Various Allotropes of Diamond Nanoparticles Generated in the Gas Phase during Hot Filament Chemical Vapor Deposition
Abstract
1. Introduction
2. Materials and Methods
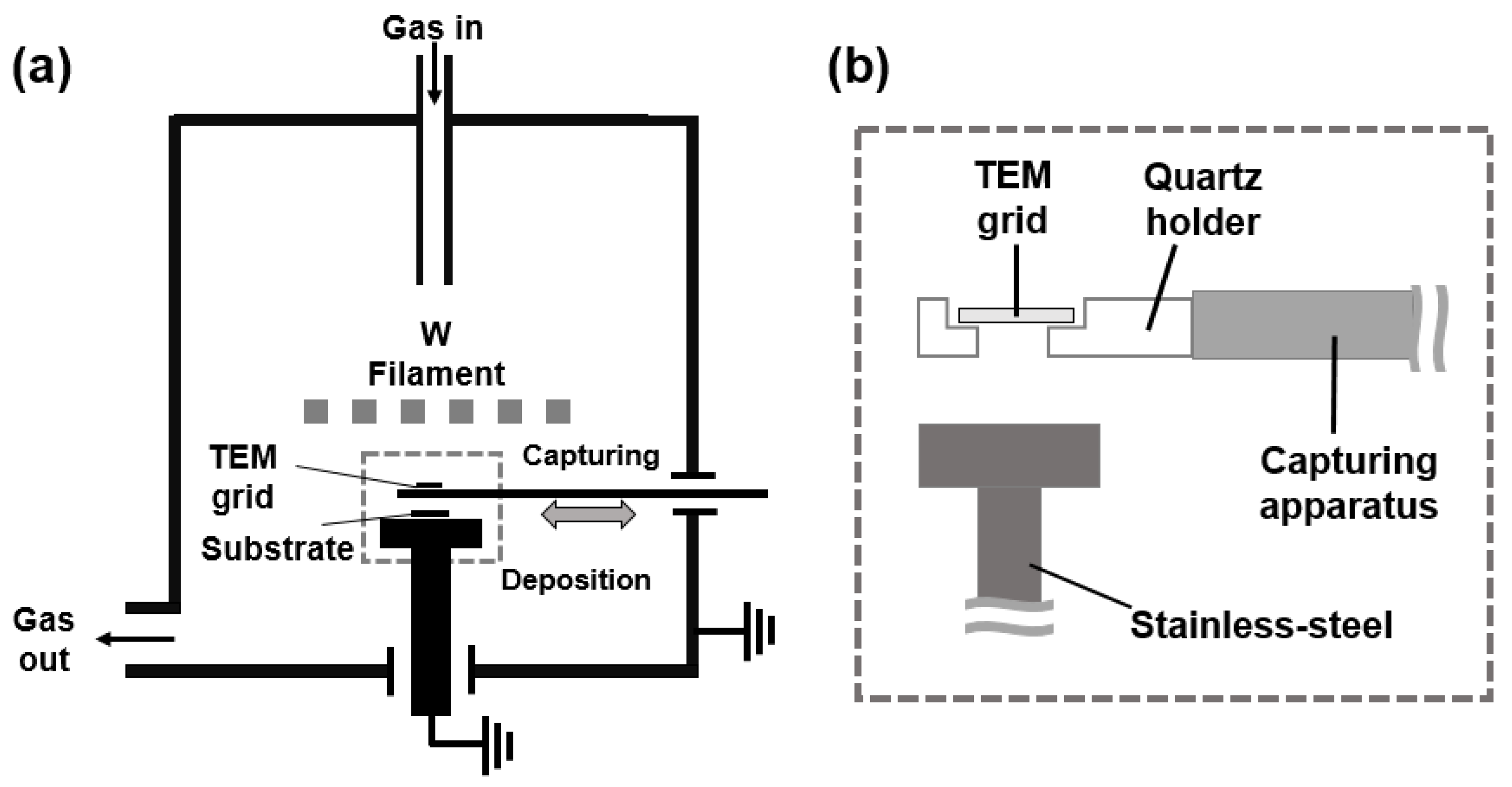
2.1. Preparation of Nanoparticles
2.2. Analysis of Nanoparticles
2.3. Current Measurement
2.4. Deposition of Diamond Particles
3. Results and Discussion
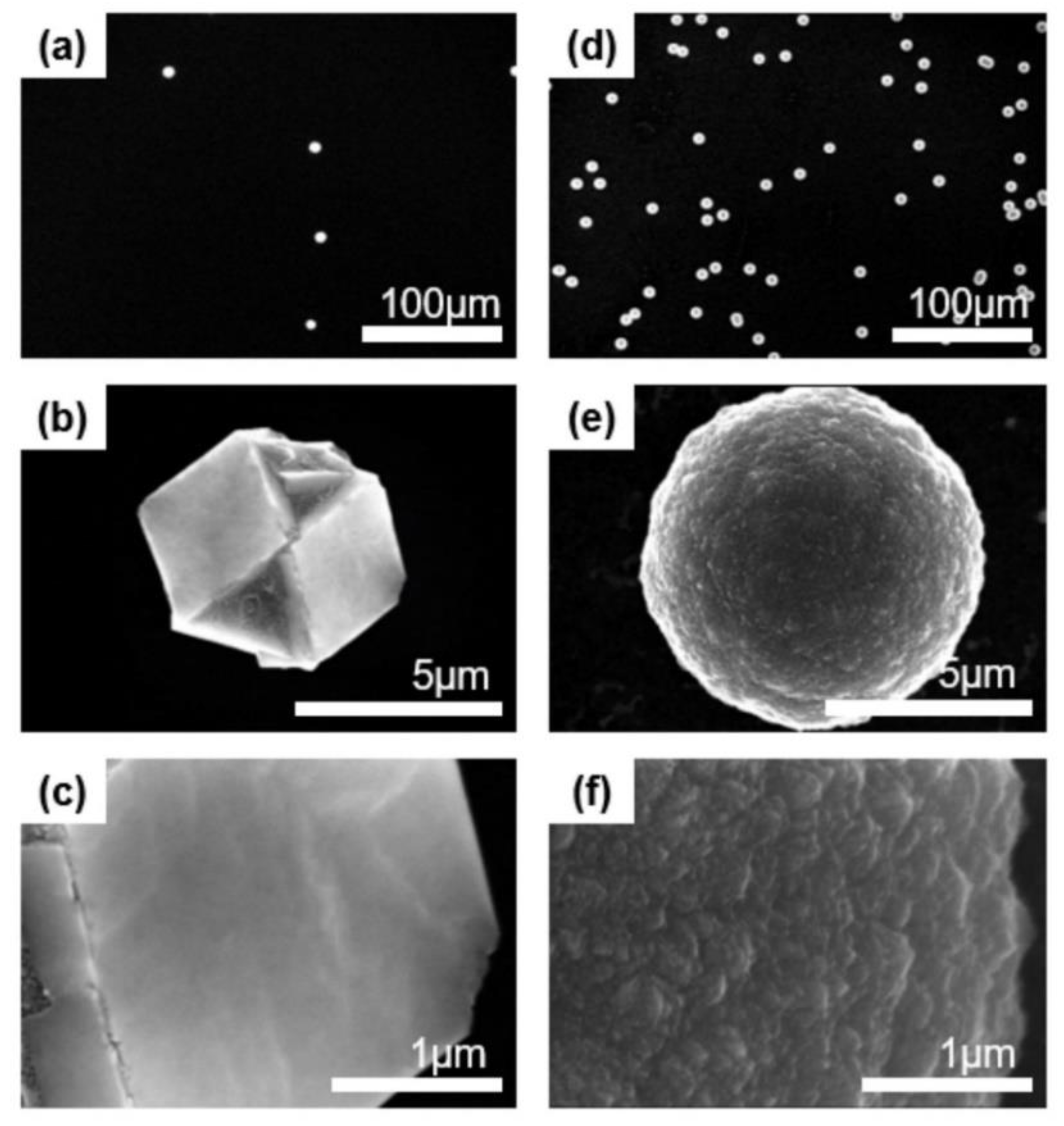
3.1. Identification of Nanoparticles
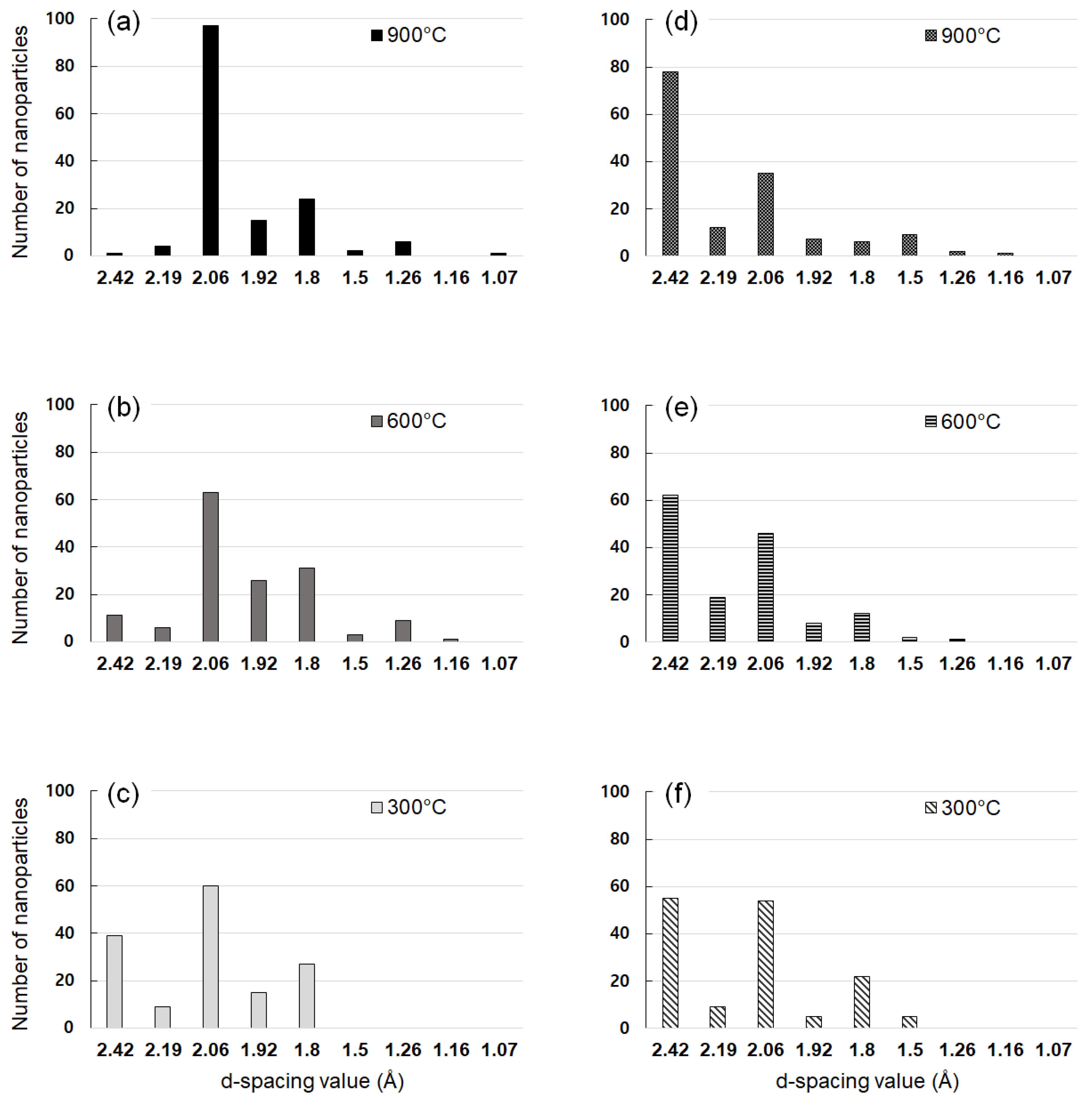
3.2. Captured Nanoparticles under Various Processing Conditions
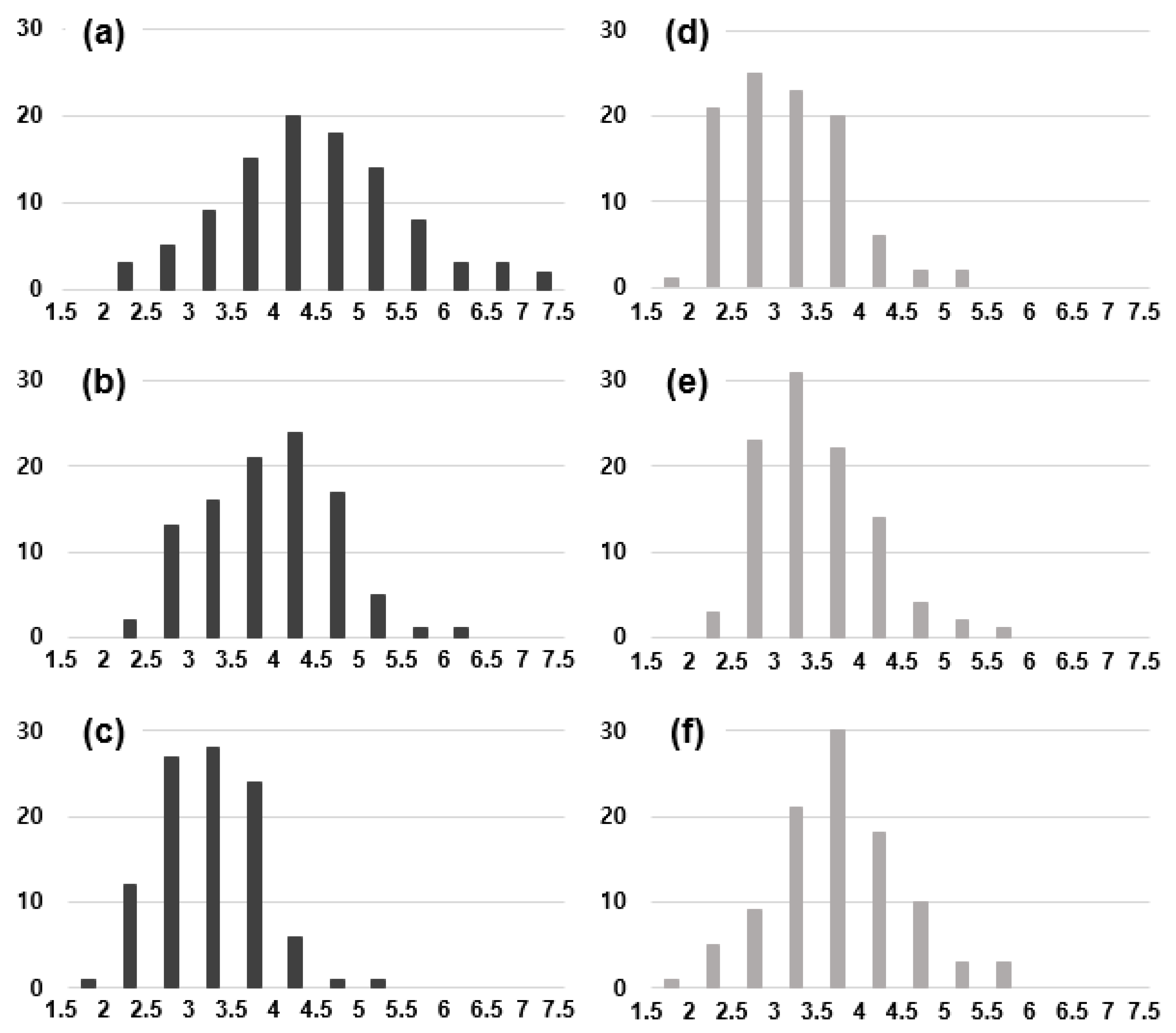
3.3. Size Dependence on the Crystal Structure of Nanoparticles
3.4. Comparing the Captured Nanoparticles and Deposited Diamond
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bundy, F.P.; Hall, H.T.; Strong, R.H. Man-made diamonds. Nature 1955, 176, 51–55. [Google Scholar] [CrossRef]
- Deshpandey, C.V.; Bunshah, R.F. Diamond and diamondlike films: Deposition processes and properties. J. Vac. Sci. Technol. A 1989, 7, 2294–2302. [Google Scholar] [CrossRef]
