Microstructure and Properties of Mechanical Alloying Al-Zr Coating by High Current Pulsed Electron Beam Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparations and Zr Coating on Al
2.2. Microstructural Characterization
2.3. Properties Testing
3. Results and Discussion
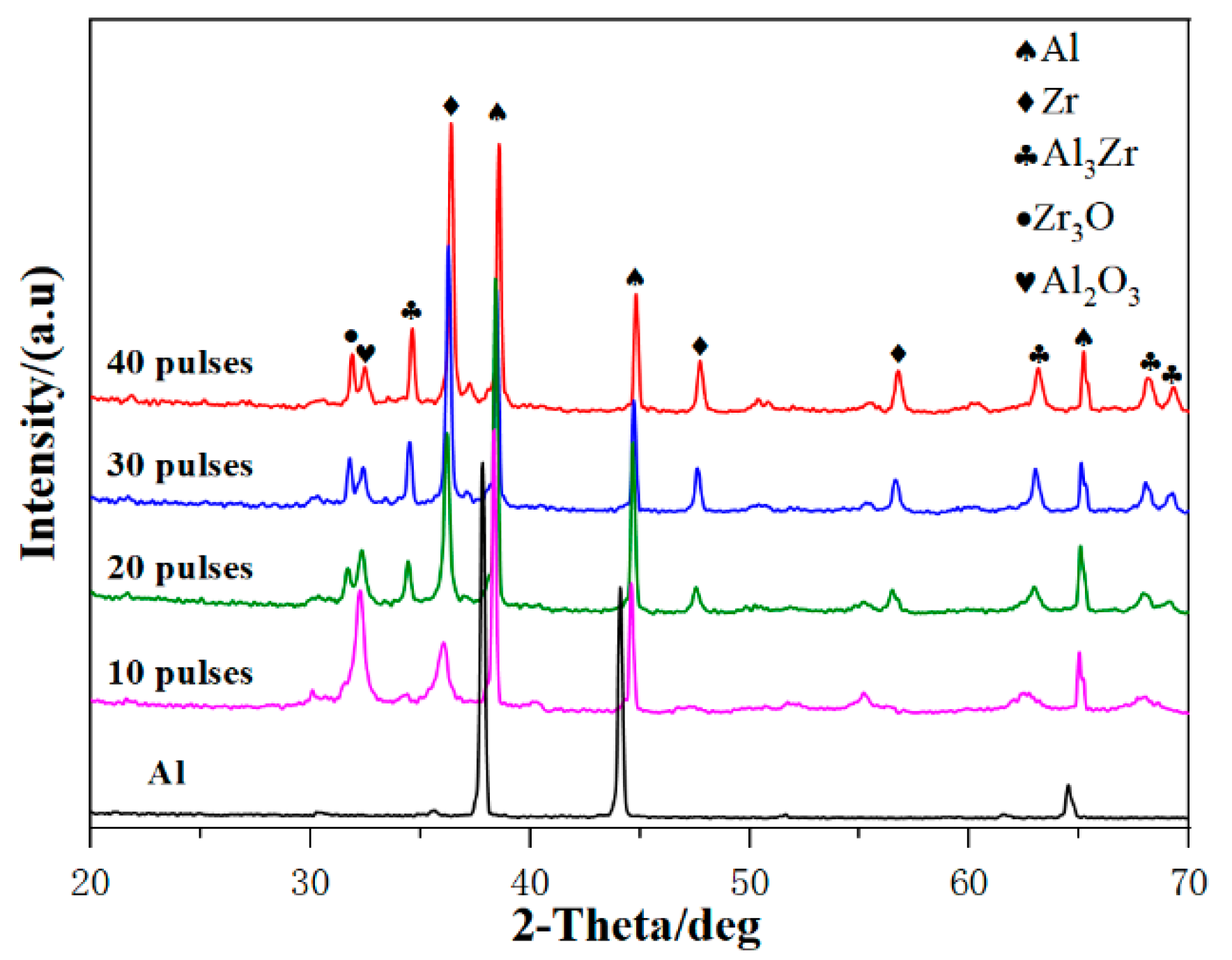
