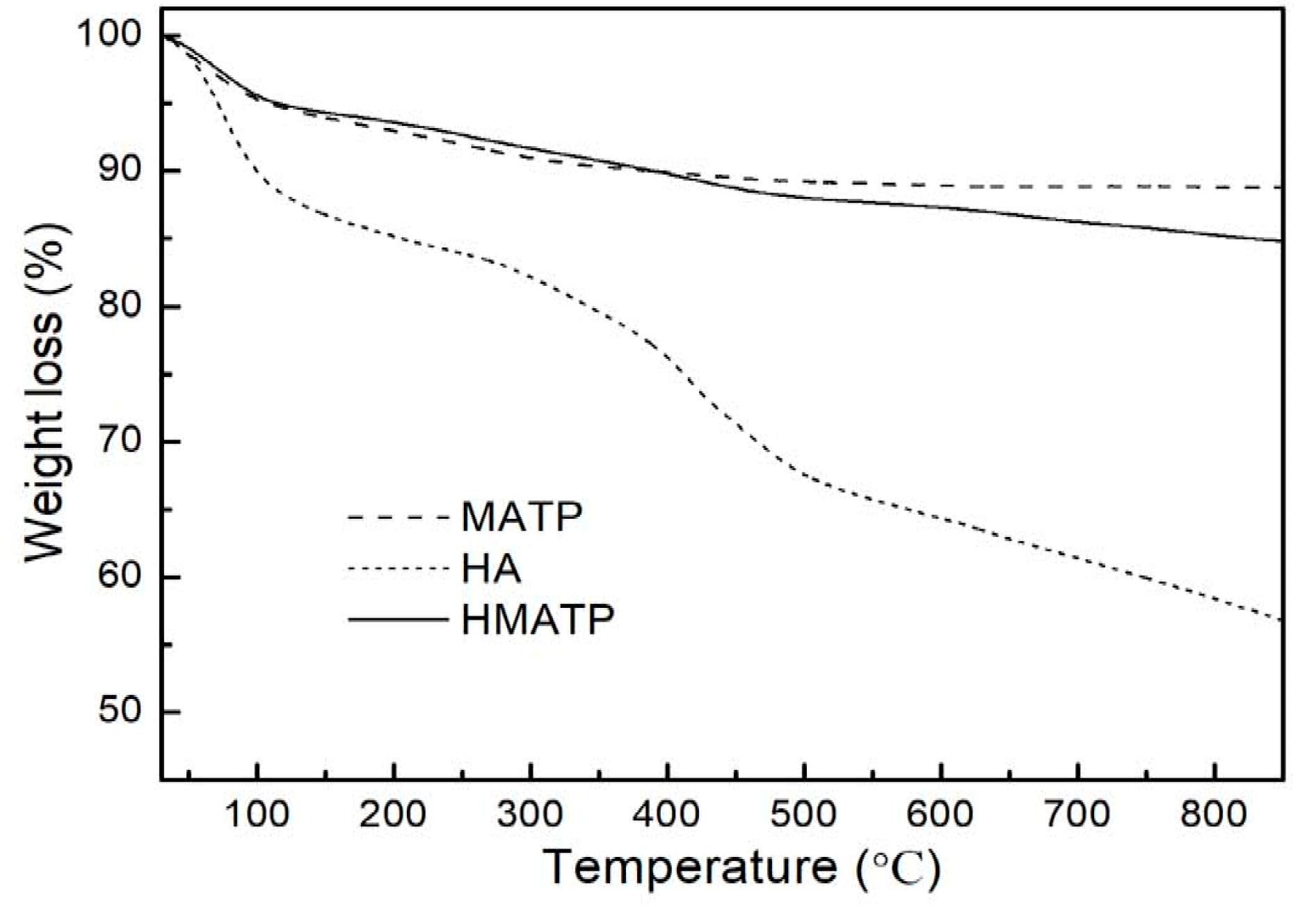
3.1. Material Characterization
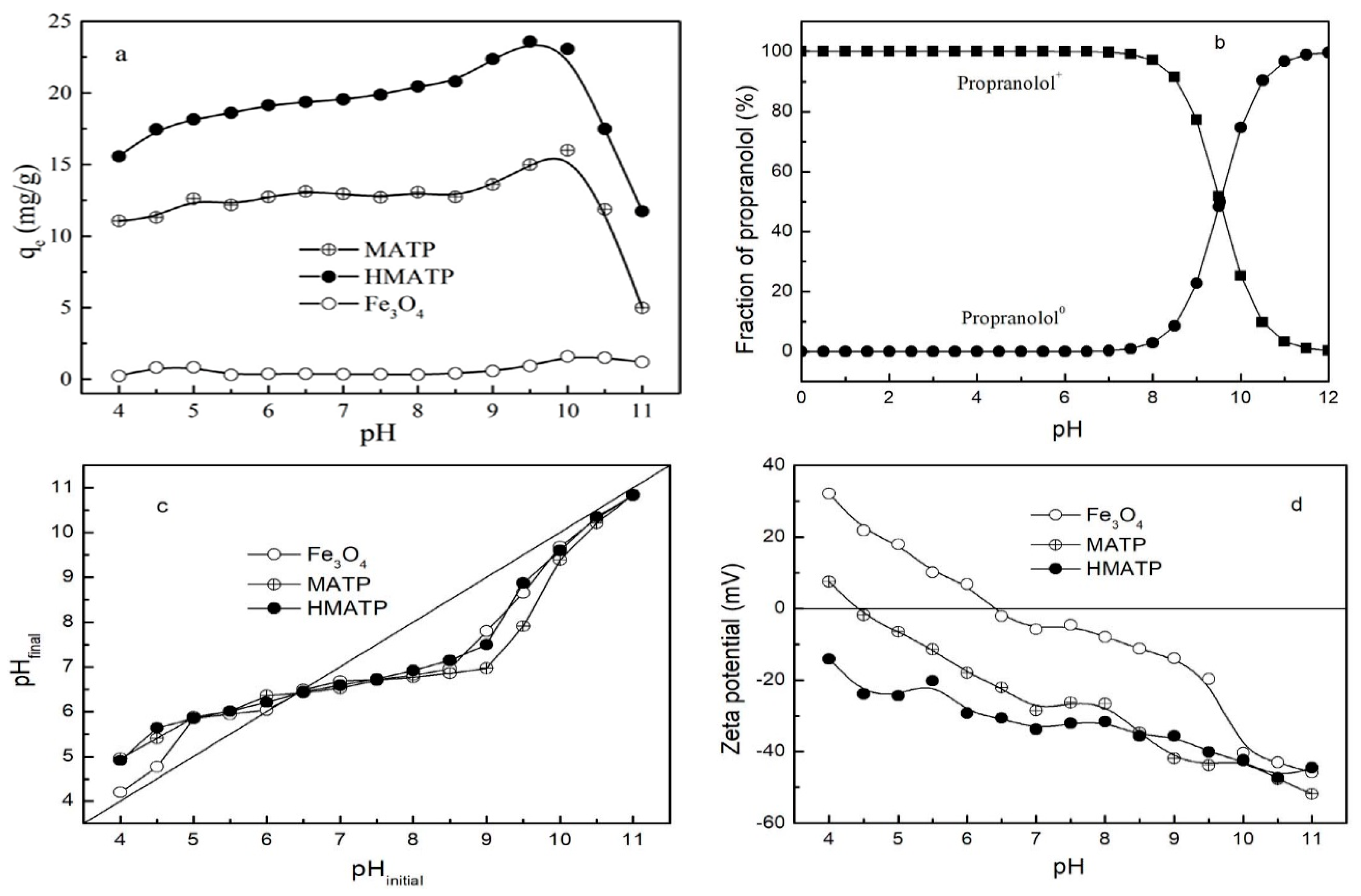
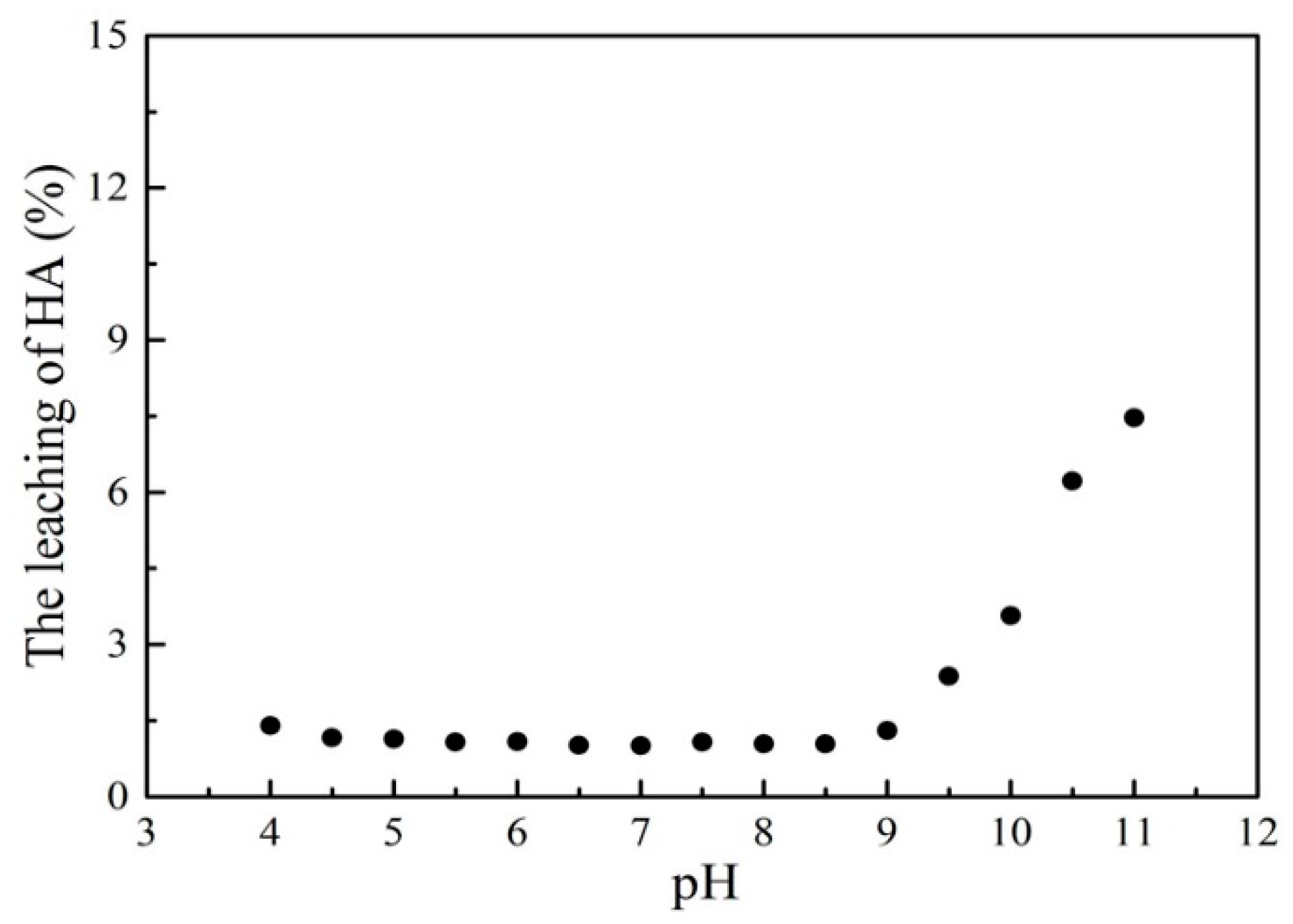
3.2. Effect of pH
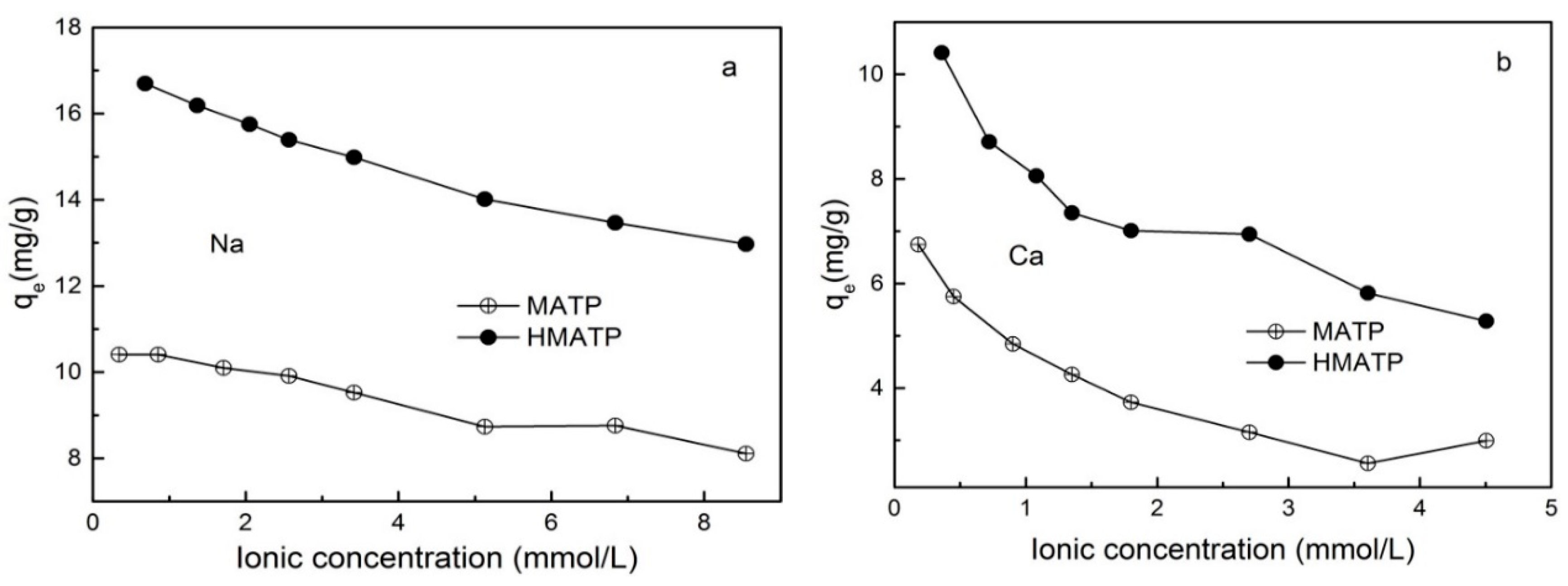
3.3. Effect of Ionic Strength
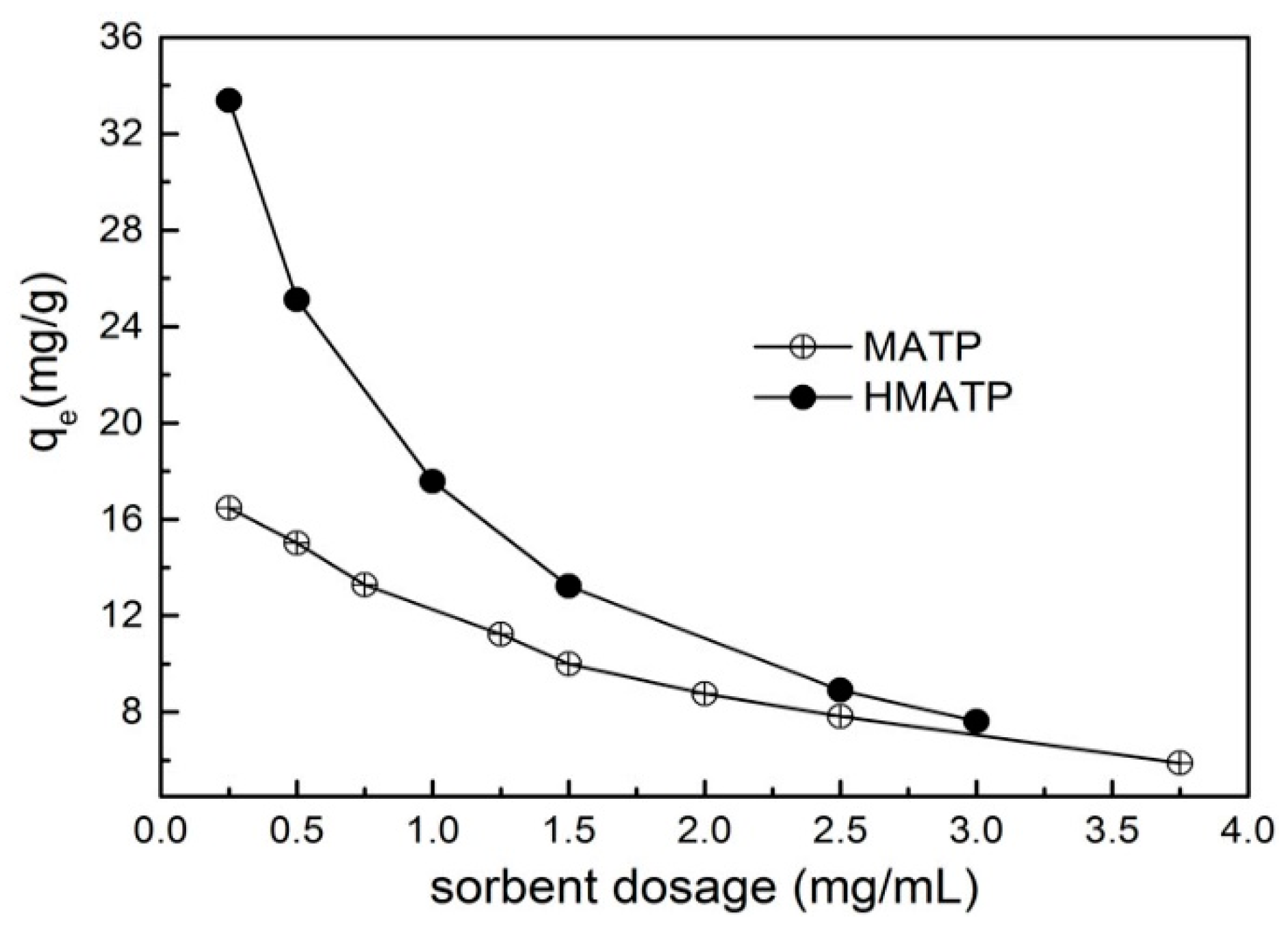
3.4. Effect of Sorbent Dosage
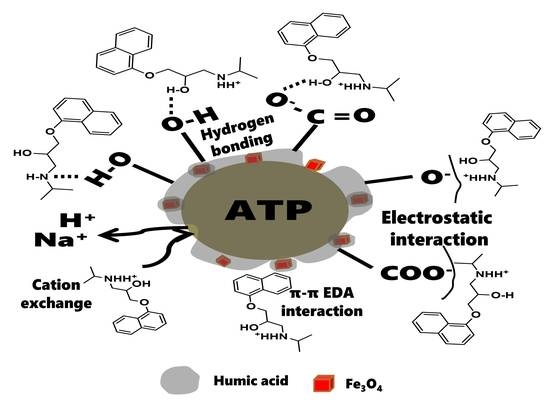
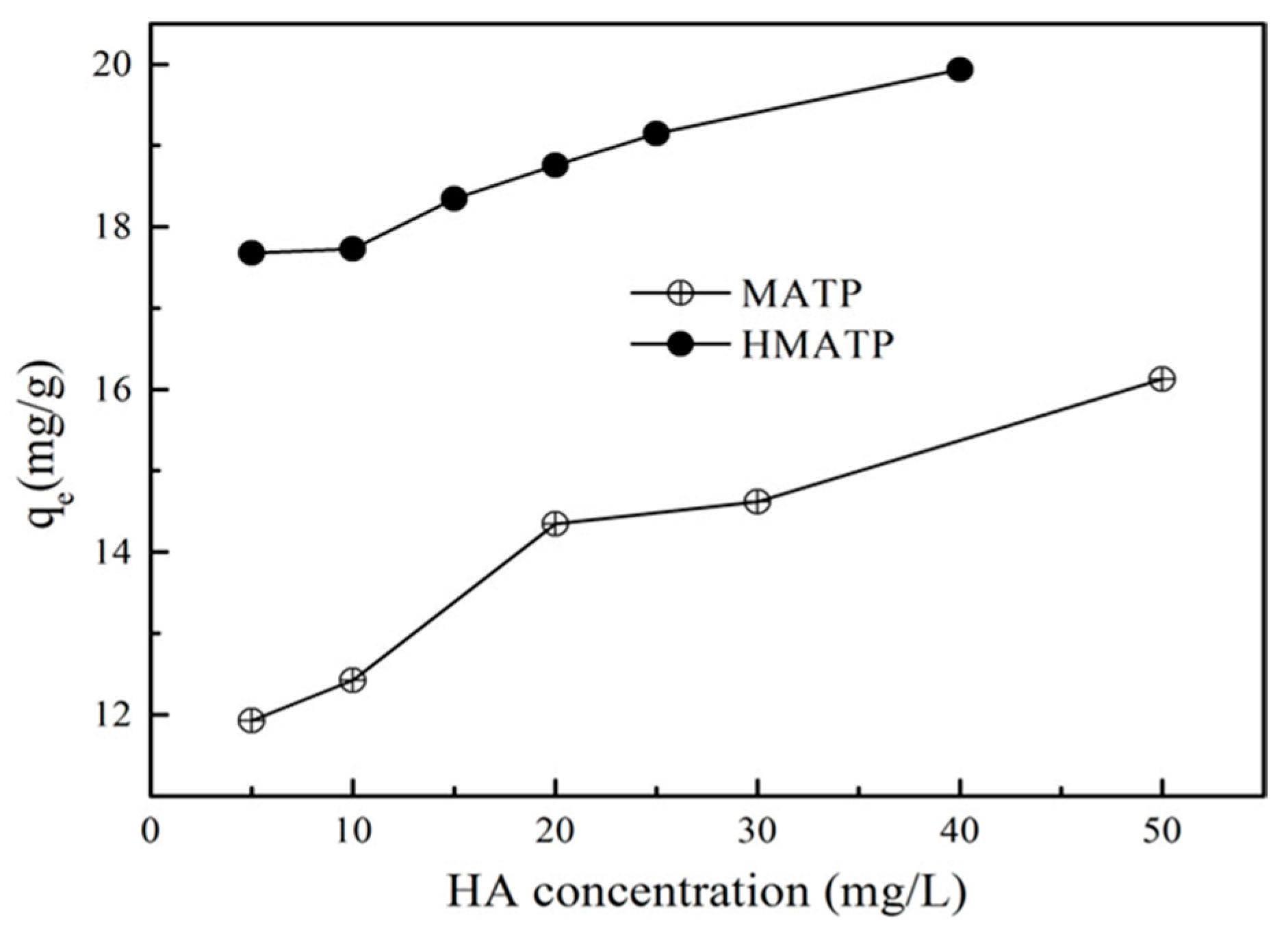
3.5. Effect of HA
3.6. Sorption isotherms
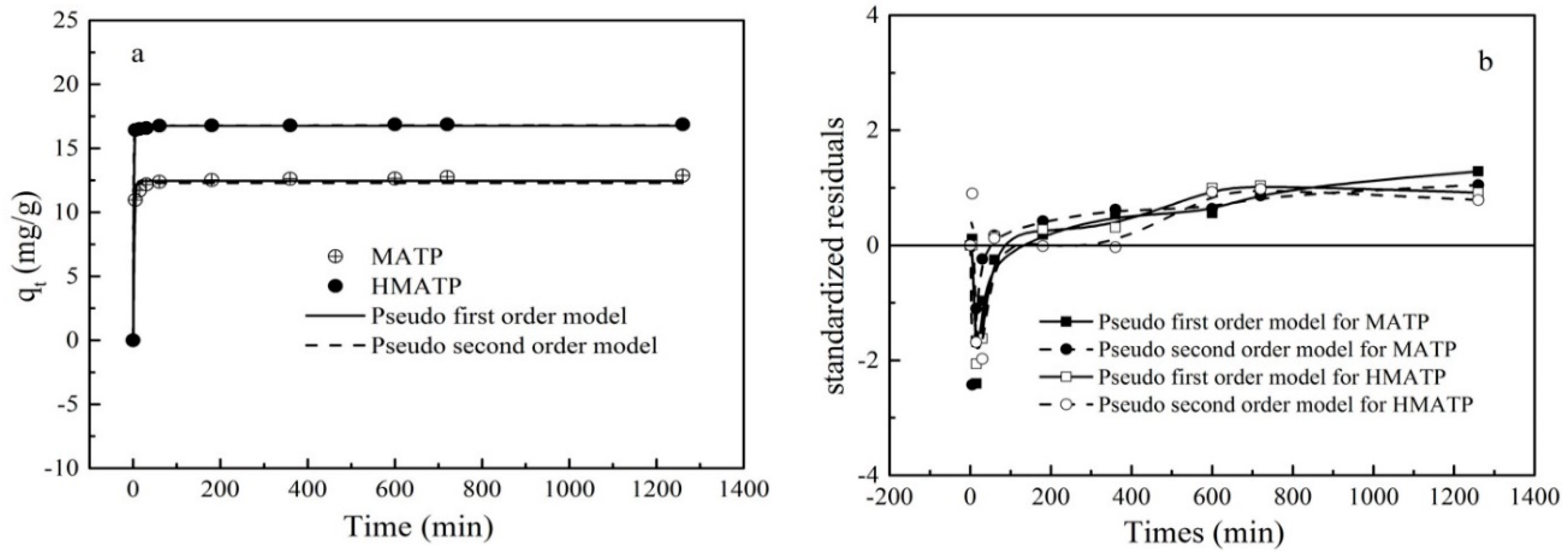
3.7. Sorption Kinetics
3.8. Material Stability
3.9. Comparison of HMATP with Other Sorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef]
- Mitchell, K.M.; Moon, T.W. Behavioral and biochemical adjustments of the zebrafish Danio rerio exposed to the β-blocker propranolol. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Escher, B.I.; Richle, P.; Schaffner, C.; Alder, A.C. Elimination of β-blockers in sewage treatment plants. Water Res. 2007, 41, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.R.; Li, Y.; Qi, Y.; Yu, X.; He, J.; Lu, Y.; Gao, X.; Deng, Y.; Song, Z. A novel treatment of waste printed circuit boards by low-temperature near-critical aqueous ammonia: debromination and preparation of nitrogen-containing fine chemicals. Waste Manage. 2019, 84, 355–363. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Occurrence, ecotoxicological effects and risk assessment of antihypertensive pharmaceutical residues in the aquatic environment—A review. Chemosphere 2015, 138, 281–291. [Google Scholar] [CrossRef]