- Angus, J.C.; Will, H.A.; Stanko, W.S. Growth of diamond seed crystals by vapor deposition. J. Appl. Phys. 1968, 39, 2915–2922. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sato, Y.; Kamo, M.; Setaka, N. Vapor deposition of diamond particles from methane. Jpn. J. Appl. Phys. 1982, 21, L183. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Detonation-synthesis nanodiamonds: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2007, 76, 339–360. [Google Scholar] [CrossRef]
- Yang, G.W.; Wang, J.B.; Liu, Q.X. Preparation of nano-crystalline diamonds using pulsed laser induced reactive quenching. J. Phys. Condens. Matter 1998, 10, 7923–7927. [Google Scholar] [CrossRef]
- Pimenov, S.M.; Shafeev, G.A.; Smolin, A.A.; Konov, V.I.; Vodolaga, B.K. Laser-induced forward transfer of ultra-fine diamond particles for selective deposition of diamond films. Appl. Surf. Sci. 1995, 86, 208–212. [Google Scholar] [CrossRef]
- Daulton, T.L.; Kirk, M.A.; Lewis, R.S.; Rehn, L.E. Production of nanodiamonds by high-energy ion irradiation of graphite at room temperature. Nucl. Instr. Meth. B 2001, 175, 12–20. [Google Scholar] [CrossRef]
- Banhart, F.; Ajayan, P.M. Carbon onions as nanoscopic pressure cells for diamond formation. Nature 1996, 382, 433–435. [Google Scholar] [CrossRef]
