Bioinstructive Micro-Nanotextured Zirconia Ceramic Interfaces for Guiding and Stimulating an Osteogenic Response In Vitro
Abstract
1. Introduction
2. Materials and Methods
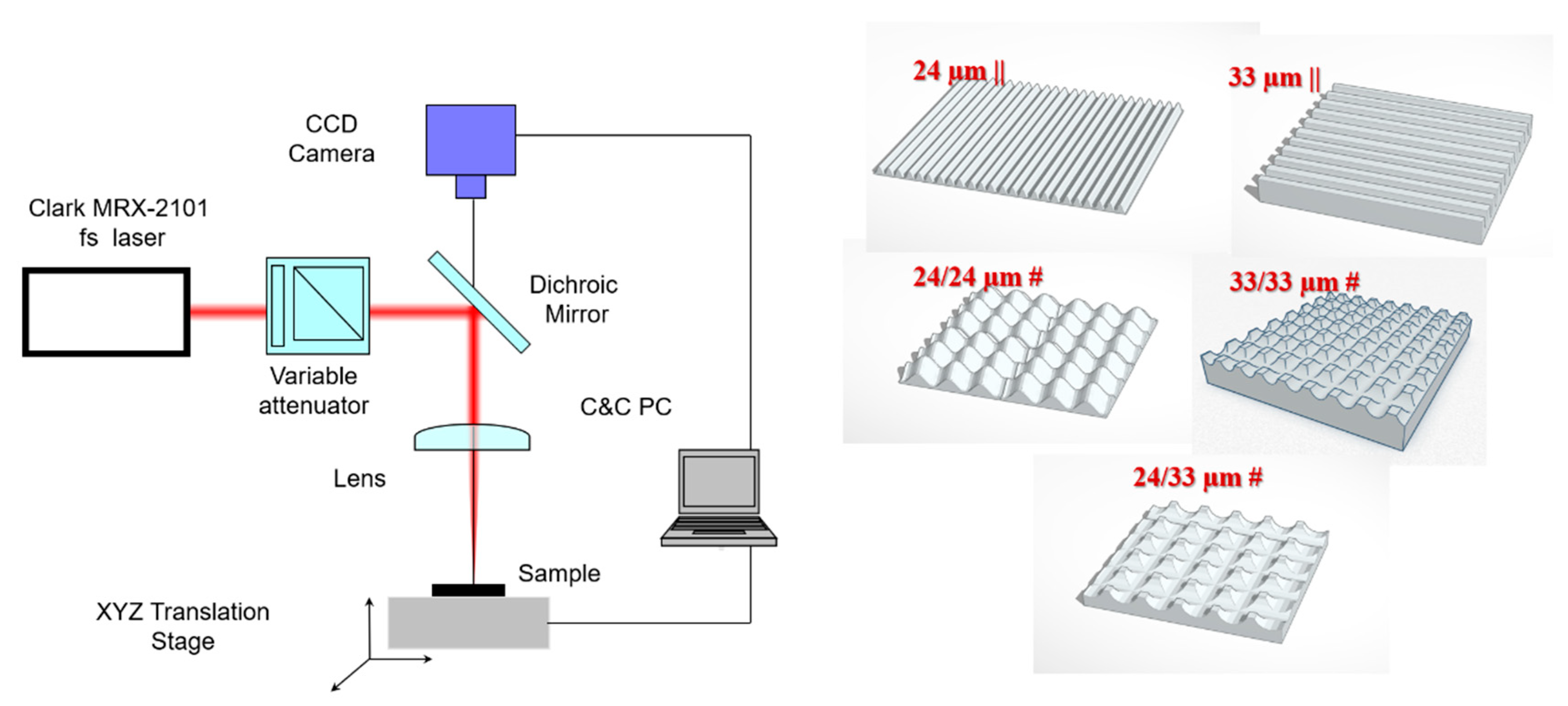
2.1. Surface Patterning of Isotropic and Anisotropic Microtopographies
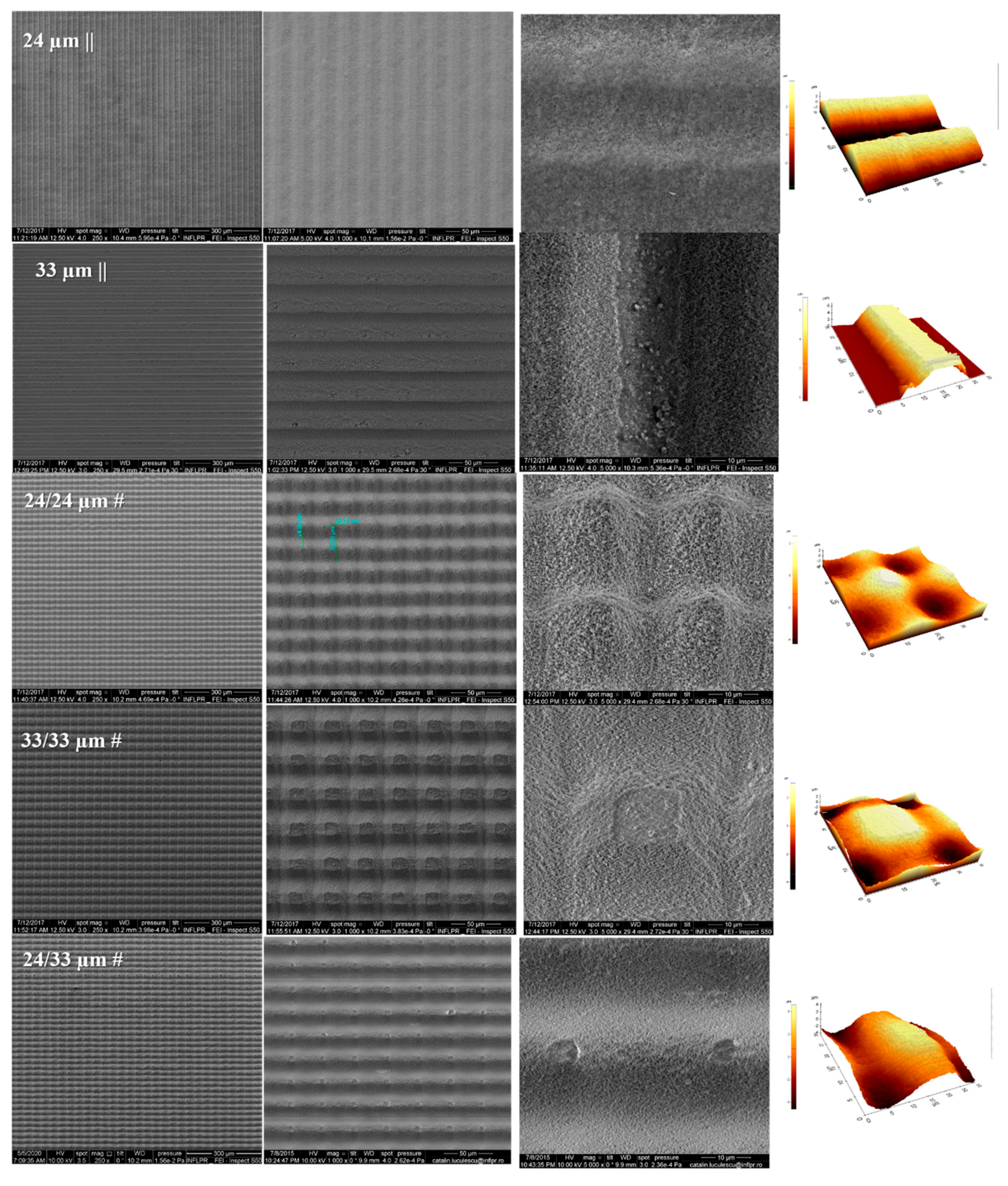
2.2. Scanning Electronic Microscopy (SEM)
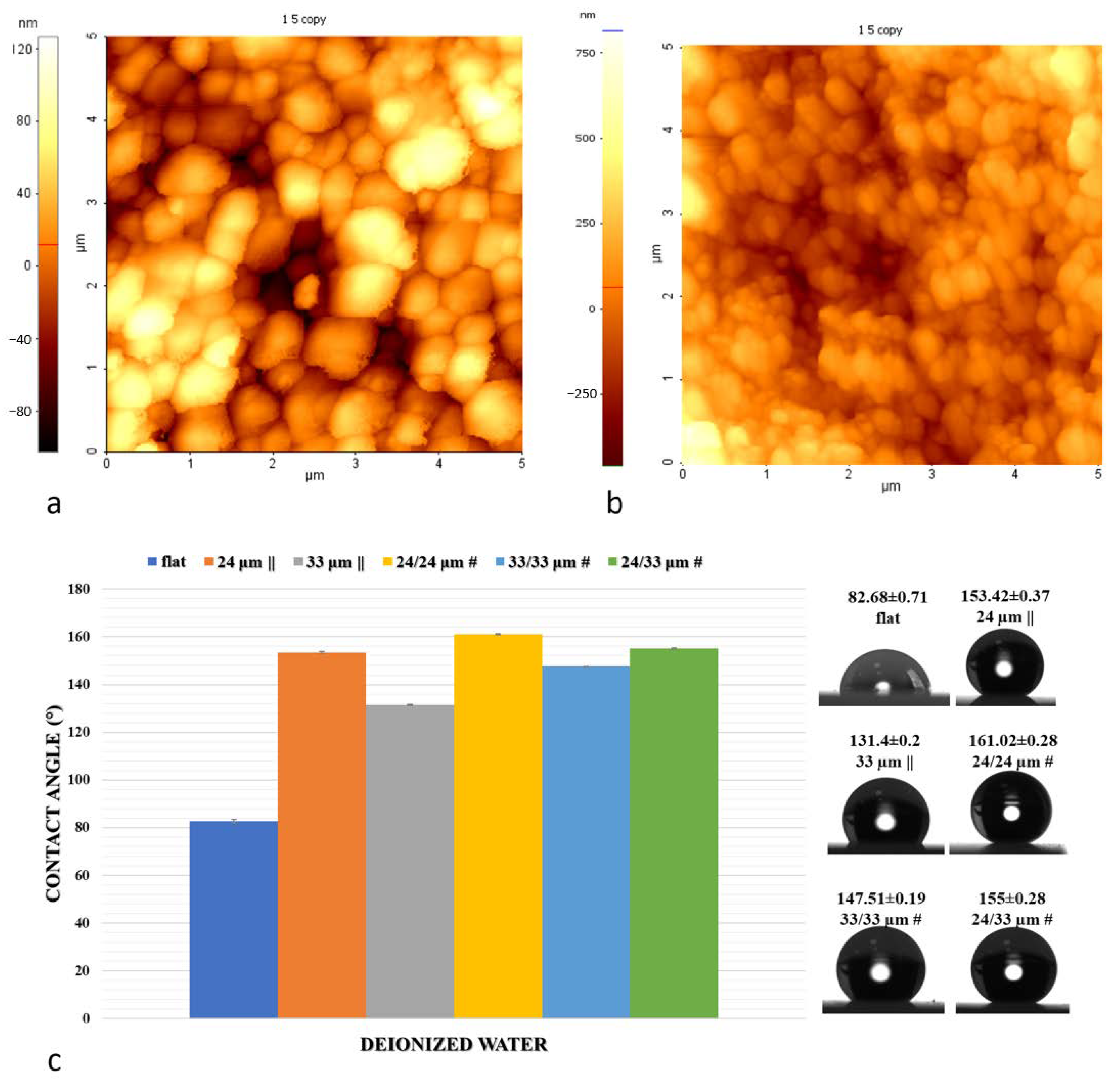
2.3. Atomic Force Microscopy (AFM) and Profilometry Measurements
2.4. Contact Angle and Surface Energy Measurements
2.5. Sterilization Procedure
2.6. Cell Culture
2.7. Osteogenic Differentiation
2.8. Microscopy Sample Processing
2.8.1. Immunofluorescence
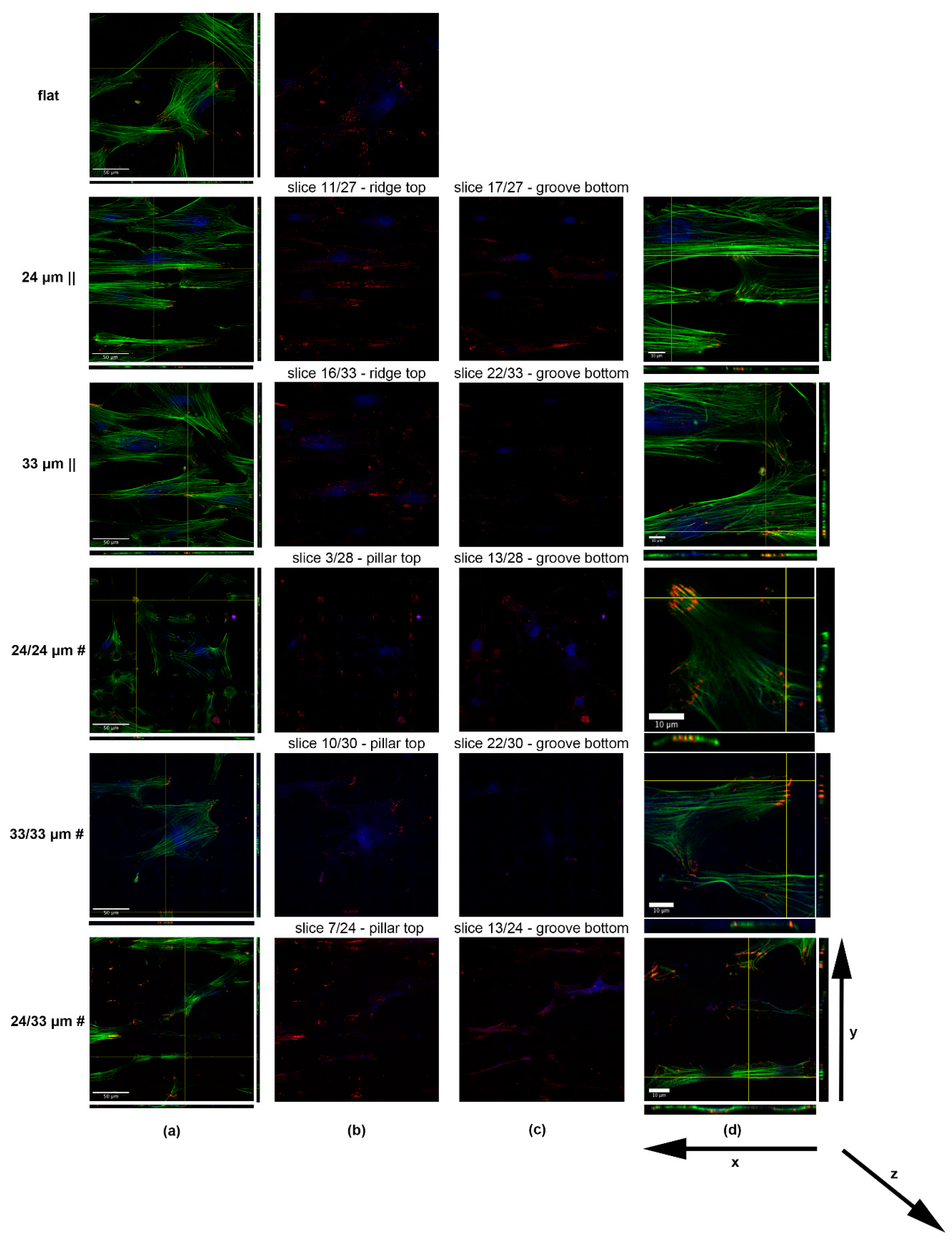
2.8.2. Confocal Microscope Imaging
2.8.3. Quantitative Image Analysis
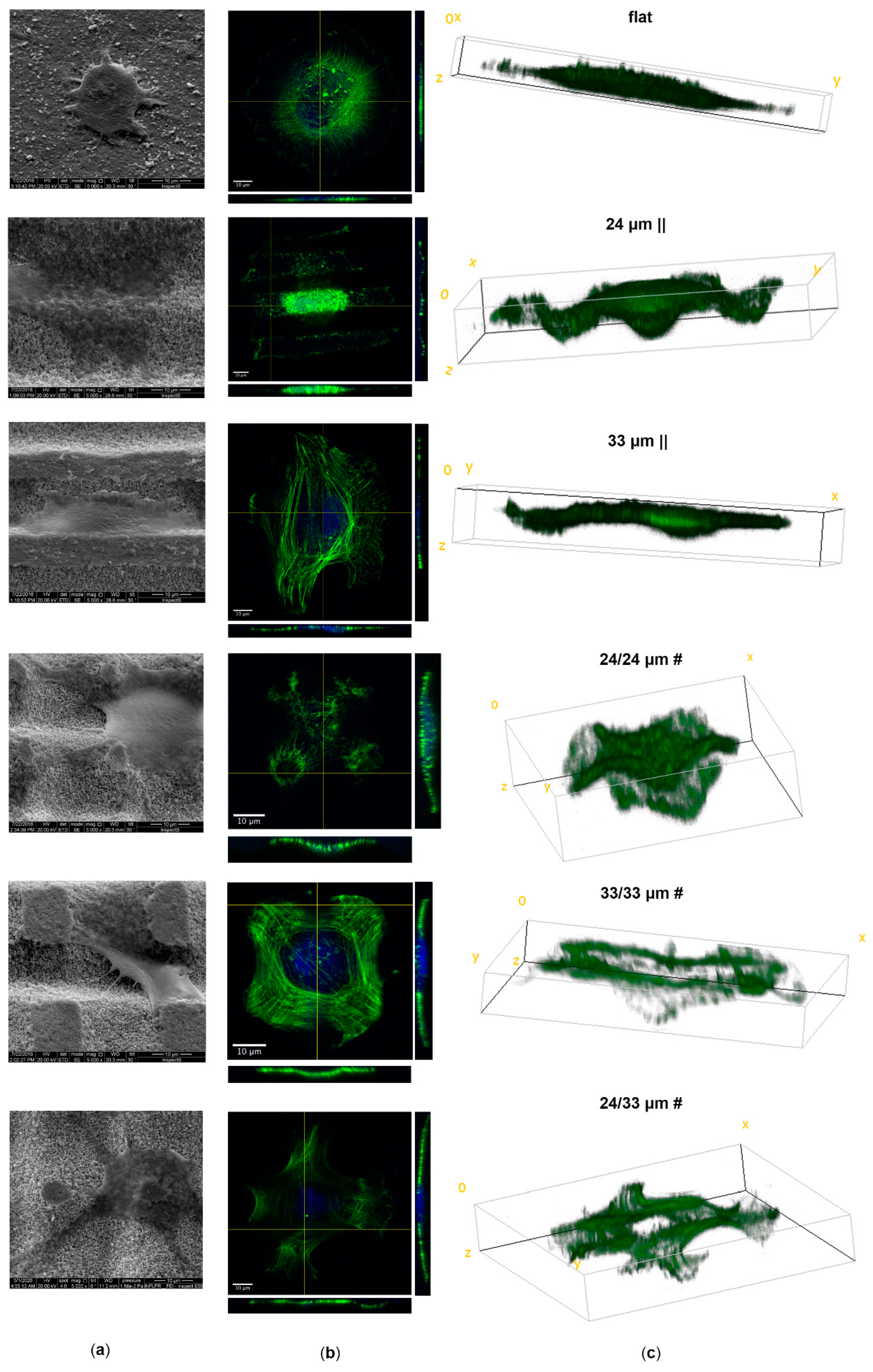
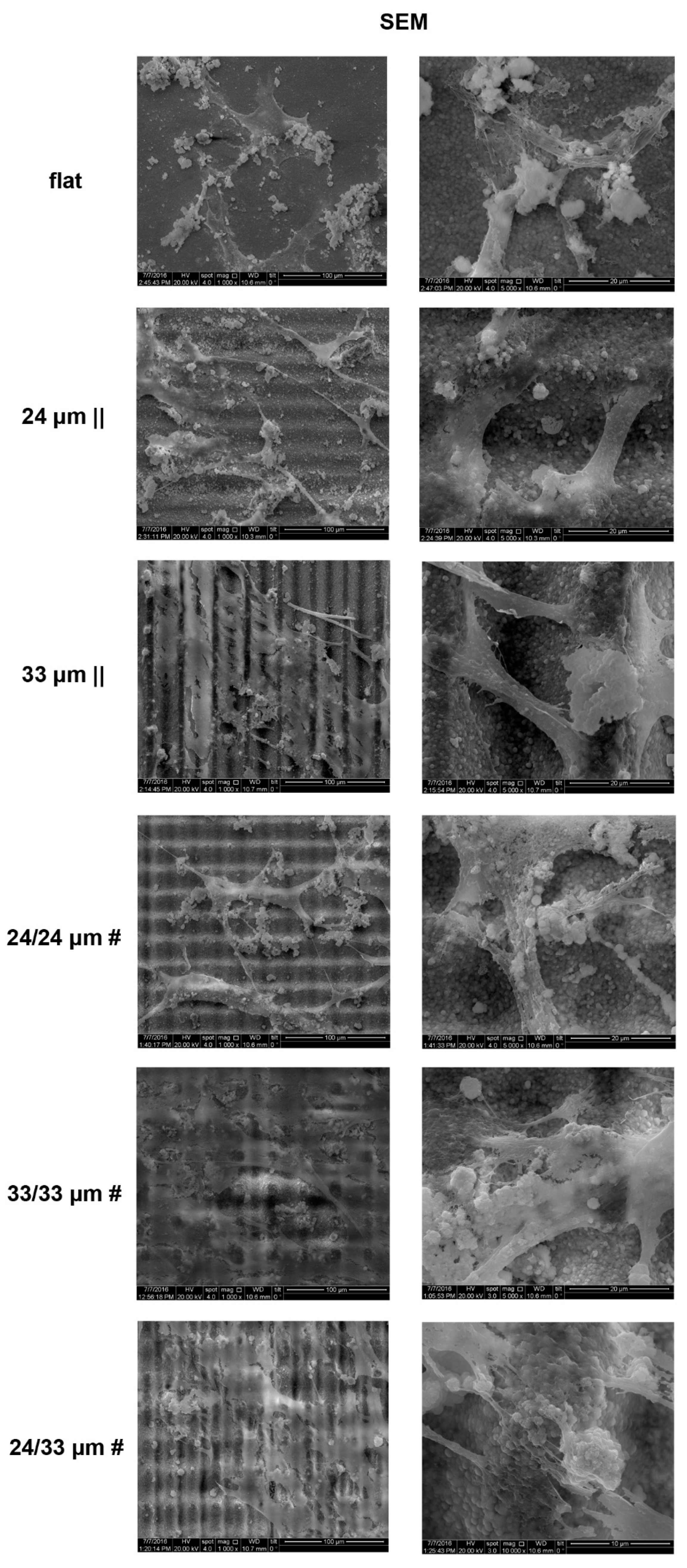
2.8.4. Cells Analysis by Scanning Electron Microscopy (SEM)
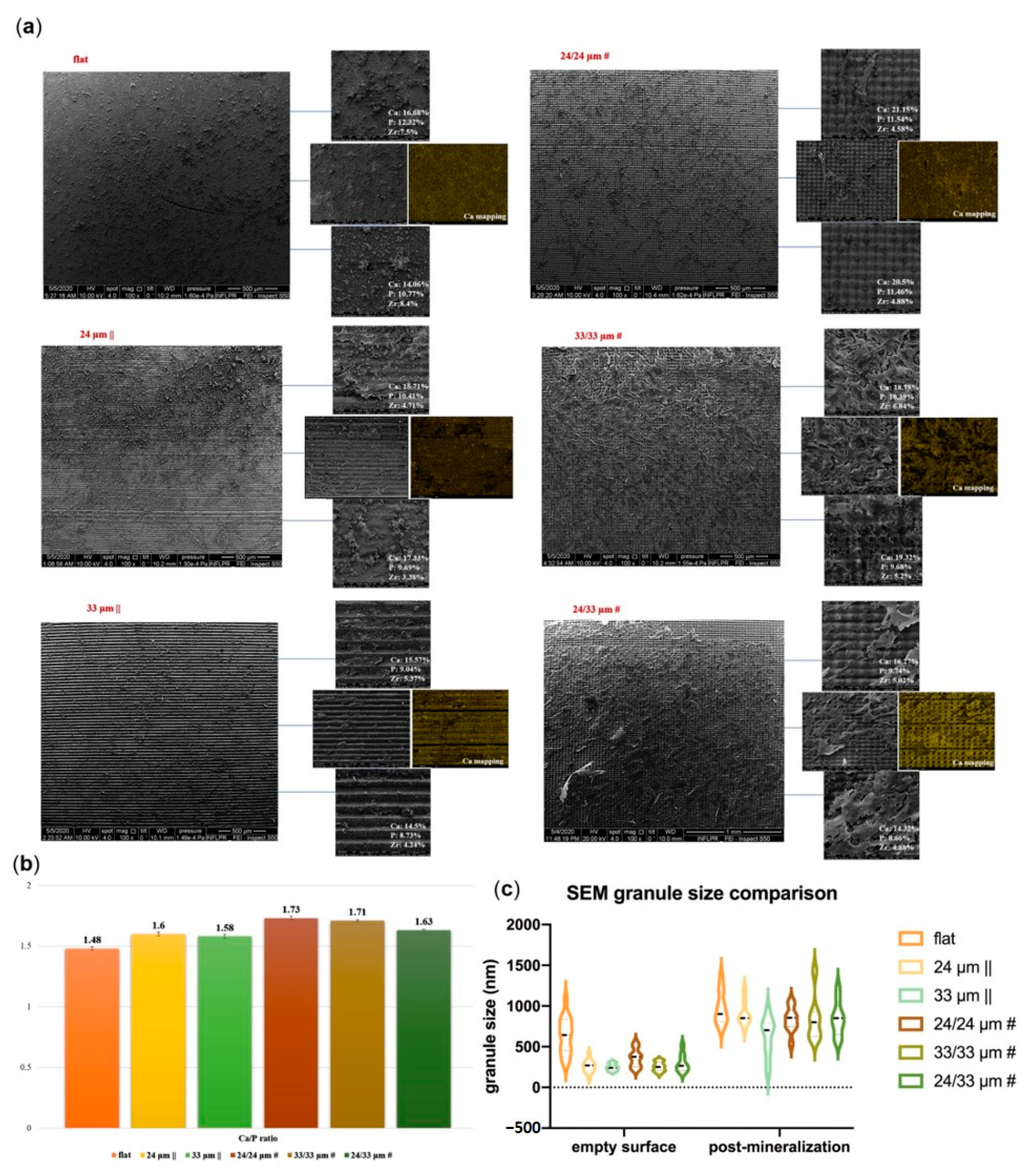
2.9. Alizarin Red Staining
2.10. Statistical Analysis
3. Results and Discussions
3.1. Design of Structure Arrangements Textured in Zirconia Ceramic Substrate and Surface Characterization
3.2. Wettability and Surface Free Energy Measurements
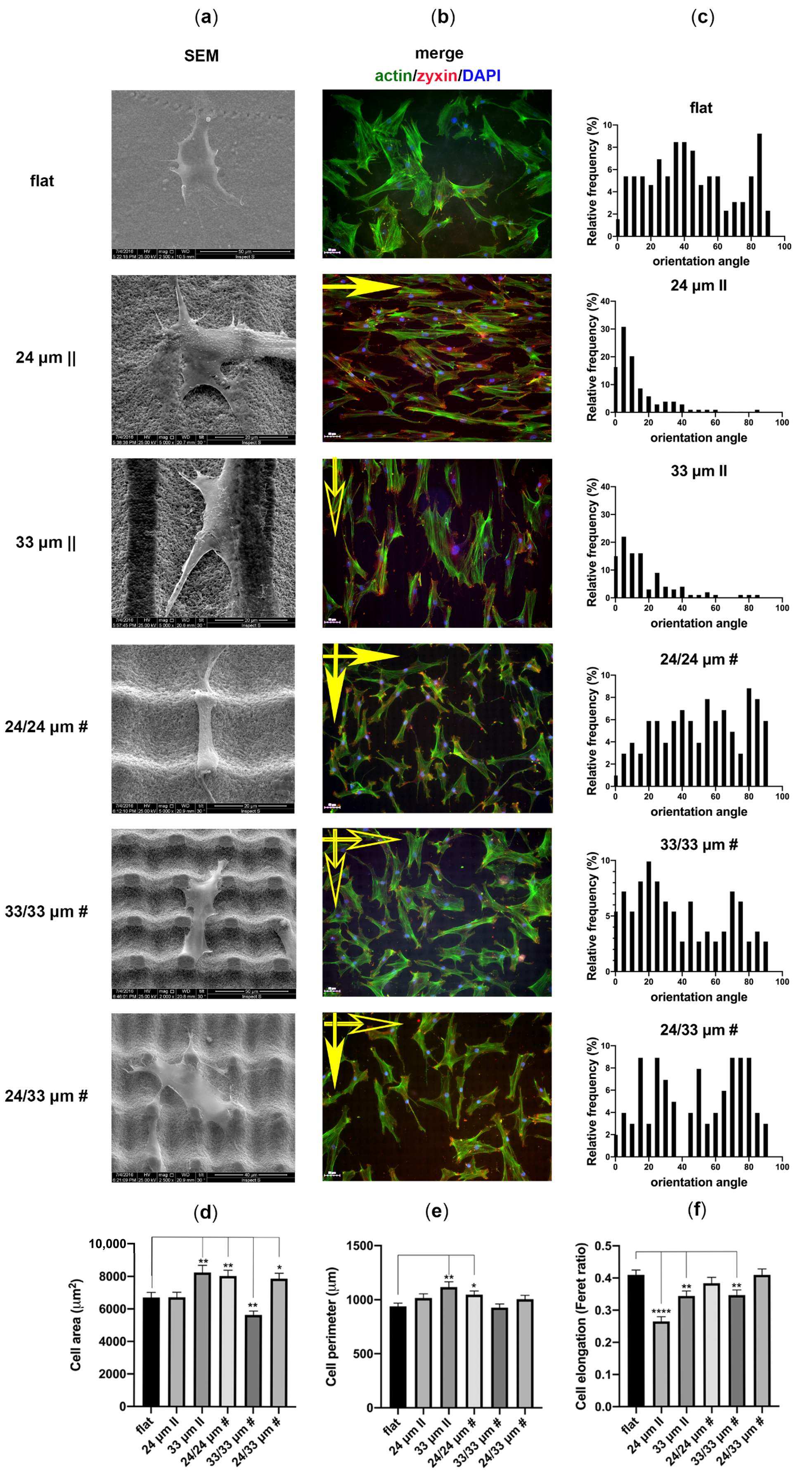
3.3. Human Mesenchymal Stem Cell Adhesion and Guidance onto Microtextured Zirconia
3.4. Human Mesenchymal Stem Cell Proliferation onto Microtextured Zirconia
3.5. Osteogenic Differentiation onto Microtextured Zirconia
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Velnar, T.; Bunc, G.; Klobucar, R.; Gradisnik, L. Biomaterials and host versus graft response: A short review. Bosn. J. Basic Med. Sci. 2016, 16, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Major, M.R.; Wong, V.W.; Nelson, E.R.; Longaker, M.T.; Gurtner, G.C. The foreign body response: At the interface of surgery and bioengineering. Plast Reconstr. Surg. 2015, 135, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Muddugangadhar, B.; Shamanna, A.; Tripathi, S.; Dikshit, S.; Divya, M.S. Biomaterials for dental implants: An overview. Int. J. Oral Implantol. Clin. Res. 2011, 2, 13–24. [Google