Impact of Quercetin Encapsulation with Added Phytosterols on Bilayer Membrane and Photothermal-Alteration of Novel Mixed Soy Lecithin-Based Liposome
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposome Preparation via the Extrusion Method
2.3. Particle Size, Polydispersity Index (PDI) and Particle Size Stability Measurement
2.4. Zeta Potential (ZP)
2.5. Encapsulation Efficiency of QU (EEQU)
2.6. Assessment of DPPH (2, 2-diphenyl-1-picrylhydrazyl) Scavenging Rate, and DPPH Scavenging Rate of Loss against UV Light
2.7. Fourier-Transform Infrared Spectroscopy (FTIR) Assay
2.8. Thermal Stability
2.9. Transmission Electron Microscopy (TEM)
2.10. Experimental Design and Statistical Analysis
3. Results and Discussion
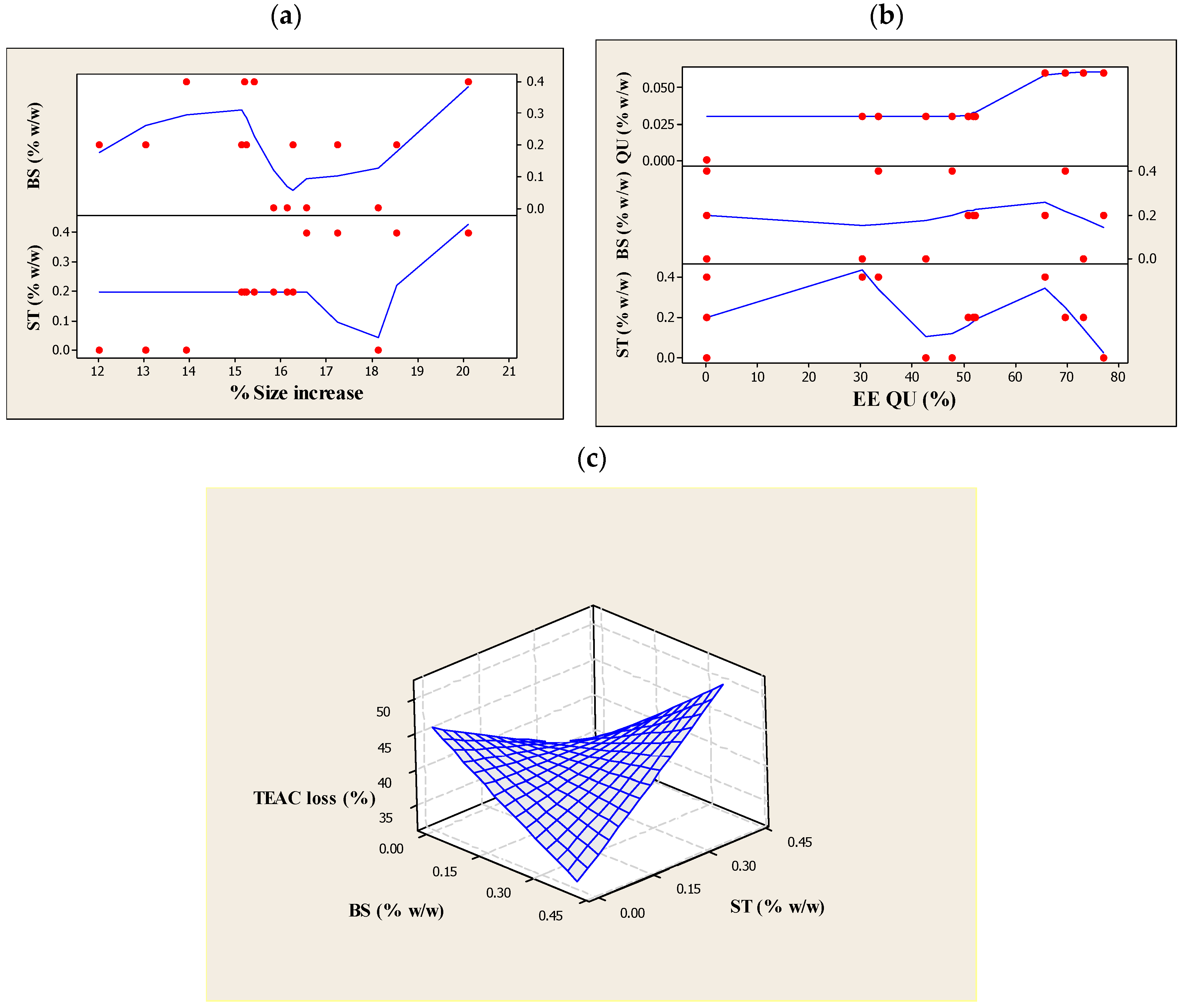
3.1. Particle Size, PDI and Particle Size Stability
3.2. ZP of the Produced Liposomes
3.3. Encapsulation Efficiency of QU (EEQU)
3.4. Assessment of TEAC, and the Percentage Loss of TEAC against UV Light
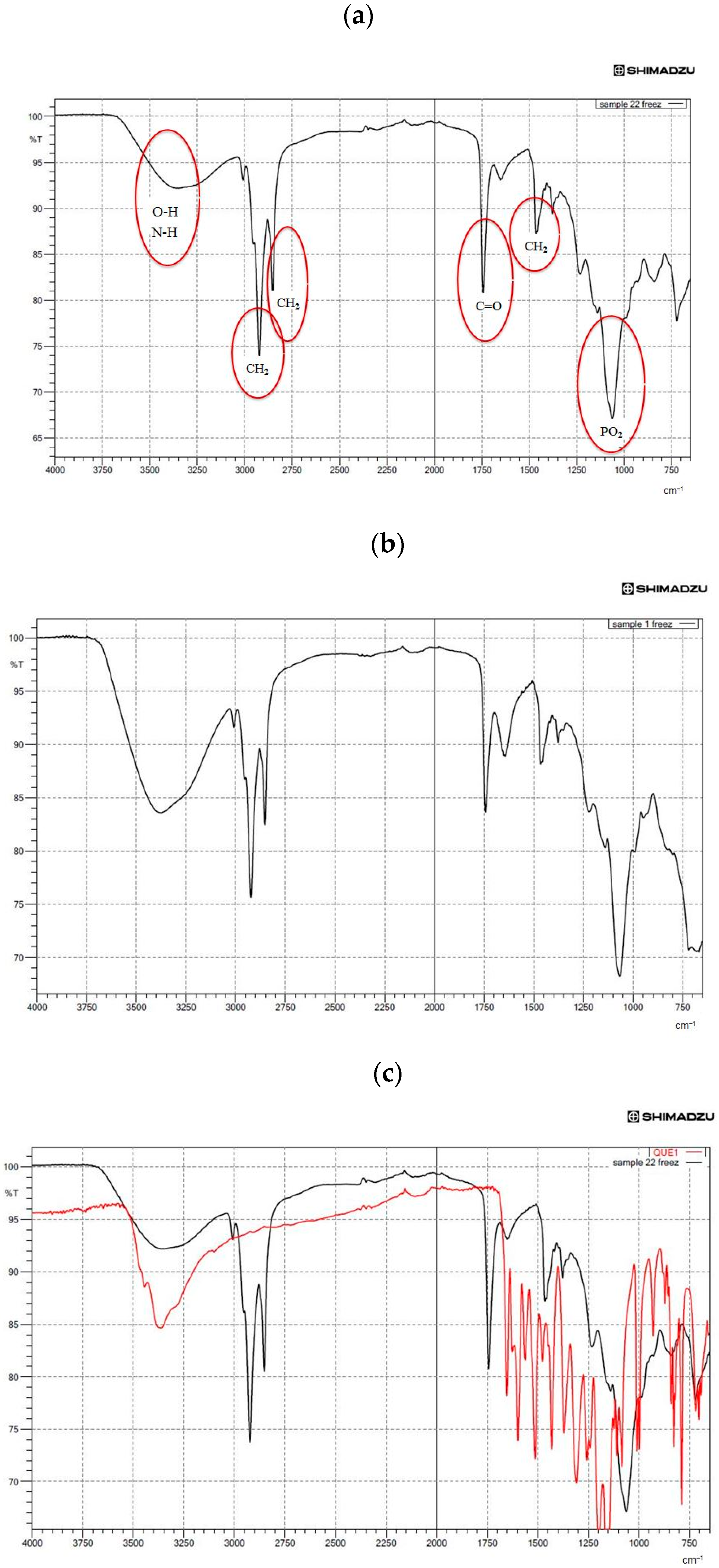
3.5. Structural Analysis by FTIR Spectroscopy
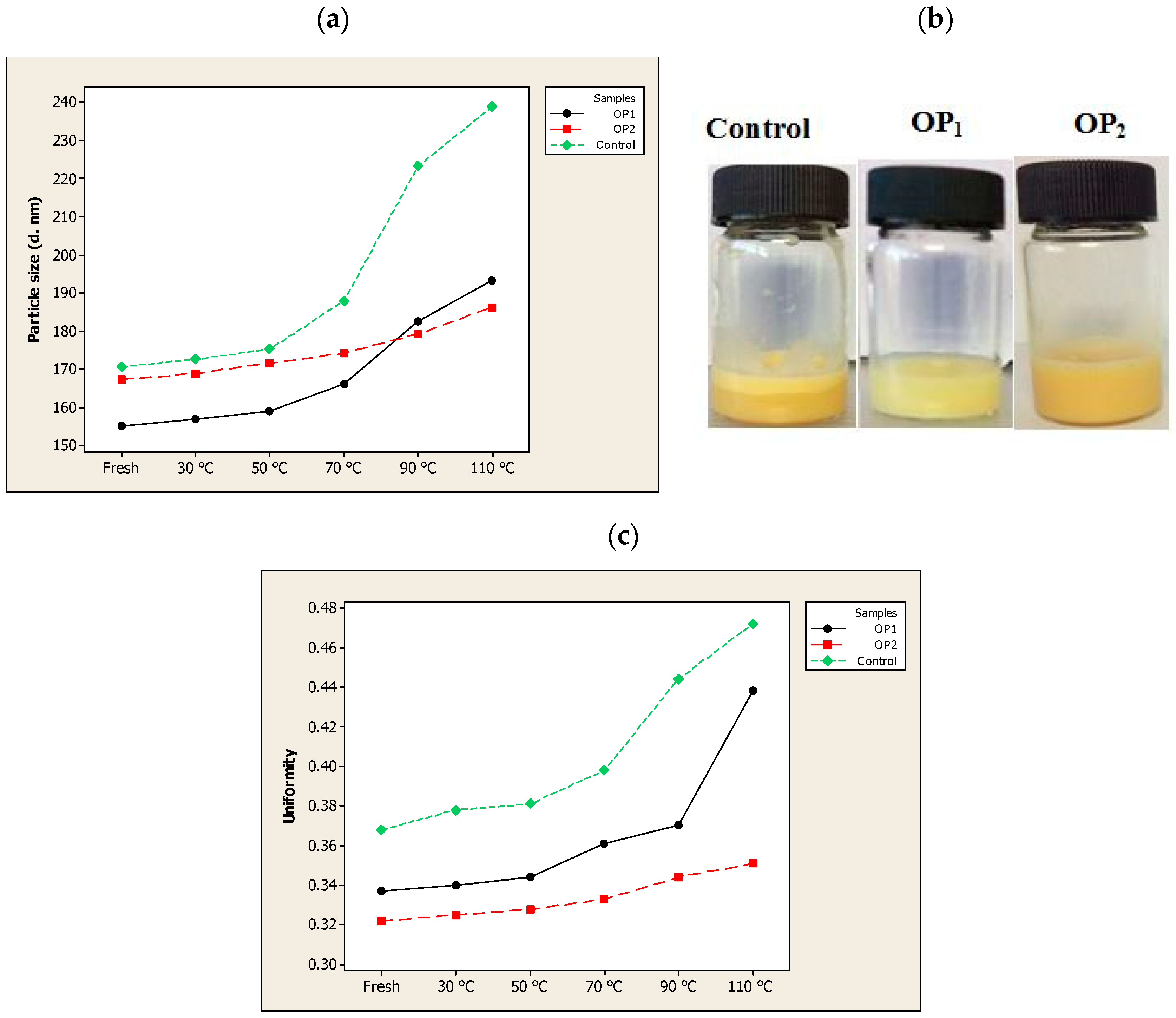
3.6. Stability of the Optimal Liposomes against Heat Treatment
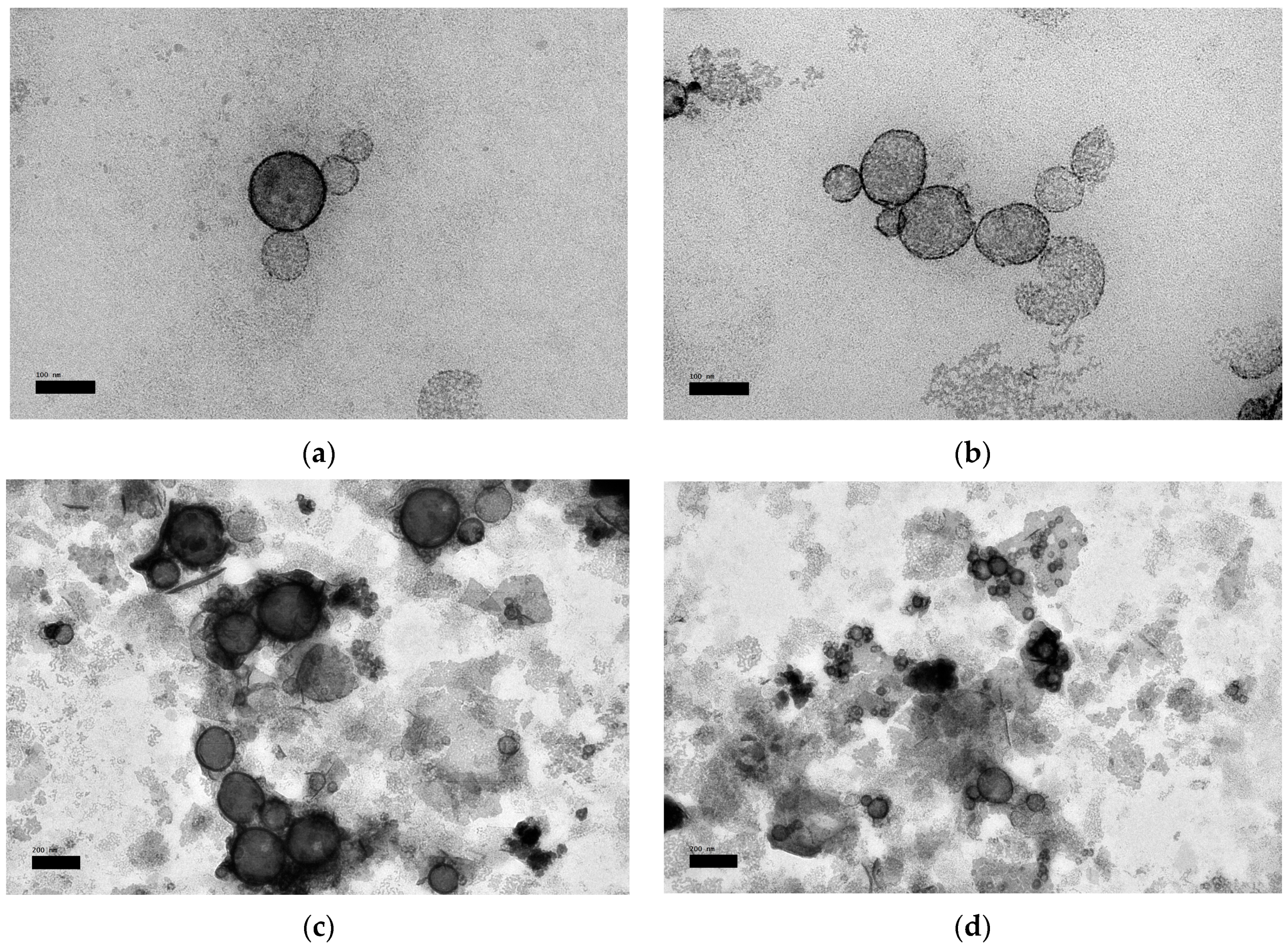
3.7. Morphological Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Inal, M.E.; Kahraman, A. The protective effect of flavonol quercetin against ultraviolet a induced oxidative stress in rats. Toxicology 2000, 154, 21–29. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Areias, F.M.; Rego, A.C.; Oliveira, C.R.; Seabra, R.M. Antioxidant effect of flavonoids after ascorbate/Fe2+-induced oxidative stress in cultured retinal cells. Biochem. Pharmacol. 2001, 62, 111–118. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug nanocrystals: In vivo performances—A review. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef]
- Frenzel, M.; Steffen-Heins, A. Impact of quercetin and fish oil encapsulation on bilayer membrane and oxidation stability of liposomes. Food Chem. 2015, 185, 48–57. [Google Scholar] [CrossRef]
- Akhtara, N.; Khan, R.A. Liposomal systems as viable drug delivery technology for skin cancer sites with an outlook on lipid-based delivery vehicles and diagnostic imaging inputs for skin conditions. Prog. Lipid Res. 2016, 64, 192–230. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.L.; Liu, C.M.; Liu, J.H.; Yang, S.B.; Zheng, H.J.; Lei, H.W.; Ruan, R.; Li, T.; Tu, Z.C. Medium-chain fatty acid nanoliposomes for easy energy supply. Nutrition 2011, 27, 700–706. [Google Scholar] [CrossRef]
- Zou, L.Q.; Peng, S.F.; Liu, W.; Gan, L.; Liu, W.L.; Liang, R.H.; Liu, C.M.; Niu, J.; Cao, Y.L.; Liu, Z. Improved in vitro digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Res. Int. 2014, 64, 492–499. [Google Scholar] [CrossRef]
