Negatively-Doped Single-Walled Carbon Nanotubes Decorated with Carbon Dots for Highly Selective NO2 Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Carbon Dots (CDs)
2.2. Preparation of Carbon Dot (CD)-Decorated SWCNT Suspensions
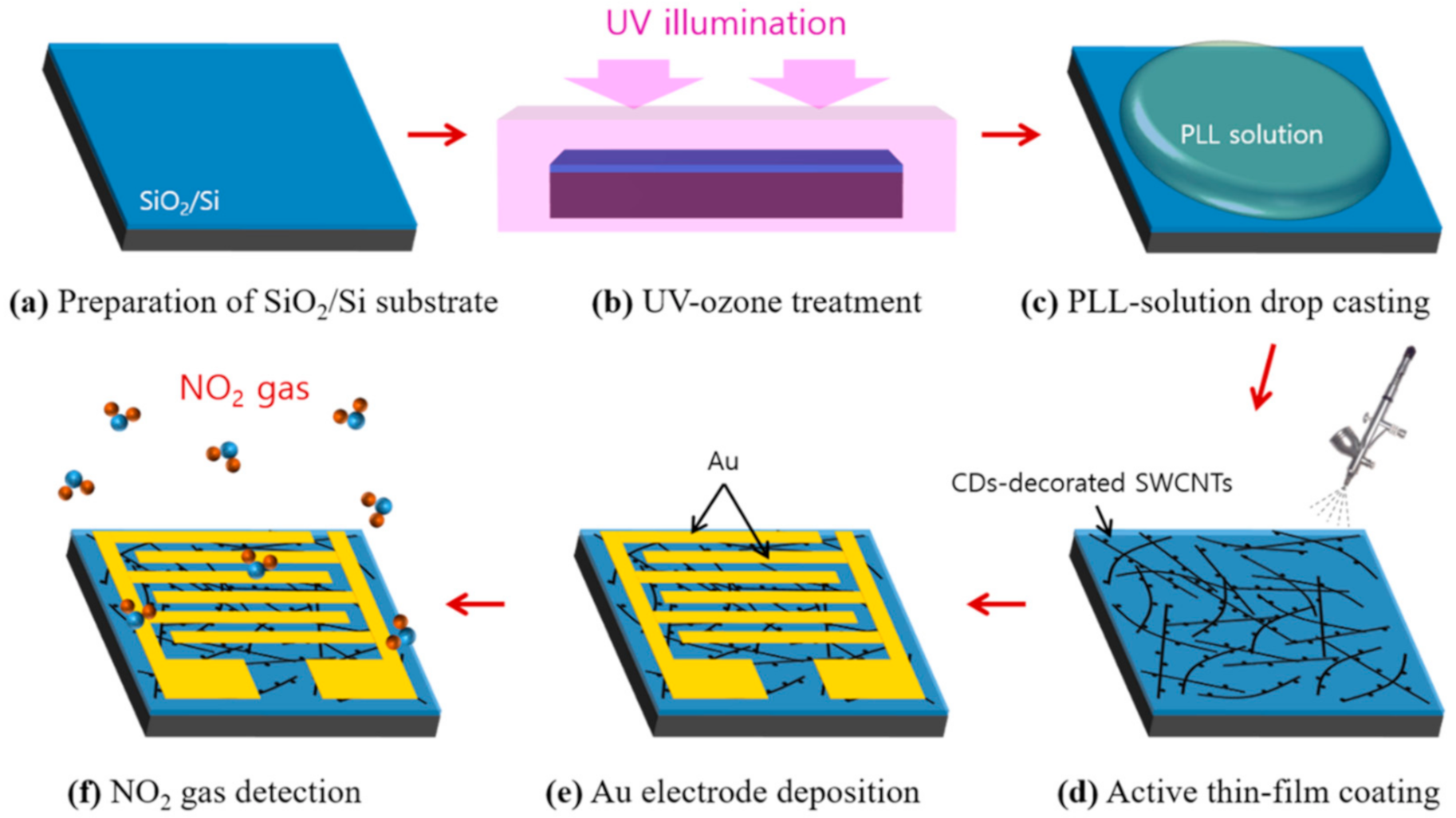
2.3. Fabrication of CD-Decorated SWCNT-Based Gas Sensors
2.4. Sensing Measurements
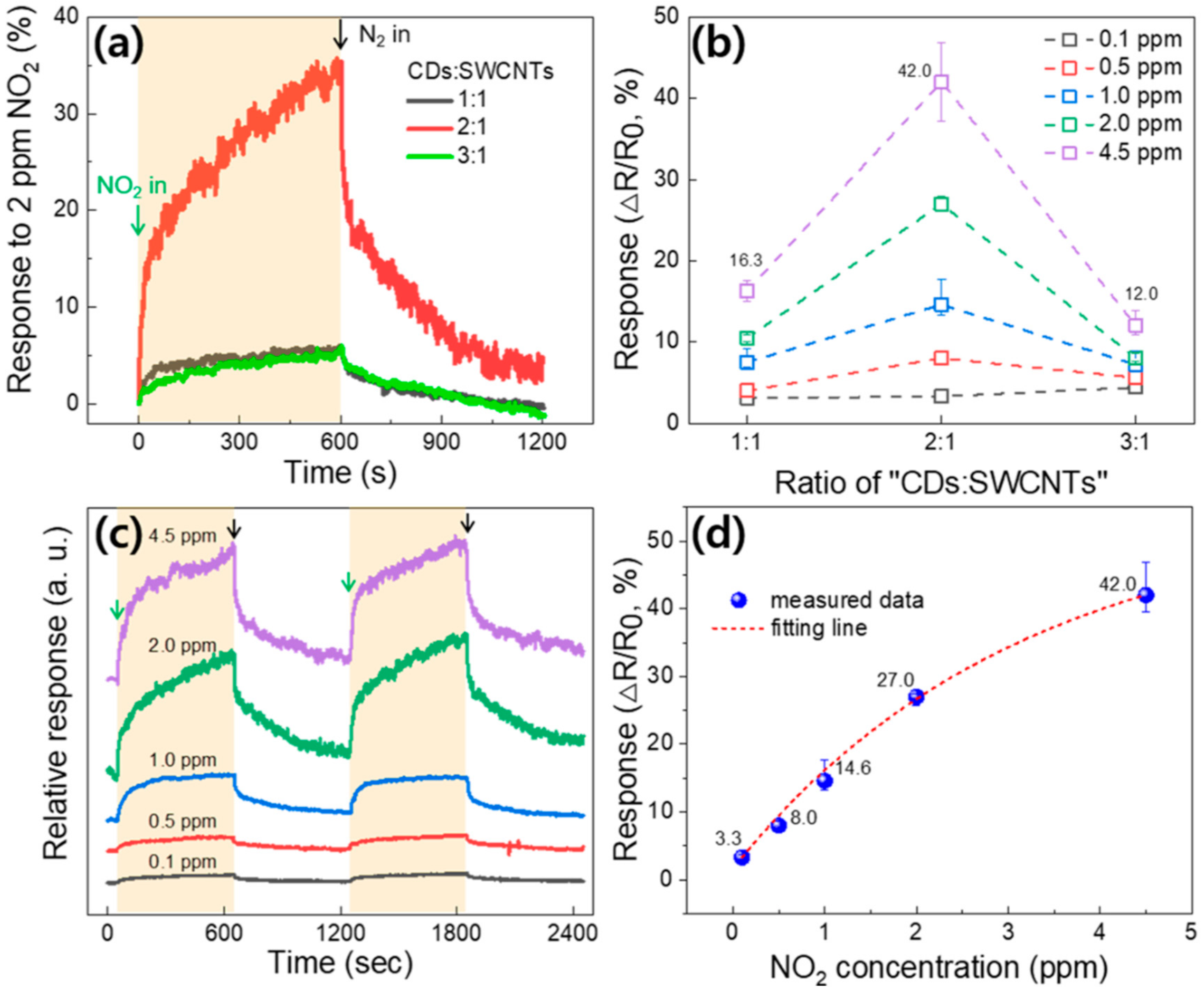
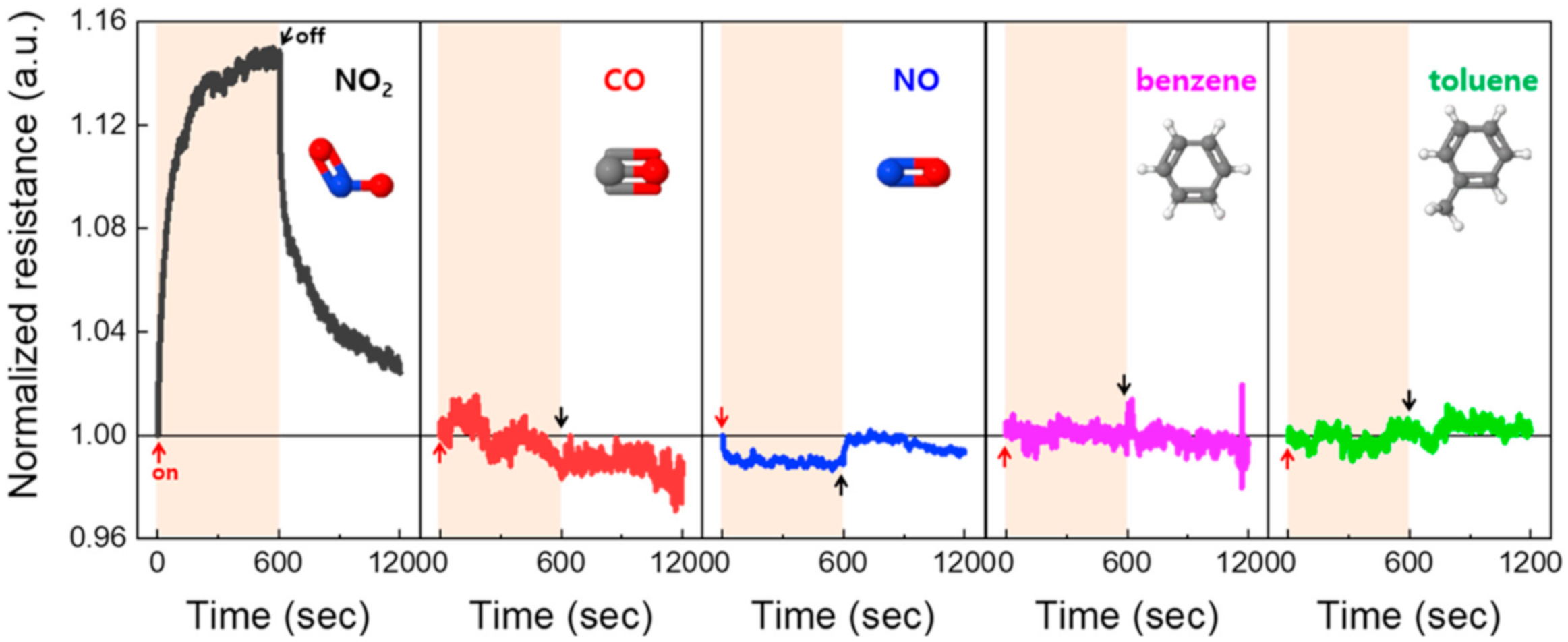
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, M.; Wu, Z.; Liu, G.; Zhao, L.; Gao, Y.; Li, S.; Zhang, B.; Yan, X.; Lu, G. Carbon dots decorated hierarchical litch-like In2O3 nanospheres for highly sensitive and selective NO2 detection. Sens. Actuators B 2020, 304, 127272. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Dong, Y.; Chi, Y.; Chen, G. Carbon quantum dot-functionalized aerogels for NO2 gas sensing. Anal. Chem. 2013, 85, 8065–8069. [Google Scholar] [CrossRef]
- Sayago, I.; Santos, H.; Horrillo, M.C.; Aleixandre, M.; Fernandez, M.J.; Terrado, E.; Tacchini, I.; Aroz, R.; Maser, W.K.; Benito, A.M.; et al. Carbon nanotube networks as gas sensors for NO2 detection. Talanta 2008, 77, 758–764. [Google Scholar] [CrossRef]
- Mane, A.A.; Moholkar, A.V. Palladium (Pd) sensitized molybdenum trioxide (MoO3) nanobelts for nitrogen dioxide (NO2) gas detection. Solid-State Electron. 2018, 139, 21–30. [Google Scholar] [CrossRef]
- Jeon, J.; Kang, B.; Byun, Y.T.; Ha, T. High-performance gas sensors based on single-walled carbon nanotube random networks for the detection of nitric oxide down to the ppb-Level. Nanoscale 2019, 11, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, X.; Bermak, A.; Fan, Z. Self-gating effect induced large performance improvement of ZnO nanocomb gas sensors. ACS Nano 2013, 7, 9318–9324. [Google Scholar] [CrossRef]
- Sun, G.; Lee, J.K.; Choi, S.; Lee, W.I.; Kim, H.W.; Lee, C. Selective oxidizing gas sensing and dominant sensing mechanism of n-CAO-decorated n-ZnO nanorod sensors. ACS Appl. Mater. Interfaces 2017, 9, 9975–9985. [Google Scholar] [CrossRef]
