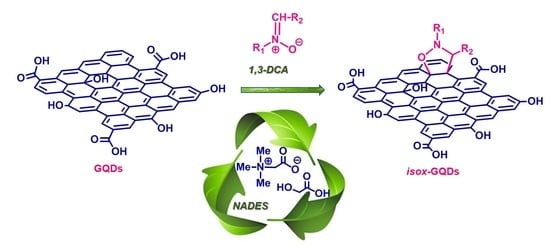
Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical, Physical, and Morphological Characterization
2.3. Synthesis of GQDs
2.4. Synthesis of NADES
2.5. Synthesis of C-(diethoxyphosphoryl)propylidene, N-benzyl nitrone 1b
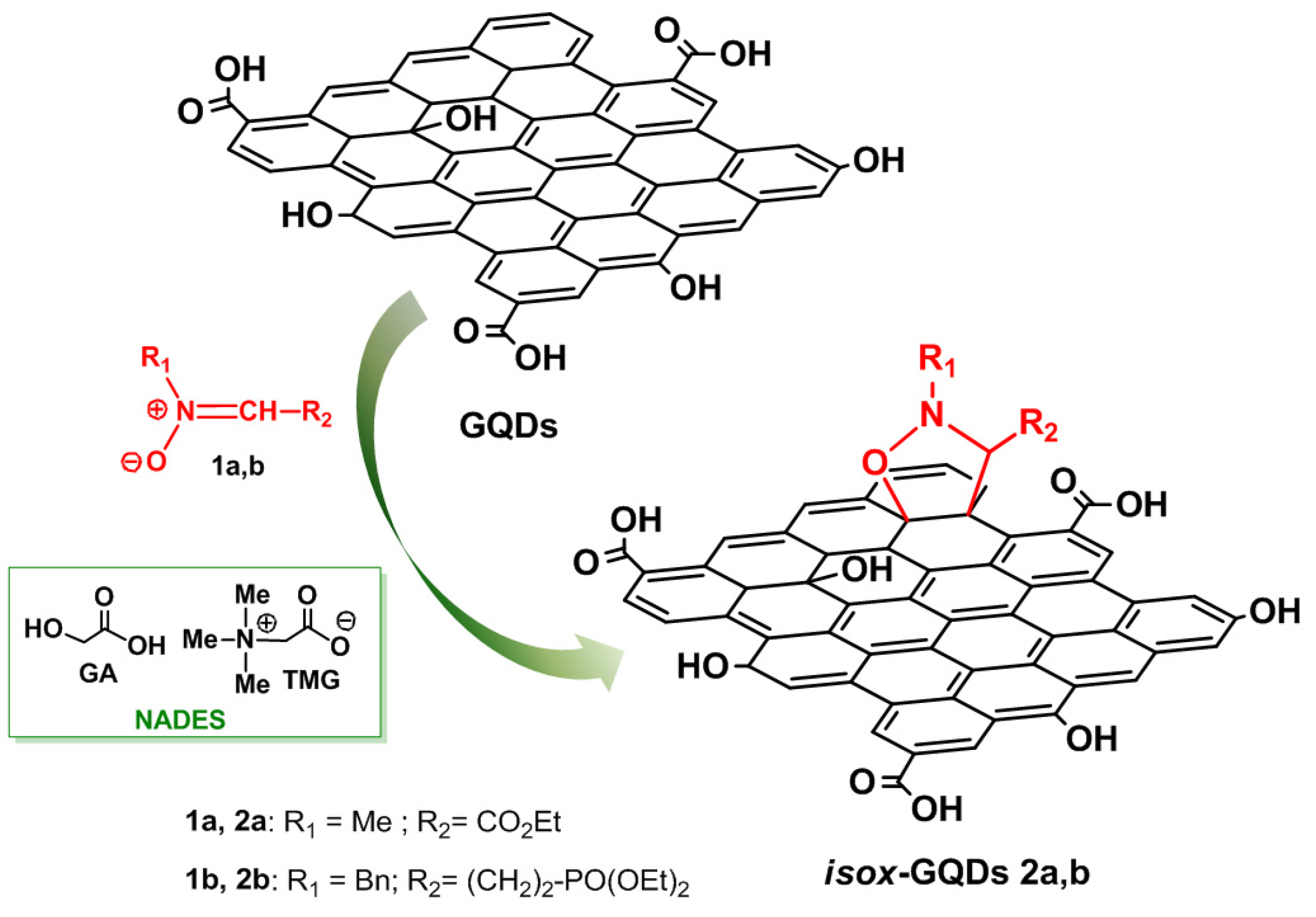
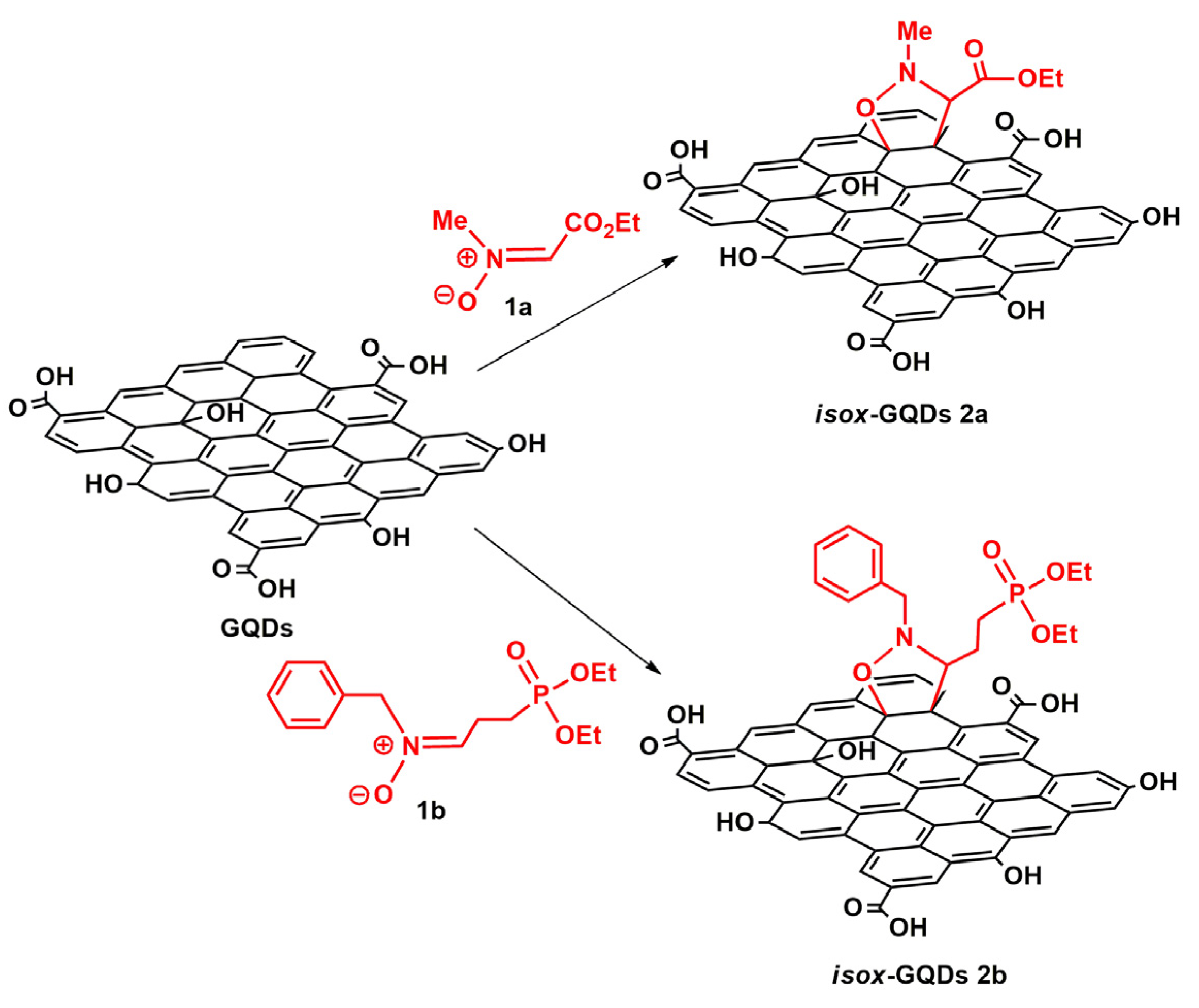
2.6. Synthesis of isox-GQDs 2a and isox-GQDs 2b
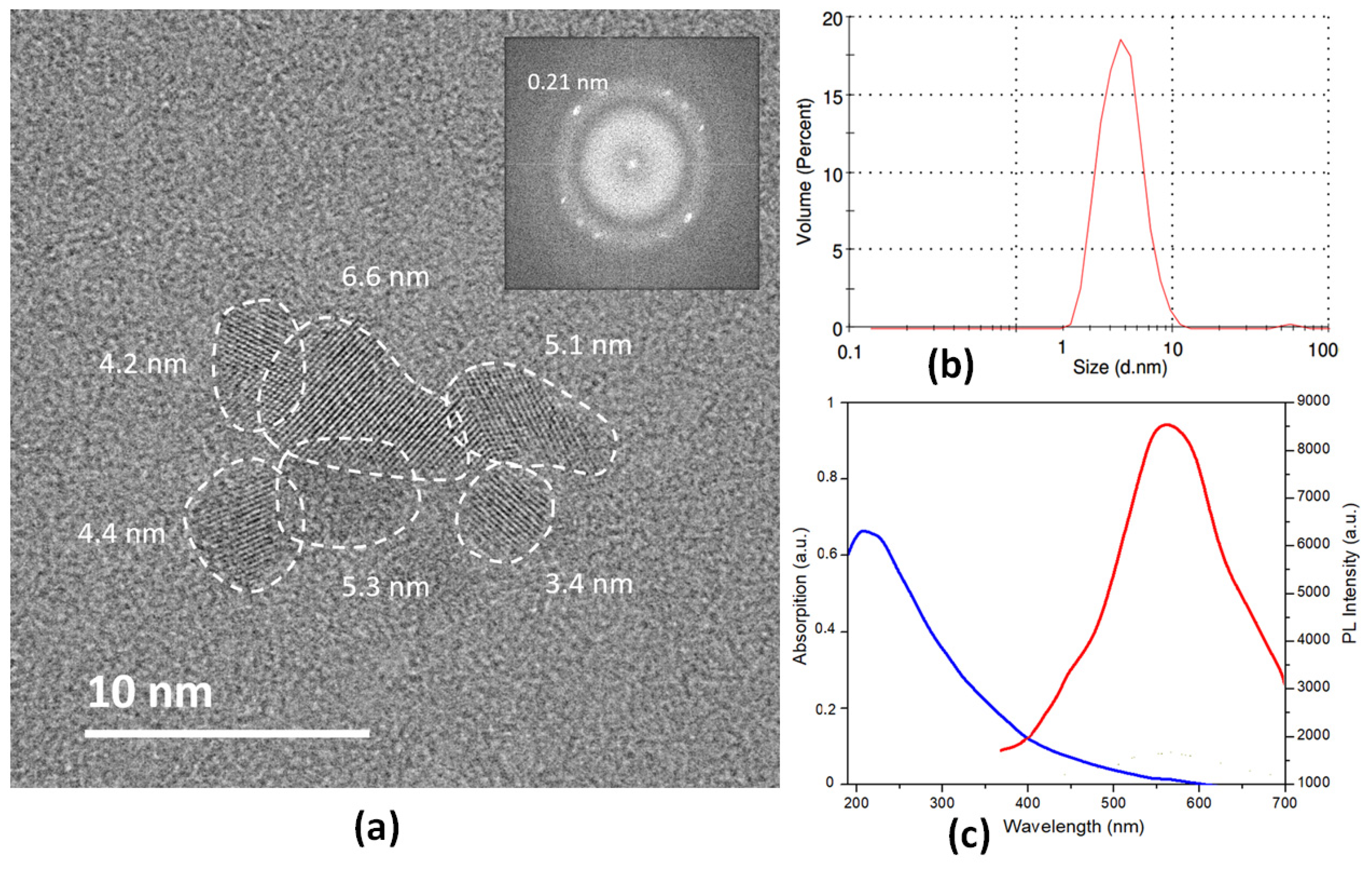
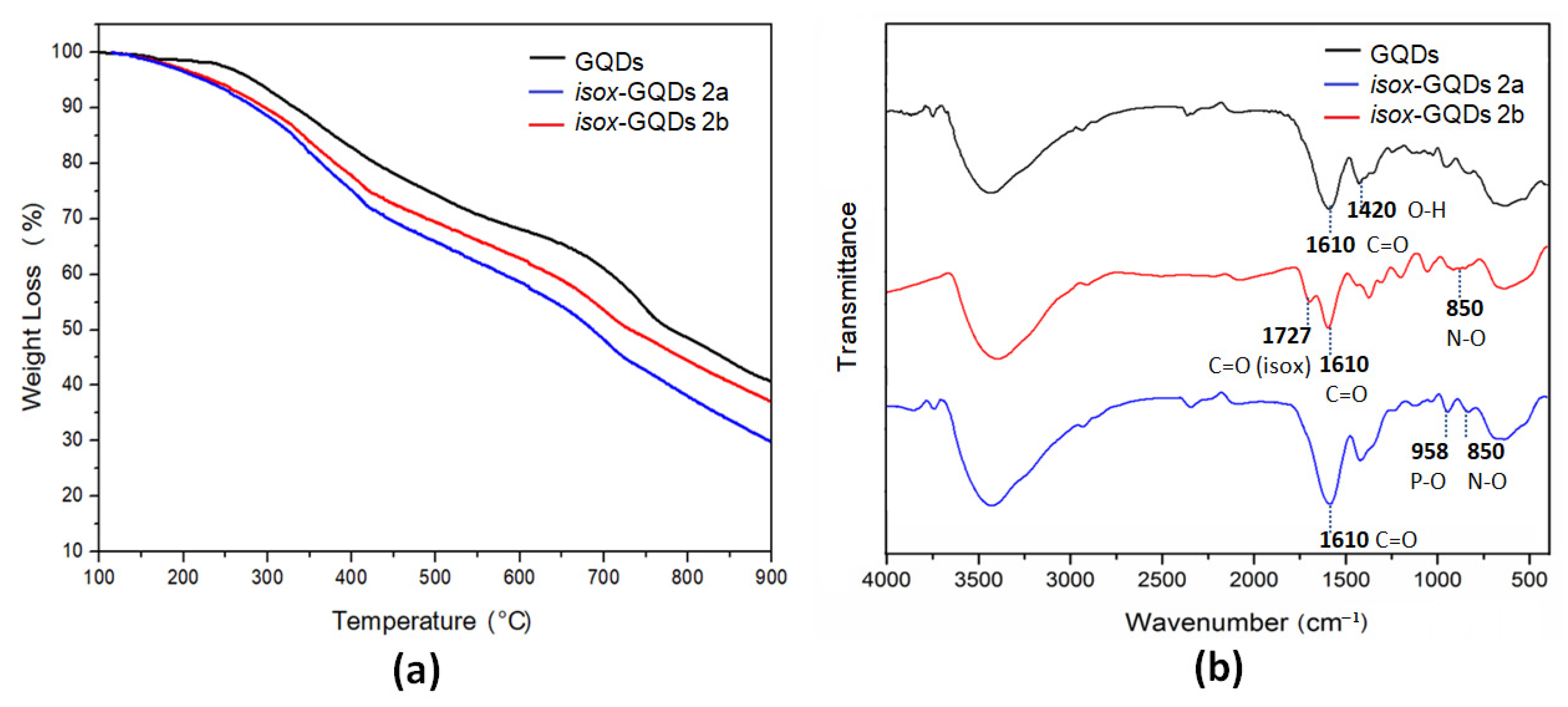
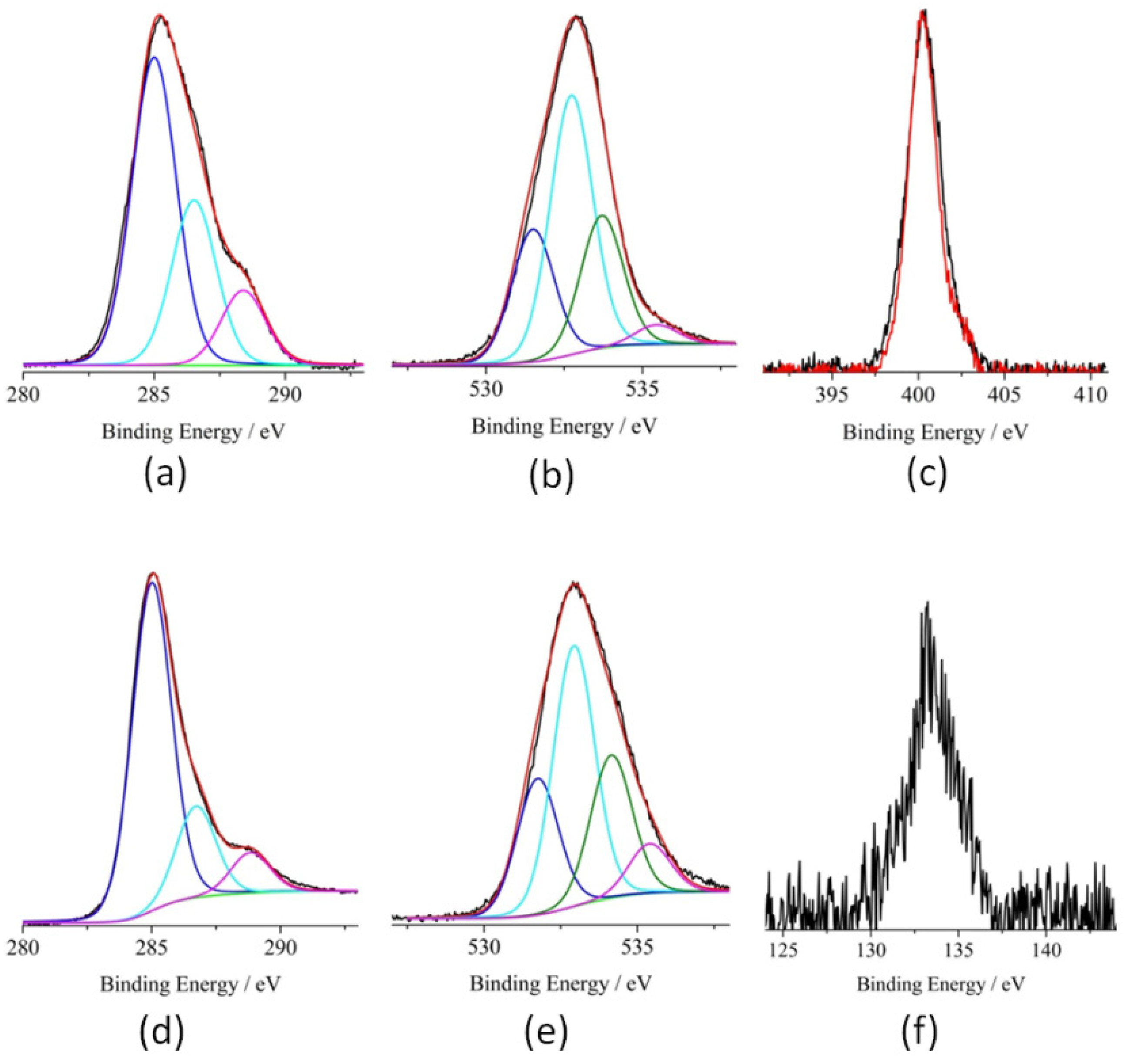
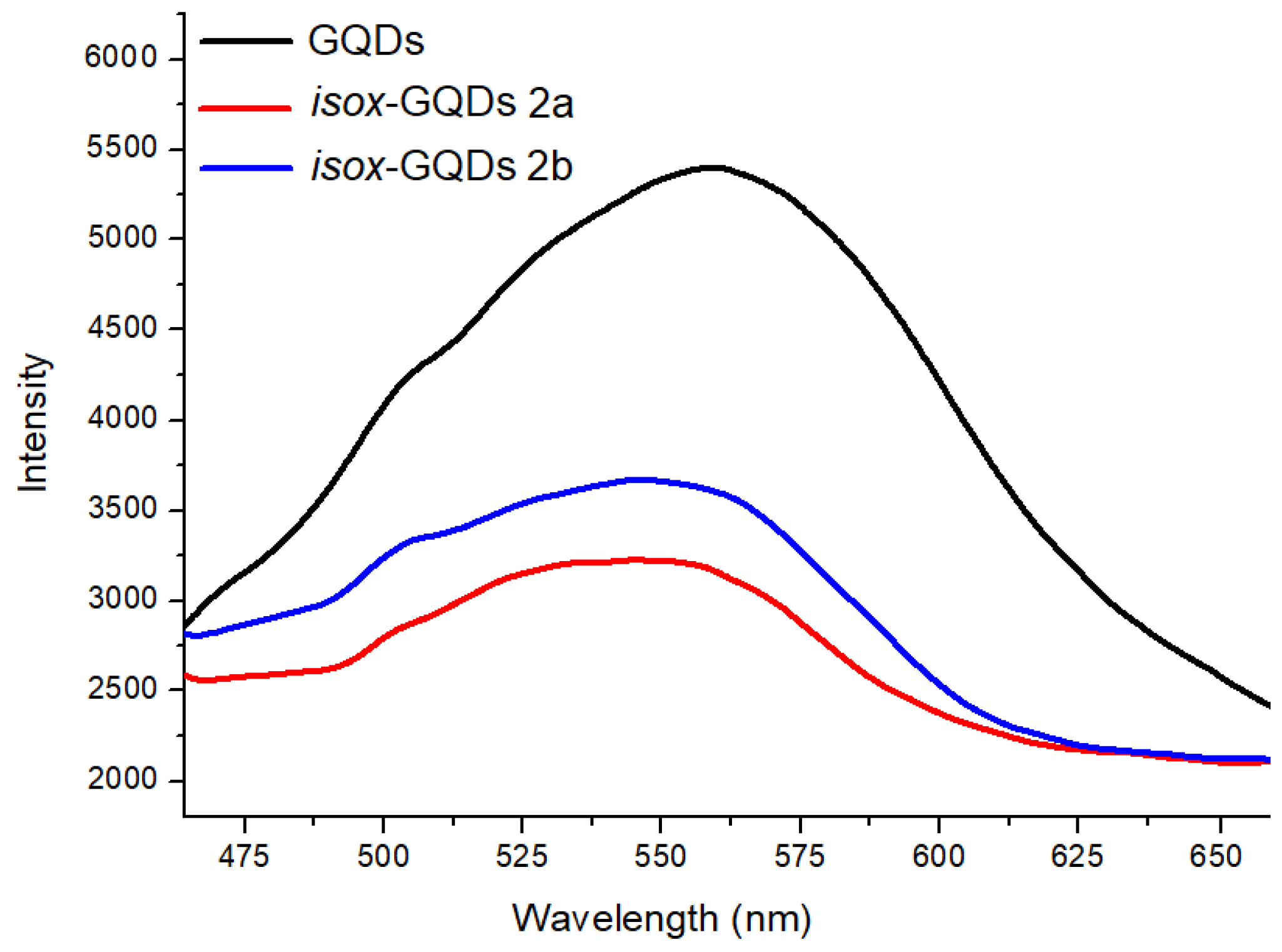
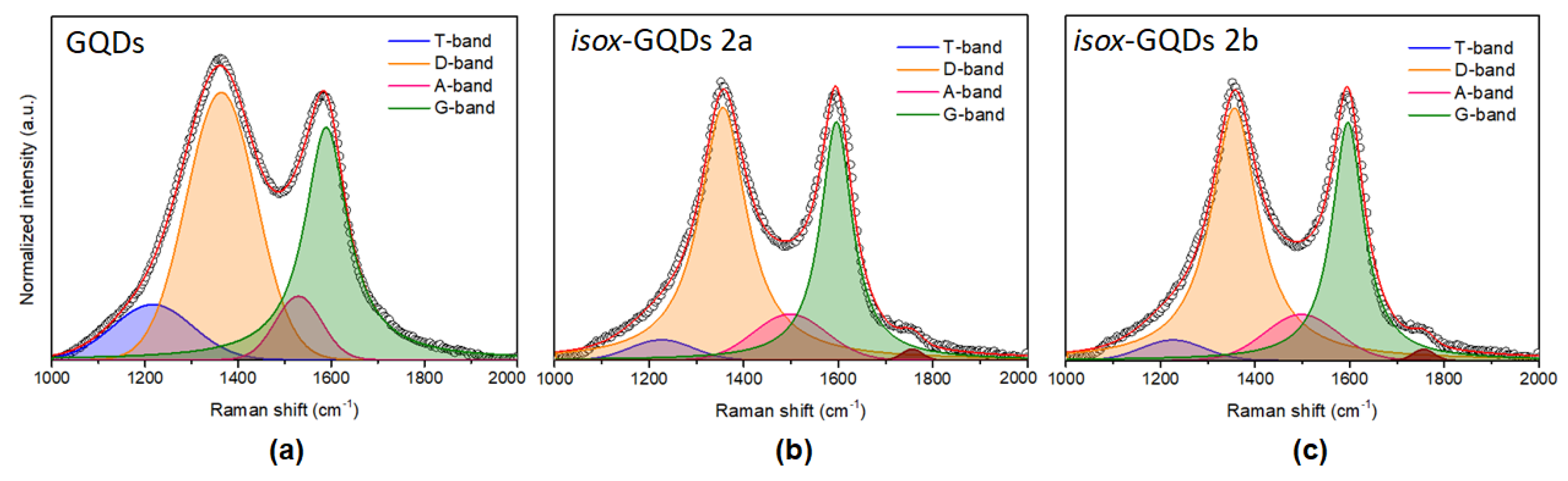
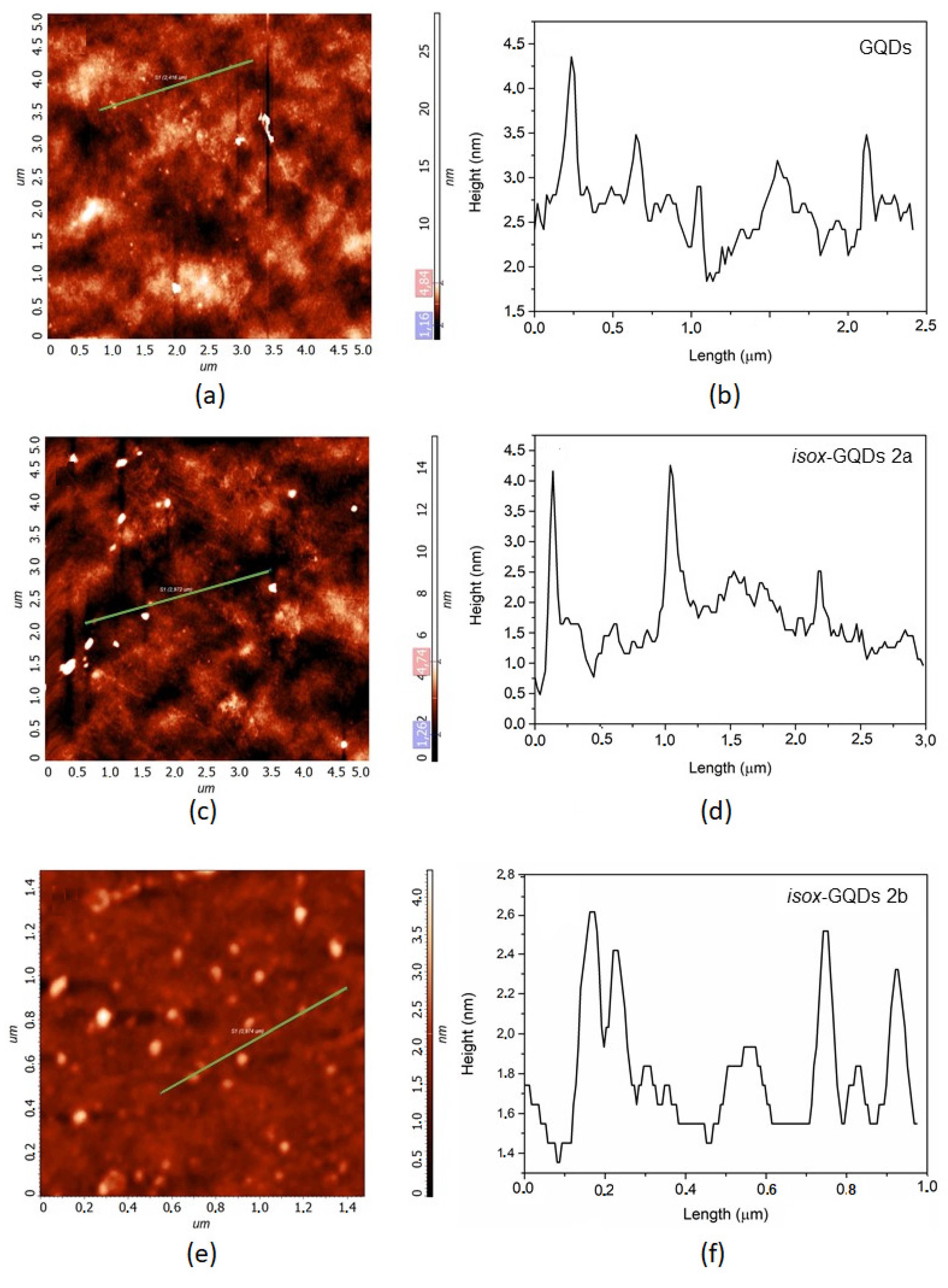
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2019, 1904362. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene Quantum Dots for Optical Bioimaging. Small 2019, 15, 1902136. [Google Scholar] [CrossRef]
- Iannazzo, D.; Ziccarelli, I.; Pistone, A. Graphene quantum dots: Multifunctional nanoplatforms for anticancer therapy. J. Mater. Chem. B 2017, 5, 6471–6489. [Google Scholar] [CrossRef]
- Iannazzo, D.; Celesti, C.; Espro, C. Recent Advances on Graphene Quantum Dots as Multifunctional Nanoplatforms for Cancer Treatment. Biotechnol. J. 2020, 1900422. [Google Scholar] [CrossRef]
- Tade, R.S.; Patil, P.O. Theranostic Prospects of Graphene Quantum Dots in Breast Cancer. ACS Biomater. Sci. Eng. 2020, 6, 5987–6008. [Google Scholar]