- Laye, C.; McClements, D.; Weiss, J. Formation of biopolymer-coated liposomes by electrostatic deposition of chitosan. J. Food Sci. 2008, 73, N7–N15. [Google Scholar] [CrossRef] [PubMed]
- Biltonen, R.L.; Lichtenberg, D. The use of differential scanning calorimetry as a tool to characterize liposome preparations. Chem. Phys. Lipids 1993, 64, 129–142. [Google Scholar] [CrossRef]
- Gao, W.; Chen, L.; Wu, F.; Yu, Z. Liquid ordered phase of binary mixtures containing dipalmitoylphosphatidylcholine and sterols. Acta Phys. Chim. Sin. 2008, 24, 1149–1154. [Google Scholar] [CrossRef]
- Hodzic, A.; Rappolt, M.; Amenitsch, H.; Laggner, P.; Pabst, G. Differential modulation of membrane structure and fluctuations by plant sterols and cholesterol. Biophys. J. 2008, 94, 3935–3944. [Google Scholar] [CrossRef] [PubMed]
- Wenz, J.J.; Barrantes, F.J. Steroid structural requirements for stabilizing or disrupting lipid domains. Biochemistry 2003, 42, 14267–14276. [Google Scholar] [CrossRef]
- Schuler, I.; Duportail, G.; Glasser, N.; Benveniste, P.; Hartmann, M.-A. Soybean phosphatidylcholine vesicles containing plant sterols: A fluorescence anisotropy study. Biochim. Biophys. Acta Biomembr. 1990, 1028, 82–88. [Google Scholar] [CrossRef]
- Halwani, M.; Yebio, B.; Suntres, Z.; Alipour, M.; Azghani, A.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef]
- Mattson, F.H.; Grundy, S.M.; Crouse, J. Optimizing the effect of plant sterols on cholesterol absorption in man. Am. J. Clin. Nutr. 1982, 35, 697–700. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Richens, J.L.; Lane, J.S.; Mather, M.L.; O’Shea, P. The interactions of squalene, alkanes and other mineral oils with model membranes; effects on membrane heterogeneity and function. J. Colloid Interface Sci. 2015, 457, 225–231. [Google Scholar] [CrossRef]
- Ott, C.; Lacatusu, I.; Badea, G.; Grafu, I.A.; Istrati, D.; Babeanu, N.; Meghea, A. Exploitation of amaranth oil fractions enriched in squalene for dual delivery of hydrophilic and lipophilic actives. Ind. Crops Prod. 2015, 77, 342–352. [Google Scholar] [CrossRef]
- Tai, K.; He, X.; Yuan, X.; Meng, K.; Gao, Y.; Yuan, F. A comparison of physicochemical and functional properties of icaritin-loaded liposomes based on different surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 218–231. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Biophys. Acta Biomembr. 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; Paris, C.; Guedon, E.; Linder, M.; Desobry, S. Liposomal nanodelivery systems using soy and marine lecithin to encapsulate food biopreservative nisin. LWT-Food Sci. Technol. 2015, 62, 341–349. [Google Scholar] [CrossRef]
