Development of Epidermal Equivalent from Electrospun Synthetic Polymers for In Vitro Irritation/Corrosion Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymers and Solvents for Electrospinning
2.2. Cell Culture
2.3. Construction of Human Epidermis Models
2.4. Test Substances
2.5. Polymer Scaffold Preparation and Characterization
2.6. Quality Control of the Tissue Model
2.6.1. Histology and Immunohistochemistry
2.6.2. Protein Expression by Western Blotting
2.6.3. Cell Viability Measurement by MTT
2.7. Applications of the Epidermis
2.7.1. Corrosion Test
2.7.2. Irritation Test
3. Results and Discussion
3.1. Fabrication of Electrospun Polymer Scaffolds
3.2. Construction and Characterization of the Epidermal Model
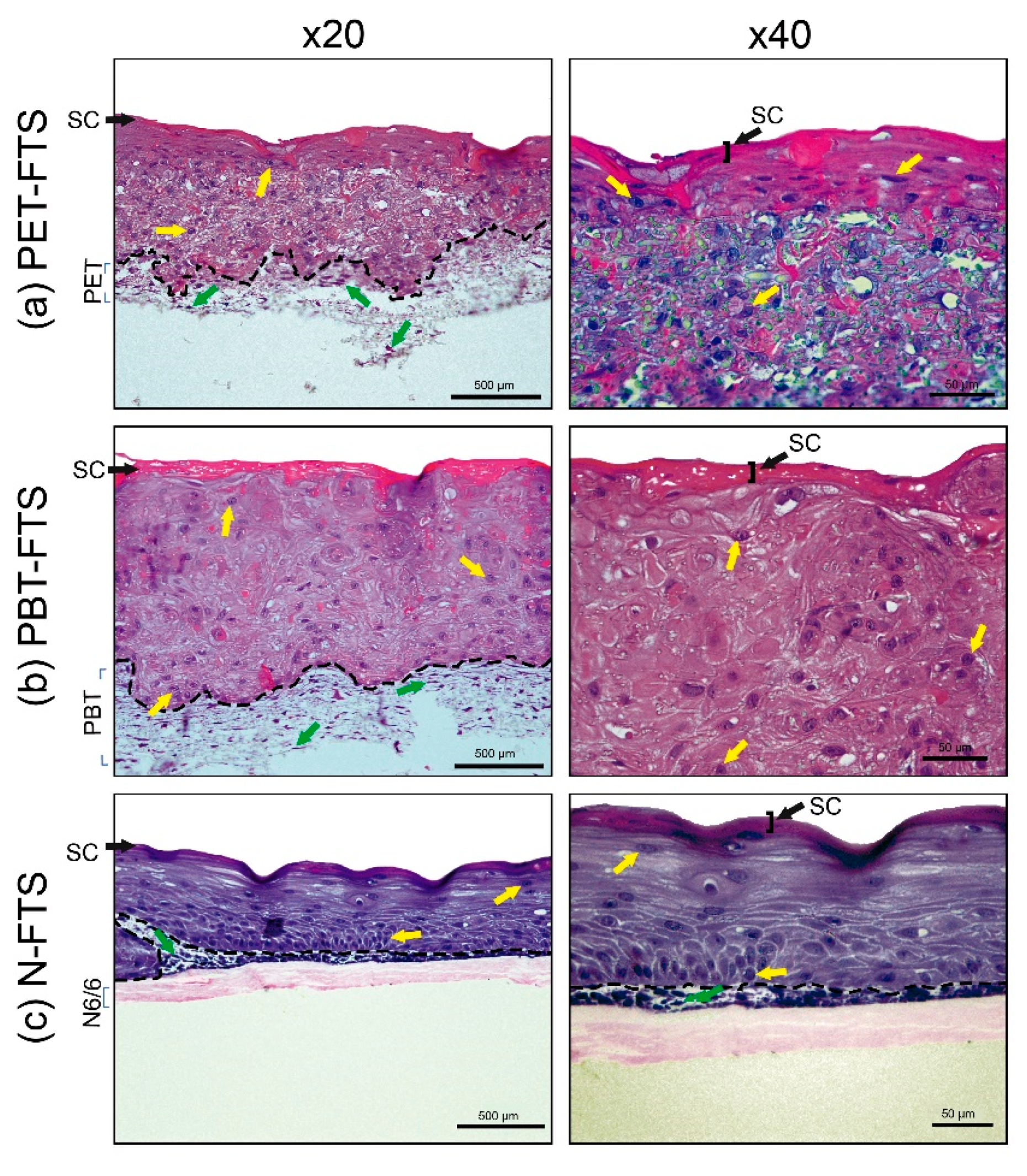
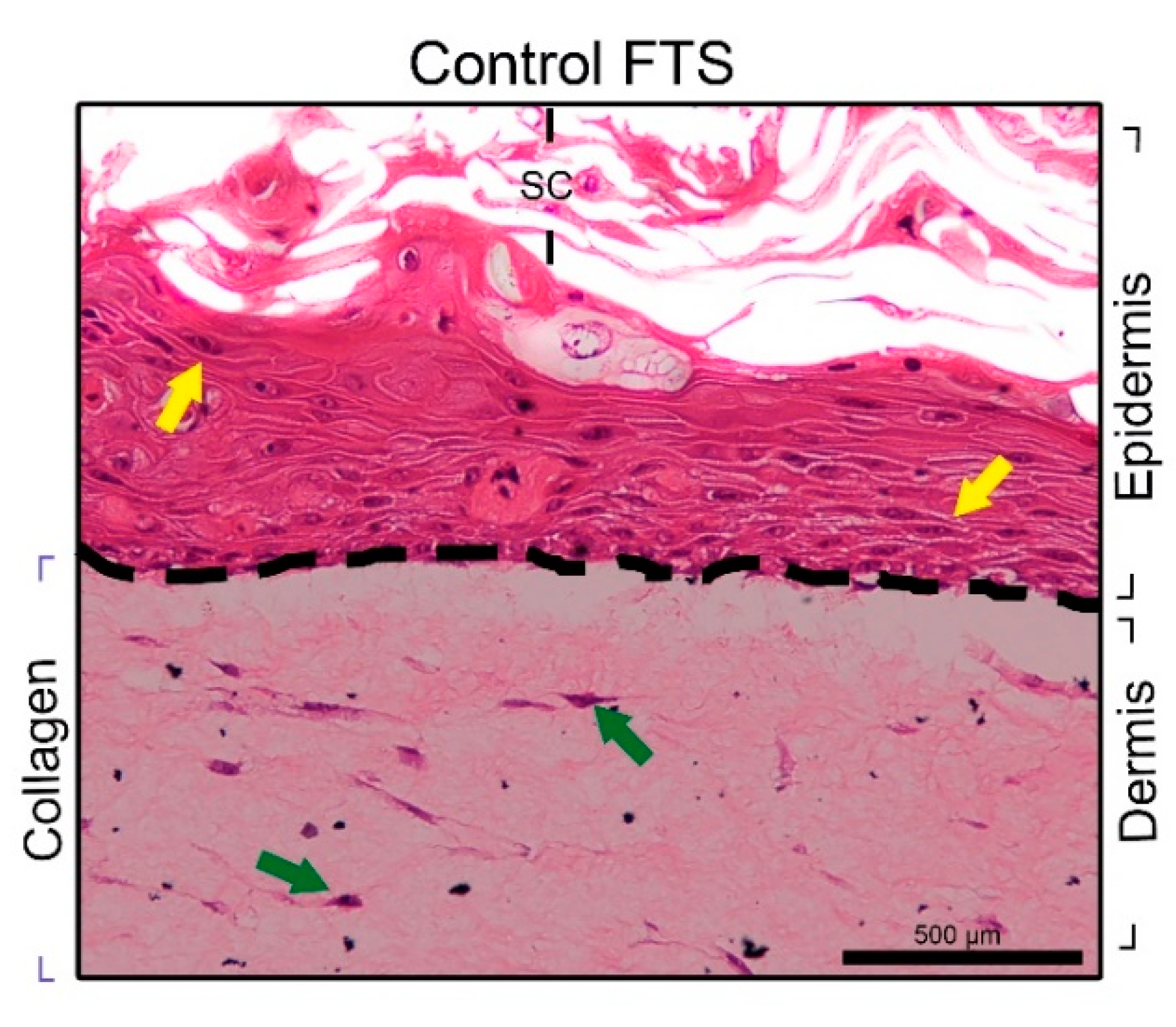
3.2.1. FTS Production
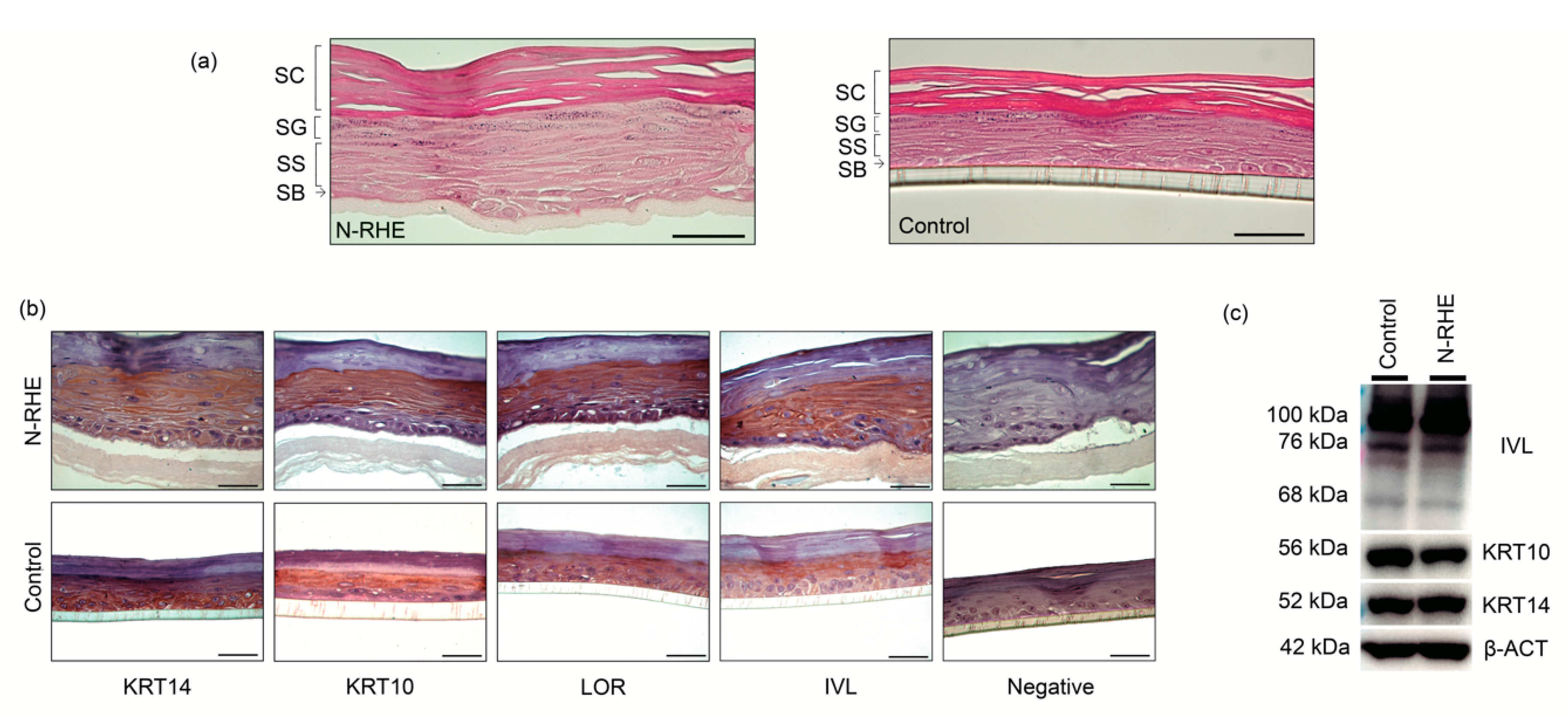
3.2.2. RHE Production
3.3. Applications of N-RHE
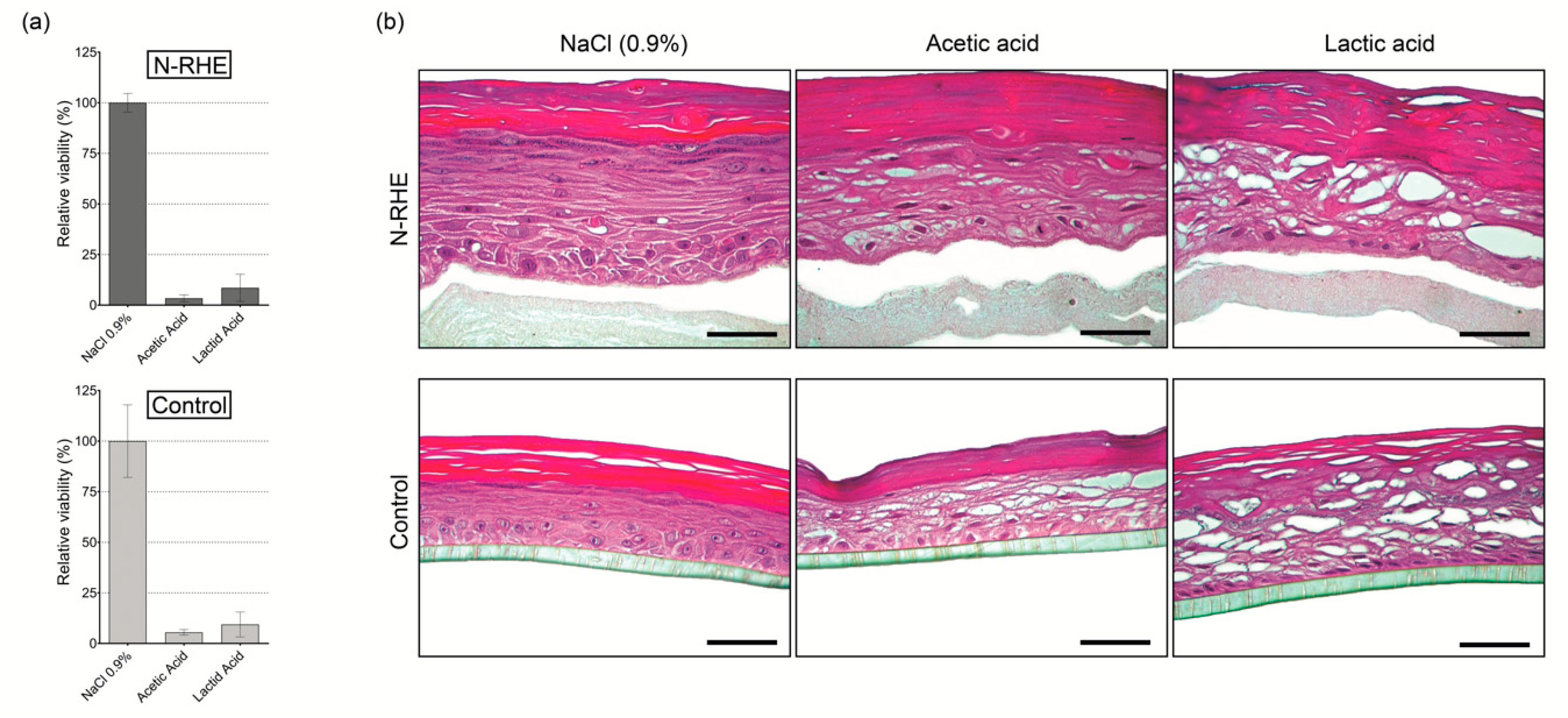
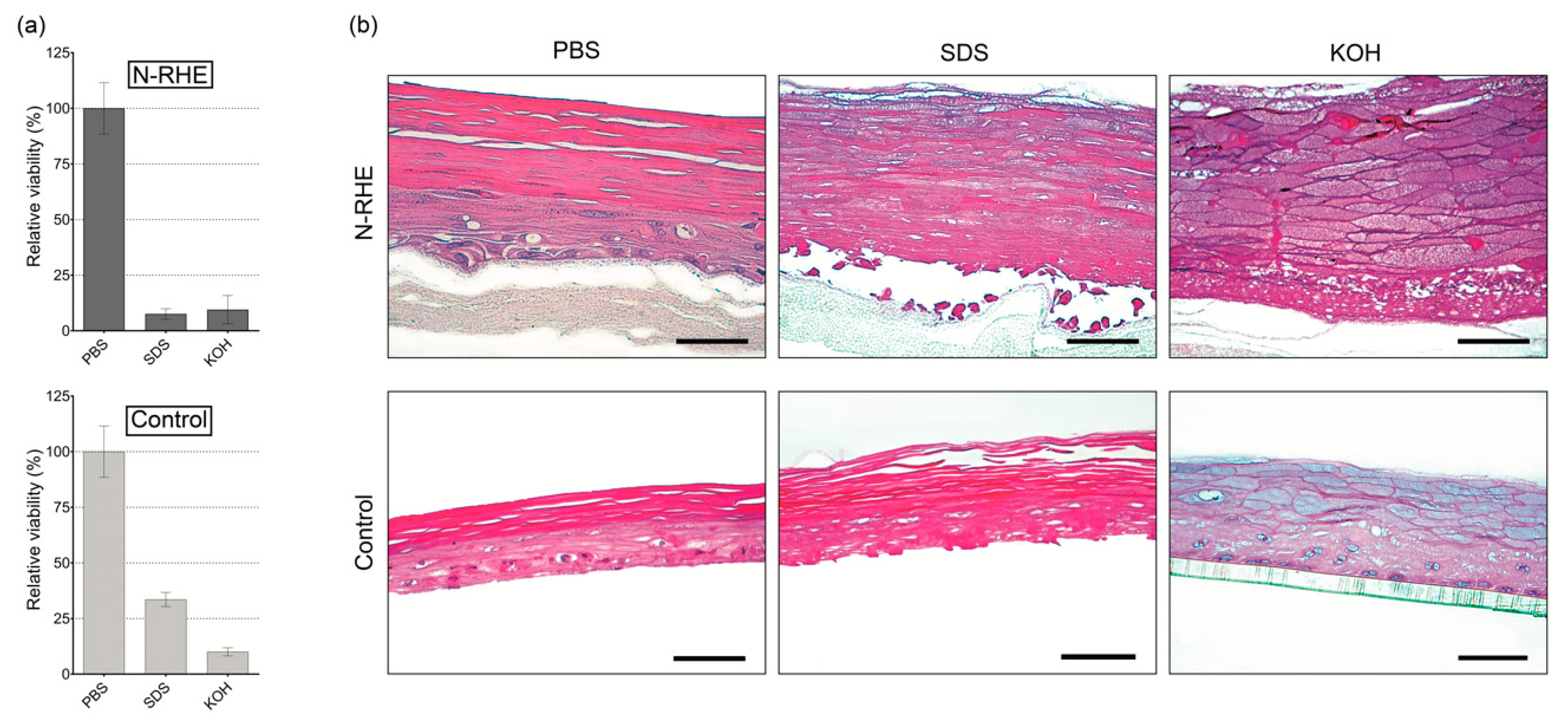
3.3.1. Corrosion Test
3.3.2. Irritation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bakr, R.O.; El-Osaily, G.H.; Bakr, R.O.; El Dine, R.S.; Fayez, A.M. Characterization and Pharmacological Evaluation of Anti-Cellulite Herbal Product(s) Encapsulated in 3D-Fabricated Polymeric Microneedles. Sci. Rep. 2020, 10, 6316. [Google Scholar] [CrossRef]
- Liu, X.; Cleary, J.; German, G.K. The global mechanical properties and multi-scale failure mechanics of heterogeneous human stratum corneum. Acta Biomater. 2016, 43, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Anjo, T.; Kaneko, A.; Senoo, Y.; Shibata, A.; Takama, H.; Yokoyama, K.; Nishito, Y.; Ono, T.; Taya, C.; et al. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat. Commun. 2017, 8, 14609. [Google Scholar] [CrossRef] [PubMed]
- Calegari, F.; Waskow, C. Stem Cells: From Basic Research to Therapy, Volume Two: Tissue Homeostasis and Regeneration during Adulthood, Applications, Legislation and Ethics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Eckert, R.L.; Crish, J.F.; Robinson, N.A. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol. Rev. 1997, 77, 397–424. [Google Scholar] [CrossRef]