- Fan, X.; Phebus, B.D.; Li, L.; Chen, S. Chemical Functionalization of Graphene Quantum Dots. Sci. Adv. Mater. 2015, 7, 1990–2010. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Galvagno, S. Functionalization methods of graphene. In Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications. Thakur, V.K., Thakur, M.K., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 510–537. [Google Scholar]
- Iannazzo, D.; Pistone, A.; Celesti, C.; Triolo, C.; Patané, S.; Giofré, S.V.; Romeo, R.; Ziccarelli, I.; Mancuso, R.; Gabriele, B.; et al. A Smart Nanovector for Cancer Targeted Drug Delivery Based on Graphene Quantum Dots. Nanomaterials 2019, 9, 282. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Ferro, S.; De Luca, L.; Monforte, A.M.; Romeo, R.; Buemi, M.R.; Pannecouque, C. Graphene Quantum Dots Based Systems As HIV Inhibitors. Bioconjugate Chem. 2018, 29, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of Graphene via 1,3- Dipolar Cycloaddition. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Scala, A.; Fazio, E.; Mineo, P.G.; Rescifina, A.; Piperno, A.; Grassi, G. Repurposing of oxazolone chemistry: Gaining access to functionalized graphene nanosheets in a top-down approach from graphite. Chem. Sci. 2015, 6, 6961–6970. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yu, W.; Xiao, Z.; Song, Y.; Long, L.; Cifuentes, M.P.; Humphrey, M.G.; Zhang, C. A 1,3-dipolar cycloaddition protocol to porphyrin-functionalized reduced graphene oxide with a push-pull motif. Nano Res. 2015, 8, 870–886. [Google Scholar] [CrossRef]
- Tagmatarchis, N.; Prato, M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions. J. Mater. Chem. 2004, 14, 437–439. [Google Scholar] [CrossRef]