- Shishiyanu, S.T.; Shishiyanu, T.S.; Lupan, O.I. Sensing characteristics of tin-doped ZnO thin films as NO2 gas sensor. Sens. Actuators B 2005, 107, 379–386. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas-sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Li, Y.-X.; Guo, Z.; Su, Y.; Jin, X.-B.; Tang, X.-H.; Huang, J.-R.; Huang, X.-J.; Li, M.-Q.; Liu, J.-H. Hierarchical morphology-dependent gas-sensing performances of three-dimensional SnO2 nanostructures. ACS Sens. 2017, 2, 102–110. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chen, H.; Li, G.-D.; Shi, Z.; Zou, H.; Zou, X. Ultrathin In2O3 nanosheets with uniform mesopores for highly sensitive nitric oxide detection. ACS Appl. Mater. Interfaces 2017, 9, 16335–16342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, D.; Zhu, Q.; Wu, J.; Xu, K.; Liao, T.; Zhang, G.; Xie, C. Effect of grain-boundaries in NIO nanosheet layers room-temperature sensing mechanism under NO2. J. Phys. Chem. C 2015, 119, 17930–17939. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Li, H.-Y.; Ding, J.-C.; Guo, X. Hierarchical flowerlike WO3 nanostructures assembled by porous nanoflakes for enhanced NO gas sensing. Sens. Actuators B 2017, 246, 225–234. [Google Scholar] [CrossRef]
- Geng, L.; Zhao, Y.; Huang, X.; Wang, S.; Zhang, S.; Wu, S. Characterization and gas sensitivity study of polyaniline/SnO2 hybrid material prepared by hydrothermal route. Sens. Actuators B 2007, 120, 568–572. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kim, J.; Byun, Y.T. Highly sensitive and selective NO2 detection by Pt nanoparticles-decorated single-walled carbon nanotubes and the underlying sensing mechanism. Sens. Actuators B 2017, 238, 1032–1042. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.-W.; Lee, J.-H.; Chung, Y.; Byun, Y.T. Gas sensing properties of defect-induced single-walled carbon nanotubes. Sens. Actuators B 2016, 228, 688–692. [Google Scholar] [CrossRef]