- Yun, Y.E.; Jung, Y.J.; Choi, Y.J.; Choi, J.S.; Cho, Y.W. Artificial skin models for animal-free testing. J. Pharm. Investig. 2018, 48, 215–223. [Google Scholar] [CrossRef]
- Lemper, M.; De Paepe, K.; Rogiers, V. Practical Problems Encountered during the Cultivation of an Open-Source Reconstructed Human Epidermis Model on a Polycarbonate Membrane and Protein Quantification. Ski. Pharmacol. Physiol. 2014, 27, 106–112. [Google Scholar] [CrossRef]
- Pedrosa, T.D.N.; De Vuyst, E.; Mound, A.; De Rouvroit, C.L.; Maria-Engler, S.; Poumay, Y. Methyl-β-cyclodextrin treatment combined to incubation with interleukin-4 reproduces major features of atopic dermatitis in a 3D-culture model. Arch. Dermatol. Res. 2016, 309, 63–69. [Google Scholar] [CrossRef]
- Kumamoto, J.; Nakanishi, S.; Makita, M.; Uesaka, M.; Yasugahira, Y.; Kobayashi, Y.; Nagayama, M.; Denda, S.; Denda, M. Mathematical-model-guided development of full-thickness epidermal equivalent. Sci. Rep. 2018, 8, 17999. [Google Scholar] [CrossRef]
- Hausmann, C.; Zoschke, C.; Wolff, C.; Darvin, M.E.; Sochorová, M.; Kováčik, A.; Wanjiku, B.; Schumacher, F.; Tigges, J.; Kleuser, B.; et al. Fibroblast origin shapes tissue homeostasis, epidermal differentiation, and drug uptake. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Catarino, C.M.; Pedrosa, T.D.N.; Pennacchi, P.C.; De Assis, S.R.; Gimenes, F.; Consolaro, M.E.L.; Barros, S.B.D.M.; Maria-Engler, S.S. Skin corrosion test: A comparison between reconstructed human epidermis and full thickness skin models. Eur. J. Pharm. Biopharm. 2018, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bogaard, E.H.V.D.; Ilic, D.; Dubrac, S.; Tomic-Canic, M.; Bouwstra, J.A.; Celli, A.; Mauro, T.M. Perspective and Consensus Opinion: Good Practices for Using Organotypic Skin and Epidermal Equivalents in Experimental Dermatology Research. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef]
- Eichner, R.; Sun, T.T.; Aebi, U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J. Cell Biol. 1986, 102, 1767–1777. [Google Scholar] [CrossRef] [Green Version]
