Influence of Dextran Molecular Weight on the Physical Properties of Magnetic Nanoparticles for Hyperthermia and MRI Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of DEX-Coated MNPs
2.3. Determination of Magnetite Concentration
2.4. Characterisation of DEX-MFs
2.5. MRI
- T1 mapping—rapid acquisition with refocused echoes (RARE) pulse sequence, with repetition time TR = 5500, 3000, 1500, 800, 400 or 200 ms, and echo time TE = 7 ms.
- T2 mapping—multi-slice multi-echo (MSME) pulse sequence, with repetition time TR = 2000 ms, starting echo time TE = 8 ms, spacing = 8 ms and 25 images.
- DEX40-MF: DEX-coated MNPs with a MW of DEX equal to 40 kDa and DHYDR = 72.03 nm.
- DEX70-MF: DEX-coated MNPs with a MW of DEX equal to 70 kDa and DHYDR = 88.37 nm.
- DEX150-MF: DEX-coated MNPs with a MW of DEX equal to 150 kDa and DHYDR = 122.2 nm.
3. Results and Discussion
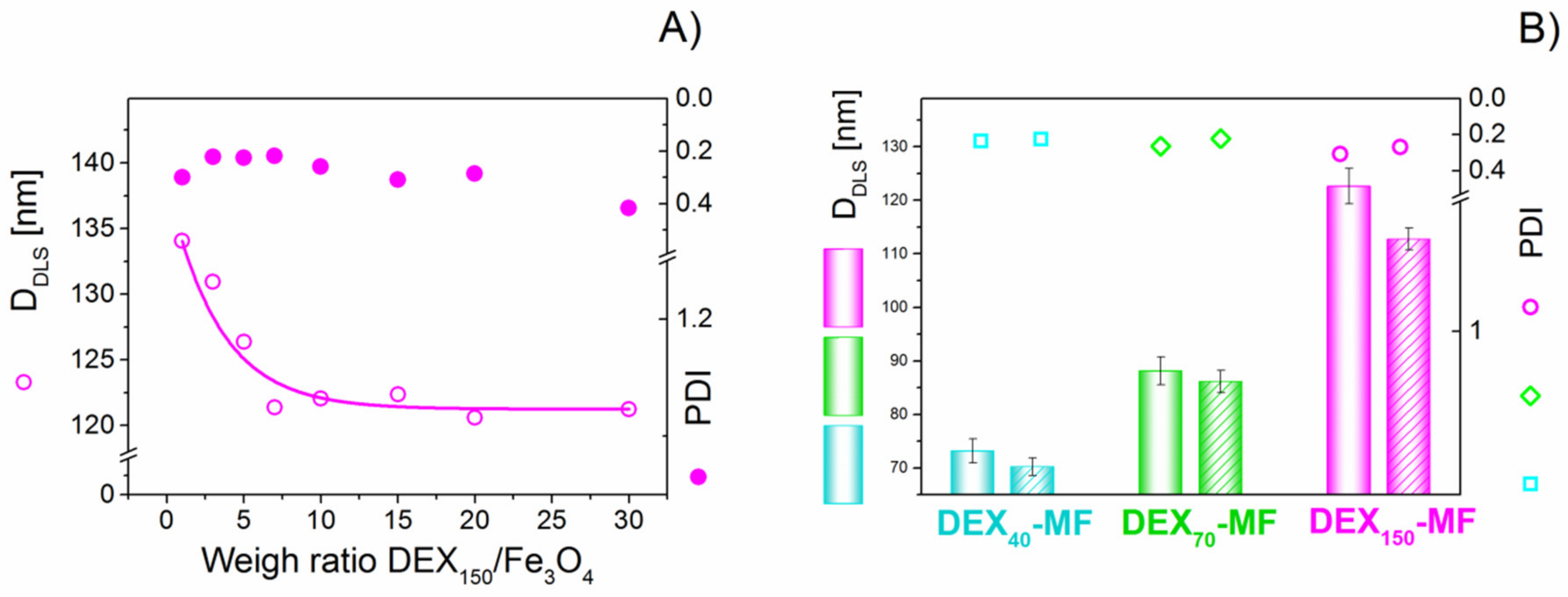
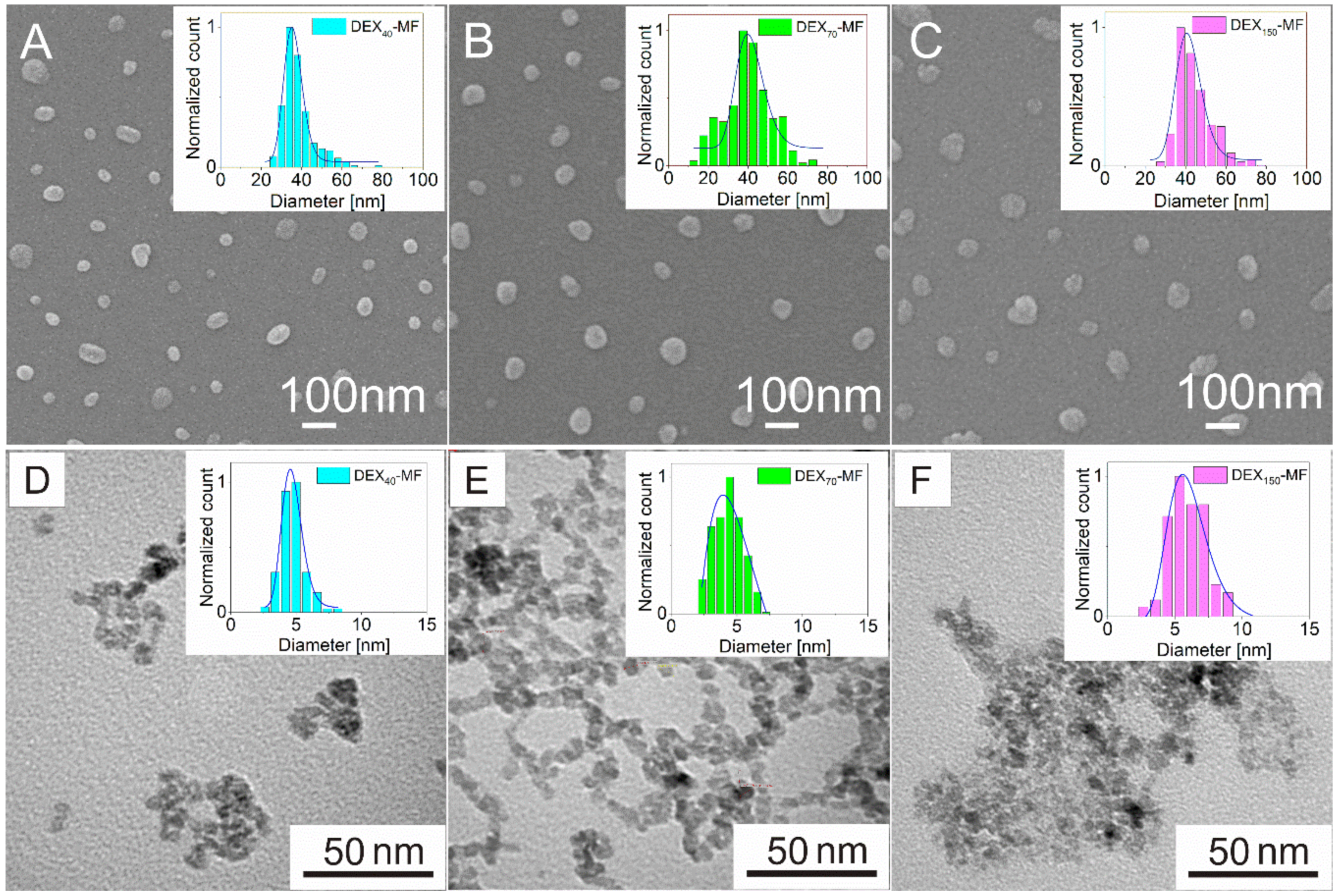
3.1. Physical Characterisation of DEX-Coated MNPs
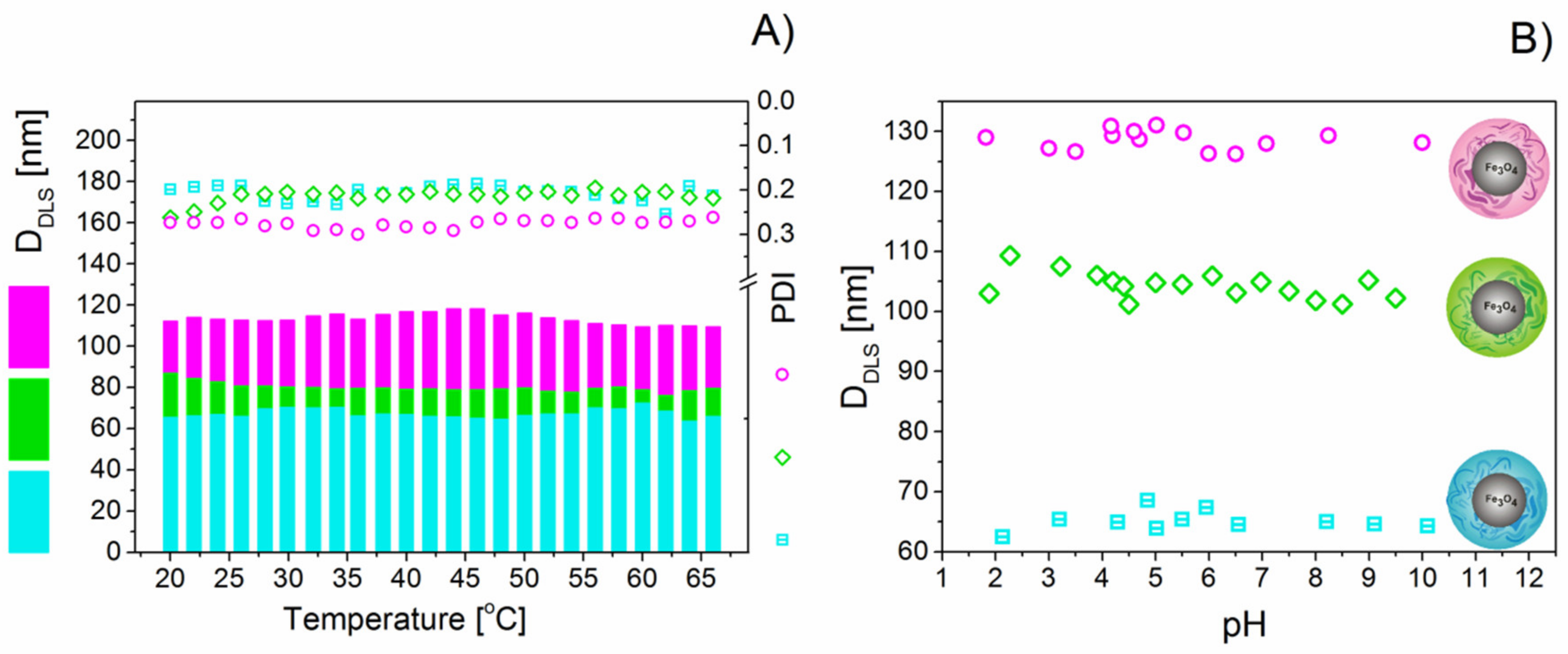
3.2. Stability of DEX-Coated MNPs
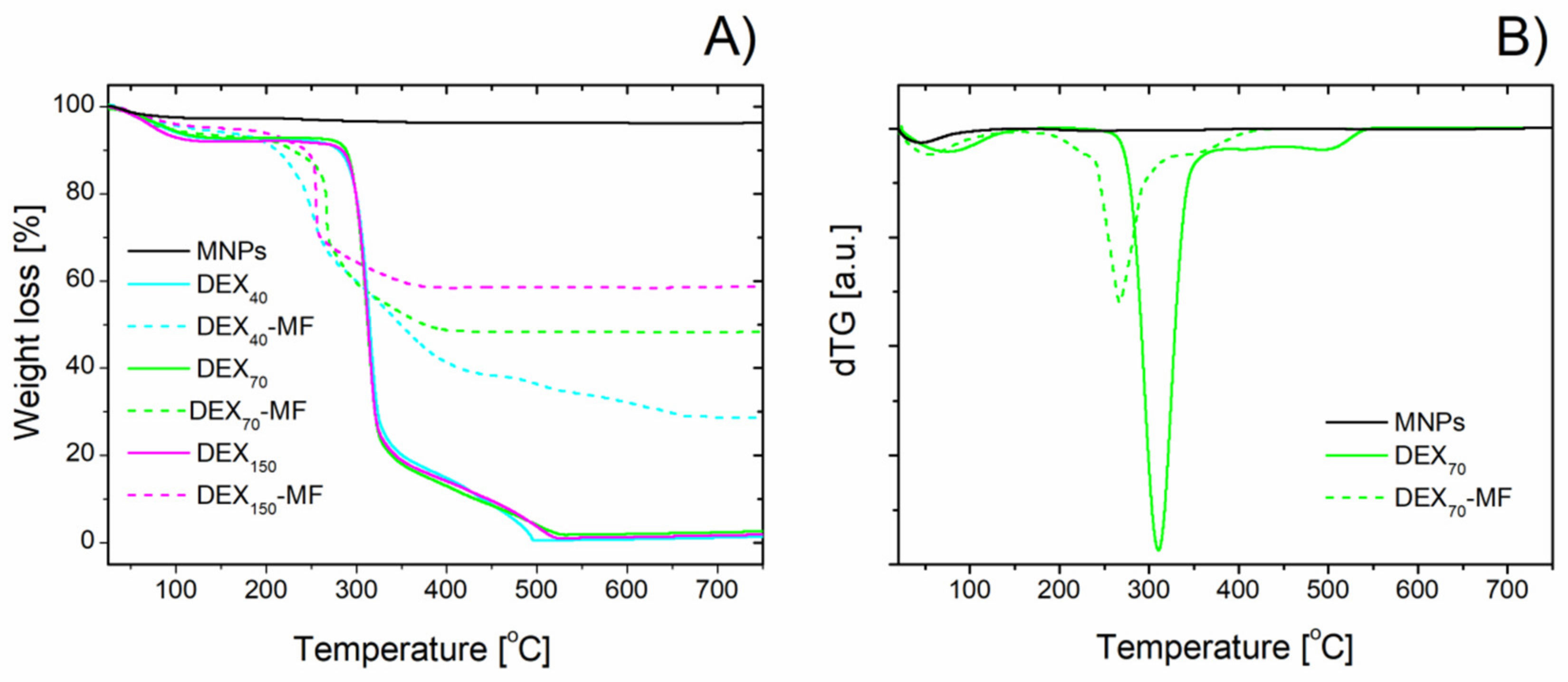
3.3. Thermogravimetric Analysis of DEX-Coated MNPs
3.4. Magnetic Properties of DEX-Coated MNPs
3.5. Magnetic Hyperthermia of DEX-Coated MNPs
3.6. MRI Relaxivity Determination of DEX-Coated MNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Varanda, L.C.; Júnior, M.J.; Júnior, W.B. Magnetic and Multifunctional Magnetic Nanoparticles in Nanomedicine: Challenges and Trends in Synthesis and Surface Engineering for Diagnostic and Therapy Applications. In Biomedical Engineering; Laskovski, A.N., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Kashkooli, F.M.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalisation of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilisation of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Shizu, C.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostic 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 14, 452001. [Google Scholar] [CrossRef] [PubMed]