- Boudou, J.P.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J.; Aubert, P.; Sennour, M.; Balasubramanian, G.; Reuter, R.; Thorel, A.; Gaffet, E. High yield fabrication of fluorescent nanodiamonds. Nanotechnology 2009, 20, 235602. [Google Scholar] [CrossRef]
- Galimov, É.M.; Kudin, A.M.; Skorobogatskii, V.N.; Plotnichenko, V.G.; Bondarev, O.L.; Zarubin, B.G.; Strazdovskii, V.V.; Aronin, A.S.; Fisenko, A.V.; Bykov, I.V.; et al. Experimental corroboration of the synthesis of diamond in the cavitation process. Dokl. Phys. 2004, 49, 150–153. [Google Scholar] [CrossRef]
- Chang, Y.R.; Lee, H.Y.; Chen, K.; Chang, C.C.; Tsai, D.S.; Fu, C.C.; Lim, T.S.; Tzeng, Y.K.; Fang, C.Y.; Han, C.C.; et al. Mass production and dynamic imaging of fluorescent nanodiamonds. Nat. Nanotechnol. 2008, 3, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Greentree, A.D.; Fairchild, B.A.; Hossain, F.M.; Prawer, S. Diamond integrated quantum photonics. Mater. Today 2008, 11, 22–31. [Google Scholar] [CrossRef]
- Vlasov, I.I.; Barnard, A.S.; Ralchenko, V.G.; Lebedev, O.I.; Kanzyuba, M.V.; Saveliev, A.V.; Konov, V.I.; Goovaerts, E. Nanodiamond photoemitters based on strong narrow-band luminescence from silicon-vacancy defects. Adv. Mater. 2009, 21, 808–812. [Google Scholar] [CrossRef]
- Ōsawa, E. Recent progress and perspectives in single-digit nanodiamond. Diam. Relat. Mater. 2007, 16, 2018–2022. [Google Scholar] [CrossRef]