- Eckert, R.L.; Rorke, E.A. Molecular biology of keratinocyte differentiation. Environ. Health Perspect. 1989, 80, 109–116. [Google Scholar] [CrossRef]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef]
- Nemes, Z.; Steinert, P.M. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Wade, R.J.; Burdick, J.A. Advances in nanofibrous scaffolds for biomedical applications: From electrospinning to self-assembly. Nano Today 2014, 9, 722–742. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Hoque, E.; Nuge, T.; Yeow, T.K.; Nordin, N. Electrospun Matrices from Natural Polymers for Skin Regeneration; Elsevier BV: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Chen, H.; Baptista, D.F.; Criscenti, G.; Crispim, J.F.; Fernandes, H.; Van Blitterswijk, C.; Truckenmüller, R.; Moroni, L. From fiber curls to mesh waves: A platform for the fabrication of hierarchically structured nanofibers mimicking natural tissue formation. Nanoscale 2019, 11, 14312–14321. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Layman, J.M.; Watkins, J.R.; Bowlin, G.L.; Matthews, J.A.; Simpson, D.G.; Wnek, G. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials 2003, 24, 907–913. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Osuchowska, P.N.; Ostrowski, R.; Sarzyński, A.; Strzelec, M.; Mierczyk, Z.; Trafny, E.A. Microstructured polyethylene terephthalate (PET) for microsieving of cancer cells. Results Phys. 2019, 15, 1–9. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribology 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Andrady, A.L. Degradation of Plastics in the Environment. In Plastics and Environmental Sustainability; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Kundu, J.; Pati, F.; Jeong, Y.H.; Cho, D.-W. Biomaterials for Biofabrication of 3D Tissue Scaffolds; Elsevier BV: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Padsalgikar, A.D. Introduction to Plastics. Plast. Med. Devices Cardiovasc. Appl. 2017, 1–29. [Google Scholar] [CrossRef]
