Growth of Lactic Acid Bacteria on Gold—Influence of Surface Roughness and Chemical Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gold Layers on a Metal Substrate
2.3. Synthesis of Polycations
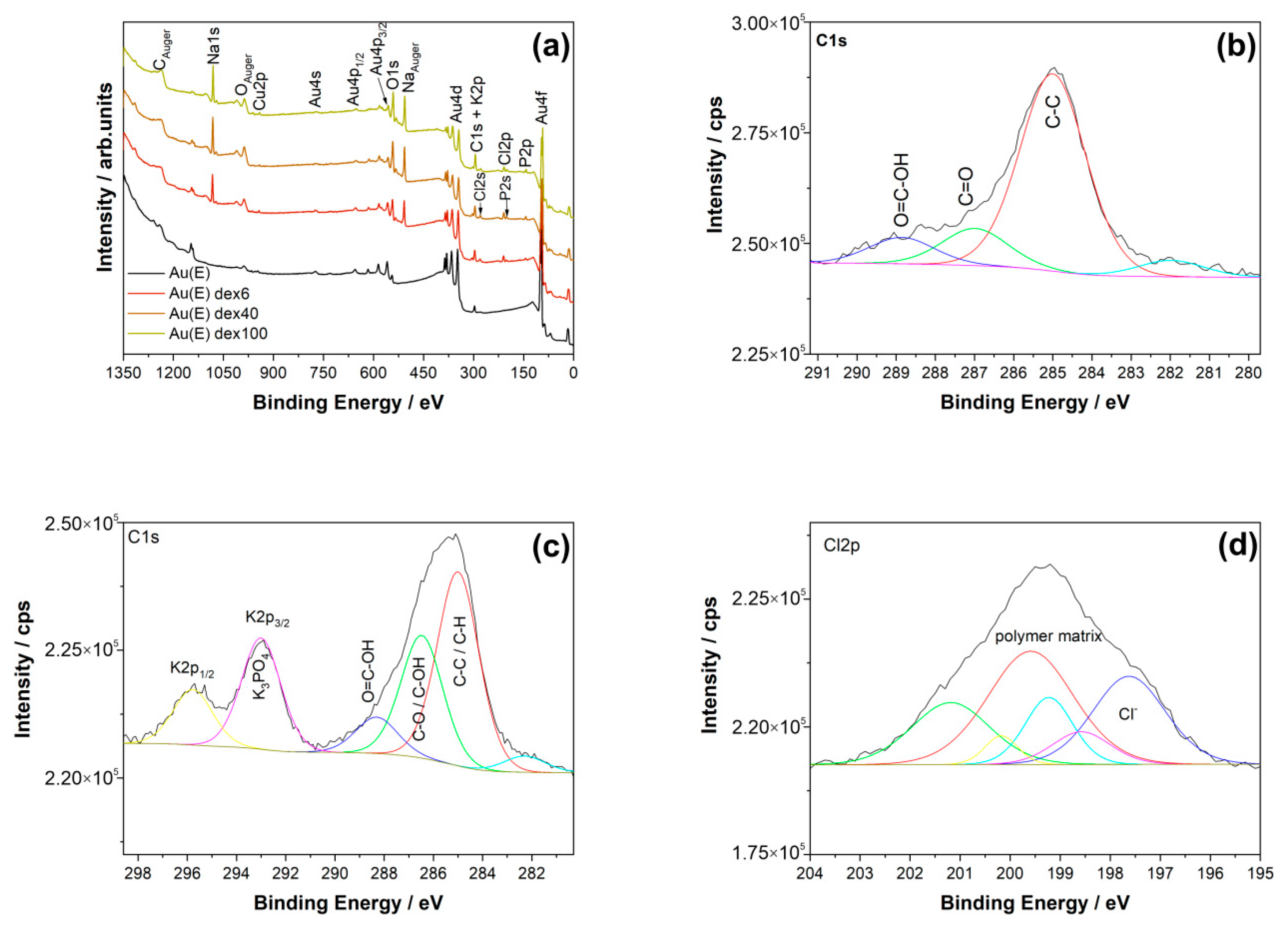
2.4. Surface Modification of Gold Substrates
2.5. Bacteria Inoculation and Cultivation
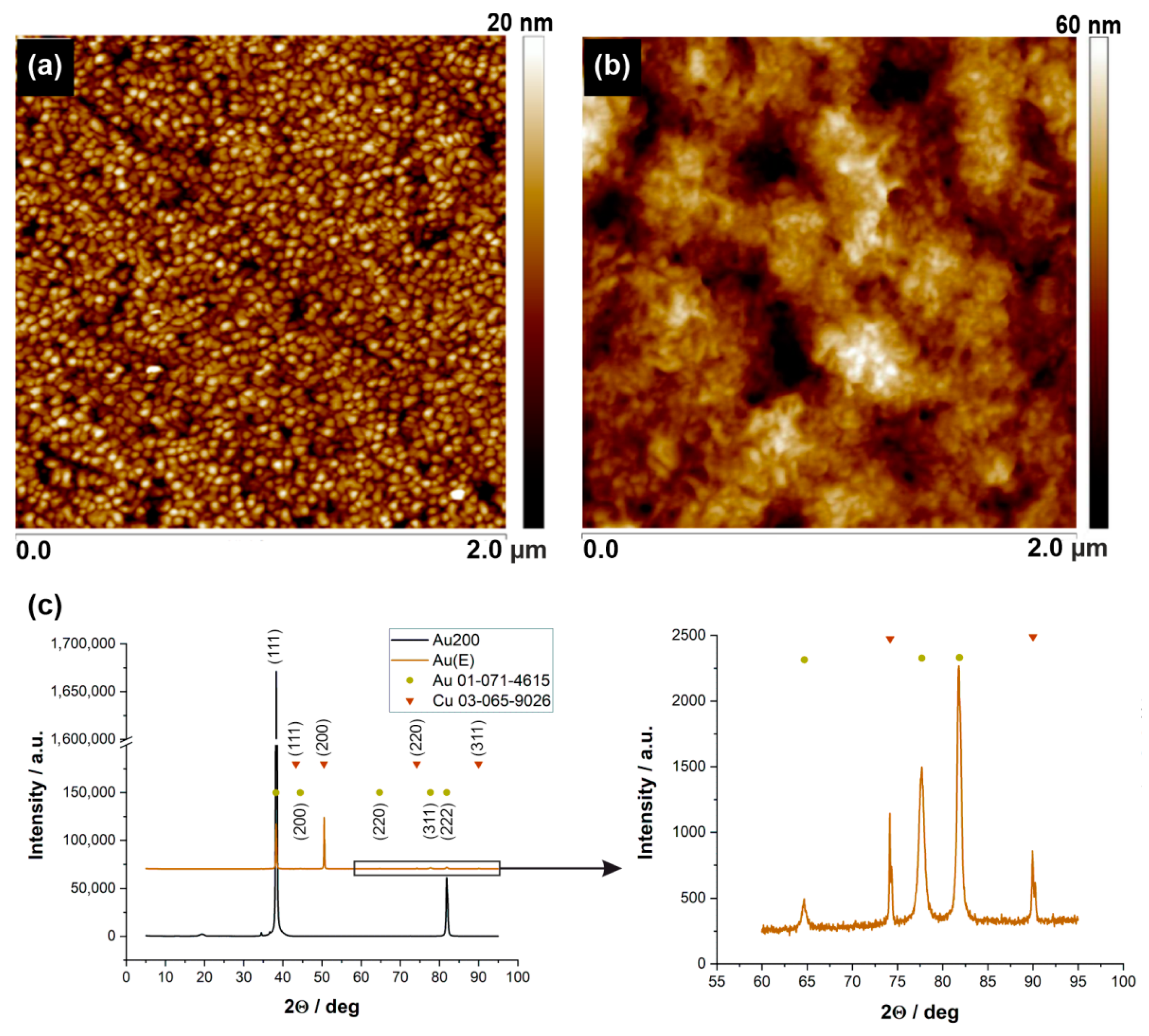
2.6. Surface Characterization
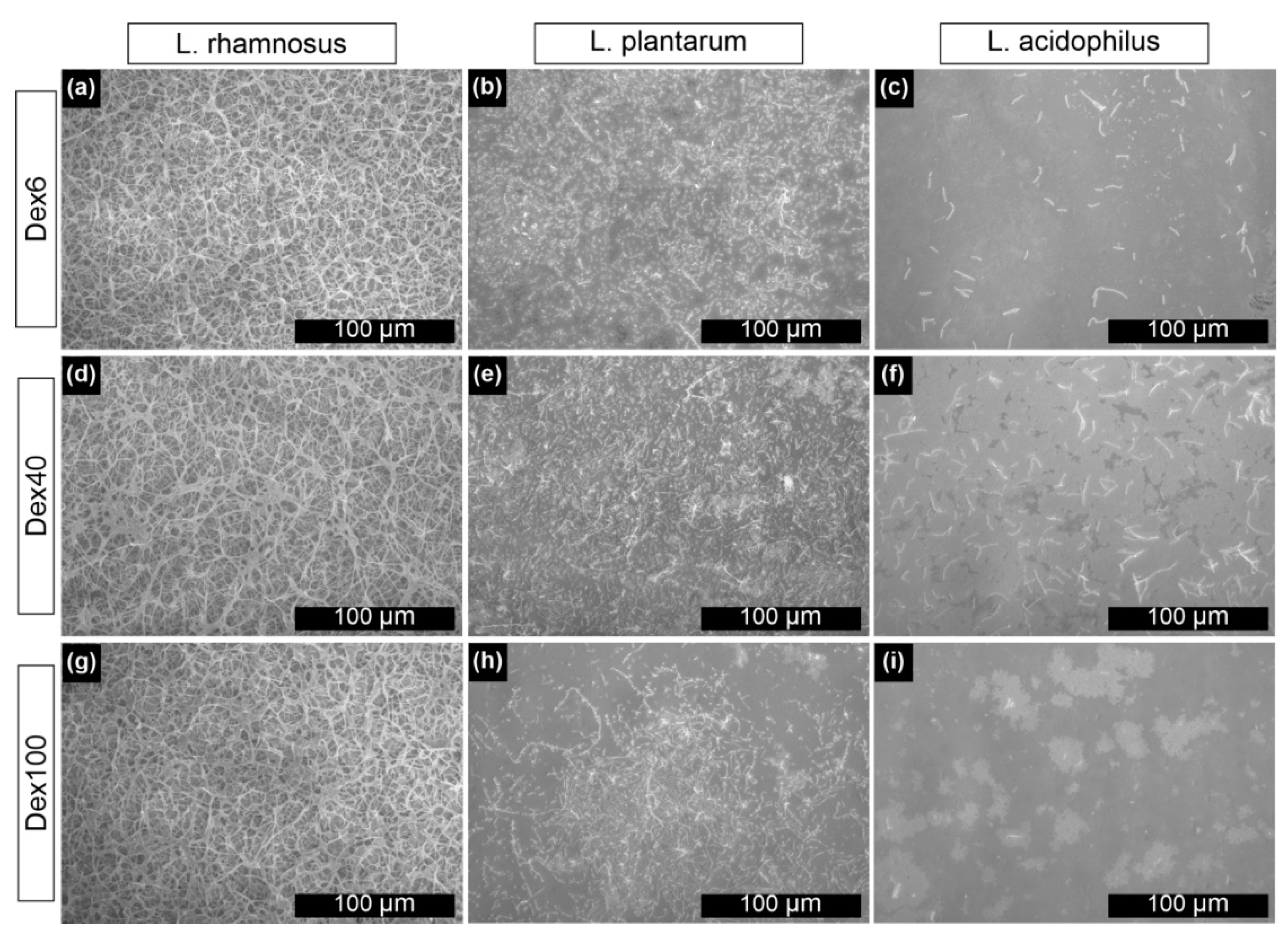
2.7. Characterization of Lactobacillus spp. Bacterial Network on Gold Surfaces
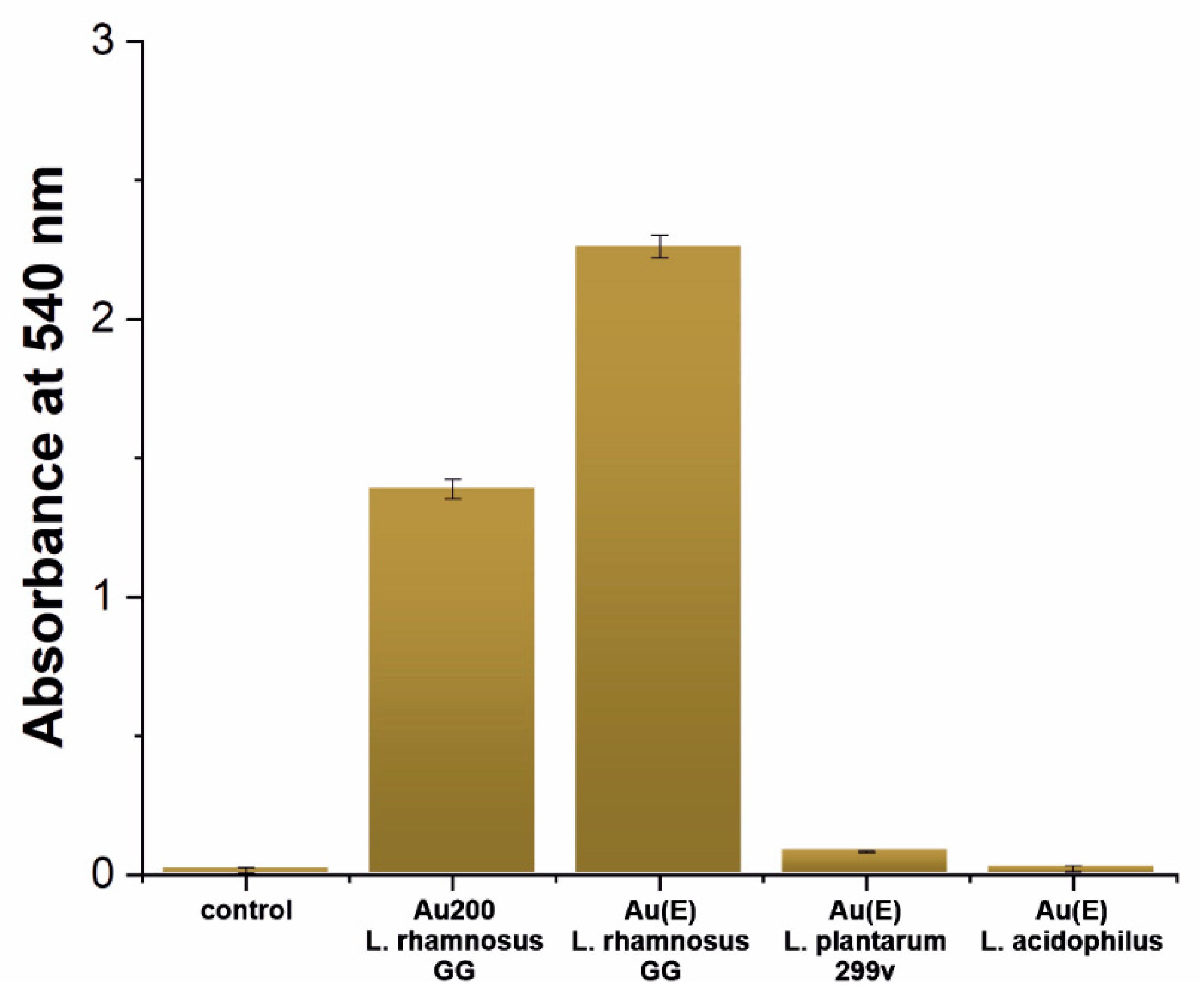
2.8. Determination of Produced Lactic Acid
3. Results
3.1. Impact of the Surface Roughness on Bacteria Adhesion
3.2. Dextran Derivatives as Additional Surface Modifying Agents
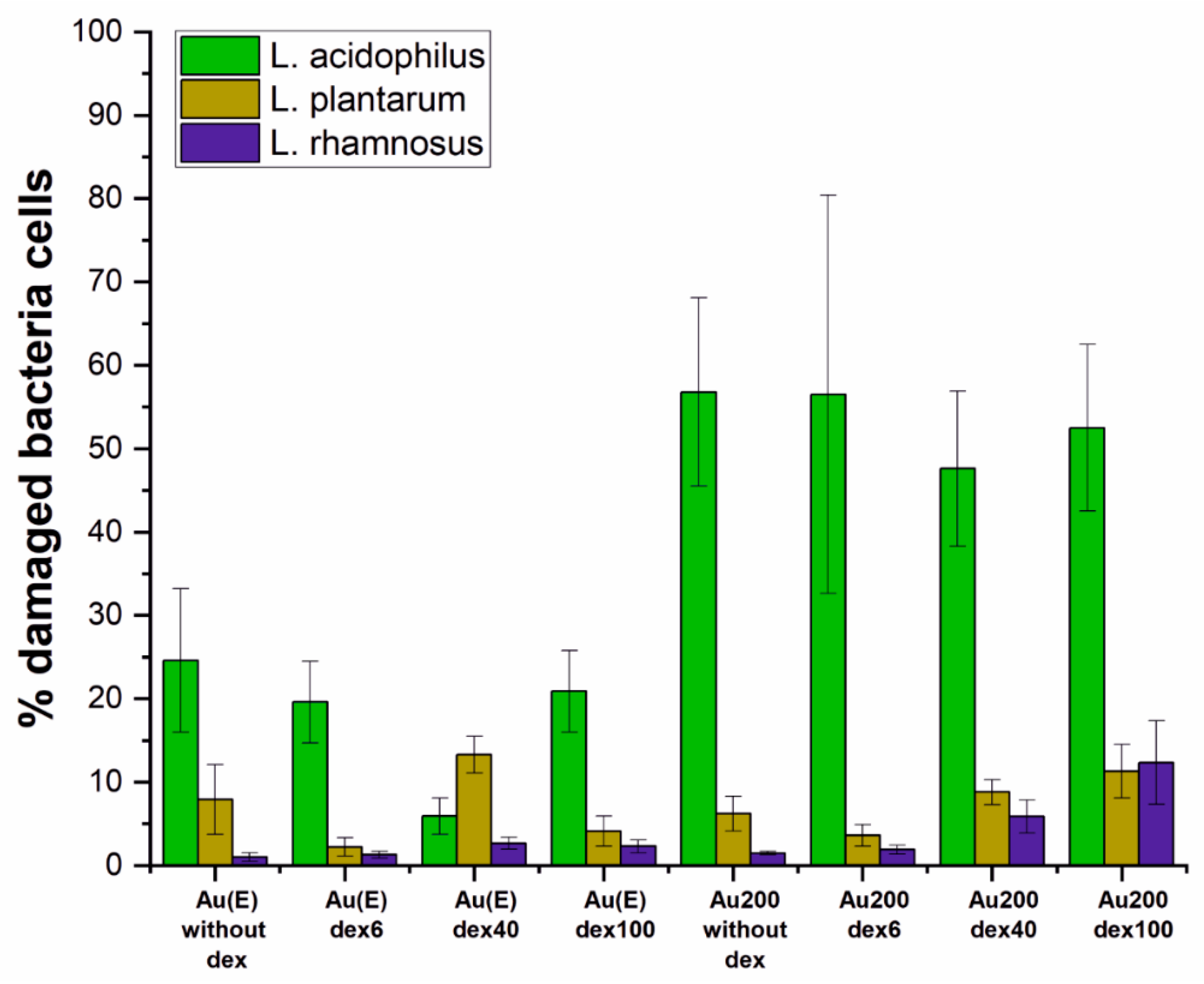
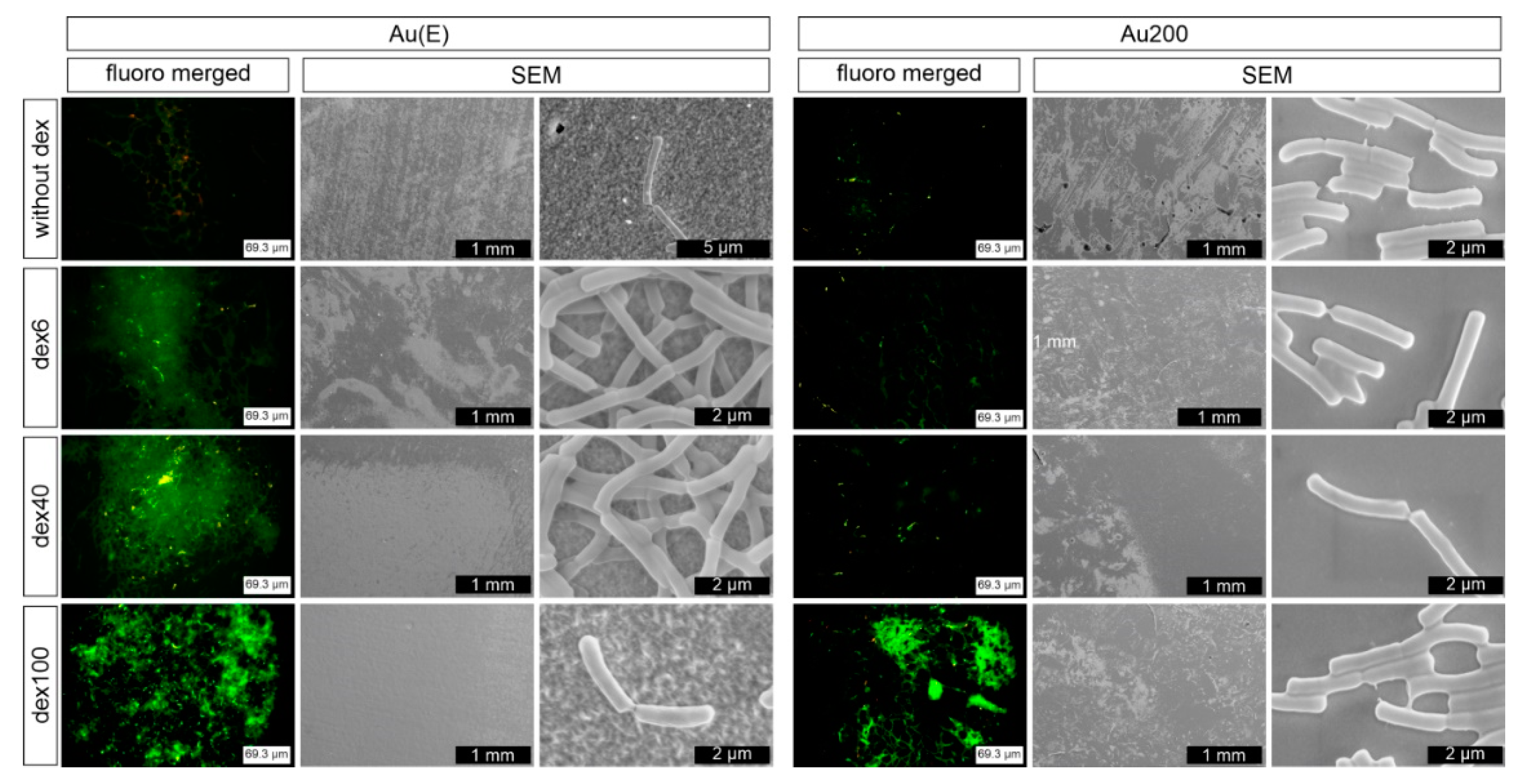
3.3. The Influence of the Surface on the Welfare of Microorganisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collart, D.; Kepner, B.; Mehrabi, S.; Robinson, L.; Mintz, E.A. Efficacy of oligodynamic metals in the control of bacteria growth in humidifier water tanks and mist droplets. J. Water Health 2006, 4, 