- Ghini, G.; Luconi, L.; Rossin, A.; Bianchini, C.; Giambastiani, G.; Cicchi, S.; Lascialfari, L.; Brandi, A.; Giannasi, A. Can nitrones functionalize carbon nanotubes? Chem. Commun. 2010, 46, 252–254. [Google Scholar] [CrossRef]
- Grassi, G.; Scala, A.; Piperno, A.; Iannazzo, D.; Lanza, M.; Milone, C.; Pistone, A.; Galvagno, S. A facile and ecofriendly functionalization of multiwalled carbon nanotubes by an old mesoionic compound. Chem. Commun. 2012, 48, 6836–6838. [Google Scholar] [CrossRef]
- Prato, M.; Suzuki, T.; Foroudian, H.; Li, Q.; Khemani, K.; Wudl, F.; Leonetti, J.; Little, R.D.; White, T.; Yamago, S.; et al. [3 + 2] and [4 + 2] Cycloadditions of fullerene C60. J. Am. Chem. Soc. 1993, 115, 1594–1595. [Google Scholar] [CrossRef]
- Akhmetov, A.R.; Tuktarov, A.R.; Popod’ko, N.R.; Dzhemilev, U.M. Cycloaddition of alkyl azides to fullerene C60 in the presence of Cu(OTf)2. Mendeleev Commun. 2015, 25, 346–347. [Google Scholar] [CrossRef]
- Martina, K.; Tagliapietra, S.; Veselov, V.V.; Cravotto, G. Green Protocols in Heterocycle Syntheses via 1,3-Dipolar Cycloadditions. Front. Chem. 2019, 7, 95. [Google Scholar] [CrossRef]
- Sekiya, R.; Uemura, Y.; Murakami, H.; Haino, T. White-Light-Emitting Edge-Functionalized Graphene Quantum Dots. Angew. Chem. 2014, 126, 5725–5729. [Google Scholar] [CrossRef]
- Qi, B.-P.; Hu, H.; Bao, L.; Zhang, Z.-L.; Tang, B.; Peng, Y.; Wang, B.-S.; Pang, D.-W. An efficient edge-functionalization method to tune the photoluminescence of graphene quantum dots. Nanoscale 2015, 7, 5969–5973. [Google Scholar] [CrossRef] [PubMed]