- Xiu, F.R.; Wang, Y.; Yu, X.; Li, Y.; Lu, Y.; Zhou, K.; He, J.; Song, Z.; Gao, X. A novel safety treatment strategy of DEHP-rich flexible polyvinyl chloride waste through low-temperature critical aqueous ammonia treatment. Sci. Total Environ. 2020, 708, 134532. [Google Scholar] [CrossRef]
- Zhou, S.F. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part II. Clin. Pharmacokinet. 2009, 48, 761–804. [Google Scholar]
- Kim, J.W.; Ishibashi, H.; Yamauchi, R.; Ichikawa, N.; Takao, Y.; Hirano, M.; Koga, M.; Arizono, K. Acute toxicity of pharmaceutical and personal care products on freshwater crustacean (Thamnocephalus platyurus) and fish (Oryzias latipes). J. Toxicol. Sci. 2009, 34, 227–232. [Google Scholar] [CrossRef]
- Ding, J.; Lu, G.; Li, S.; Nie, Y.; Liu, J. Biological fate and effects of propranolol in an experimental aquatic food chain. Sci. Total Environ. 2015, 532, 31–39. [Google Scholar] [CrossRef]
- Ding, J.; Lu, G.; Liu, J.; Yang, H.; Li, Y. Uptake, depuration, and bioconcentration of two pharmaceuticals, roxithromycin and propranolol, in Daphnia magna. Ecotoxicol. Environ. Saf. 2016, 126, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-blockers in the environment: Part II. Ecotoxicity study. Sci. Total Environ. 