Scholar]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable materials and metallic implants—A review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials: Design, Development and Biomedical Applications, Nanotechnology Applications for Tissue Engineering; William Andrew Publishing: Norwich, NY, USA, 2015; pp. 21–44. ISBN 9780323328890. [Google Scholar]
- Katti, K.S.; Verma, D.; Katti, D.R. Materials for joint replacement. In Joint Replacement Technology; Revell, P.A., Ed.; Woodhead: Cambridge, UK, 2008; pp. 81–104. [Google Scholar]
- Dinu, M.; Franchi, S.; Pruna, V.; Cotrut, C.M.; Secchi, V.; Santi, M.; Titorencu, I.; Battocchio, C.; Iucci, G.; Vladescu, A. Ti-Nb-Zr system and its surface biofunctionalization for biomedical applications. Titan. Med. Dent. Appl. 2018, 44, 175–200. [Google Scholar]
- van Hoof, M.; Wigren, S.; Blechert, J.I.; Molin, M.; Andersson, H.; Mateijsen, D.; Bom, S.J.; Calmels, M.; van der Rijt, A.J.; Flynn, M.C. A multinational cost-consequence analysis of a bone conduction hearing implant system—A randomized trial of a conventional vs. a less invasive treatment with new abutment technology. Front. Neurol. 2020, 11, 106. [Google Scholar] [CrossRef]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Filgueira, L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening—Current concepts. J. Biomed. Mater. Res. A 2009, 91, 1252–1262. [Google Scholar] [CrossRef]
- Loeffler, H.; Jonitz-Heincke, A.; Peters, K.; Mueller-Hilke, B.; Fiedler, T.; Bader, R.; Klinder, A. Comparison of inflammatory effects in THP-1 monocytes and macrophages after exposure to metal ions. Materials 2020, 13, 1150. [Google Scholar] [CrossRef]
- Gauta, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef]
- Vitti, R.; Catelan, A.; Amaral, M.; Pacheco, R. Zirconium in dentistry, advanced dental. In Advanced Dental Biomaterials; Woodhead: Cambridge, UK, 2019; pp. 317–345. [Google Scholar]