- Molcan, M.; Gojzewski, H.; Skumiel, A.; Dutz, S.; Kovac, J.; Kubovcikova, M.; Kopcansky, P.; Vekas, L.; Timko, M. Energy losses in mechanically modified bacterial magnetosomes. J. Phys. D Appl. Phys. 2016, 49, 365002. [Google Scholar] [CrossRef]
- Ortega, D.; Pankhurst, Q.A. Magnetic Hyperthermia, in Nanoscience: Volume 1: Nanostructures through Chemistry; O’Brien, P., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 60–88. [Google Scholar]
- Frtus, A.; Smolkova, B.; Uzhytchak, M.; Lunova, M.; Jirsa, M.; Kubinova, S.; Dejneka, A.; Lunov, O. Analysing the mechanisms of iron oxide nanoparticles interactions with cells: A road from failure to success in clinical applications. J. Control. Release 2020, 328, 59–77. [Google Scholar] [CrossRef]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Inter. J. Hyperth. 2019, 36, 686–700. [Google Scholar] [CrossRef]
- Babic, M.; Horak, D.; Molcan, M.; Timko, M. Heat generation of surface-modified magnetic γ-Fe2O3 nanoparticles in applied alternating magnetic field. J. Phys. D Appl. Phys. 2017, 50, 345002. [Google Scholar] [CrossRef]
- Osaci, M.; Cacciola, M. About the influence of the colloidal magnetic nanoparticles coating on the specific loss power in magnetic hyperthermia. J. Magn. Magn. Mater. 2020, 519, 167451. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. Correlation between effects of the particle size and magnetic field strength on the magnetic hyperthermia efficiency of dextran-coated magnetite nanoparticles. Mater. Sci. Eng. C 2020, 117, 111274. [Google Scholar] [CrossRef] [PubMed]
- Antal, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Khmara, I.; Lucanska, D.; Jelenska, L.; Vidlickova, I.; Zatovicova, M.; Pastorekova, S.; et al. D,L-Lysine functionalised Fe3O4 nanoparticles for detection of cancer cells. Colloids Surf. B Biointerfaces 2018, 1, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Hornowski, T.; Antal, I.; Timko, M.; Józefczak, A. Magneto-ultrasonic heating with nanoparticles. J. Magn. Magn. Mater. 2019, 474, 400–405. [Google Scholar] [CrossRef]