2014, 493, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.; Hui, M.; Li, V.; Lorenzi, V.; de la Paz, N.; Cheng, S.H.; Lai-Chan, L.; Schlenk, D. Effects of propranolol on heart rate and development in Japanese medaka (Oryzias latipes) and zebrafish (Danio rerio). Aquat. Toxicol. 2012, 122–123, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, V.; Mehinto, A.C.; Denslow, N.D.; Schlenk, D. Effects of exposure to the β-blocker propranolol on the reproductive behavior and gene expression of the fathead minnow, Pimephales promelas. Aquat. Toxicol. 2012, 116–117, 8–15. [Google Scholar] [CrossRef]
- Massarsky, A.; Trudeau, V.L.; Moon, T.W. β-blockers as endocrine disruptors: The potential effects of human β-blockers on aquatic organisms. J. Exp. Zool. A Ecol. Genet. Physiol. 2011, 315A, 251–265. [Google Scholar] [CrossRef]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-blockers in the environment: Part I. Mobility and hydrolysis study. Sci. Total Environ. 2014, 493, 1112–1121. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Zhang, Z.; Bao, Y.; Liu, F.; Wu, D.; Wang, Y. Biological effects and bioaccumulation of pharmaceutically active compounds in crucian carp caged near the outfall of a sewage treatment plant. Environ. Sci. Processes Impacts 2015, 17, 54–61. [Google Scholar] [CrossRef]
- Nanaki, S.G.; Kyzas, G.Z.; Tzereme, A.; Papageorgiou, M.; Kostoglou, M.; Bikiaris, D.N.; Lambropoulou, D.A. Synthesis and characterization of modified carrageenan microparticles for the removal of pharmaceuticals from aqueous solutions. Colloids Surf. B 2015, 127, 256–265. [Google Scholar] [CrossRef]