- Turner, S.; Lebedev, O.L.; Shenderova, O.; Vlasov, I.I.; Verbeeck, J.; Tendeloo, G.V. Determination of size, morphology, and nitrogen impurity location in treated detonation nanodiamond by transmission electron microscopy. Adv. Funct. Mater. 2009, 19, 2116–2124. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, K.S.; Hwang, N.M. Gas phase generation of diamond nanoparticles in the hot filament chemical vapor deposition reactor. Carbon 2016, 106, 289–294. [Google Scholar] [CrossRef]
- Vora, H.; Moravec, T.J. Structural investigation of thin films of diamondlike carbon. J. Appl. Phys. 1981, 52, 6151–6157. [Google Scholar] [CrossRef]
- Peng, J.L.; Orwa, J.O.; Jiang, B.; Prawer, S.; Bursill, L.A. Nano-crystals of c-diamond, n-diamond and i-carbon grown in carbon-ion implanted fused quartz. Int. J. Mod. Phys. B 2001, 15, 3107–3123. [Google Scholar] [CrossRef]
- Yamada, K.; Sawaoka, A.B. Very small spherical crystals of distorted diamond found in a detonation product of explosive/graphite mixtures and their formation mechanism. Carbon 1994, 32, 665–673. [Google Scholar] [CrossRef]
