Simple New Method for the Preparation of La(IO3)3 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results
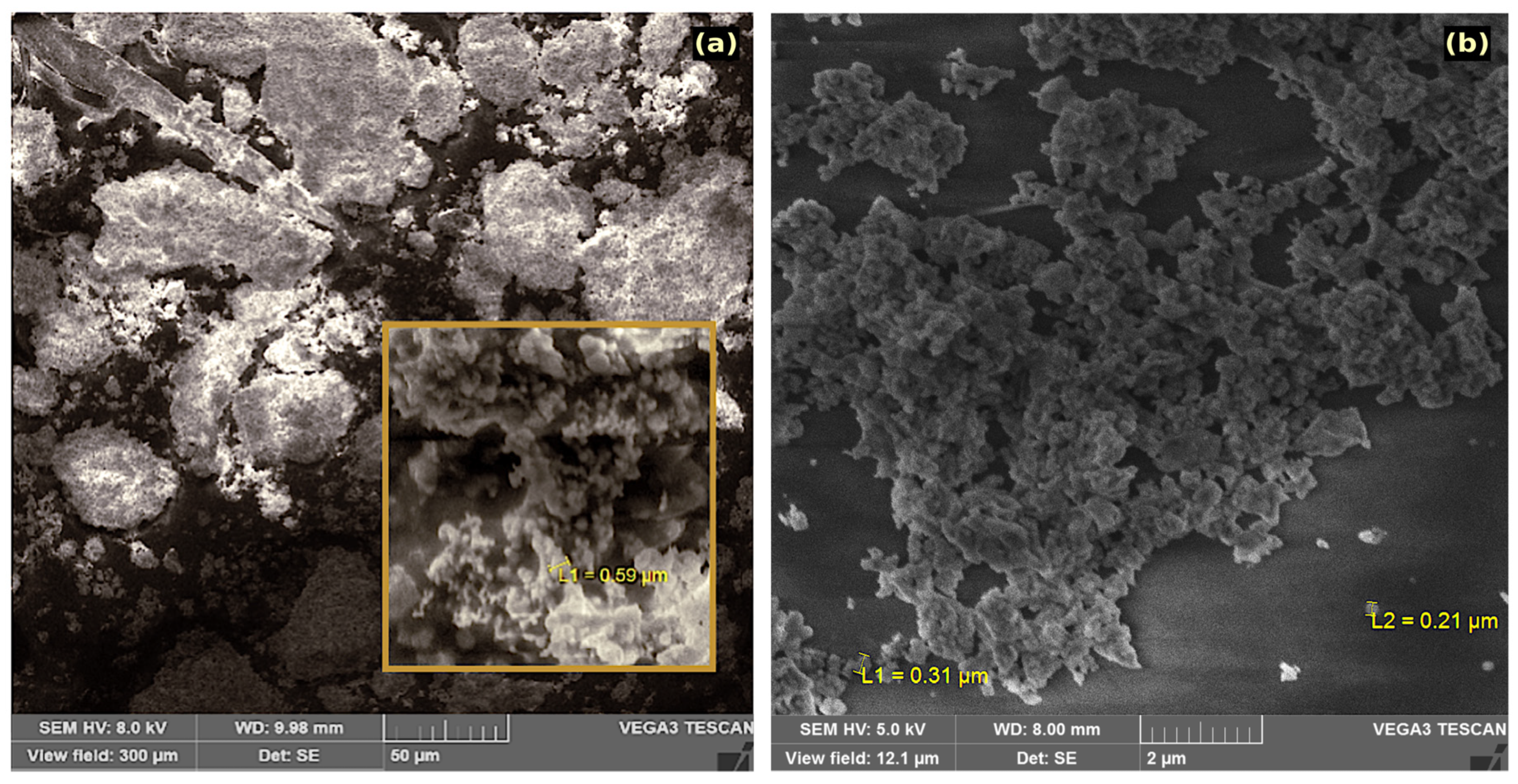
3.1. Morphology and Composition
3.2. Powder X-ray Diffraction
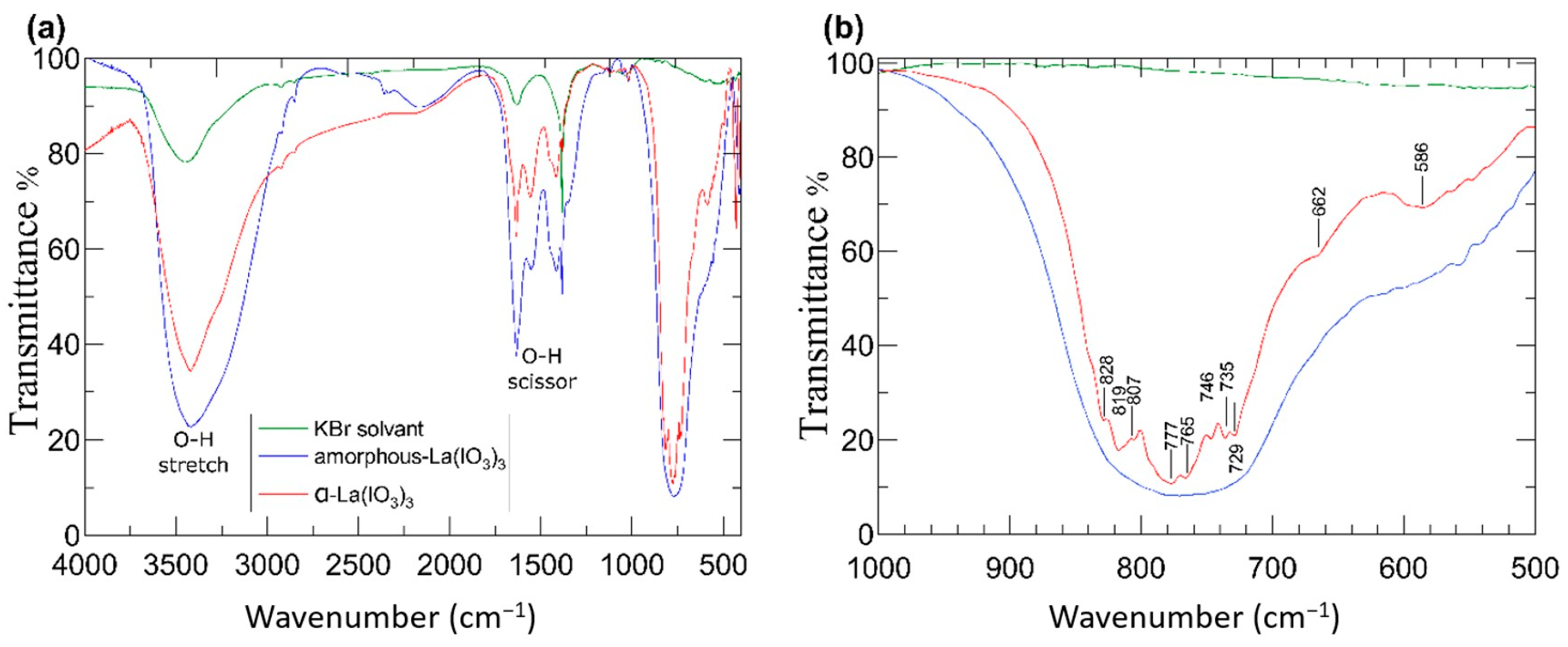
3.3. Fourier-Transform Infrared Spectroscopy
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Yang, L.; Chen, Z.G.; Shin, D.M. Application of nanotechnology in cancer therapy and imaging. CA Cancer J. Clin. 2008, 58, 97. [Google Scholar] [CrossRef]
- Chan, W.C.; Nie, S. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science 1998, 281, 2016. [Google Scholar] [CrossRef]
- Thorek, D.L.; Chen, A.K.; Czupryna, J.; Tsourkas, A. Superparamagnetic Iron Oxide Nanoparticle Probes for Molecular Imaging. Biomed. Eng. 2006, 34, 23. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, S.; Kang, E.; Kim, K.; Choi, K.; Kwon, I.C. New Generation of Multifunctional Nanoparticles for Cancer Imaging and Therapy. Adv. Funct. Mater. 2009, 19, 1553–1566. [Google Scholar] [CrossRef]
- Regny, S.; Riporto, J.; Mugnier, Y.; Le Dantec, R.; Kodjikian, S.; Pairis, S.; Gautier-Luneau, I.; Dantelle, G. Microwave Synthesis and Up-Conversion Properties of SHG-Active α-(La, Er)(IO3)3 Nanocrystals. Inorg. Chem. 2019, 58, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Riporto, J.; Demierre, A.; Kilin, V.; Balciunas, T.; Schmidt, C.; Campargue, G.; Urbain, M.; Baltuska, A.; Le Dantec, R.; Wolf, J.P.; et al. Bismuth ferrite dielectric nanoparticles excited at telecom wavelengths as multicolor sources by second, third, and fourth harmonic generation. Nanoscale 2018, 10, 8146–8152. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.L.; Hu, C.L.; Kong, F.; Mao, J.G. BiFSeO3: An Excellent SHG Material Designed by Aliovalent Substitution. J. Am. Chem. Soc. 2016, 138, 9433. [Google Scholar] [CrossRef] [PubMed]
- Chi, E.O.; Ok, K.M.; Porter, Y.; Halasyamani, P.S. Na2Te3Mo3O16: A New Molybdenum Tellurite with Second-Harmonic Generating and Pyroelectric Properties. Chem. Mater. 2006, 18, 2070–2074. [Google Scholar] [CrossRef]
- Liang, F.; Kang, L.; Lin, Z.; Wu, Y. Mid-Infrared Nonlinear Optical Materials Based on Metal Chalcogenides: Structure–Property Relationship. Cryst. Growth Des. 2017, 17, 2254. [Google Scholar] [CrossRef]