- Kutzner, S.; Schaffer, M.; Licha, T.; Worch, E.; Börnick, H. Sorption of organic cations onto silica surfaces over a wide concentration range of competing electrolytes. J. Colloid Interface Sci. 2016, 484, 229–236. [Google Scholar] [CrossRef]
- Ali, I.; Alothman, Z.A.; Alwarthan, A. Uptake of propranolol on ionic liquid iron nanocomposite adsorbent: Kinetic, thermodynamics and mechanism of adsorption. J. Mol. Liq. 2017, 236, 205–213. [Google Scholar] [CrossRef]
- Gazpio, C.; Sánchez, M.; Isasi, J.R.; Vélaz, I.; Martín, C.; Martínez-Ohárriz, C.; Zornoza, A. Sorption of pindolol and related compounds by a β-cyclodextrin polymer: Isosteric heat of sorption. Carbohydr. Polym. 2008, 71, 140–146. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Derome, C.; Buleté, A.; Vulliet, E.; Bressy, A.; Varrault, G.; Chebbo, G.; Rocher, V. Removal of emerging micropollutants from wastewater by activated carbon adsorption: Experimental study of different activated carbons and factors influencing the adsorption of micropollutants in wastewater. J. Environ. Chem. Eng. 2016, 4, 1102–1109. [Google Scholar] [CrossRef]
- Nam, S.W.; Choi, D.J.; Kim, S.K.; Her, N.; Zoh, K.D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef]