- Bauer, S.; von der Mark, P.K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Brezavšček, M.; Fawzy, A.; Bächle, M.; Tuna, T.; Fischer, J.; Att, W. The effect of UV treatment on the osteoconductive capacity of zirconia-based materials. Materials 2016, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Vinas, M.; Anglada, M. Strength and fracture toughness of zirconia dental ceramics. Dent. Mater. 2018, 34, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Adanez, M.H.; Att, W. Current status of zirconia implants in dentistry: Preclinical tests. J. Prosthodont. Res. 2019, 63, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Noumbissi, S.; Kunrath, M.F. Nano modified zirconia dental implants: Advances and the frontiers for rapid osseointegration. Med. Devices Sens. 2020, 3, e10076. [Google Scholar] [CrossRef]
- Hiew, V.V.; Simat, S.F.B.; Teoh, P.L. The advancement of biomaterials in regulating stem cell fate. Stem. Cell Rev. Rep. 2018, 14, 43–57. [Google Scholar] [CrossRef]
- McMurray, R.J.; Dalby, M.J.; Tsimbouri, P.M. Using biomaterials to study stem cell mechanotransduction, growth and differentiation. J. Tissue Eng. Regen. Med. 2015, 9, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res Ther. 2016, 7, 188. [Google Scholar] [CrossRef]
- Griffin, M.F.; Butler, P.E.; Seifalian, A.M.; Kalaskar, D.M. Control of stem cell fate by engineering their micro and nanoenvironment. World J. Stem Cells 2015, 7, 37–50. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Dische, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Ren, Y.; Effler, J.C.; Norstrom, M.; Luo, T.; Firtel, R.A.; Iglesias, P.A.; Rock, R.S.; Robinson, D.N. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr. Biol. 2009, 19, 1421–1428. [Google Scholar] [CrossRef]
- Costa, P.; Almeida, F.V.; Connelly, J.T. Biophysical signals controlling cell fate decisions: How do stem cells really feel? Int. J. Biochem. Cell Biol. 2012, 44, 2233–2237. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, L.; Yu, S.; Simmons, C.A. Mesenchymal stem cell mechanobiology and emerging experimental platforms. J. R Soc. Interface 2013, 10, 20130179. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Ingber, D.E. Micromechanical control of cell and tissue development: Implications for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Worley, K.; Certo, A.; Wan, L.Q. Geometry—Force control of stem cell fate. BioNanoScience 2013, 3, 43–51. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef]