- Langley-Hobbs, S. Sutures and General Surgical Implants; Elsevier BV: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Pennacchi, P.C.; De Almeida, M.E.S.; Gomes, O.L.A.; Faião-Flores, F.; Crepaldi, M.C.D.A.; Dos Santos, M.F.; Barros, S.B.D.M.; Maria-Engler, S.S. Glycated Reconstructed Human Skin as a Platform to Study the Pathogenesis of Skin Aging. Tissue Eng. Part A 2015, 21, 2417–2425. [Google Scholar] [CrossRef]
- Immich, A.; Pennacchi, P.; Naves, A.; Felisbino, S.; Boemo, R.; Maria-Engler, S.; Catalani, L.H. Improved tympanic membrane regeneration after myringoplastic surgery using an artificial biograft. Mater. Sci. Eng. C 2017, 73, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, T.D.N.; Catarino, C.M.; Pennacchi, P.C.; De Assis, S.R.; Gimenes, F.; Consolaro, M.E.L.; Barros, S.B.D.M.; Maria-Engler, S.S. A new reconstructed human epidermis for in vitro skin irritation testing. Toxicol. Vitr. 2017, 42, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Dossanova, A.; Lozovoy, V.; Manekenova, K.; Lozovaya, Y.; Seidakhmetov, M.; Dossanov, B.; Omarov, T.; Botabaeva, A.; Shakeeva, A.; Baubekov, Z. Histological and morphological characteristics of the prepuce of penis skin structure in different age groups. J. Pediatr. Urol. 2018, 14, 280.e1–280.e6. [Google Scholar] [CrossRef] [PubMed]
- Hossy, B.H.; Leitão, A.A.D.C.; Torres, R.B.; Ramos-e-Silva, M.; Miguel, N.C.D.O.; De Pádula, M. Histological observation of hairless mice skin after exposure to Simulated Solar Light: Comparison between the histological findings with different methodologies and 3R principle correlations. Burns 2018, 44, 359–369. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Waring, G.O.; Akor, C.; Castellano-Sanchez, A.A.; Bennett, K. Evaluation of hematoxylin and eosin and special stains for the detection of acanthamoeba keratitis in penetrating keratoplasties. Am. J. Ophthalmol. 2003, 136, 520–526. [Google Scholar] [CrossRef]
- Ozawa, A.; Sakaue, M. New decolorization method produces more information from tissue sections stained with hematoxylin and eosin stain and masson-trichrome stain. Ann. Anat. Anat. Anz. 2020, 227, 151431. [Google Scholar] [CrossRef] [PubMed]