- Chang, H.Y.; Kim, S.H.; Halasyamani, P.S.; Ok, K.M. Alignment of Lone Pairs in a New Polar Material: Synthesis, Characterization, and Functional Properties of Li2Ti(IO3)6. J. Am. Chem. Soc. 2009, 131, 2426–2427. [Google Scholar] [CrossRef]
- Phanon, D.; Gautier-Luneau, I. Promising Material for Infrared Nonlinear Optics: NaI3O8 Salt Containing an Octaoxotriiodate(V) Anion Formed from Condensation of [IO3]− Ions. Angew. Chem. Int. Ed. 2007, 46, 8488. [Google Scholar] [CrossRef]
- Phanon, D.; Mosset, A.; Gautier-Luneau, I. New materials for infrared non-linear optics. Syntheses, structural characterisations, second harmonic generation and optical transparency of M(IO3)3 metallic iodates. J. Mater. Chem. 2007, 17, 1123. [Google Scholar] [CrossRef]
- Benghia, A.; Hebboul, Z.; Chikhaoui, R.; Khaldoun Lefkaier, I.; Chouireb, A.; Goumri-Said, S. Effect of iodic acid concentration in preparation of zinc iodate: Experimental characterization of Zn(IO3)2, and its physical properties from density functional theory. Vacuum 2020, 181, 109660. [Google Scholar] [CrossRef]
- Liang, A.; Rahman, S.; Rodriguez-Hernandez, P.; Muñoz, A.; Manjón, F.J.; Nenert, G.; Errandonea, D. High-Pressure Raman Study of Fe(IO3)3: Soft-Mode Behavior Driven by Coordination Changes of Iodine Atoms. J. Phys. Chem. C 2020, 124, 21329–21337. [Google Scholar] [CrossRef]
- Hu, C.L.; Mao, J.G. Recent advances on second-order NLO materials based on metal iodates. Coord. Chem. Rev. 2015, 288, 1–17. [Google Scholar] [CrossRef]
- Jia, Y.J.; Chen, Y.G.; Guo, Y.; Guan, X.F.; Li, C.; Li, B.; Liu, M.M.; Zhang, X.M. LiMII(IO3)3 (MII=Zn and Cd): Two Promising Nonlinear Optical Crystals Derived from a Tunable Structure Model of α-LiIO3. Angew. Chem. Int. Ed. 2019, 58, 17194–17198. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Pracht, G.; Lange, N.; Lutz, H.D. Z. Zinkiodate – Schwingungsspektren (IR, Raman) und Kristallstruktur von Zn(IO3)2 · 2H2O. Anorg. Allg. Chem. 2000, 626, 208. [Google Scholar] [CrossRef]
- Liang, A.; Rahman, S.; Saqib, H.; Rodriguez-Hernandez, P.; Muñoz, A.; Nénert, G.; Yousef, I.; Popescu, C.; Errandonea, D. First-Order Isostructural Phase Transition Induced by High Pressure in Fe(IO3)3. J. Phys. Chem. C 2020, 124, 8669–8679. [Google Scholar] [CrossRef]