- Salmasi, S.; Kalaskar, D.M.; Yoon, W.W.; Blunn, G.W.; Seifalian, A.M. Role of nanotopography in the development of tissue engineered 3D organs and tissues using mesenchymal stem cells. World J. Stem Cells 2015, 26, 266–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Gordon, A.; Qian, W.; Chen, W. Engineering nanoscale stem cell niche: Direct stem cell behavior at cell-matrix interface. Adv. Healthc. Mater. 2015, 4, 1900–1914. [Google Scholar] [CrossRef]
- Anderson, H.J.; Sahoo, J.K.; Ulijn, R.V.; Dalby, M.J. Mesenchymal stem cell fate: Applying biomaterials for control of stem cell behavior. Front. Bioeng. Biotechnol. 2016, 4, 38. [Google Scholar] [CrossRef]
- Mahmoodi, N.; Hooshmand, T.; Heidari, S.; Khoshro, K. Effect of sandblasting, silica coating, and laser treatment on the microtensile bond strength of a dental zirconia ceramic to resin cements. Lasers Med. Sci. 2015, 31, 205–211. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A.; Negri, B.; Fernández, M.P.; de Val, J.E.M.S.; Gómez-Moreno, G.; Romanos, G.E. Histological and histomorphometric evaluation of zirconia dental implants modified by femtosecond laser versus titanium implants: An experimental study in fox hound dogs. Clin. Implant Dent. Relat. Res. 2015, 17, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Dinca, V.; Sima, L.; Rusen, L.; Lippert, T.; Bonciu, A.; Dinescu, M.; Farsari, M. Bio-interfaces engineering using laser-based methods for controlled regulation of mesenchymal stem cell response in vitro. In Recent Advances in Biopolymers; IntechOpen: London, UK, 2016; pp. 221–251. [Google Scholar]
- Delgado-Ruíz, R.A.; Moreno, G.G.; Aguilar-Salvatierra, A.; Markovic, A.; Mate-Sánchez, J.E.; Calvo-Guirado, J.L. Human fetal osteoblast behavior on zirconia dental implants and zirconia disks with microstructured surfaces. An experimental in vitro study. Clin. Oral Implants Res. 2016, 27, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Yilbas, B.S. Laser treatment of zirconia surface for improved surface hydrophobicity. J. Alloys Compd. 2015, 625, 208–215. [Google Scholar] [CrossRef]