- Hieda, D.S.; Carvalho, L.A.D.C.; De Mello, B.V.; De Oliveira, E.A.; De Assis, S.R.; Wu, J.; Du-Thumm, L.; Da Silva, C.L.V.; Roubicek, D.A.; Maria-Engler, S.S.; et al. Air Particulate Matter Induces Skin Barrier Dysfunction and Water Transport Alteration on a Reconstructed Human Epidermis Model. J. Investig. Dermatol. 2020, 140, 2343–2352.e3. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Jakob, C.A.; Aebi, M.; Tuor, U. The MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] Assay Is a Fast and Reliable Method for Colorimetric Determination of Fungal Cell Densities. Appl. Environ. Microbiol. 1999, 65, 3727–3729. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-C.; Hsu, H.-C.; Lin, C.-H.; Wu, C.-Y.; Chen, W.; Lai, H.-M. Testing Method Development and Validation for in Vitro Skin Irritation Testing (SIT) by Using Reconstructed Human Epidermis (RhE) Skin Equivalent—EPiTRI®; Springer: Singapore, 2018. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- OECD. Test Guideline No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. Test No. 417 Toxicokinet. 2020, 4, 1–25. [Google Scholar] [CrossRef]
- OCDE. Section 4 Health Effects OECD/OCDE 431 OECD Guideline for Testing of Chemicals In Vitro Skin Corrosion: Reconstructed Human Epidermis (RhE) Test Method; No. 431; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Tavares, R.S.N.; Engler, S.S.M.; Colepicolo, P.; Debonsi, H.M.; Schäfer-Korting, M.; Marx, U.; Gaspar, L.; Zoschke, C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [Green Version]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Brohem, C.A.; Cardeal, L.B.D.S.; Tiago, M.; Soengas, M.S.; Barros, S.B.D.M.; Maria-Engler, S.S. Artificial skin in perspective: Concepts and applications. Pigment. Cell Melanoma Res. 2011, 24, 35–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poumay, Y.; Dupont, F.; Marcoux, S.; Leclercq-Smekens, M.; Coquette, A. A simple reconstructed human epidermis: Preparation of the culture model and utilization in in vitro studies. Arch. Dermatol. Res. 2004, 296, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Astashkina, A.; Grainger, D.W. Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv. Drug Deliv. Rev. 2014, 69, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, N.J.; Edwards, R.J.; Fritsche, E.; Goebel, C.; Aeby, P.; Scheel, J.; Reisinger, K.; Ouédraogo, G.; Duche, D.; Eilstein, J.; et al. Use of Human In Vitro Skin Models for Accurate and Ethical Risk Assessment: Metabolic Considerations. Toxicol. Sci. 2013, 133, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hokmabad, V.R.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef]
- Bataillon, M.; Lelièvre, D.; Chapuis, A.; Thillou, F.; Autourde, J.B.; Durand, S.; Boyera, N.; Rigaudeau, A.-S.; Besné, I.; Pellevoisin, C. Characterization of a New Reconstructed Full Thickness Skin Model, T-Skin™, and its Application for Investigations of Anti-Aging Compounds. Int. J. Mol. Sci. 2019, 20, 2240. [Google Scholar] [CrossRef] [Green Version]
- Purkis, P.E.; Steel, J.B.; MacKenzie, I.C.; Nathrath, W.B.; Leigh, I.M.; Lane, E.B. Antibody markers of basal cells in complex epithelia. J. Cell Sci. 1990, 97, 39–50. [Google Scholar]
- Roger, M.; Fullard, N.; Costello, L.; Bradbury, S.; Markiewicz, E.; O’Reilly, S.; Darling, N.; Ritchie, P.; Määttä, A.; Karakesisoglou, I.; et al. Bioengineering the microanatomy of human skin. J. Anat. 2019, 234, 438–455. [Google Scholar] [CrossRef]
- Yang, P.-C.; Mahmood, T. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef]
- Cau, L.; Pendaries, V.; Lhuillier, E.; Thompson, P.R.; Serre, G.; Takahara, H.; Méchin, M.-C.; Simon, M. Lowering relative humidity level increases epidermal protein deimination and drives human filaggrin breakdown. J. Dermatol. Sci. 2017, 86, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, B.; Liu, J.; Liu, Y.; Hu, L.; Liu, Y.; Panayi, A.C.; Zhou, W.; Liu, G. The Designer Antimicrobial Peptide A-hBD-2 Facilitates Skin Wound Healing by Stimulating Keratinocyte Migration and Proliferation. Cell. Physiol. Biochem. 2018, 51, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Pendaries, V.; Malaisse, J.; Pellerin, L.; Le Lamer, M.; Nachat, R.; Kezic, S.; Schmitt, A.