- Kumar, L.R.; Chatterjee, N.; Tejpal, C.; Vishnu, K.; Anas, K.; Asha, K.; Anandan, R.; Mathew, S. Evaluation of chitosan as a wall material for microencapsulation of squalene by spray drying: Characterization and oxidative stability studies. Int. J. Biol. Macromol. 2017, 104, 1986–1995. [Google Scholar] [CrossRef]
- Batzri, S.; Korn, E.D. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta Biomembr. 1973, 298, 1015–1019. [Google Scholar] [CrossRef]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-coated nanoliposomes. LWT-Food Sci. Technol. 2012, 85, 37–44. [Google Scholar] [CrossRef]
- Awah, F.M.; Uzoegwu, P.N.; Ifeonu, P.; Oyugi, J.O.; Rutherford, J.; Yao, X.; Fehrmann, F.; Fowke, K.R.; Eze, M.O. Free radical scavenging activity, phenolic contents and cytotoxicity of selected Nigerian medicinal plants. Food Chem. 2012, 131, 1279–1286. [Google Scholar] [CrossRef]
- Niki, E. Assessment of Antioxidant Capacity in vitro and in vivo: A review. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Effect of liposomal encapsulation on the recovery and antioxidant properties of green tea catechins incorporated into a hard low-fat cheese following in vitro simulated gastrointestinal digestion. Food Bioprod. Process. 2016, 100, 238–245. [Google Scholar] [CrossRef]
- Alexander, M.; Lopez, A.A.; Fang, Y.; Corredig, M. Incorporation of phytosterols in soy phospholipids nanoliposomes: Encapsulation efficiency and stability. LWT-Food Sci. Technol. 2012, 47, 427–436. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Preparation of liposomes using supercritical carbon dioxide technology: Effects of phospholipids and sterols. Food Res. Int. 2015, 77, 63–72. [Google Scholar] [CrossRef]
- Mannock, D.A.; Lewis, R.N.; McMullen, T.P.; McElhaney, R.N. The effect of variations in phospholipid and sterol structure on the nature of lipid–sterol interactions in lipid bilayer model membranes. Chem. Phys. Lipids 2010, 163, 403–448. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Hope, M.J.; Cullis, P.R. Generation of large unilamellar vesicles from long-chain saturated phosphatidylcholines by extrusion technique. Biochim. Biophys. Acta Biomembr. 1989, 986, 200–206. [Google Scholar] [CrossRef]
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef]
- Yin, M.C.; Faustman, C. Influence of temperature, pH, and phospholipid composition upon the stability of myoglobin and phospholipid: A liposome model. J. Agric. Food Chem. 1993, 41, 853–857. [Google Scholar] [CrossRef]
- Murari, R.; Murari, M.P.; Baumann, W.J. Sterol Orientations in Phosphatidylcholine Liposomes as Determined by Deuterium NMR. Biochemistry 1986, 25, 1062–1067. [Google Scholar] [CrossRef]