- Bica, D.; Vekas, L.; Avdeev, M.V.; Marinica, O.; Socoliuc, V.; Balasoiu, M.; Garamus, V.M. Sterically stabilised water based magnetic fluids: Synthesis, structure and properties. J. Magn. Magn. Mater. 2007, 311, 17–21. [Google Scholar] [CrossRef]
- Araújo-Neto, R.P.; Silva-Freitas, E.L.; Carvalho, J.F.; Pontes, T.R.F.; Silva, K.L.; Damasceno, I.H.M.; Egito, E.S.T.; Dantas, A.L.; Morales, M.A.; Carriço, A.S. Monodisperse sodium oleate coated magnetite high susceptibility nanoparticles for hyperthermia applications. J. Magn. Magn. Mater. 2014, 364, 72–79. [Google Scholar] [CrossRef]
- Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Antal, I.; Csach, K.; Kopcansky, P.; Vidlickova, I.; Csaderova, L.; Pastorekova, S.; et al. Preparation of poly-l-lysine functionalised magnetic nanoparticles and their influence on viability of cancer cells. J. Magn. Magn. Mater. 2017, 427, 114–121. [Google Scholar] [CrossRef]
- Kubovcikova, M.; Koneracká, M.; Strbak, O.; Molcan, M.; Zavisova, V.; Antal, I.; Khmara, I.; Lucanska, D.; Tomco, L.; Barathova, M.; et al. Poly-L-lysine designed magnetic nanoparticles for combined hyperthermia, magnetic resonance imaging and cancer cell detection. J. Magn. Magn. Mater. 2019, 475, 316–326. [Google Scholar] [CrossRef]
- Babic, M.; Horak, D.; Trchova, M.; Jendelova, P.; Glogarova, K.; Lesna, P.; Herynek, V.; Hajek, M.; Sykova, E. Poly(l-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjugate. Chem. 2008, 19, 740–750. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Kovac, J.; Kubovcikova, M.; Antal, I.; Kopcansky, P.; Bednarikova, M.; Muckova, M. The cytotoxicity of iron oxide nanoparticles with different modifications evaluated in vitro. J. Magn. Magn. Mater. 2015, 380, 85–89. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Gabelova, A.; Svitkova, B.; Ursinyova, M.; Kubovcikova, M.; Antal, I.; Khmara, I.; Jurikova, A.; Molcan, M.; et al. Effect of magnetic nanoparticles coating on cell proliferation and uptake. J. Magn. Magn. Mater. 2016, 380, 85–89. [Google Scholar] [CrossRef]
- Kubovcikova, M.; Antal, I.; Koneracka, M.; Zavisova, V.; Jurikova, A.; Siposova, K.; Gazova, Z.; Kovac, J.; Kovarik, M.; Kupka, D.; et al. Magnetic nanoparticles modified with polyethylene glycol. Magnetohydrodynamics 2013, 49, 282–286. [Google Scholar] [CrossRef]