- Vinas, M.; Morillas, J.; Moreno, P.; Anglada, M. Evaluation of damage in front of starting notches induced by ultra-short pulsed laser ablation for the determination of fracture toughness in zirconia. J. Eur. Ceram. Soc. 2017, 37, 5127–5131. [Google Scholar] [CrossRef]
- Stanciuc, A.; Flamant, Q.; Sprecher, C.; Alini, M.; Anglada, M.; Peroglio, M. Femtosecond laser multi-patterning of zirconia for screening of cell-surface interactions. J. Eur. Ceram. Soc. 2018, 38, 939–948. [Google Scholar] [CrossRef]
- Zhou, H.; Li, C.; Zhou, Z.; Cao, R.; Chen, Y.; Zhang, S.; Wang, G.; Xiao, S.; Li, Z.; Xiao, P. Femtosecond laser-induced periodic surface microstructure on dental zirconia ceramic. Mater. Lett. 2018, 229, 74–77. [Google Scholar] [CrossRef]
- Pae, A.; Lee, H.; Kim, H.-S.; Kwon, Y.-D.; Woo, Y.-H. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed. Mater. 2009, 4, 025005. [Google Scholar] [CrossRef]
- Gnilitskyi, I.; Pogorielov, M.; Viter, R.; Ferraria, A.M.; Carapeto, A.P.; Oleshko, O.; Orazi, L.; Mishchenko, O. Cell and tissue response to nanotextured Ti6Al4V and Zr implants using high-speed femtosecond laser-induced periodic surface structures. Nanomedicine 2019, 21, 102036. [Google Scholar] [CrossRef]
- Carvalho, A.; Vilar, R.; Fernandes, M.H.; Monteiro, F.J. Human bone marrow derived mesenchymal stem cells modulation by micro/nanostructured ATZ surfaces treated with a femtosecond laser. Front. Bioeng. Biotechnol 2016. [Google Scholar] [CrossRef]
- Carvalho, A.; Cangueiro, L.; Oliveira, V.; Vilar, R.; Fernandes, M.H.; Monteiro, F.J. Femtosecond laser microstructured Alumina toughened Zirconia: A new strategy to improve osteogenic differentiation of hMSCs. Appl. Surf. Sci. 2018, 435, 1237–1245. [Google Scholar] [CrossRef]
- Wilkinson, A.; Hewitt, R.N.; McNamara, L.E.; McCloy, D.; Meek, R.M.D.; Dalby, M.J. Biomimetic microtopography to enhance osteogenesis in vitro. Acta Biomater. 2011, 7, 2919–2925. [Google Scholar] [CrossRef] [PubMed]