- Dufourc, E.J. Sterols and membrane dynamics: A review. J. Chem. Biol. 2008, 1, 63–77. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Bastiat, G.; Jin, C.; Keyvanloo, A.; Lafleur, M. Influence of the nature of the sterol on the behavior of palmitic acid/sterol mixtures and their derived liposomes. Biochim. Biophys. Acta Biomembr. 2010, 1798, 1144–1152. [Google Scholar] [CrossRef]
- Schuler, I.; Milon, A.; Nakatani, Y.; Ourisson, G.; Albrecht, A.-M.; Benveniste, P.; Hartman, M.-A. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA 1991, 88, 6926–6930. [Google Scholar] [CrossRef]
- Khelashvilia, G.; Harries, D. How sterol tilt regulates properties and organization of lipid membranes and membrane insertions. Chem. Phys. Lipids 2013, 169, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.; McElhaney, R.N. A comparative differential scanning calorimetry study of the effects of cholesterol and various oxysterols on the thermotropic phase behavior of dipalmitoylphosphatidylcholine bilayer membranes. Chem. Phys. Lipids 2016, 195, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wassall, S.R.; Stillwell, W. Docosahexaenoic acid domains: The ultimate non-raft membrane domain. Chem. Phys. Lipids 2008, 153, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, H.; Lin, Z.; Chen, D.; Zhu, Y.; Hou, S.; Shi, X. Quercetin deformable liposome: Preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2013, 127, 8–17. [Google Scholar] [CrossRef]
- Toniazzo, T.; Peres, M.S.; Ramos, A.P.; Pinho, S.C. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017, 19, 17–25. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef]
- Webb, M.S.; Irving, T.C.; Steponkus, P.L. Effects of plant sterols on the hydration and phase behavior of DOPE/DOPC mixtures. Biochim. Biophys. Acta Biomembr. 1995, 1239, 226–238. [Google Scholar] [CrossRef][Green Version]
- Driscoll, D.F. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959. [Google Scholar] [CrossRef]
- Cui, Z.K.; Lafleur, M. Lamellar self-assemblies of single-chain amphiphiles and sterols and their derived liposomes: Distinct compositions and distinct properties. Colloids Surf. B 2014, 114, 177–185. [Google Scholar] [CrossRef]
- McIntosh, T.J.; McDaniel, R.V.; Simon, S.A. Induction of an interdigitated gel phase in fully hydrated phosphatidylcholine bilayers. Biochim. Biophys. Acta Biomembr. 1983, 731, 109–114. [Google Scholar] [CrossRef]
- Tasi, L.M.; Liu, D.Z.; Chen, W.Y. Microcalorimetric investigation of the interaction of polysorbate surfactants with unilamellar phosphatidylcholines liposomes. Colloids Surf. A Physicochem. Eng. Asp. 2003, 213, 7–14. [Google Scholar] [CrossRef]