- Antal, I.; Kubovcikova, M.; Zavisova, V.; Koneracka, M.; Pechanova, O.; Barta, A.; Cebova, M.; Antal, V.; Diko, P.; Zduriencikova, M.; et al. Magnetic poly(d,l-lactide) nanoparticles loaded with aliskiren: A promising tool for hypertension treatment. J. Magn. Magn. Mater. 2015, 380, 280–284. [Google Scholar] [CrossRef]
- Strbak, O.; Antal, I.; Gogola, D.; Baciak, L.; Kubovcikova, M.; Koneracka, M.; Zavisova, V.; Krafcik, A.; Masarova-Kozelova, M.; Kopcansky, P.; et al. Measurement of the magnetite nanoparticles’ relaxivity during encapsulation into polylactide carriers. Measurement 2017, 104, 89–92. [Google Scholar] [CrossRef]
- Rahayu, L.B.H.; Wulandari, I.O.; Santjojo, D.H.; Sabarudin, A. Synthesis and characterization of Fe3O4 nanoparticles using polyvinyl alcohol (PVA) as capping agent and glutaraldehyde (GA) as crosslinker. Mater. Sci. Eng. 2018, 299, 012062. [Google Scholar] [CrossRef]
- Khmara, I.; Strbak, O.; Zavisova, V.; Koneracka, M.; Kubovcikova, M.; Antal, I.; Kavecansky, V.; Lucanska, D.; Dobrota, D.; Kopcansky, P. Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging. J. Magn. Magn. Mater. 2019, 474, 319–325. [Google Scholar] [CrossRef]
- Li, G.Y.; Jiang, Y.R.; Huang, K.L.; Ding, P.; Chen, J. Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. J. Alloys Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Siposova, K.; Pospiskova, K.; Bednarikova, Z.; Safarik, I.; Safarikova, M.; Kubovcikova, M.; Kopcansky, P.; Gazova, Z. The molecular mass of dextran used to modify magnetite nanoparticles affects insulin amyloid aggregation. J. Magn. Magn. Mater. 2017, 427, 48–53. [Google Scholar] [CrossRef]
- Majeed, J.; Pradhan, L.; Ningthoujam, R.S.; Vatsa, R.K.; Bahadur, D.; Tyagi, A.K. Enhanced specific absorption rate in silanol functionalised Fe3O4 core–shell nanoparticles: Study of Fe leaching in Fe3O4 and hyperthermia in L929 and HeLa cells. Colloids Surf. B Biointerfaces 2014, 122, 396–403. [Google Scholar] [CrossRef]