- Choi, S.-W.; Byun, Y.T. The effect of platinum precursor concentrations on chlorine sensing characteristics of platinum nanoparticles-loaded single walled carbon nanotubes. Appl. Surf. Sci. 2018, 433, 480–486. [Google Scholar] [CrossRef]
- Lee, D.-J.; Choi, S.-W.; Byun, Y.T. Room temperature monitoring of hydrogen peroxide vapor using platinum nanoparticles-decorated single-walled carbon nanotube networks. Sens. Actuators B 2018, 256, 744–750. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kim, B.-M.; Oh, S.-H.; Byun, Y.T. Selective detection of chlorine at room temperature utilizing single-walled carbon nanotubes functionalized with platinum nanoparticles synthesized via ultraviolet irradiation. Sens. Actuators B 2017, 249, 414–422. [Google Scholar] [CrossRef]
- Lee, J.-S.; Jeong, D.-W.; Byun, Y.T. Porphyrin nanofiber/single-walled carbon nanotube nanocomposite-based sensors for monitoring hydrogen peroxide vapor. Sens. Actuators B 2020, 306, 127518. [Google Scholar] [CrossRef]
- Yaqoob, U.; Phan, D.-T.; Uddin, A.S.M.I.; Chung, G.-S. Highly flexible room temperature NO2 sensor based on MWCNTs-WO3 nanoparticles hybrid on a PET substrate. Sens. Actuators B 2015, 221, 760–768. [Google Scholar] [CrossRef]
- Hong, J.; Lee, S.; Seo, J.; Pyo, S.; Kim, J.; Lee, T. A highly sensitive hydrogen sensor with gas selectivity using a PMMA membrane-coated Pd nanoparticle/single-layer graphene hybrid. Sens. ACS Appl. Mater. Interfaces 2015, 7, 3354–3561. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.G.; Kim, D.H.; Lee, H.M.; Kim, T.; Choi, J.H.; Seo, D.; Yoo, J.-B.; Hong, S.-H.; Kang, T.-J.; Kim, Y.H. Highly sensitive NO2 gas sensor based on ozone treated graphene. Sens. Actuators B 2012, 166-167, 172–176. [Google Scholar] [CrossRef]