149–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic antibacterial effects of metallic nanoparticle combinations. Sci. Rep. 2019, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Wang, Y.; Jiang, H.; Wang, X. Advances and challenges in metallic nanomaterial synthesis and antibacterial applications. J. Mater. Chem. B 2020, 8, 4764–4777. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Babauta, J.; Renslow, R.; Lewandowski, Z.; Beyenal, H. Electrochemically active biofilms: Facts and fiction. A review. Biofouling 2012, 28, 789–812. [Google Scholar] [CrossRef]
- Yu, P.; Wang, C.; Zhou, J.; Jiang, L.; Xue, J.; Li, W. Influence of surface properties on adhesion forces and attachment of Streptococcus mutans to zirconia in vitro. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Preedy, E.; Perni, S.; Nipiĉ, D.; Bohinc, K.; Prokopovich, P. Surface roughness mediated adhesion forces between borosilicate glass and Gram-positive bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PLoS ONE 2011, 6, e25029. [Google Scholar] [CrossRef]
- Wu, S.; Altenried, S.; Zogg, A.; Zuber, F.; Maniura-Weber, K.; Ren, Q. Role of the surface nanoscale roughness of stainless steel on bacterial adhesion and microcolony formation. ACS Omega 2018, 3, 6456–6464. [Google Scholar] [CrossRef]
- Richter, H.; McCarthy, K.; Nevin, K.P.; Johnson, J.P.; Rotello, V.M.; Lovley, D.R. Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir 2008, 24, 4376–4379. [Google Scholar] [CrossRef]
- Reith, F.; Etschmann, B.; Grosse, C.; Moors, H.; Benotmane, M.A.; Monsieurs, P.; Grass, G.; Doonan, C.; Vogt, S.; Lai, B.; et al. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl. Acad. Sci. USA 2009, 106, 17757–17762. [Google Scholar] [CrossRef]
- Jarosz, M.; Grudzień, J.; Kamiński, K.; Gawlak, K.; Wolski, K.; Nowakowska, M.; Sulka, G.D. Novel bioelectrodes based on polysaccharide modified gold surfaces and electrochemically active Lactobacillus rhamnosus GG biofilms. Electrochim. Acta 2019, 296, 999–1008. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Lactobacillus rhamnosus GG: An updated strategy to use microbial products to promote health. Funct. Food Rev. 2012, 4, 77–84. [Google Scholar]
- Deepika, G.; Karunakaran, E.; Hurley, C.R.; Biggs, C.A.; Charalampopoulos, D. Influence of fermentation conditions on the surface properties and adhesion of Lactobacillus rhamnosus GG. Microb. Cell Fact. 2012, 11, 1–12. [Google Scholar] [CrossRef]