-M.; Paul, C.; Poumay, Y.; Serre, G.; et al. Knockdown of Filaggrin in a Three-Dimensional Reconstructed Human Epidermis Impairs Keratinocyte Differentiation. J. Investig. Dermatol. 2014, 134, 2938–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafino, A.; Nicotera, G.; Andreola, F.; Giovannini, D.; Zonfrillo, M.; Sferrazza, G.; Calcaterra, A.; De Angelis, C.; Camponeschi, C.; Pierimarchi, P. Synergistic antiproliferative and differentiating effect of 2,4-monofurfurylidene-tetra-O-methylsorbitol and 4,6-dimethyl-2-(3,4,5-trimethoxyphenylamino)pyrimidine on primary and immortalized keratinocytes. Biomed. Pharmacother. 2018, 107, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Karvinen, S.; Pasonen-Seppanen, S.; Hyttinen, J.M.T.; Pienimaki, J.-P.; Torronen, K.; Jokela, T.A.; Tammi, M.I.; Tammi, R. Keratinocyte Growth Factor Stimulates Migration and Hyaluronan Synthesis in the Epidermis by Activation of Keratinocyte Hyaluronan Synthases 2 and 3. J. Biol. Chem. 2003, 278, 49495–49504. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, H.J.; Tabaksblat, E.M.; Enghild, J.J.; Dahl, R. Human skin keratins are the major proteins in exhaled breath condensate. Eur. Respir. J. 2008, 31, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Robinson, N.A.; Lacelle, P.T.; Eckert, R.L. Involucrin Is a Covalently Crosslinked Constituent of Highly Purified Epidermal Corneocytes: Evidence for a Common Pattern of Involucrin Crosslinking in Vivo and in Vitro. J. Investig. Dermatol. 1996, 107, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Netzlaff, F.; Lehr, C.-M.; Wertz, P.W.; Schaefer, U.F. The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef]
- Alépée, N.; Grandidier, M.; Cotovio, J. Sub-categorisation of skin corrosive chemicals by the EpiSkin™ reconstructed human epidermis skin corrosion test method according to UN GHS: Revision of OECD Test Guideline 431. Toxicol. Vitr. 2014, 28, 131–145. [Google Scholar] [CrossRef]
- Basketter, D.; Jirova, D.; Kandárová, H. Review of skin irritation/corrosion hazards on the basis of human data: A regulatory perspective. Interdiscip. Toxicol. 2012, 5, 98–104. [Google Scholar] [CrossRef]