- Choi, S.-J.; Ryu, W.-H.; Kim, S.-J.; Cho, H.-J.; Kim, I.-D. Bi-functional co-sensitization of graphene oxide sheets and Ir nanoparticles on p-Type Co3O4 nanofibers for selective acetone detection. J. Mater. Chem. B 2014, 2, 7160–7167. [Google Scholar] [CrossRef] [PubMed]
- Septiani, N.L.W.; Yuliarto, B. Review-the development of gas sensor based on carbon nanotubes. J. Electrochem. Soc. 2016, 163, B97. [Google Scholar] [CrossRef]
- Ellis, J.E.; Star, A. Carbon nanotube based gas sensors toward breath analysis. ChemPlusChem 2016, 81, 1248–1265. [Google Scholar] [CrossRef]
- Goldoni, A.; Alijani, V.; Sangaletti, L.; D’Arsie, L. Advanced promising routes of carbon/metal oxides hybrid in sensors: A review. Electrochim. Acta 2018, 266, 139–150. [Google Scholar] [CrossRef]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent develpments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, B.; Wang, X.; Luo, C. Effect of plasma treatment on multi-walled carbon nanotubes for the detection of H2S and SO2. Sensors 2012, 12, 9375–9385. [Google Scholar] [CrossRef]
- Zhao, W.; Fam, D.W.H.; Yin, Z.; Sun, T.; Tan, H.T.; Liu, W.; Tok, A.I.Y.; Boey, Y.C.F.; Zhang, H.; Hng, H.H.; et al. A carbon monoxide gas sensor using oxygen plasma modified carbon nanotubes. Nanotechnology 2012, 23, 425502. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Hahm, M.G.; Kim, D.-H.; Kim, A.R.; Kahng, Y.H.; Park, S.-W.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; et al. Graphene-based gas sensor: Metal decoration effect and application to a flexible device. J. Mater. Chem. C 2014, 2, 5280–5285. [Google Scholar] [CrossRef]
- Evans, G.P.; Buckley, D.J.; Skipper, N.T.; Parkin, I.P. Single-walled carbon nanotube composite inks for printed gas sensors: Enhanced detection of NO2, NH3, EtOH and acetone. RSC Adv. 2014, 4, 51395–51403. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kim, J.; Lee, J.-H.; Byun, Y.T. Remarkable improvement of CO-sensing performances in single-walled carbon nanotubes due to modification of the conducting channel by functionalization of Au nanoparticles. Sens. Actuators B 2016, 232, 625–632. [Google Scholar] [CrossRef]