- Jozefczak, A.; Hornowski, T.; Skumiel, A.; Zavisova, V.; Koneracka, M.; Tomasovicova, N.; Timko, M.; Kopcansky, P.; Kelani, H.N. Effect of the molecular weight of poly(ethylene glycol) on the properties of biocompatible magnetic fluids. Int. J. Thermophys. 2012, 33, 640–652. [Google Scholar] [CrossRef]
- Maia, J.; Evangelista, M.B.; Gil, H.; Ferreira, L. Dextran-based materials for biomedical applications. Res. Signpost 2014, 37661, 31–53. [Google Scholar]
- Linh, P.H.; Phuc, N.X.; Hong, L.V.; Uyen, L.L.; Chien, N.V.; Nam, P.H.; Quy, N.T.; Nhung, H.T.M.; Phong, P.T.; Lee, I.J. Dextran coated magnetite high susceptibility nanoparticles for hyperthermia applications. J. Magn. Magn. Mater. 2018, 460, 128–136. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef]
- Portet, D.; Denizot, B.; Rump, E.; Lejeune, J.J.; Jallet, P. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. J. Colloid. Interface Sci. 2001, 238, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.Y.; Feng, B.; Chen, L.L.; Liu, G.H.; Li, Z.H.; Zheng, Y.; Wei, D.G. Synthesis, characterisation and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Engin. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Molday, R.S.; Mackenzie, D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J. Immunol. Methods 1982, 52, 353–367. [Google Scholar] [CrossRef]
- Woods, J.; Mellon, M. Thiocyanate Method for Iron: A Spectrophotometric Study. Determination of iron by thiocyanate colorimetry. Ind. Eng. Chem. Anal. Ed. 1941, 13, 551–554. [Google Scholar] [CrossRef]
- Skumiel, A.; Kaczmarek, K.; Flak, D.; Rajnak, M.; Antal, I.; Brzakala, H. The influence of magnetic nanoparticle concentration with dextran polymers in agar gel on heating efficiency in magnetic hyperthermia. J. Mol. Liq. 2020, 304, 112734. [Google Scholar] [CrossRef]
- Larsen, E.K.U.; Nielsen, T.; Wittenborn, T.; Rydtoft, L.M.; Lokanathan, A.R.; Hansen, L.; Ostergaard, L.; Kingshott, P.; Howard, K.A.; Besenbacher, F.; et al. Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale 2012, 4, 2352–2361. [Google Scholar] [CrossRef]