- Kutzner, S.; Schaffer, M.; Börnick, H.; Licha, T.; Worch, E. Sorption of the organic cation metoprolol on silica gel from its aqueous solution considering the competition of inorganic cations. Water Res. 2014, 54, 273–283. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Koltsakidou, A.; Nanaki, S.G.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of beta-blockers from aqueous media by adsorption onto graphene oxide. Sci. Total Environ. 2015, 537, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fitzgerald, N.M.; Albert, Z.; Schnabl, A.; Jiang, W.T. Contrasting mechanisms of metoprolol uptake on kaolinite and talc. Chem. Eng. J. 2015, 272, 48–57. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, F.; Liu, B.; Hu, X.; Sun, C. Sorptive removal of β-blocker propranolol from aqueous solution by modified attapulgite: Effect factors and sorption mechanisms. Chem. Eng. J. 2011, 174, 571–578. [Google Scholar] [CrossRef]
- Pan, D.; Fan, Q.; Fan, F.; Tang, Y.; Zhang, Y.; Wu, W. Removal of uranium contaminant from aqueous solution by chitosan@attapulgite composite. Sep. Purif. Technol. 2017, 177, 86–93. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Su, Z.; Li, F.; Wen, F. Attapulgite—Fe3O4 magnetic nanoparticles via co-precipitation technique. Appl. Surf. Sci. 2008, 255, 2020–2025. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y.; Nie, W.; Gao, X.; Zhang, L.; Yang, P.; Tan, X. Fast removal of propranolol from water by attapulgite/graphene oxide magnetic ternary composites. Materials (Basel) 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Price, N.T.; Gracia Pinilla, M.Á.; O’Shea, K.E. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Res. 2017, 123, 353–360. [Google Scholar] [CrossRef]