- Marichy, C.; Russo, P.A.; Latino, M.; Tessonnier, J.-P.; Willinger, M.-G.; Donato, N.; Neri, G.; Pinna, N. Tin dioxide-carbon heterostructures applied to gas sensing: Structure-dependent properties and general sensing mechanism. J. Phys. Chem. C 2013, 117, 19729–19739. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Q.; Chen, X.; Yang, W.; Wu, Z.; Xu, X.; Jiang, M.; Zhou, Z. Enhanced performance of core-shell structured polyaniline at helical carbon nanotube hybrids for ammonia gas sensor. Appl. Phys. Lett. 2014, 105, 203109. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhang, Y.; Zhang, C.; Zhang, T. High performance room temperature NO2 sensors based on reduced graphene oxide-multiwalled carbon nanotubes-tin oxide nanoparticles hybrids. Sens. Actuators B 2015, 211, 318–324. [Google Scholar] [CrossRef]
- Tung, T.T.; Pham-Huu, C.; Janowska, I.; Kim, T.Y.; Castro, M.; Feller, J.-F. Hybrid films of graphene and carbon nanotubes for high performance chemical and temperature sensing applications. Small 2015, 11, 3485–3493. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.-G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 2015, 82, 304–313. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Shen, Y.; Wang, M.; Li, J. Poly-l-lysine functionalization of single-walled carbon nanotubes. J. Phys. Chem. B 2004, 108, 15343–15346. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchundan, R.; Sethuraman, M.G.; Shim, J.-J.; Lee, Y.R. Microwave assisted green synthesis of fluorescent N-doped carbon dots: Cytotoxicity and bio-imaging applications. J. Photochem. Photobiol. B 2016, 161, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Duong, D.L.; Lee, S.M.; Lee, Y.H. Origin of unipolarity in carbon nanotubes field effect transistors. J. Mater. Chem. 2012, 22, 1994–1997. [Google Scholar] [CrossRef]