- Bentria, B.; Benbertal, D.; Bagieu-Beucher, M.; Mosset, A.; Zaccaro, J. Crystal engineering strategy for quadratic nonlinear optics. Part II: Hg(IO3)2. Solid State Sci. 2003, 5, 359. [Google Scholar] [CrossRef]
- Hebboul, Z.; Galez, C.H.; Benbertal, D.; Beauquis, S.; Mugnier, Y.; Benmakhlouf, A.; Bouchenafa, M.; Errandonea, D. Synthesis, Characterization, and Crystal Structure Determination of a New Lithium Zinc Iodate Polymorph LiZn(IO3)3. Crystals 2019, 9, 464. [Google Scholar] [CrossRef]
- Ok, K.M.; Halasyamani, P.S. New Metal Iodates: Syntheses, Structures, and Characterizations of Noncentrosymmetric La(IO3)3 and NaYI4O12 and Centrosymmetric β-Cs2I4O11 and Rb2I6O15(OH)2·H2O. Inorg. Chem. 2005, 44, 9353–9359. [Google Scholar] [CrossRef] [PubMed]
- Taouti, M.B.; Suffren, Y.; Leynaud, O.; Benbertal, D.; Brenier, A.; Gautier-Luneau, I. Structures, Thermal Behaviors, and Luminescent Properties of Anhydrous Lanthanum Iodate Polymorphs. Inorg. Chem. 2015, 54, 3608–3618. [Google Scholar] [CrossRef] [PubMed]
- Taouti, M.B.; Gacemi, A.; Benbertal, D.; Gautier-Luneau, I. Crystal structure of lanthanum triiodate iodic acid, La(IO3)3(HIO3). Z. Kristallogr. New Cryst. Struct. 2008, 223, 179–180. [Google Scholar] [CrossRef]
- Bachir, B.; Djamal, B.; Zoulikha, H.; Muriel, B.-B.; Alain, M. Polymorphism of Anhydrous Cadmium Iodate Structure of ε-Cd(IO3)2. Z. Anorg. Chem. 2005, 63, 894–901. [Google Scholar] [CrossRef]
- Hebboul, Z.; Benbertal, D. Synthesis and Characterization of New Anhydrous Cadmium Iodate zeta polymorph ζ-Cd(IO3)2. J. Mater. Environ. Sci. 2018, 9, 565. [Google Scholar] [CrossRef]
- Pereira, A.L.J.; Sans, J.A.; Manjón, F.J.; Errandonea, D.; García-Domene, B.; Miquel-Veyrat, A.; Beltrán, A.; Gracia, L.; Gomis, O.; Muñoz, A.; et al. Structural study of α-Bi2O3 under pressure. J. Phys. Condens. Matter 2013, 25, 475402. [Google Scholar] [CrossRef]
- Panchal, V.; Errandonea, D.; Segura, A.; Rodriguez-Hernandez, P.; Muñoz, A.; Lopez-Moreno, S.; Bettinelli, M. The electronic structure of zircon-type orthovanadates: Effects of high-pressure and cation substitution. J. Appl. Phys. 2011, 110, 043723. [Google Scholar] [CrossRef]