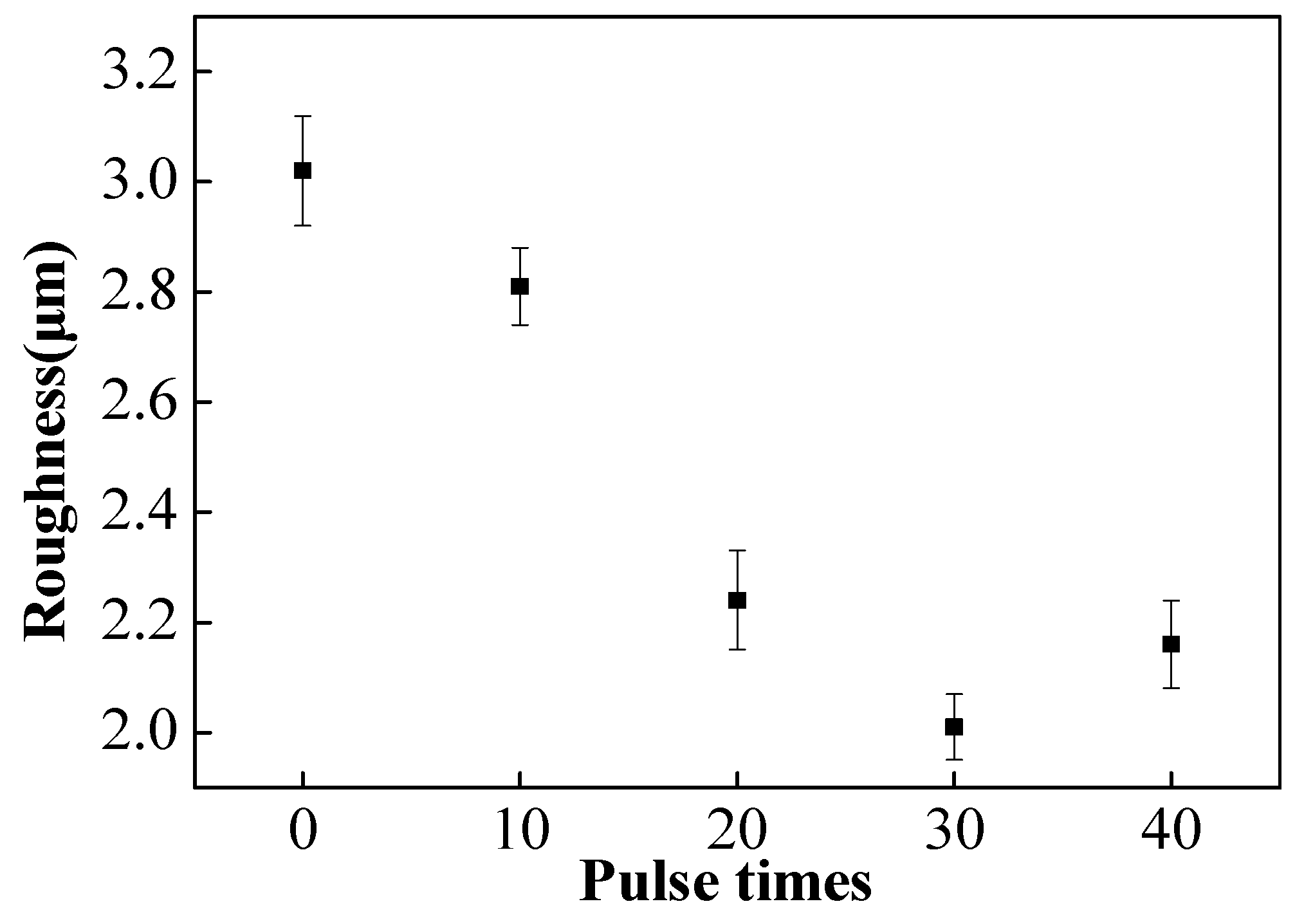
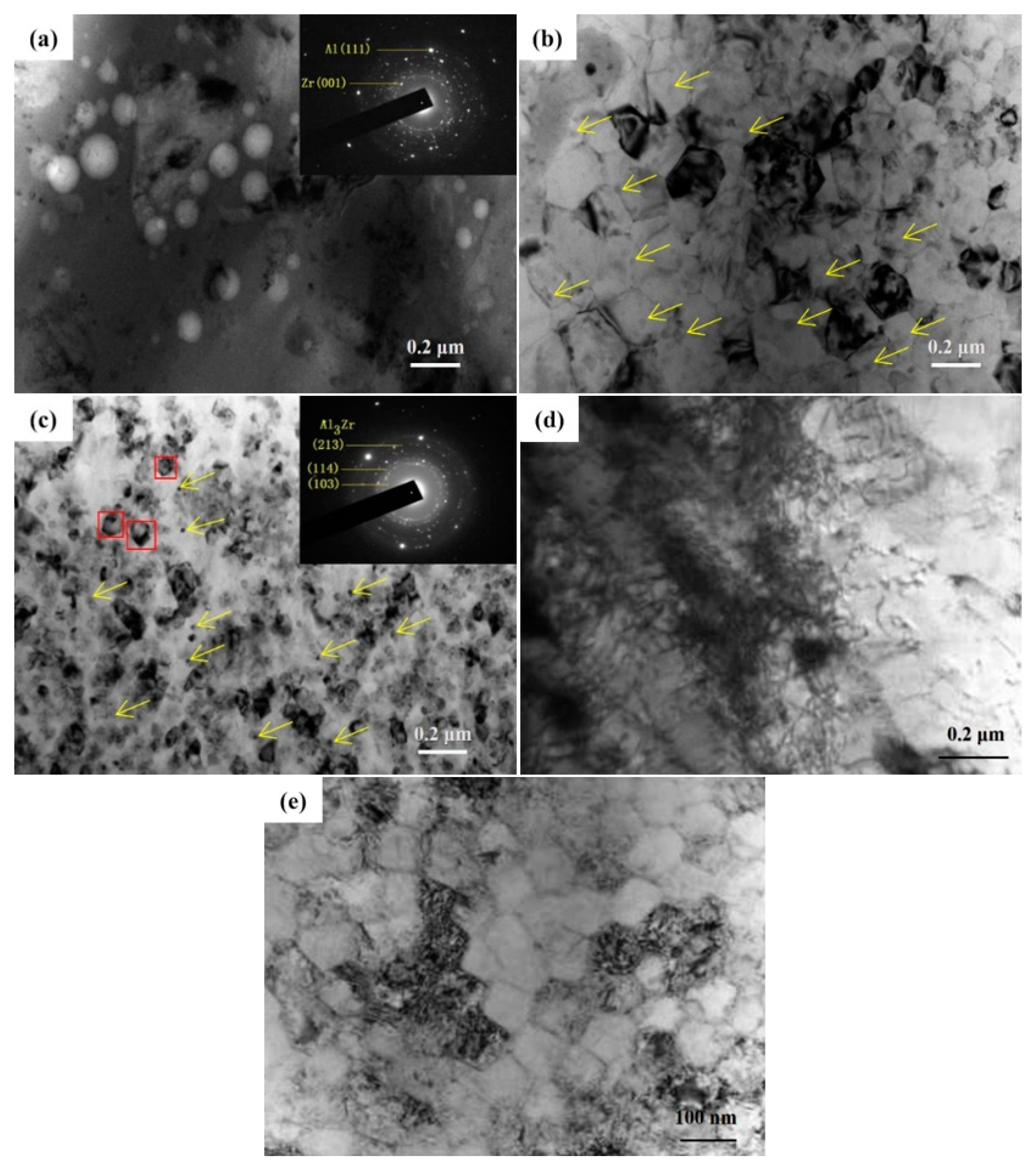
3.1. Microstructure Characterization
3.2. Microstructure Characterization
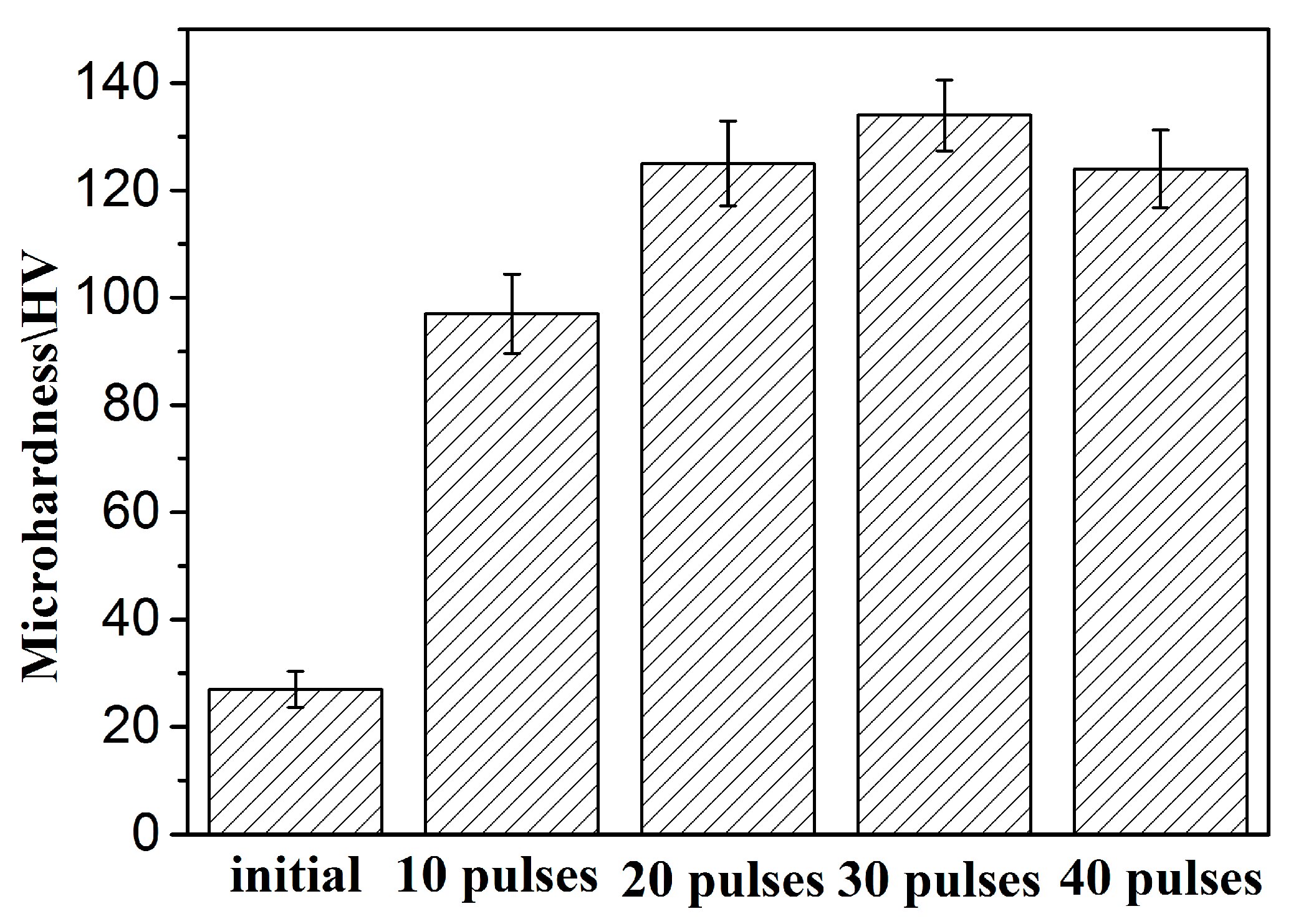
- The main strengthening mechanism of samples irradiated with 10 pulses was the dispersion strengthening of nanoscale Zr particles.
- After 20 and 30 pulses of irradiation, the Al grains were significantly refined, and the solid solubility of Zr in Al was greatly improved. Therefore, the fine-grain strengthening and solid-solution strengthening were the main strengthening mechanisms at this time.
- The HCPEB alloyed sample showed well dispersed Al3Zr phases. Moreover, the defect structures and nano gains were also observed in the alloying layer after 30-pulsed irradiation. Thus, the combined effects of the nanoscale Al3Zr phases, nanograins, and defect structures are responsible for the hardness improvement for 30-pulsed irradiated samples.