- Rusen, L.; Cazan, M.; Mustaciosu, C.; Filipescu, M.; Simion, S.; Zamfirescu, M.; Dinca, V.; Dinescu, M. Tailored topography control of biopolymer surfaces by ultrafast lasers for cell-substrate studies. Appl. Surf. Sci. 2014, 302, 256–261. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige aspekte der benetzungstheorie und ihre anwendung auf die untersuchung und veränderung der oberflächeneigenschaften von polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Scarisoreanu, N.D.; Craciun, F.; Ion, V.; Birjega, R.; Bercea, A.; Dinca, V.; Dinescu, M.; Sima, L.E.; Icriverz, M.; Roseanu, A.; et al. Lead-free piezoelectric (Ba,Ca)(Zr,Ti)O-3 thin films for biocompatible and flexible devices. ACS Appl. Mater. Interfaces 2017, 9, 266–278. [Google Scholar] [CrossRef]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. A 2010, 95, 1203–1214. [Google Scholar] [CrossRef]
- Cazan, M.; Sima, L.E.; Dinca, V.; Dinescu, M.; Popescu, C.R. Maple coating of Zirconia with PEG-PCL embedded cyprinol for ear implant applications. Rom. J. Biochem. 2010, 50, 13–14. [Google Scholar]
- Crowder, S.W.; Leonardo, V.; Whittaker, T.; Papathanasiou, P.; Stevens, M.M. Material cues as potent regulators of epigenetics and stem cell function. Cell Stem Cell 2016, 18, 39–52. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M. Role of materials surface topography on mammalian cell response. Int. Mater. Rev. 2011, 56, 243–266. [Google Scholar] [CrossRef]
- Im, B.; Lee, S.; Oh, N.; Lee, M.; Kang, J.; Leesungbok, R.; Lee, S.C.; Ahn, S.J.; Park, J.S. Texture direction of combined microgrooves and submicroscale topographies of titanium substrata influence adhesion, proliferation, and differentiation in human primary cells. Arch. Oral Biol. 2012, 57, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kusk, J.P.; Zscharnack, M.; Loeffler, M.; Galle, J. Spatial organization of mesenchymal stem cells in vitro—Results from a new individual cell-based model with podia. PLoS ONE 2011, 6, 21960. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, Y.; Nava, A.C.; Schweizerhof, S.; van Dongen, M.; Haraszti, T.; Köhler, J.; Zhang, H.; Windoffer, R.; Mourran, A.; Möller, M.; et al. Cellular responses to beating hydrogels to investigate mechanotransduction. Nat. Commun. 2019, 10, 4027. [Google Scholar] [CrossRef] [PubMed]