- Cadena, P.G.; Pereira, M.A.; Cordeiro, R.B.; Cavalcanti, I.M.; Neto, B.B.; Maria do Carmo, C.; Lima Filho, J.L.; Silva, V.L.; Santos-Magalhães, N.S. Nanoencapsulation of quercetin and resveratrol into elastic liposomes. Biochim. Biophys. Acta Biomembr. 2013, 1828, 309–316. [Google Scholar] [CrossRef]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Krajewski-Bertrand, M.A.; Milon, A.; Hartmann, M.A. Deuterium-NMR investigation of plant sterol effects on soybean phosphatidylcholine acyl chain ordering. Chem. Phys. Lipids 1992, 63, 235–241. [Google Scholar] [CrossRef]
- Demel, R.; Bruckdorfer, K.; Van Deenen, L. Structural requirements of sterols for the interaction with lecithin at the air-water interface. Biochim. Biophys. Acta Biomembr. 1972, 255, 311–320. [Google Scholar] [CrossRef]
- Ohvo-Rekilä, H.; Ramstedt, B.; Leppimäki, P.; Slotte, J.P. Cholesterol interactions with phospholipids in membranes: A review. Prog. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef]
- Silvius, J.R. Role of cholesterol in lipid raft formation: Lessons from lipid model systems. Biochim. Biophys. Acta Biomembr. 2003, 1610, 174–183. [Google Scholar] [CrossRef]
- Bernsdorff, C.; Winter, R. Differential properties of the sterols cholesterol, ergosterol, β-sitosterol, trans-7-dehydrocholesterol, stigmasterol and lanosterol on DPPC bilayer order. J. Phys. Chem. B 2003, 107, 10658–10664. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Wang, Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J. Agric. Food Chem. 2012, 60, 836–843. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, T.T.; Teng, Z.; Chen, P.; Sun, J.; Wang, Q. Encapsulation of indole-3-carbinol and 3, 3′-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. 2013, 139, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlęga, B.; Gruszecki, W.I.; Misiak, L.; Paduch, R.; Piersiak, T.; Zarzyka, B.; Pawelec, J.; Gawron, A. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X. Binding, stability, and antioxidant activity of quercetin with soy protein isolate particles. Food Chem. 2015, 188, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mishurov, D.; Voronkin, A.; Roshal, A.; Bogatyrenko, S. Influence of structure 3,5,7,3′,4′—Pentahydroxyflavone-based polymer films on their optical transparency. Opt. Mater. 2017, 64, 166–170. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L. Cholesterol autoxidation 1981–1986. Chem. Phys. Lipids 1987, 44, 87–125. [Google Scholar] [CrossRef]
- Smith, L.L. Review of progress in sterol oxidations: 1987–1995. Lipids 1996, 31, 453–487. [Google Scholar] [CrossRef]
- Soupas, L.; Huikko, L.; Lampi, A.M.; Piironen, V. Oxidative stability of phytosterols in some food applications. Eur. Food Res. Technol. 2006, 222, 266. [Google Scholar] [CrossRef]
- Fošnarič, M.; Iglič, A.; May, S. Influence of rigid inclusions on the bending elasticity of a lipid membrane. Phys. Rev. E 2006, 74, 051503. [Google Scholar] [CrossRef]