- Sengupta, R.; Altermann, E.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Polak-Berecka, M.; Waśko, A.; Paduch, R.; Skrzypek, T.; Sroka-Bartnicka, A. The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus. Antonie Van Leeuwenhoek 2014, 106, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Berríos, P.; Fuentes, J.A.; Salas, D.; Carreño, A.; Aldea, P.; Fernández, F.; Trombert, A.N. Inhibitory effect of biofilm-forming Lactobacillus kunkeei strains against virulent Pseudomonas aeruginosa in vitro and in honeycomb moth (Galleria mellonella) infection model. Benef. Microbes 2018, 9, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ibarreche, M.; Castellano, P.; Leclercq, A.; Vignolo, G. Control of Listeria monocytogenes biofilms on industrial surfaces by the bacteriocin-producing Lactobacillus sakei CRL1862. FEMS Microbiol. Lett. 2016, 363, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; García, A. Biofilm forming Lactobacillus: New challenges for the development of probiotics. Microorganisms 2016, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Nojima, N.; Furukawa, S.; Ogihara, H.; Morinaga, Y. Steric microstructure of mixed-species biofilm formed by interaction between Lactobacillus plantarum ML11-11 and Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2019, 83, 2386–2389. [Google Scholar] [CrossRef]
- Al-Ani, A.; Boden, A.; Al Kobaisi, M.; Pingle, H.; Wang, P.Y.; Kingshott, P. The influence of PEG-thiol derivatives on controlling cellular and bacterial interactions with gold surfaces. Appl. Surf. Sci. 2018, 462, 980–990. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef]
- Parreira, P.; Magalhaes, A.; Gonaçalves, I.C.; Gomes, J.; Vidal, R.; Reis, C.A.; Leckband, D.E.; Martins, M.C.L. Effect of surface chemistry on bacterial adhesion, viability, and morphology. J. Biomed. Mater. Res. Part A 2011, 99, 344–353. [Google Scholar] [CrossRef]
- Kamiński, K.; Kaczor-Kamińska, M.; Irska, I.; Popioek, I.; Szczubiaka, K.; Nowakowska, M. New long-term action insulin formulations obtained using polycations for heparin neutralization. Bio-Algorithms Med-Syst. 2019, 15, 1–12. [Google Scholar] [CrossRef]