- Zhu, W.; Song, H.; Zhang, L.; Weng, Y.; Su, Y.; Lv, Y. Fabrication of fluorescent nitrogen-rich graphene quantum dots by tin(IV) catalytic carbonization of ethanolamine. RSC Adv. 2015, 5, 60085–60089. [Google Scholar] [CrossRef]
- Robinson, J.A.; Snow, E.S.; Badescu, S.C.; Reinecke, T.L.; Perkins, F.K. Role of defects in single-walled carbon nanotube chemical sensors. Nano Lett. 2006, 6, 1747–1751. [Google Scholar] [CrossRef]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P.; Chouksey, A.; Lal, M.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sens. Actuators B 2017, 240, 1134–1140. [Google Scholar] [CrossRef]
- Hoffmann, M.W.G.; Prades, J.D.; Mayrhofer, L.; Hernandez-Ramirez, F.; Jarvi, T.T.; Moseler, M.; Waag, A.; Shen, H. Highly selective SAM-nanowire hybrid NO2 sensor: Insight into charge transfer dynamics and alignment of frontier molecular orbitals. Adv. Funct. Mater. 2014, 24, 595–602. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, N.; Lee, J.-S.; Byun, Y.T. Negatively-Doped Single-Walled Carbon Nanotubes Decorated with Carbon Dots for Highly Selective NO2 Detection. Nanomaterials 2020, 10, 2509. https://doi.org/10.3390/nano10122509
Lim N, Lee J-S, Byun YT. Negatively-Doped Single-Walled Carbon Nanotubes Decorated with Carbon Dots for Highly Selective NO2 Detection. Nanomaterials. 2020; 10(12):2509. https://doi.org/10.3390/nano10122509
Chicago/Turabian StyleLim, Namsoo, Jae-Sung Lee, and Young Tae Byun. 2020. "Negatively-Doped Single-Walled Carbon Nanotubes Decorated with Carbon Dots for Highly Selective NO2 Detection" Nanomaterials 10, no. 12: 2509. https://doi.org/10.3390/nano10122509
APA StyleLim, N., Lee, J.-S., & Byun, Y. T. (2020). Negatively-Doped Single-Walled Carbon Nanotubes Decorated with Carbon Dots for Highly Selective NO2 Detection. Nanomaterials, 10(12), 2509. https://doi.org/10.3390/nano10122509