- SalehAbd, O.; Ashraf, E.; Sherief, H. Zirconia based ceramics, some clinical and biological aspects: Review. Future Dent. J. 2016, 2, 55–64. [Google Scholar]
- Afzal, A. Implantable zirconia bioceramics for bone repair and replacement: A chronological review. Mater. Express 2014, 4. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, D.H.; Leeban, E.J.; Lee, S.-H. Study of cellular behaviors on concave and convex microstructures fabricated from elastic PDMS membranes. Lab Chip 2009, 9, 2043–2049. [Google Scholar] [CrossRef]
- Assoian, R.K.; Bade, N.D.; Cameron, C.V.; Stebe, K.J. Cellular sensing of micron-scale curvature: A frontier in understanding the microenvironment. Open Biol. 2019, 9, 190155. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. Emerging ceramic-based materials for dentistry. J. Dent. Res. 2014, 93, 1235–1242. [Google Scholar] [CrossRef]
- Strickstrock, M.; Rothe, H.; Grohmann, S.; Hildebrand, G.; Zylla, I.M.; Liefeith, K. Influence of surface roughness of dental zirconia implants on their mechanical stability, cell behavior and osseointegration. BioNanoMaterials 2017, 18. [Google Scholar] [CrossRef]
- González-Martín, M.L.; Labajos-Broncano, L.; Jańczuk, B.; Bruque, J.M. Wettability and surface free energy of zirconia ceramics and their constituents. J. Mater. Sci. 1999, 34, 5923–5926. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J.; Chian, K.S. Osteoblast cell adhesion on a laser modified zirconia based bioceramic. J. Mater. Sci. Mater. Med. 2005, 16, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Jensen, C.C.; Chaturvedi, A.; Yoshigi, M.; Beckerle, M.C. Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin regulators. Mol. Biol. Cell 2012, 23, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Sigaut, L.; von Bilderling, C.; Bianchi, M.; Burdisso, J.E.; Gastaldi, L.; Pietrasanta, L.I. Live cell imaging reveals focal adhesions mechanoresponses in mammary epithelial cells under sustained equibiaxial stress. Sci. Rep. 2018, 8, 9788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Castañeda Ocampo, O.; Guimarães, C.F.; van Kooten, T.G.; van Rijn, P.; Kühn, P.T. Screening platform for cell contact guidance based on inorganic biomaterial micro/nanotopographical gradients. ACS Appl. Mater. Interfaces 2017, 9, 31433–31445. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef]


| Samples Type | γ_d | γ_p | γ_t | |
|---|---|---|---|---|
| Non-Irradiated Control | Flat | 26.35 | 5.17 | 31.52 |
| Anisotropic patterns | 24 µm || | 47.61 | 12.63 | 60.24 |
| 33 µm || | 49.29 | 8.77 | 58.06 | |
| Isotropic patterns | 24/24 µm# | 47.43 | 17.75 | 65.18 |
| 33/33 µm # | 48.75 | 14.63 | 63.38 | |
| 24/33 µm # | 46.73 | 13.16 | 59.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elena Sima, L.; Bonciu, A.; Baciu, M.; Anghel, I.; Dumitrescu, L.N.; Rusen, L.; Dinca, V. Bioinstructive Micro-Nanotextured Zirconia Ceramic Interfaces for Guiding and Stimulating an Osteogenic Response In Vitro. Nanomaterials 2020, 10, 2465. https://doi.org/10.3390/nano10122465
Elena Sima L, Bonciu A, Baciu M, Anghel I, Dumitrescu LN, Rusen L, Dinca V. Bioinstructive Micro-Nanotextured Zirconia Ceramic Interfaces for Guiding and Stimulating an Osteogenic Response In Vitro. Nanomaterials. 2020; 10(12):2465. https://doi.org/10.3390/nano10122465
Chicago/Turabian StyleElena Sima, Livia, Anca Bonciu, Madalina Baciu, Iulia Anghel, Luminita Nicoleta Dumitrescu, Laurentiu Rusen, and Valentina Dinca. 2020. "Bioinstructive Micro-Nanotextured Zirconia Ceramic Interfaces for Guiding and Stimulating an Osteogenic Response In Vitro" Nanomaterials 10, no. 12: 2465. https://doi.org/10.3390/nano10122465
APA StyleElena Sima, L., Bonciu, A., Baciu, M., Anghel, I., Dumitrescu, L. N., Rusen, L., & Dinca, V. (2020). Bioinstructive Micro-Nanotextured Zirconia Ceramic Interfaces for Guiding and Stimulating an Osteogenic Response In Vitro. Nanomaterials, 10(12), 2465. https://doi.org/10.3390/nano10122465