- Woutersen, A.; Emmerichs, U.; Bakker, H. Femtosecond mid-IR pump-probe spectroscopy of liquid water: Evidence for a two-component structure. Science 1997, 278, 658–660. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Herman, C.J.; Groves, M.J. The influence of free fatty acid formation on the pH of phospholipid-stabilized triglyceride emulsions. Pharm. Res. 1993, 10, 774–776. [Google Scholar] [CrossRef] [PubMed]
| Composition | Ingredients (%) |
|---|---|
| Phosphatidylcholine | 19–21 |
| Phosphatidylethanolamine | 8–20 |
| Inositol phosphatides | 20–21 |
| Other phosphatides | 5–11 |
| Soybean oil | 33–35 |
| Carbohydrates, free | 2–5 |
| Moisture | 1 |
| Formulation Code | Runs | Quercetin, QU (X1, % w/w) | β-Sitosterol, βS (X2, % w/w) | Stigmasterol, ST (X3, % w/w) |
|---|---|---|---|---|
| F1 | 1 | 0 | 0.2 | 0.4 |
| F2 | 2 | 0 | 0.2 | 0 |
| F3 | 3 | 0.06 | 0.2 | 0.4 |
| F4 | 4 | 0.03 | 0.4 | 0.4 |
| F5 | 5 | 0 | 0.4 | 0.2 |
| F6 | 6 | 0.06 | 0.2 | 0 |
| F7 | 7 * | 0.03 | 0.2 | 0.2 |
| F8 | 8 | 0.06 | 0.4 | 0.2 |
| F9 | 9 | 0.03 | 0.4 | 0 |
| F10 | 10 * | 0.03 | 0.2 | 0.2 |
| F11 | 11 | 0 | 0 | 0.2 |
| F12 | 12 * | 0.03 | 0.2 | 0.2 |
| F13 | 13 | 0.03 | 0 | 0.4 |
| F14 | 14 | 0.06 | 0 | 0.2 |
| F15 | 15 | 0.03 | 0 | 0 |
| Formulation Code | Particle Size, d (nm) | TEAC (µM) | ||
|---|---|---|---|---|
| - | Before | After | Before | After |
| F1 | 178 ± 1.45 | 211 ± 1.71 | 119.28 | 63.56 |
| F2 | 158 ± 2.36 | 177 ± 5.36 | 130.41 | 73.01 |
| F3 | 174 ± 1.78 | 204 ± 6.84 | 166.7 | 110.33 |
| F4 | 184 ± 4.12 | 221 ± 9.27 | 70.17 | 33.14 |
| F5 | 175 ± 6.19 | 202 ± 1.65 | 110.4 | 60.42 |
| F6 | 161 ± 3.24 | 182 ± 5.14 | 177.2 | 123.35 |
| F7 | 164 ± 8.17 | 189 ± 3.75 | 159.56 | 95.37 |
| F8 | 171 ± 4.67 | 197 ± 6.54 | 171.12 | 115.88 |
| F9 | 165 ± 3.39 | 188 ± 9.12 | 116.48 | 78.04 |
| F10 | 165 ± 7.51 | 190 ± 3.20 | 162.99 | 96.83 |
| F11 | 164 ± 4.84 | 190 ± 9.41 | 129.45 | 67.92 |
| F12 | 166 ± 9.22 | 193 ± 1.43 | 159.8 | 93.55 |
| F13 | 169 ± 7.43 | 197 ± 4.30 | 110.9 | 72.82 |
| F14 | 161 ± 5.36 | 187 ± 7.83 | 174.95 | 122.61 |
| F15 | 160 ± 4.55 | 189 ± 1.51 | 131.61 | 72.87 |
| Formulation Code | PDI | ZP (mV) | Particle Size Increase (%) | TEAC Loss (%) | EEQU (%) |
|---|---|---|---|---|---|
| F1 | 0.180 ± 0.0049 | −27.94 ± 1.33 | 18.53 | 46.71 | - |
| F2 | 0.177 ± 0.0028 | −33.33 ± 0.81 | 12.02 | 44.01 | - |
| F3 | 0.192 ± 0.0035 | −42.47 ± 2.51 | 17.24 | 33.81 | 65.74 ± 1.82 |
| F4 | 0.253 ± 0.0063 | −30.35 ± 1.32 | 20.1 | 52.77 | 33.41 ± 2.19 |
| F5 | 0.199 ± 0.0063 | −23.28 ± 2.63 | 15.42 | 45.27 | - |
| F6 | 0.177 ± 0.0035 | −39.76 ± 0.67 | 13.04 | 30.38 | 77.13 ± 2.84 |
| F7 | 0.181 ± 0.0028 | −33.49 ± 1.81 | 15.24 | 40.22 | 50.76 ± 0. 67 |
| F8 | 0.188 ± 0.0056 | −42.82 ± 0.41 | 15.2 | 32.28 | 69.59 ± 3.20 |
| F9 | 0.176 ± 0.0042 | −36.35 ± 0.94 | 13.93 | 33 | 47.76 ± 0.91 |