- Kamiński, K.; Stalińska, K.; Niziołek, A.; Wróbel, M.; Nowakowska, M.; Kaczor-Kamińska, M. Cell proliferation induced by modified cationic dextran. Bio-Algorithms Med-Syst. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Kamiński, K.; Płonka, M.; Ciejka, J.; Szczubiałka, K.; Nowakowska, M.; Lorkowska, B.; Korbut, R.; Lach, R. Cationic derivatives of dextran and hydroxypropylcellulose as novel potential heparin antagonists. J. Med. Chem. 2011, 54, 6586–6596. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar] [CrossRef]
- Chissoe, W.F.; Vezey, E.L.; Skvarla, J.J. Hexamethyldisilazane as a drying agent for pollen scanning electron microscopy. Biotech. Histochem. 1994, 69, 192–198. [Google Scholar] [CrossRef]
- Kamiński, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef]
- Nguyen, D.H.K.; Pham, V.T.H.; Truong, V.K.; Sbarski, I.; Wang, J.; Balčytis, A.; Juodkazis, S.; Mainwaring, D.E.; Crawford, R.J.; Ivanova, E.P. Role of topological scale in the differential fouling of: Pseudomonas aeruginosa and Staphylococcus aureus bacterial cells on wrinkled gold-coated polystyrene surfaces. Nanoscale 2018, 10, 5089–5096. [Google Scholar] [CrossRef]