- Bucknum, M.J.; Pickard, C.J.; Stamatin, I.; Castro, E.A. On the structure of i-carbon. J. Theor. Comput. Chem. 2006, 5, 175–185. [Google Scholar] [CrossRef]
- Gamarnik, M.Y. Size-related stabilization of diamond nanoparticles. Nanostruct. Mater. 1996, 7, 651–658. [Google Scholar] [CrossRef]
- Barnard, A.S.; Russo, S.P.; Snook, I.K. Coexistence of bucky diamond with nanodiamond and fullerene carbon phases. Phys. Rev. B 2003, 68, 073406. [Google Scholar] [CrossRef]
- Jeon, I.D.; Park, C.J.; Kim, D.Y.; Hwang, N.M. Effect of methane concentration on size of charged clusters in the hot filament diamond CVD process. J. Cryst. Growth 2001, 223, 6–14. [Google Scholar] [CrossRef]
- Hwang, N.M.; Hahn, J.H.; Yoon, D.Y. Charged cluster model in the low pressure synthesis of diamond. J. Cryst. Growth 1996, 162, 55–68. [Google Scholar] [CrossRef]
- Sundman, B.; Jansson, B.; Andersson, J.O. The thermo-calc databank system. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Teng, F.; Wang, J.; An, X.; Lu, B.; Su, Y.; Gong, C.; Zhang, P.; Zhang, Z.; Xie, E. Single-phase tungsten carbide nanopillar arrays prepared by chemical vapor deposition. RSC Adv. 2012, 2, 7403–7405. [Google Scholar] [CrossRef]
- Krishnan, K.S.; Jain, S.C. Thermionic constants of graphite. Nature 1952, 169, 702–703. [Google Scholar] [CrossRef]