- Sykora, R.E.; Ok, K.M.; Halasyamani, P.S.; Wells, D.M.; Albrecht-Schmitt, T.E. New One-Dimensional Vanadyl Iodates: Hydrothermal Preparation, Structures, and NLO Properties of A[VO2(IO3)2] (A = K, Rb) and A[(VO)2(IO3)3O2] (A = NH4, Rb, Cs). Chem. Mater. 2002, 14, 2741. [Google Scholar] [CrossRef]
- Sykora, R.E.; Ok, K.M.; Halasyamani, P.S.; Albrecht-Schmitt, T.E. Structural Modulation of Molybdenyl Iodate Architectures by Alkali Metal Cations in AMoO3(IO3) (A = K, Rb, Cs): A Facile Route to New Polar Materials with Large SHG Responses. J. Am. Chem. Soc. 2002, 124, 1951. [Google Scholar] [CrossRef]
- Shehee, T.C.; Sykora, R.E.; Ok, K.M.; Halasyamani, P.S.; Albrecht-Schmitt, T.E. Hydrothermal Preparation, Structures, and NLO Properties of the Rare Earth Molybdenyl Iodates, RE(MoO2)(IO3)4(OH) [RE = Nd, Sm, Eu]. Inorg. Chem. 2003, 42, 457. [Google Scholar] [CrossRef]
- Crettez, J.M.; Gard, R.; Remoissenet, M. Near and far infrared investigations from α and β lithium iodate crystals. Solid State Commun. 1972, 11, 951–954. [Google Scholar] [CrossRef]
- Sykora, R.E.; Khalifah, P.; Assefa, Z.; Albrecht-Schmitt, T.E.; Haire, R.G. Magnetism and Raman spectroscopy of the dimeric lanthanide iodates Ln(IO3)3 (Ln=Gd, Er) and magnetism of Yb(IO3)3. J. Solid State Chem. 2008, 181, 1867–1875. [Google Scholar] [CrossRef]
- Errandonea, D.; Munoz, A.; Rodriguez-Hernandez, P.; Proctor, J.E.; Sapina, F.; Bettinelli, M. Theoretical and Experimental Study of the Crystal Structures, Lattice Vibrations, and Band Structures of Monazite-Type PbCrO4, PbSeO4, SrCrO4, and SrSeO4. Inorg. Chem. 2015, 54, 7524–7535. [Google Scholar] [CrossRef] [PubMed]


| Polymorph | Synthesis Conditions | SG | Unit-Cell Parameters | Ref. |
|---|---|---|---|---|
| α-La(IO3)3 | Hydrothermal treatment, 220 °C (4 days): La2O3 + 14 HIO3 in water | Cc | a = 12.526(2) Å, b = 7.0939(9) Å, c = 27.823(4) Å, β = 101.975(4)° | [21] |
| Hydrothermal treatment, 220 °C (4 days): LaCl3∙6H2O + 4 HIO3 in water | a = 12.4920 Å, b = 7.0720 Å, c = 27.7270 Å, β = 102° | [22] | ||
| Nanocrystals Microwave-assisted hydrothermal method, 250 °C (1 h) LaCl3 + 3 HIO3 in water | a = 12.5454 Å, b = 7.0939 Å, c = 27.8304 Å, β = 102.044° | [5] | ||
| Nanocrystals Present work | a = 12.57(1) Å, b = 7.102(7) Å, c = 27.69(3) Å, β = 101.7(1)° | This work | ||