- Tiecco, M.; Cappellini, F.; Nicoletti, F.; Del Giacco, T.; Germani, R.; Di Profio, P. Role of the hydrogen bond donor component for a proper development of novel hydrophobic deep eutectic solvents. J. Mol. Liq. 2019, 281, 423–430. [Google Scholar] [CrossRef]
- Lu, J.; Li, W.-T.; Ma, E.-Q.; Mo, L.-P.; Zhang, Z.-H. Inside Back Cover: Superparamagnetic CuFeO2 Nanoparticles in Deep Eutectic Solvent: An Efficient and Recyclable Catalytic System for the Synthesis of Imidazo[1,2-α]pyridines. ChemCatChem 2014, 6, 2854–2859. [Google Scholar] [CrossRef]
- Müller, C.R.; Meiners, I.; de Domínguez María, P. Highly enantioselective tandem enzyme–organocatalyst crossed aldol reactions with acetaldehyde in deep-eutectic-solvents. RSC Adv. 2014, 4, 46097–46101. [Google Scholar]
- Perez, J.M.; Ramòn, D.J. Synthesis of 3,5-disubstituted isoxazoles and isoxazolines in deep eutectic solvents. ACS Sustain. Chem. Eng. 2015, 3, 2343–2349. [Google Scholar] [CrossRef]
- Pinto Martins, M.A.; Caneppele Paveglio, G.; Valvassori Rodrigues, L.; Piccinin Frizzo, C.; Zanatta, N.; Gauze Bonacorso, H. Promotion of 1,3-dipolar cycloaddition between azides and β-enaminones by deep eutectic solvents. New J. Chem. 2016, 40, 5989–5992. [Google Scholar] [CrossRef]
- Curti, F.; Tiecco, M.; Pirovano, V.; Germani, R.; Caselli, A.; Rossi, E.; Abbiati, G. p-TSA-Based DESs as “Active Green Solvents” for Microwave Enhanced Cyclization of 2-Alkynyl-(hetero)-arylcarboxylates: An Alternative Access to 6-Substituted 3,4-Fused 2-Pyranones. Eur. J. Org. Chem. 2019, 9, 1904–1914. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Requejo, P.F.; Kroon, M.C. Aliphatic−Aromatic Separation Using Deep Eutectic Solvents as Extracting Agents. Ind. Eng. Chem. Res. 2015, 54, 11404–11412. [Google Scholar] [CrossRef]
- Iannazzo, D.; Brunaccini, E.; Giofrè, S.V.; Piperno, A.; Romeo, G.; Ronsisvalle, S.; Chiacchio, M.A.; Lanza, G.; Chiacchio, U. Competitive Formation of Enaminones and 3-Amino-2(5H)-furanones from the Isoxazolidine System: A Combined Synthetic and Quantum Chemical Study. Eur. J. Org. Chem. 2010, 5897–5905. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Tiecco, M.; Zappacosta, R.; Boncompagni, S.; Di Profio, P.; Ettorre, V.; Fontana, A.; Germani, R.; Siani, G. Novel zwitterionic Natural Deep Eutectic Solvents as environmentally friendly media for spontaneous self-assembly of gold nanoparticles. J. Mol. Liq. 2018, 268, 371–375. [Google Scholar] [CrossRef]
- Donato, M.G.; Galvagno, S.; Messina, G.; Milone, C.; Pistone, A.; Santangelo, S. Optimisation of gas mixture composition for the preparation of high quality MWCNT by catalytically assisted CVD. Diam. Relat. Mater. 2007, 16, 1095–1100. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Gulino, A. Structural and electronic characterization of self-assembled molecular nanoarchitectures by X-rayphotoelectron spectroscopy. Anal. Bioanal. Chem. 2013, 405, 1479–1495. [Google Scholar] [CrossRef]
- Briggs, D.; Grant, J.T. Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; IM Publications: Chichester, UK; Surface Spectra Ltd.: Manchester, UK, 2003. [Google Scholar]