- Pellevoisin, C.; Videau, C.; Briotet, D.; Grégoire, C.; Tornier, C.; Alonso, A.; Rigaudeau, A.S.; Bouez, C.; Seyler, N. SkinEthic™ RHE for in vitro evaluation of skin irritation of medical device extracts. Toxicol. Vitr. 2018, 50, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Mateeva, V.; Angelova-Fischer, I. Irritant Contact Dermatitis. In Applied Dermatotoxicology; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 11–39. [Google Scholar]

| Solution Concentration (w/v) % | Solvent Mixture (v/v) |
|---|---|
| PET (20% and 30%) | HFP:DCM (10:0, 7:3 and 1:1) |
| PBT (20% and 30%) | HFP:DCM (10:0, 7:3 and 1:1) |
| N6/6 (12.5%) | FAc:CHCl3 (7.5:2.5) |
| Polymer | Process Parameters | ||
|---|---|---|---|
| Voltage (kV) | Flow Rate (mL·h−1) | Tip-to-Collector Distance (cm) | |
| PET | 20 | 12 | 30 |
| PBT | 20 | 12 | 30 |
| N6/6 | 20 | 2 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarena, D.E.M.; Matsuyama, L.S.A.S.; Maria-Engler, S.S.; Catalani, L.H. Development of Epidermal Equivalent from Electrospun Synthetic Polymers for In Vitro Irritation/Corrosion Testing. Nanomaterials 2020, 10, 2528. https://doi.org/10.3390/nano10122528
Camarena DEM, Matsuyama LSAS, Maria-Engler SS, Catalani LH. Development of Epidermal Equivalent from Electrospun Synthetic Polymers for In Vitro Irritation/Corrosion Testing. Nanomaterials. 2020; 10(12):2528. https://doi.org/10.3390/nano10122528
Chicago/Turabian StyleCamarena, Denisse Esther Mallaupoma, Larissa Satiko Alcântara Sekimoto Matsuyama, Silvya Stuchi Maria-Engler, and Luiz Henrique Catalani. 2020. "Development of Epidermal Equivalent from Electrospun Synthetic Polymers for In Vitro Irritation/Corrosion Testing" Nanomaterials 10, no. 12: 2528. https://doi.org/10.3390/nano10122528
APA StyleCamarena, D. E. M., Matsuyama, L. S. A. S., Maria-Engler, S. S., & Catalani, L. H. (2020). Development of Epidermal Equivalent from Electrospun Synthetic Polymers for In Vitro Irritation/Corrosion Testing. Nanomaterials, 10(12), 2528. https://doi.org/10.3390/nano10122528