- Park, N.J.; Park, S.G.; Hwang, N.M.; Ihm, J.S.; Tejima, S.; Nakamura, H. First-principles study of the effect of charge on the stability of a diamond nanocluster surface. Phys. Rev. B 2004, 69, 195411. [Google Scholar] [CrossRef]
- Lai, L.; Barnard, A.S. Charge-induced restructuring and decomposition of bucky-diamonds. J. Mater. Chem. 2012, 22, 13141–13147. [Google Scholar] [CrossRef]

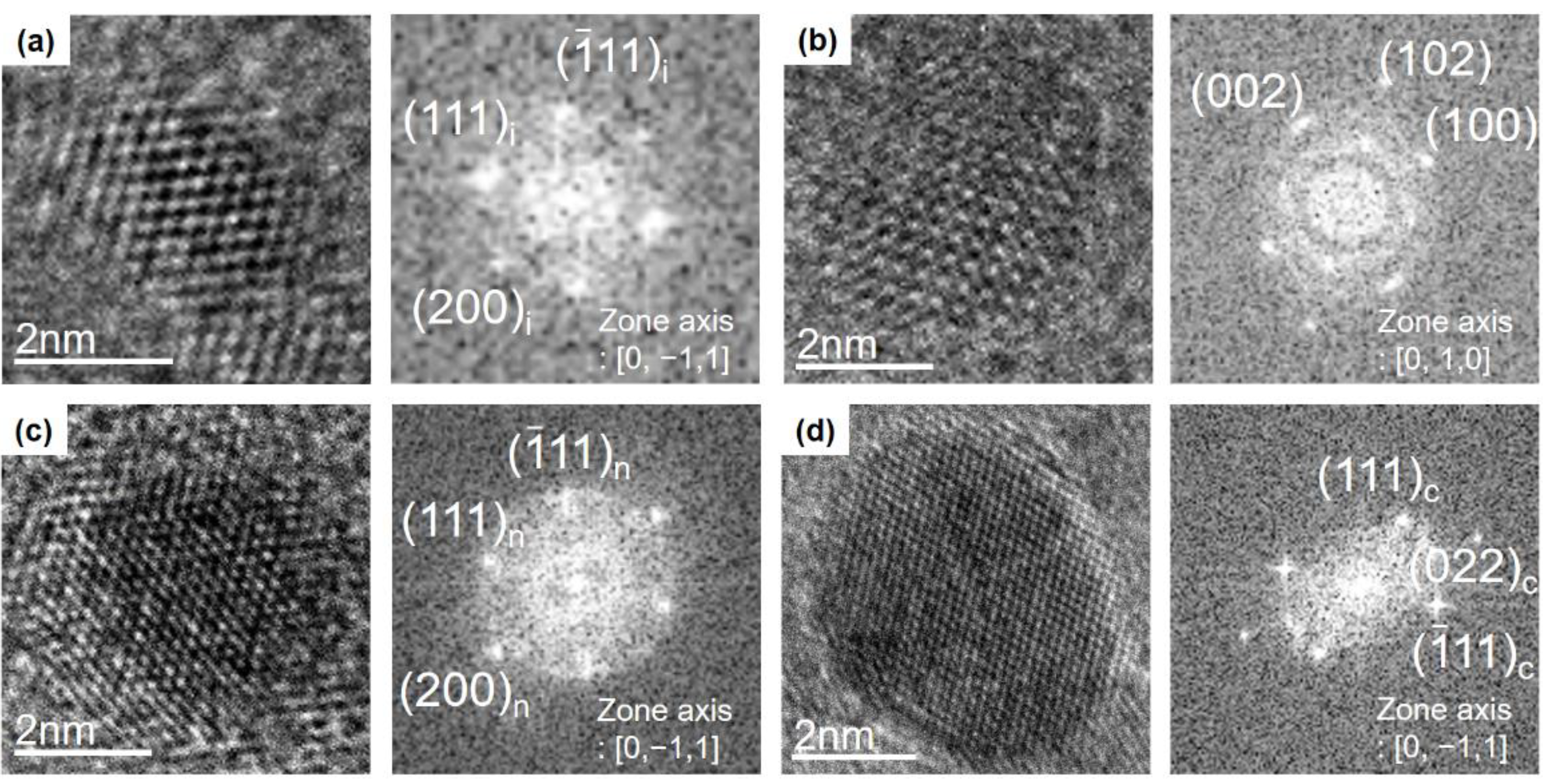

| Cubic Diamond (Observed) | Cubic Diamond (JCPDS6-0675) | n-Diamond (Observed) | n-Diamond (JCPDS43-1104) | Hexagonal Diamond (Observed) | Hexagonal Diamond (JCPDS19-0268) | i-Carbon (Observed) | i-Carbon [18] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d | hkl | d | hkl | I/I111 | d | hkl | d | hkl | I/I111 | d | hkl | d | hkl | I/I111 | d | hkl | d | hkl |
| 2.42 | 111 | 2.46 | 111 | |||||||||||||||
| 2.16 | 100 | 2.19 | 100 | 100 | ||||||||||||||
| 2.06 | 111 | 2.06 | 111 | 100 | 2.06 | 111 | 2.06 | 111 | 100 | 2.06 | 002 | 2.06 | 002 | 100 | 2.10 | 200 * | 2.12 | 200* |
| 1.93 | 101 | 1.92 | 101 | 50 | ||||||||||||||
| 1.78 | 200 * | 1.78 | 200 * | 100 | 1.73 | 211 | 1.74 | 211 | ||||||||||
| 1.50 | 102 | 1.50 | 102 | 25 | 1.47 | 220 | 1.50 | 220 | ||||||||||