- Cardellini, F.; Tiecco, M.; Germani, R.; Cardinali, G.; Corte, L.; Roscini, L.; Spreti, N. Novel zwitterionic deep eutectic solvents from trimethylglycine and carboxylic acids: Characterization of their properties and their toxicity. RSC Adv. 2014, 4, 55990–56002. [Google Scholar] [CrossRef]
- Lavin, J.G.; Subramoney, S.; Ruoff, R.; Berber, S.; Tomanek, D. Scrolls and nested tubes in multiwall carbon nanotubes. Carbon 2002, 40, 1123–1130. [Google Scholar] [CrossRef]
- Iannazzo, D.; Piperno, A.; Romeo, G.; Romeo, R.; Chiacchio, U.; Rescifina, A.; Balestrieri, E.; Macchi, B.; Mastino, A.; Cortese, R. 3-Amino-2(5H)furanones as inhibitors of subgenomic hepatitis C virus RNA replication. Bioorg. Med. Chem. 2008, 16, 9610–9615. [Google Scholar] [CrossRef]
- Camper, N.; Scott, C.J.; Migaud, M. Synthesis of an analogue of the bisphosphonate drug Ibandronate for targeted drug-delivery therapeutic strategies. New J. Chem. 2010, 34, 949–955. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Afshari, R.; Ramón, D.J.; Varma, R.S. Deep eutectic solvents: Cutting-edge applications in cross-coupling reactions. Green Chem. 2020, 22, 3668–3692. [Google Scholar]
- Uceta, H.; Vizuete, M.; Carrillo, J.R.; Barrejón, M.; Fierro, G.J.L.; Prieto, M.P.; Langa, F. Cycloaddition of Nitrile Oxides to Graphene: A Theoretical and Experimental Approach. Chem. Eur. J. 2019, 25, 14644–14650. [Google Scholar] [CrossRef]