- LaConte, L.E.W.; Nitin, N.; Zurkiya, O.; Caruntu, D.; O’Connor, C.J.; Hu, X.; Bao, G. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J. Magn. Reason. Imaging 2007, 26, 1634–1641. [Google Scholar] [CrossRef]
- Xue, W.; Liu, Y.; Zhang, N.; Yao, Y.; Ma, P.; Wen, H.; Huang, S.; Luo, Y.; Fan, H.M. Effects of core size and PEG coating layer of iron oxide nanoparticles on the distribution and metabolism in mice. Int. J. Nanomed. 2018, 13, 5719–5731. [Google Scholar] [CrossRef] [PubMed]
- Shaterbadi, Z.; Nabiyouni, G.; Soleymani, M. High impact of in situ dextran coating on biocompatibility, stability and magnetic properties of iron oxide nanoparticles. Mater. Sci. Eng. C 2017, 75, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Liedl, T.; Duhr, S.; Kudera, S.; Zanella, M.; Lin, C.A.J.; Chang, W.H.; Braun, D.; Parak, W.J. Size determination of (bio)conjugated water-soluble colloidal nanoparticles: A comparison of different techniques. J. Phys. Chem. C 2007, 111, 11552–11559. [Google Scholar] [CrossRef]
- Carp, O.; Patron, L.; Culita, D.C.; Budrugeac, P.; Feder, M.; Diamandescu, L. Thermal analysis of two types of dextran-coated magnetite. J. Therm. Anal. Calorim. 2010, 101, 181–187. [Google Scholar] [CrossRef]
- Koneracka, M.; Antosova, A.; Zavisova, V.; Lancz, G.; Gazova, Z.; Siposova, K.; Jurikova, A.; Csach, K.; Kovac, J.; Tomasovicova, N.; et al. Characterization of Fe3O4 magnetic nanoparticles modified with dextran and investigation of their interaction with protein amyloid aggregates. Acta Phys. Polonica A 2010, 118, 983–985. [Google Scholar] [CrossRef]
- Butterworth, M.D.; Illum, L.; Davis, S.S. Preparation of ultrafine silica- and PEG-coated magnetite particles. Colloids Surf. A Physicochem. Eng. Asp. 2001, 179, 93–102. [Google Scholar] [CrossRef]
- Jurikova, A.; Csach, K.; Miskuf, J.; Koneracka, M.; Zavisova, V.; Kubovcikova, M.; Kopcansky, P.; Muckova, M. Thermal properties of magnetic nanoparticles modified with polyethylene glycol. IEEE Trans. Magn. 2013, 49, 236–239. [Google Scholar] [CrossRef]
- Yoona, M.; Kima, Y.M.; Kima, Y.; Volkova, V.; Songa, H.J.; Parkb, Y.J.; Vasilyakc, S.L.; Parka, I.-W. Magnetic properties of iron nanoparticles in a polymer film. J. Magn. Magn. Mater. 2003, 265, 357–362. [Google Scholar] [CrossRef]