- Tripathi, P.; Beaussart, A.; Alsteens, D.; Dupres, V.; Claes, I.; Von Ossowski, I.; De Vos, W.M.; Palva, A.; Lebeer, S.; Vanderleyden, J.; et al. Adhesion and nanomechanics of pili from the probiotic lactobacillus rhamnosus GG. ACS Nano 2013, 7, 3685–3697. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. In Microbial Cell Factories; BioMed Central: London, UK, 2014; Volume 13, pp. 1–16. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]


| Solution | Zeta Potential/mV | ||
|---|---|---|---|
| Dex6 | Dex40 | Dex100 | |
| Water solution | 24.00 ± 6.35 | 31.70 ± 1.45 | 27.40 ± 3.25 |
| PBS solution | −4.85 ± 0.84 | −4.93 ± 0.33 | −6.99 ± 0.45 |
| Sample Name | L. rhamnosus | L. plantarum | L. acidophilus |
|---|---|---|---|
| Concentration nmol·µL−1 ± 5.7 nmol·µL−1 | Concentration nmol·µL−1 ± 4.5 nmol·µL−1 | Concentration nmol·µL−1 ± 0.15 nmol·µL−1 | |
| Au(E) | 12.6 | 8.9 | 0.35 |
| Au(E) dex6 | 10.4 | 10.2 | * |
| Au(E) dex40 | 14.2 | 9.5 | * |
| Au(E) dex100 | 24.2 | 12.2 | 0.42 |
| Au200 | 36.5 | 9.3 | 0.21 |
| Au200 dex6 | 24.6 | 12.1 | * |
| Au200 dex40 | 42.1 | 5.4 | * |