| F10 | 0.207 ± 0.0035 | −33.36 ± 2.16 | 15.15 | 40.59 | 52.19 ± 1.39 |
| F11 | 0.211 ± 0.0063 | −34.05 ± 1.39 | 15.85 | 47.53 | - |
| F12 | 0.228 ± 0.0091 | −33.71 ± 0.80 | 16.26 | 41.45 | 51.86 ± 3.44 |
| F13 | 0.194 ± 0.0035 | −35.29 ± 2.18 | 16.56 | 34.33 | 30.22 ± 0.49 |
| F14 | 0.219 ± 0.0084 | −39.05 ± 0. 73 | 16.14 | 29.91 | 73.19 ± 2.87 |
| F15 | 0.230 ± 0.0028 | −30.88 ± 1.39 | 18.12 | 44.63 | 42.51 ± 3.17 |
| Source | Particle Size (nm) | ZP (mV) | EEQU (%) | TEAC (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | F Ratio | p Value | Coefficient | F Ratio | p Value | Coefficient | F Ratio | p Value | Coefficient | F Ratio | p Value | |
| Model | - | - | - | - | - | - | - | - | - | - | - | - |
| Linear | - | - | - | - | - | - | - | - | - | - | - | - |
| b1 | −0.000 | 0.00 | 1.000 c | −130.33 | 5.03 | 0.075 c | 586.82 | 28.79 | 0.003 a | −190.4 | 0.23 | 0.653 c |
| b2 | −8.125 | 0.79 | 0.414 c | −2.32 | 0.07 | 0.801 c | 69.34 | 17.86 | 0.008 | 240.8 | 16.21 | 0.010 |
| b3 | 11.875 | 1.69 | 0.250 c | −3.64 | 0.17 | 0.693 c | 49.79 | 9.21 | 0.029 | 233.7 | 15.26 | 0.011 |
| Quadratic | - | - | - | - | - | - | - | - | - | - | - | - |
| b11 | 555.556 | 0.47 | 0.522 c | 2187.50 | 8.04 | 0.036 b | 3880.09 | 7.15 | 0.044 c | 14891.2 | 7.92 | 0.037 b |
| b22 | 56.250 | 9.59 | 0.027 b | −17.22 | 0.98 | 0.367 c | −151.70 | 21.57 | 0.006 | −692.6 | 33.83 | 0.002 a |
| b33 | 56.250 | 9.59 | 0.027 b | 9.66 | 0.31 | 0.602 c | −176.51 | 29.21 | 0.003 a | −644.7 | 29.31 | 0.003 |
| Interaction | - | - | - | - | - | - | - | - | - | - | - | - |
| b12 | −41.667 | 0.13 | 0.735 c | 605.83 | 29.69 | 0.003 a | −117.92 | 0.32 | 0.597 c | 634.2 | 0.69 | 0.444 c |
| b13 | −291.667 | 6.28 | 0.054 c | 337.50 | 9.21 | 0.029 | −166.67 | 0.63 | 0.462 c | 26.2 | 0.00 | 0.974 c |
| b23 | 62.500 | 12.82 | 0.016 a | −65.06 | 15.22 | 0.011 | −12.88 | 0.17 | 0.699 c | −160.0 | 1.96 | 0.221 c |
| Lack of fit | - | 2.58 | 0.291 c | - | 94.12 | 0.011 | - | 18.07 | 0.053 c | - | 37.41 | 0.026 |
| R2 | 98.73% | - | - | 97.71% | - | - | 99.35% | - | - | 96.88% | - | - |
| Adj-R2 | 96.43% | - | - | 93.59% | - | - | 98.18% | - | - | 91.27% | - | - |
| precision | - | - | - | - | - | - | - | - | - | - | - | - |
| Source | PDI | Particle Size Increase (%) | TEAC Loss (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | F Ratio | p Value | Coefficient | F Ratio | p Value | Coefficient | F Ratio | p Value | |
| Model | - | - | - | - | - | - | - | - | - |
| Linear | - | - | - | - | - | - | - | - | - |
| b1 | 1.1903 | 2.50 | 0.174 c | 84.92 | 3.46 | 0.122 c | −134.06 | 4.79 | 0.080 c |
| b2 | −0.2946 | 6.81 | 0.048 b | −20.65 | 9.09 | 0.030 b | −41.30 | 20.20 | 0.006 |
| b3 | −0.0452 | 0.16 | 0.705 c | −3.11 | 0.21 | 0.669c | −30.80 | 11.23 | 0.020 |