| β-La(IO3)3 | Thermal decomposition at 490 °C: La(IO3)3-(HIO3) or La(IO3)3-(HIO3)1.33 | P21 | a = 7.2539(4) Å, b = 8.5360(5) Å, c = 13.5018(7) Å, β = 97.499(2)° | [22] |
| γ-La(IO3)3 | Reversible transition from β-La(IO3)3 at 140 °C. Cannot be recovered at room temperature | P21/c | a = 7.3427(9) Å, b = 8.684(1) Å, c = 13.741(2) Å, β = 99.913(8)° | [22] |
| δ-La(IO3)3 | Thermal decomposition at 300 °C: La(IO3)3-(HIO3) Thermal decomposition at 340 °C La(IO3)3-(HIO3)1.33 | Pmmm | a = 10.3646(6) Å, b = 10.3758(6) Å, c =15.4933(6) Å | [22] |
| Nanocrystals Present work | a = 10.35(1) Å, b = 10.36(1) Å, c =15.45(2) Å | This work |
| Reaction | Reagents | Temperature, Time of Reaction | Yield % | Product 1 | Heat Treatment Temp, Time | Product 2 |
|---|---|---|---|---|---|---|
| A | La(NO3)3∙6H2O + 3NaIO3 (0.43g) (0.59g) | Room temperature, spontaneous | 84.5 | amorphous-La(IO3)3 | 400 °C, 2 h | Nanopowder α-La(IO3)3 |
| B | La(NO3)3∙6H2O + 3HIO3 (0.43g) (0.53g) | 60 °C, 3 days | 56.6 | amorphous-La(IO3)3 | 400 °C, 2 h | Nanopowder δ-La(IO3)3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hebboul, Z.; Ghozlane, A.; Turnbull, R.; Benghia, A.; Allaoui, S.; Liang, A.; Errandonea, D.; Touhami, A.; Rahmani, A.; Lefkaier, I.K. Simple New Method for the Preparation of La(IO3)3 Nanoparticles. Nanomaterials 2020, 10, 2400. https://doi.org/10.3390/nano10122400
Hebboul Z, Ghozlane A, Turnbull R, Benghia A, Allaoui S, Liang A, Errandonea D, Touhami A, Rahmani A, Lefkaier IK. Simple New Method for the Preparation of La(IO3)3 Nanoparticles. Nanomaterials. 2020; 10(12):2400. https://doi.org/10.3390/nano10122400
Chicago/Turabian StyleHebboul, Zoulikha, Amira Ghozlane, Robin Turnbull, Ali Benghia, Sara Allaoui, Akun Liang, Daniel Errandonea, Amina Touhami, Abdellah Rahmani, and Ibn Khaldoun Lefkaier. 2020. "Simple New Method for the Preparation of La(IO3)3 Nanoparticles" Nanomaterials 10, no. 12: 2400. https://doi.org/10.3390/nano10122400
APA StyleHebboul, Z., Ghozlane, A., Turnbull, R., Benghia, A., Allaoui, S., Liang, A., Errandonea, D., Touhami, A., Rahmani, A., & Lefkaier, I. K. (2020). Simple New Method for the Preparation of La(IO3)3 Nanoparticles. Nanomaterials, 10(12), 2400. https://doi.org/10.3390/nano10122400