| Au200 dex100 | 20.4 | 14.4 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudzień, J.; Jarosz, M.; Kamiński, K.; Kobasa, M.; Wolski, K.; Kozieł, M.; Pisarek, M.; Sulka, G.D. Growth of Lactic Acid Bacteria on Gold—Influence of Surface Roughness and Chemical Composition. Nanomaterials 2020, 10, 2499. https://doi.org/10.3390/nano10122499
Grudzień J, Jarosz M, Kamiński K, Kobasa M, Wolski K, Kozieł M, Pisarek M, Sulka GD. Growth of Lactic Acid Bacteria on Gold—Influence of Surface Roughness and Chemical Composition. Nanomaterials. 2020; 10(12):2499. https://doi.org/10.3390/nano10122499
Chicago/Turabian StyleGrudzień, Joanna, Magdalena Jarosz, Kamil Kamiński, Mirosława Kobasa, Karol Wolski, Marcin Kozieł, Marcin Pisarek, and Grzegorz D. Sulka. 2020. "Growth of Lactic Acid Bacteria on Gold—Influence of Surface Roughness and Chemical Composition" Nanomaterials 10, no. 12: 2499. https://doi.org/10.3390/nano10122499
APA StyleGrudzień, J., Jarosz, M., Kamiński, K., Kobasa, M., Wolski, K., Kozieł, M., Pisarek, M., & Sulka, G. D. (2020). Growth of Lactic Acid Bacteria on Gold—Influence of Surface Roughness and Chemical Composition. Nanomaterials, 10(12), 2499. https://doi.org/10.3390/nano10122499