- Tan, L.; Wang, X.X.; Tan, X.; Mei, H.; Chen, C.; Hayat, T.; Alsaedi, A.; Wen, T.; Lu, S.; Wang, X.K. Bonding properties of humic acid with attapulgite and its influence on U(VI) sorption. Chem. Geol. 2017, 464, 91–100. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G. Bin Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; López, R.; Gondar, D.; Antelo, J.; Fiol, S.; Arce, F. Adsorption of paraquat on goethite and humic acid-coated goethite. J. Hazard. Mater. 2010, 183, 664–668. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Ge, J.; Yang, C.; Dang, Z.; Liu, S.; Gao, L. Sorption and photodegradation of tylosin and sulfamethazine by humic acid-coated goethite. RSC Adv. 2015, 5, 100464–100471. [Google Scholar] [CrossRef]
- Radwan, E.K.; Abdel Ghafar, H.H.; Moursy, A.S.; Langford, C.H.; Bedair, A.H.; Achari, G. Adsorptive removal of hazardous organic water pollutants by humic acid–carbon hybrid materials: Kinetics and isotherm study. Desalin. Water Treat. 2017, 80, 297–305. [Google Scholar] [CrossRef]
- Singhal, P.; Jha, S.K.; Pandey, S.P.; Neogy, S. Rapid extraction of uranium from sea water using Fe3O4 and humic acid coated Fe3O4 nanoparticles. J. Hazard. Mater. 2017, 335, 152–161. [Google Scholar] [CrossRef]