- Ferrándiz-Saperas, M.; Ghisolfi, A.; Cazorla-Amorós, D.; Nájera, C.; Sansano, J.M. Multilayer graphene functionalized through thermal 1,3-dipolar cycloadditions with imino esters: A versatile platform for supported ligands in catalysis. Chem. Commun. 2019, 55, 7462–7465. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, J.; Kaminker, R.; Motiei, L.; de Ruiter, G.; Morozov, M.; Lupo, F.; Gulino, A.; Van der Boom, M.E. Linear vs Exponential Formation of Molecular-Based Assemblies. J. Am. Chem. Soc. 2010, 132, 9295–9297. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Han, J.; Yang, C.T.; Cheng, C.M.; Luo, Y.M.; Wang, X.L. A category of hierarchically porous tin (IV) phosphonate backbone with the implication for radioanalytical separation. Chem. Eng. J. 2016, 302, 368–376. [Google Scholar] [CrossRef]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Malviya, R.; Sharma, P.K. Measurement Techniques and Pharmaceutical Applications of Zeta Potential: A Review. J. Drug Deliv. Ther. 2014, 4, 33–40. [Google Scholar]
- Huang, C.L.; Huang, C.C.; Mai, F.D.; Yen, C.L.; Tzing, S.H.; Hsieh, H.T.; Ling, Y.C.; Chang, J.Y. Application of paramagnetic graphene quantum dots as a platform for simultaneous dual-modality bioimaging and tumor-targeted drug delivery. J. Mater. Chem. B 2015, 3, 651–664. [Google Scholar] [CrossRef]
- Rajender, G.; Giri, P.K. Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 10852. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Kumar, G.S.; Roy, R.; Sen, D.; Ghorai, U.K.; Thapa, R.; Mazumder, N.; Saha, S.; Chattopadhyay, K.K. Amino-functionalized graphene quantum dots: Origin of tunable heterogeneous photoluminescence. Nanoscale 2014, 6, 3384. [Google Scholar] [CrossRef]