| 1.25 | 220 | 1.26 | 220 | 25 | 1.25 | 220 | 1.26 | 220 | 100 | 1.26 | 110 | 75 | 1.28 | 311 | ||||
| 1.17 | 103 | 50 | ||||||||||||||||
| 1.07 | 311 | 16 | 1.07 | 311 | 100 | 1.07 | 112 | 50 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Kim, D.-S.; Kim, K.-S.; Hwang, N.-M. Various Allotropes of Diamond Nanoparticles Generated in the Gas Phase during Hot Filament Chemical Vapor Deposition. Nanomaterials 2020, 10, 2504. https://doi.org/10.3390/nano10122504
Kim H-Y, Kim D-S, Kim K-S, Hwang N-M. Various Allotropes of Diamond Nanoparticles Generated in the Gas Phase during Hot Filament Chemical Vapor Deposition. Nanomaterials. 2020; 10(12):2504. https://doi.org/10.3390/nano10122504
Chicago/Turabian StyleKim, Hwan-Young, Da-Seul Kim, Kun-Su Kim, and Nong-Moon Hwang. 2020. "Various Allotropes of Diamond Nanoparticles Generated in the Gas Phase during Hot Filament Chemical Vapor Deposition" Nanomaterials 10, no. 12: 2504. https://doi.org/10.3390/nano10122504
APA StyleKim, H.-Y., Kim, D.-S., Kim, K.-S., & Hwang, N.-M. (2020). Various Allotropes of Diamond Nanoparticles Generated in the Gas Phase during Hot Filament Chemical Vapor Deposition. Nanomaterials, 10(12), 2504. https://doi.org/10.3390/nano10122504