| Quadratic | - | - | - | - | - | - | - | - | - |
| b11 | −18.2407 | 3.34 | 0.127 c | −1037.50 | 2.93 | 0.148 c | −2478.24 | 9.29 | 0.028 b |
| b22 | 0.3833 | 2.91 | 0.149 c | 25.91 | 3.61 | 0.116 c | 5.61 | 0.09 | 0.771 c |
| b33 | −0.1854 | 0.68 | 0.447 c | 14.78 | 1.18 | 0.328 c | 5.11 | 0.08 | 0.791 c |
| Interaction | - | - | - | - | - | - | - | - | - |
| b12 | −0.7917 | 0.30 | 0.606 c | −21.25 | 0.06 | 0.817 c | 192.92 | 2.71 | 0.161 c |
| b13 | 0.5000 | 0.12 | 0.742 c | −96.25 | 1.21 | 0.321 | 30.42 | 0.07 | 0.806 c |
| b23 | 0.7063 | 10.71 | 0.022 a | 48.31 | 13.60 | 0.014 a | 187.94 | 15.22 | 0.000 a |
| Lack of fit | - | 0.23 | 0.870 c | - | 4.15 | 0.200 c | - | 7.61 | 0.118 c |
| R2 | 80.52% | - | - | 90.96% | - | - | 98.60% | - | - |
| Adj−R2 | 45.46% | - | - | 74.69% | - | - | 96.08% | - | - |
| CV | - | - | - | - | - | - | - | - | - |
| Adequate | - | - | - | - | - | - | - | - | - |
| precision | - | - | - | - | - | - | - | - | - |
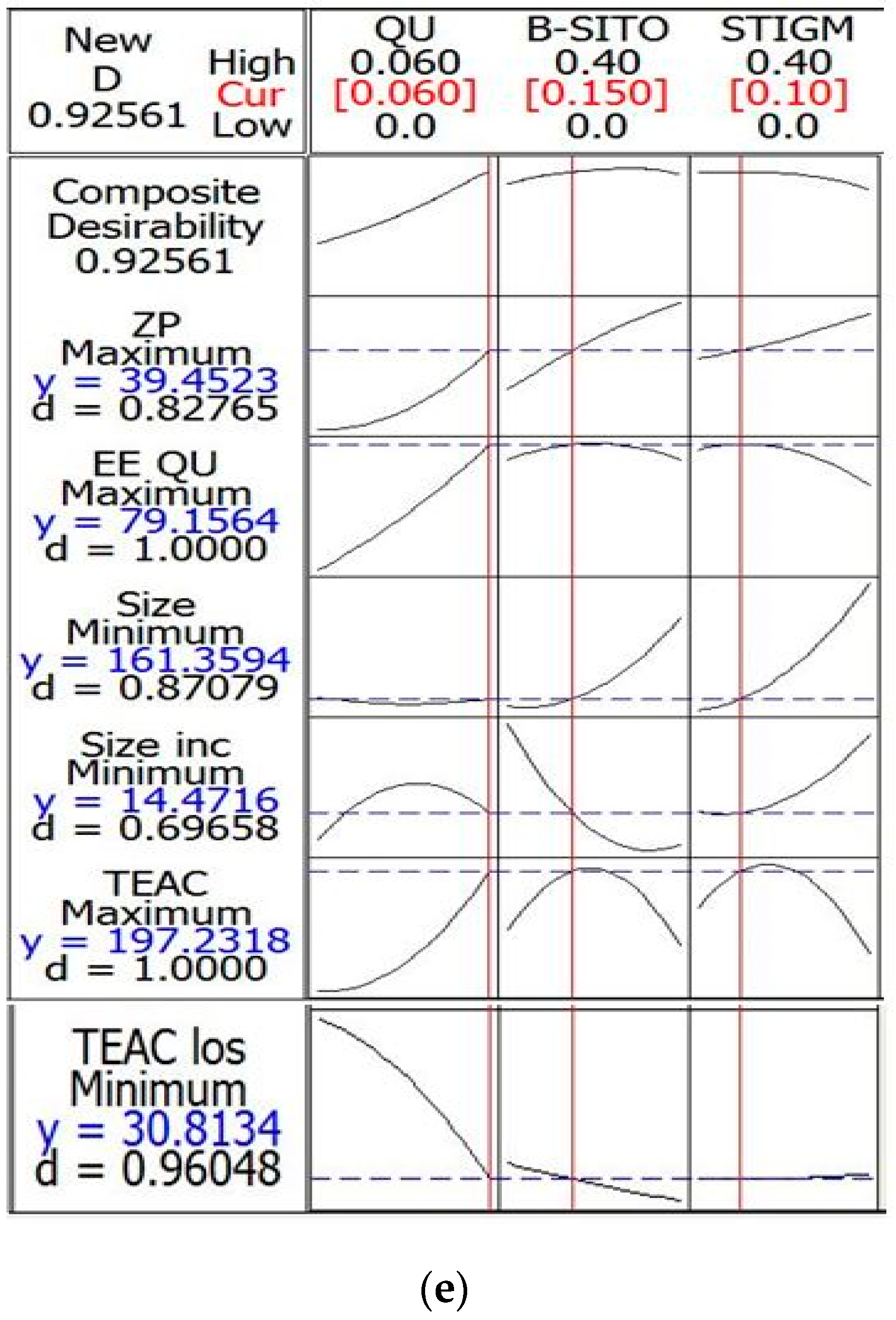
| Response Variables | Experimental Value | Predicted Value | Desirability |
|---|---|---|---|
| Particle size (nm) ± SD | 161.69 ± 1.98 | 161.35 | 0.870 |
| ZP (mV) EE (%) | −37.85 ± 0.48 79.36 ± 2.93 | −39.45 79.15 | 0.827 1 |
| TEAC (µM) | 198.00 ± 3.40 | 197.23 | 1 |
| Particle size increase (%) | 16.28 ± 1.03 | 14.47 | 0.696 |
| TEAC loss (%) | 32.54 ± 1.93 | 30.81 | 0.960 |
| Composite | 0.925 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toopkanloo, S.P.; Tan, T.B.; Abas, F.; Alharthi, F.A.; Nehdi, I.A.; Tan, C.P. Impact of Quercetin Encapsulation with Added Phytosterols on Bilayer Membrane and Photothermal-Alteration of Novel Mixed Soy Lecithin-Based Liposome. Nanomaterials 2020, 10, 2432. https://doi.org/10.3390/nano10122432
Toopkanloo SP, Tan TB, Abas F, Alharthi FA, Nehdi IA, Tan CP. Impact of Quercetin Encapsulation with Added Phytosterols on Bilayer Membrane and Photothermal-Alteration of Novel Mixed Soy Lecithin-Based Liposome. Nanomaterials. 2020; 10(12):2432. https://doi.org/10.3390/nano10122432
Chicago/Turabian StyleToopkanloo, Sahar Pakbaten, Tai Boon Tan, Faridah Abas, Fahad A. Alharthi, Imededdine Arbi Nehdi, and Chin Ping Tan. 2020. "Impact of Quercetin Encapsulation with Added Phytosterols on Bilayer Membrane and Photothermal-Alteration of Novel Mixed Soy Lecithin-Based Liposome" Nanomaterials 10, no. 12: 2432. https://doi.org/10.3390/nano10122432
APA StyleToopkanloo, S. P., Tan, T. B., Abas, F., Alharthi, F. A., Nehdi, I. A., & Tan, C. P. (2020). Impact of Quercetin Encapsulation with Added Phytosterols on Bilayer Membrane and Photothermal-Alteration of Novel Mixed Soy Lecithin-Based Liposome. Nanomaterials, 10(12), 2432. https://doi.org/10.3390/nano10122432