- Modafferi, V.; Fiore, M.; Fazio, E.; Patanè, S.; Triolo, C.; Santangelo, S.; Ruffo, R.; Neri, F.; Musolino, M.G. Synthesis and characterization of Fe2O3/reduced graphene oxide nanocomposite as a high-performance anode material for sodium-ion batteries. Model. Meas. Control B 2018, 87, 129–134. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giofrè, S.V.; Tiecco, M.; Celesti, C.; Patanè, S.; Triolo, C.; Gulino, A.; Spitaleri, L.; Scalese, S.; Scuderi, M.; Iannazzo, D. Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent. Nanomaterials 2020, 10, 2549. https://doi.org/10.3390/nano10122549
Giofrè SV, Tiecco M, Celesti C, Patanè S, Triolo C, Gulino A, Spitaleri L, Scalese S, Scuderi M, Iannazzo D. Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent. Nanomaterials. 2020; 10(12):2549. https://doi.org/10.3390/nano10122549
Chicago/Turabian StyleGiofrè, Salvatore V., Matteo Tiecco, Consuelo Celesti, Salvatore Patanè, Claudia Triolo, Antonino Gulino, Luca Spitaleri, Silvia Scalese, Mario Scuderi, and Daniela Iannazzo. 2020. "Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent" Nanomaterials 10, no. 12: 2549. https://doi.org/10.3390/nano10122549
APA StyleGiofrè, S. V., Tiecco, M., Celesti, C., Patanè, S., Triolo, C., Gulino, A., Spitaleri, L., Scalese, S., Scuderi, M., & Iannazzo, D. (2020). Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent. Nanomaterials, 10(12), 2549. https://doi.org/10.3390/nano10122549