- Khmara, I.; Molcan, M.; Antosova, A.; Bednarikova, Z.; Zavisova, V.; Kubovcikova, M.; Jurikova, A.; Girman, V.; Baranovicova, E.; Koneracka, M.; et al. Bioactive properties of chitosan stabilised magnetic nanoparticles—Focus on hyperthermic and anti-amyloid activities. J. Magn. Magn. Mater. 2020, 513, 167056. [Google Scholar] [CrossRef]
- Skumiel, A.; Leszczyński, B.; Molcan, M.; Timko, M. The comparison of magnetic circuits used in magnetic hyperthermia. J. Magn. Magn. Mater. 2016, 420, 177–184. [Google Scholar] [CrossRef]
- Kostevsek, N. A review on the optimal design of magnetic nanoparticle-based T2 MRI contrast agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hemanth Kumar, B.S.; Khushu, S.; Tripathi, R.P.; Gangenahalli, G. Increased transverse relaxivity in ultrasmall superparamagnetic iron oxide nanoparticles used as MRI contrast agent for biomedical imaging. Contrast Media Mol. Imaging 2016, 11, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Laurent, S.; Jo, Y.S.; Roch, A.; Mikhaylova, M.; Bhujwalla, Z.M.; Muller, R.N.; Muhammed, M. A high-performance magnetic resonance imaging T2 contrast agent. Adv. Mater. 2007, 19, 1874–1878. [Google Scholar] [CrossRef]
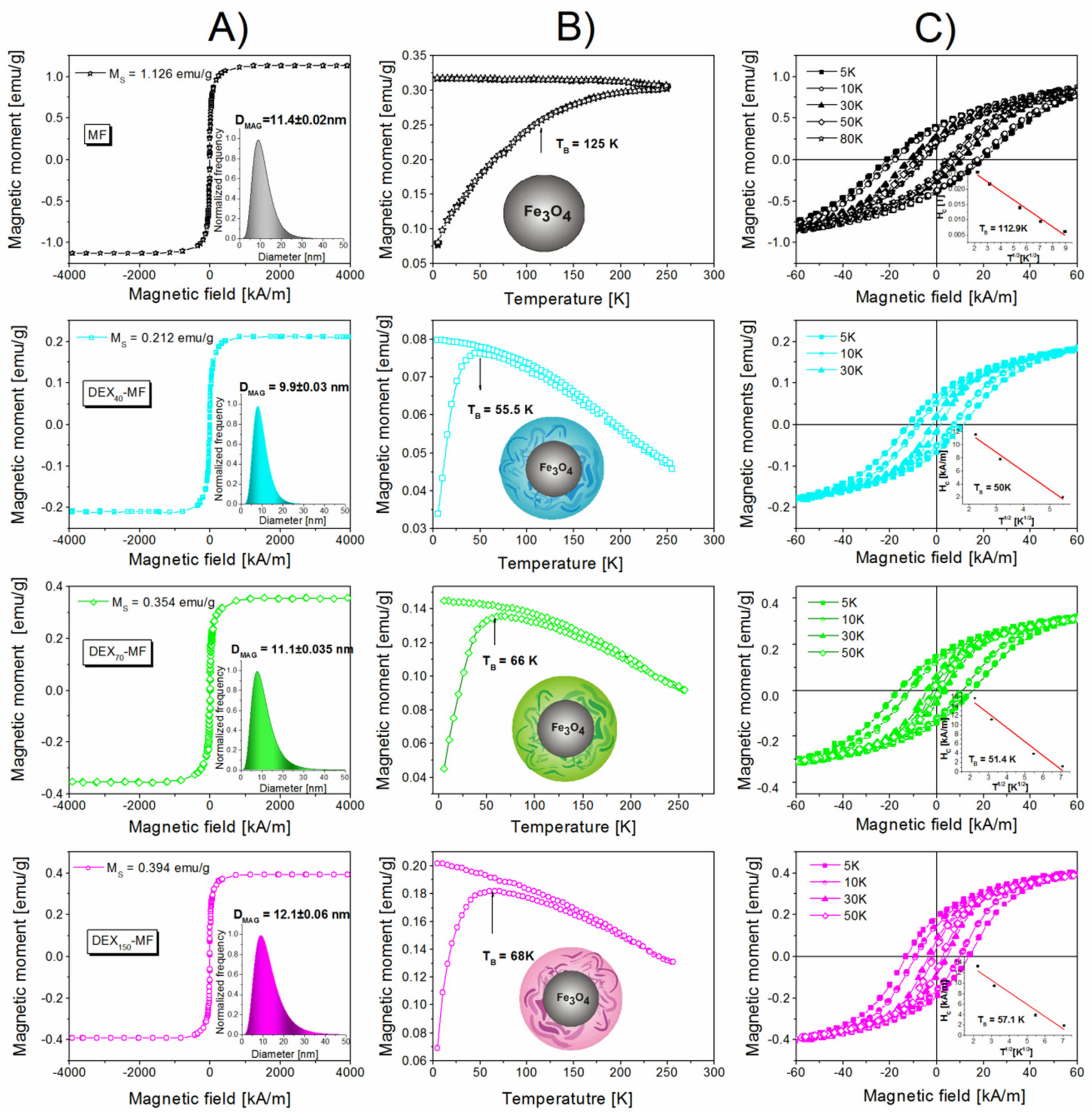
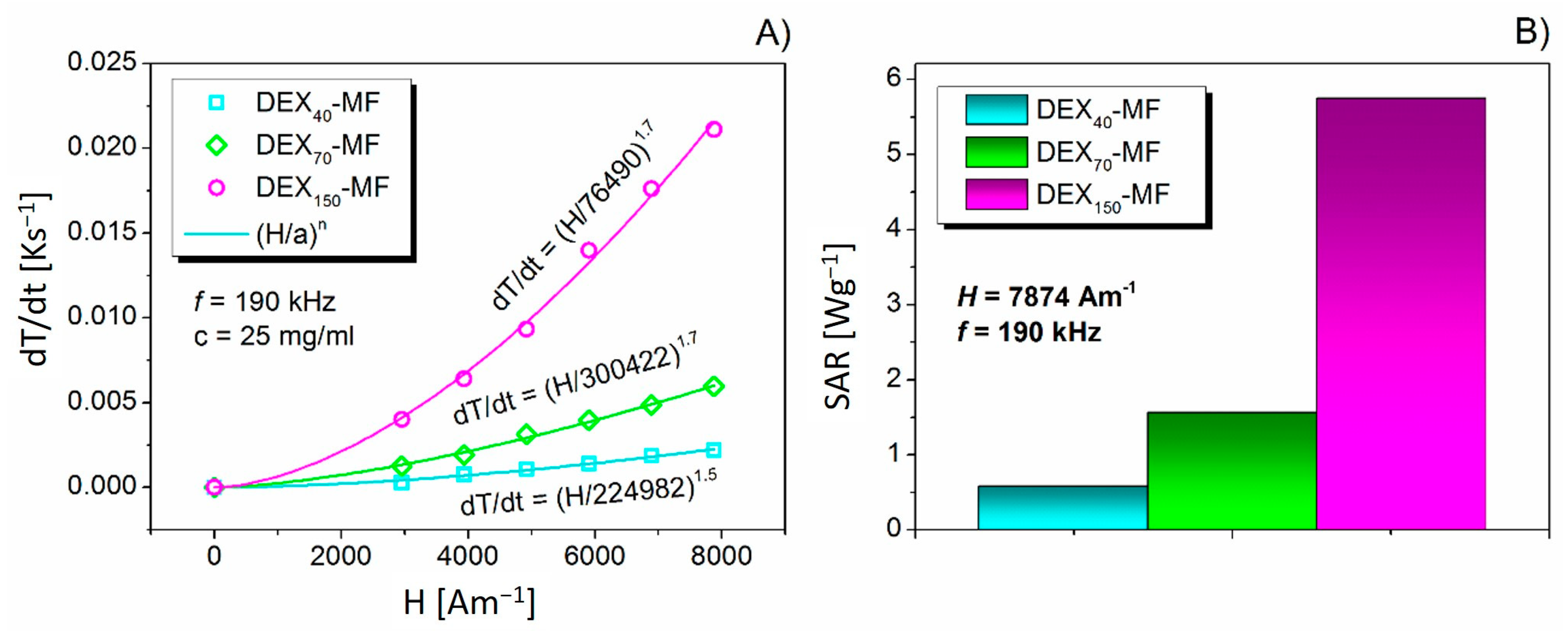
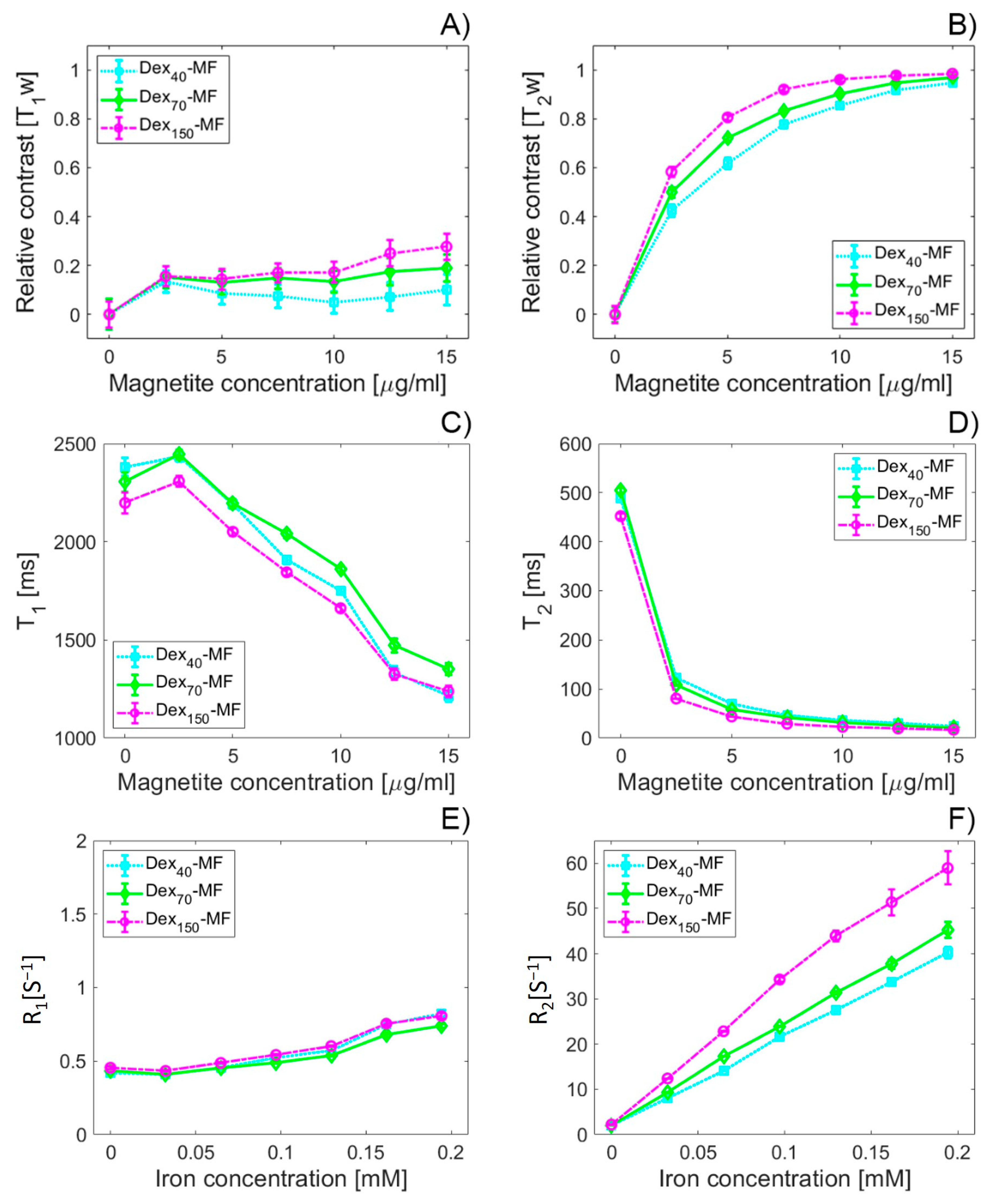
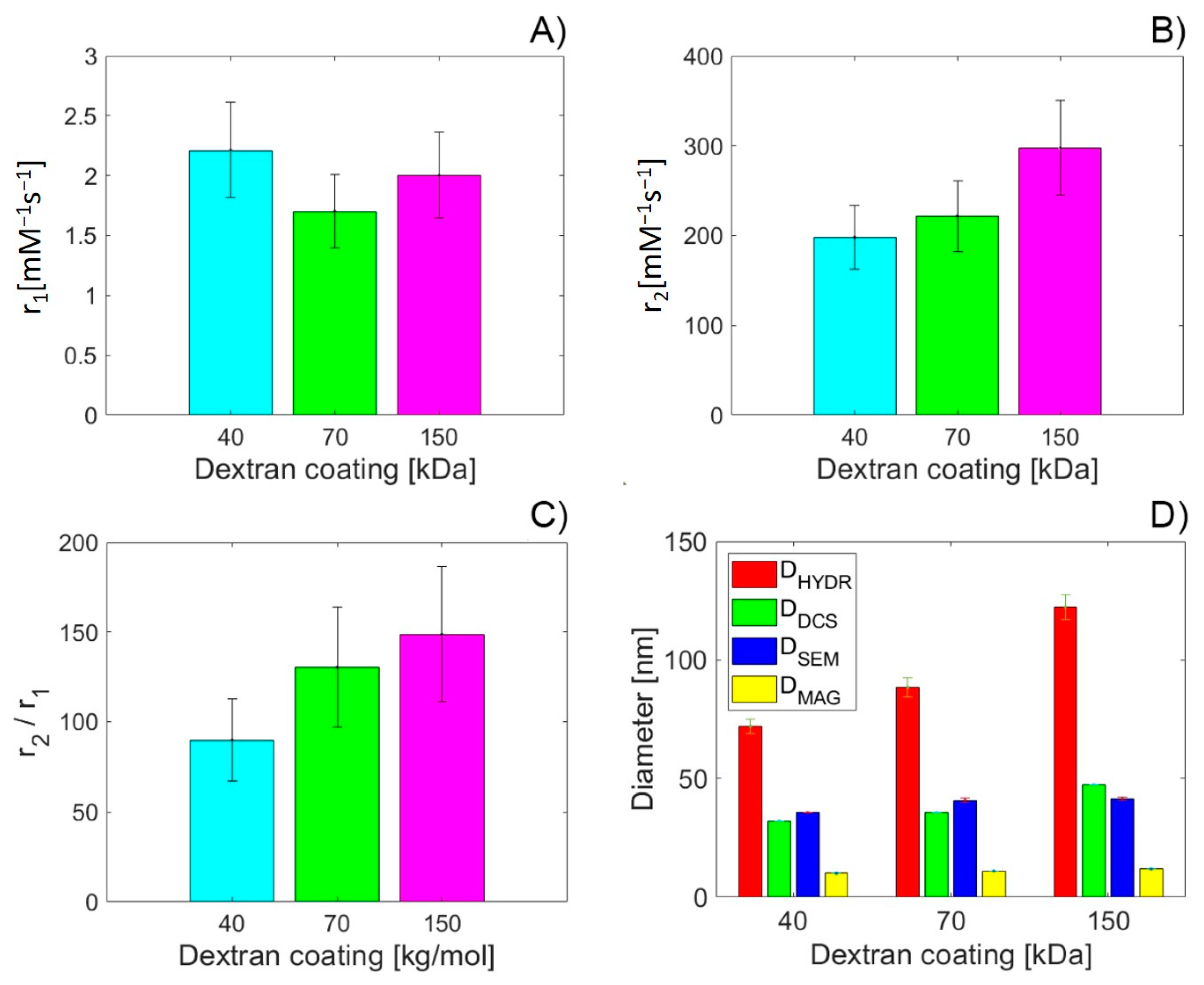
| Sample | Adsorbed DEXx [mg DEX/ mg Fe3O4] | ζ [mV] | DDCS [nm] | DSEM [nm] | MS [emu/g] | DMAG [nm] | TB * [K] | TB ** [K] | IEP | SAR [W/g] |
|---|---|---|---|---|---|---|---|---|---|---|
| DEX40-MF | 2.37 | −0.7 ± 0.17 | 32.3 | 35.8 ± 7.8 | 0.212 | 10.1 ± 0.1 | 56 | 49 | 5.2 | 0.6 |
| DEX70-MF | 0.99 | −1 ± 0.133 | 35.8 | 40.9 ± 11.7 | 0.354 | 11.1 ± 0.1 | 66 | 51 | 5.0 | 1.6 |
| DEX150-MF | 0.64 | −1.3 ± 0.4 | 47.4 | 41.5 ± 8.6 | 0.394 | 12.0 ± 0.2 | 68 | 57 | 5.5 | 5.9 |
| Sample | b | a | SSE | R2 [%] | adj-R2 [%] | RMSE |
|---|---|---|---|---|---|---|
| r1 | ||||||
| DHYDR | −0.002 | 2.179 | 0.1249 | 4.91 | −90 | 0.3535 |