- Hu, X.; Liu, B.; Deng, Y.; Chen, H.; Luo, S.; Sun, C.; Yang, P.; Yang, S. Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution. Appl. Catal. B-Environ. 2011, 107, 274–283. [Google Scholar] [CrossRef]
- Xu, J.-M.; Li, W.; Yin, Q.-F.; Zhu, Y.-L. Direct electrochemistry of Cytochrome c on natural nano-attapulgite clay modified electrode and its electrocatalytic reduction for H2O2. Electrochim. Acta 2007, 52, 3601–3606. [Google Scholar] [CrossRef]
- Meng, J.H.; Yang, G.Q.; Yan, L.M.; Wang, X.Y. Synthesis and characterization of magnetic nanometer pigment Fe3O4. Dyes Pigments 2005, 66, 109–113. [Google Scholar] [CrossRef]
- Ye, C.; Hu, S.; Yan, W.; Duan, J.; Jing, C. Insights into propranolol adsorption on TiO2: Spectroscopic and molecular modeling study. J. Phys. Chem. C 2013, 117, 5785–5791. [Google Scholar] [CrossRef]
- Del Mar Orta, M.; Martín, J.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of propranolol onto montmorillonite: Kinetic, isotherm and pH studies. Appl. Clay Sci. 2019, 173, 107–114. [Google Scholar] [CrossRef]
- Wang, M.; Liao, L.; Zhang, X.; Li, Z. Adsorption of low concentration humic acid from water by palygorskite. Appl. Clay Sci. 2012, 67–68, 164–168. [Google Scholar] [CrossRef]
- Ye, X.; Jalel, A.; Josephine, M.H. Erroneous Application of Pseudo-Second-Order Adsorption Kinetics Model: Ignored Assumptions and Spurious Correlations. Ind. Eng. Chem. Res. 2018, 57, 2705–2709. [Google Scholar]

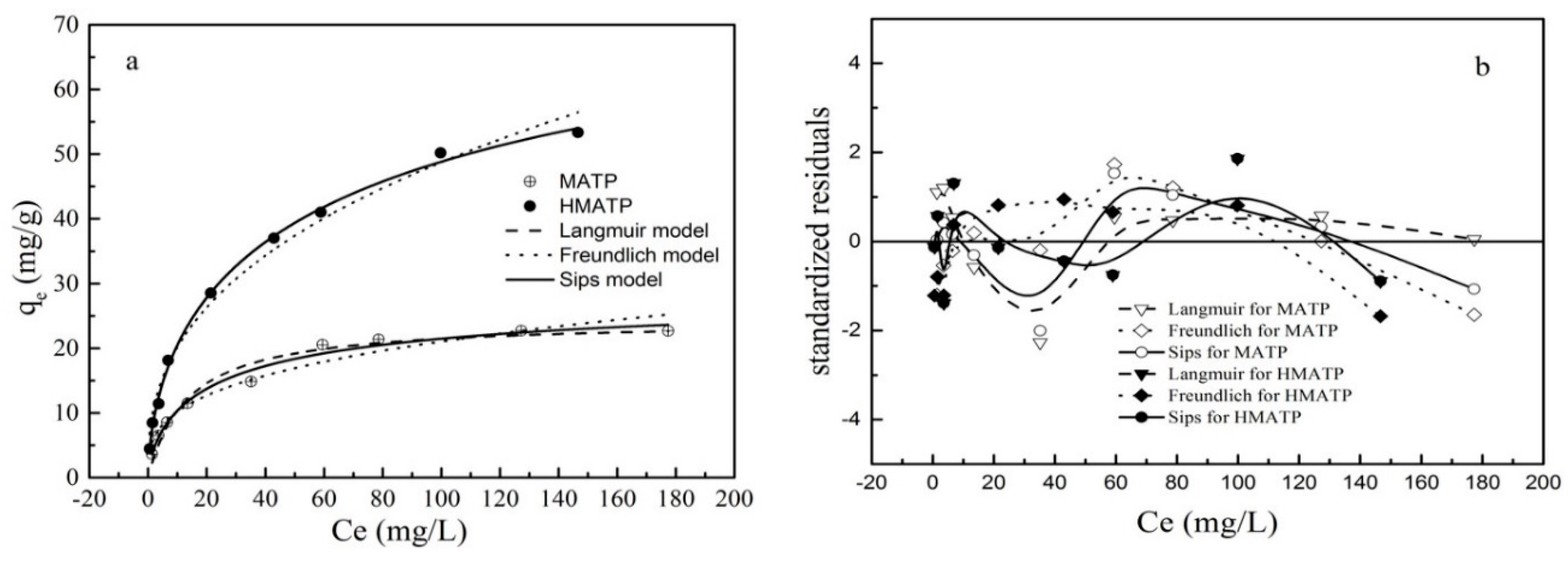