| DDCS | −0.004 | 2.122 | 0.1295 | 1.47 | −97 | 0.36 |
| DSEM | −0.06 | 4.39 | 0.057 | 56.42 | 12.83 | 0.2393 |
| DMAG | −0.12 | 3.275 | 0.11 | 19.11 | −61.78 | 0.326 |
| r2 | ||||||
| DHYDR | 2.01 | 49.36 | 50.38 | 99.06 | 98.12 | 7.1 |
| DDCS | 6.55 | −13.09 | 0.112 | 100 | 100 | 0.3335 |
| DSEM | 12.38 | −248.9 | 2346 | 56.18 | 12.36 | 48.43 |
| DMAG | 51.56 | −331.7 | 549.6 | 89.73 | 79.47 | 23.44 |
| r2/r1 | ||||||
| DHYDR | 1.08 | 21.24 | 295 | 83.77 | 67.55 | 17.18 |
| DDCS | 3.32 | −4.89 | 443.2 | 75.63 | 51.26 | 21.05 |
| DSEM | 9.41 | −248 | 80.28 | 95.59 | 91.17 | 8.96 |
| DMAG | 31.18 | −222.2 | 61.92 | 96.59 | 93.19 | 7.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strbak, O.; Antal, I.; Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Molcan, M.; Jurikova, A.; Hnilicova, P.; Gombos, J.; et al. Influence of Dextran Molecular Weight on the Physical Properties of Magnetic Nanoparticles for Hyperthermia and MRI Applications. Nanomaterials 2020, 10, 2468. https://doi.org/10.3390/nano10122468
Strbak O, Antal I, Khmara I, Koneracka M, Kubovcikova M, Zavisova V, Molcan M, Jurikova A, Hnilicova P, Gombos J, et al. Influence of Dextran Molecular Weight on the Physical Properties of Magnetic Nanoparticles for Hyperthermia and MRI Applications. Nanomaterials. 2020; 10(12):2468. https://doi.org/10.3390/nano10122468
Chicago/Turabian StyleStrbak, Oliver, Iryna Antal, Iryna Khmara, Martina Koneracka, Martina Kubovcikova, Vlasta Zavisova, Matus Molcan, Alena Jurikova, Petra Hnilicova, Jan Gombos, and et al. 2020. "Influence of Dextran Molecular Weight on the Physical Properties of Magnetic Nanoparticles for Hyperthermia and MRI Applications" Nanomaterials 10, no. 12: 2468. https://doi.org/10.3390/nano10122468
APA StyleStrbak, O., Antal, I., Khmara, I., Koneracka, M., Kubovcikova, M., Zavisova, V., Molcan, M., Jurikova, A., Hnilicova, P., Gombos, J., Kadasova, N., & Dobrota, D. (2020). Influence of Dextran Molecular Weight on the Physical Properties of Magnetic Nanoparticles for Hyperthermia and MRI Applications. Nanomaterials, 10(12), 2468. https://doi.org/10.3390/nano10122468