| Materials | Langmuir Equation | Freundlich Equation | Sips Equation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (L/g) | n1 | R2 | qm (mg/g) | n2 | b | R2 | |
| MATP | 24.33 | 0.075 | 0.964 | 4.94 | 3.18 | 0.945 | 29.83 | 1.46 | 0.04 | 0.979 |
| HMATP | 92.42 | 0.072 | 0.997 | 8.33 | 2.61 | 0.990 | 92.42 | 1.68 | 0.01 | 0.997 |
| Materials | qexp (mg/g) | Pseudo-First-Order Equation | Pseudo-Second-Order Equation | ||||
|---|---|---|---|---|---|---|---|
| K1 (1/min) | qcal (mg/g) | R2 | K2 (g/(mg·min)) | qcal (mg/g) | R2 | ||
| MATP | 12.87 | 0.419 | 12.463 | 0.992 | 0.094 | 12.291 | 0.976 |
| HMATP | 16.87 | 0.797 | 16.752 | 0.999 | 0.459 | 16.796 | 0.999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Li, Y. Surface-Bound Humic Acid Increased Propranolol Sorption on Fe3O4/Attapulgite Magnetic Nanoparticles. Nanomaterials 2020, 10, 205. https://doi.org/10.3390/nano10020205
Deng Y, Li Y. Surface-Bound Humic Acid Increased Propranolol Sorption on Fe3O4/Attapulgite Magnetic Nanoparticles. Nanomaterials. 2020; 10(2):205. https://doi.org/10.3390/nano10020205
Chicago/Turabian StyleDeng, Yuehua, and Yani Li. 2020. "Surface-Bound Humic Acid Increased Propranolol Sorption on Fe3O4/Attapulgite Magnetic Nanoparticles" Nanomaterials 10, no. 2: 205. https://doi.org/10.3390/nano10020205
APA StyleDeng, Y., & Li, Y. (2020). Surface-Bound Humic Acid Increased Propranolol Sorption on Fe3O4/Attapulgite Magnetic Nanoparticles. Nanomaterials, 10(2), 205. https://doi.org/10.3390/nano10020205