- The decrease of the hardness after 40-pulsed irradiation was related to the exfoliation of the Zr coating and the release of residual stresses [30].
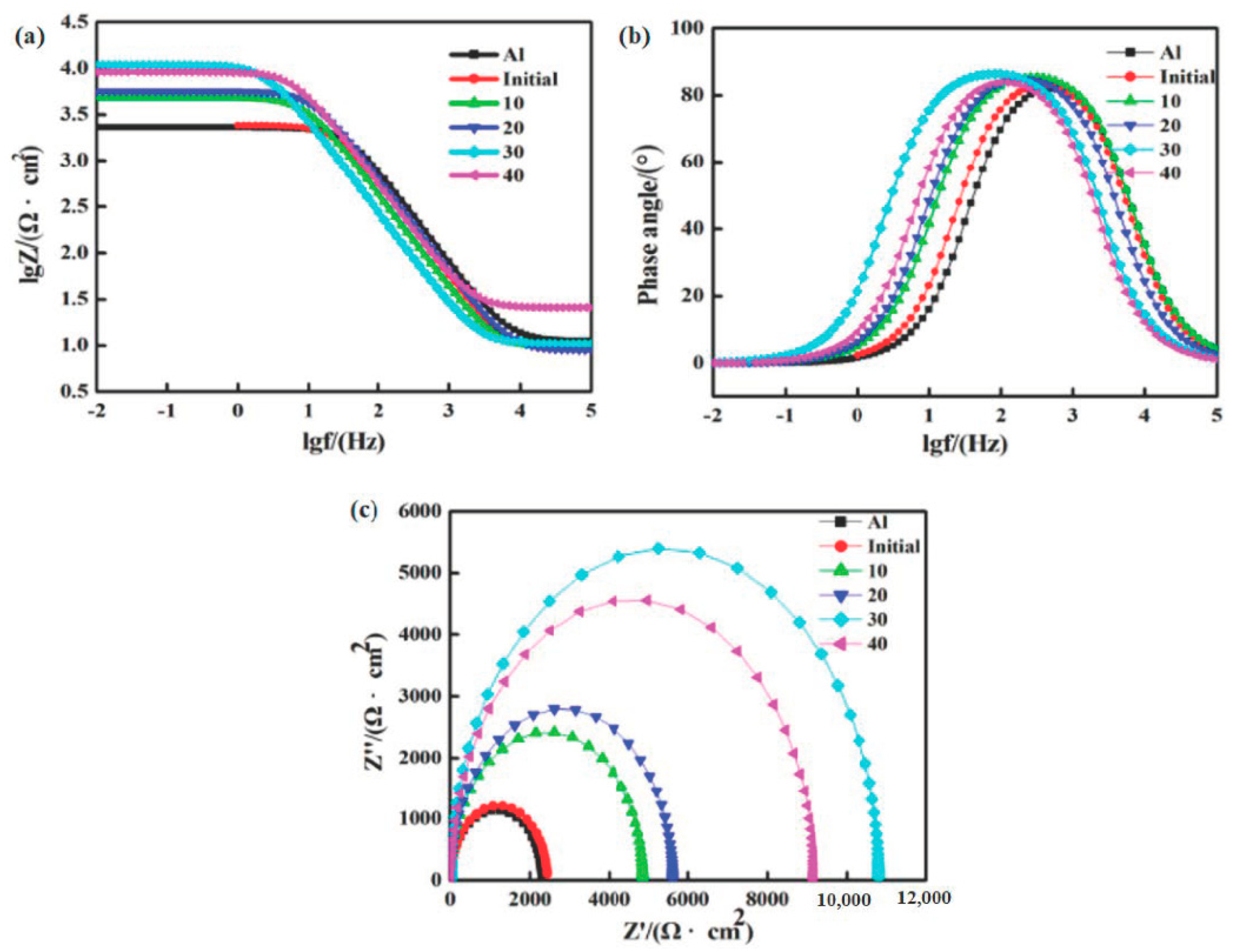
3.3. Corrosion Performance Analysis
- After HCPEB irradiation, high-density crystal defects were formed inside the crystal grains. A large number of crystalline defects can provide a channel for oxygen ions to enter the sample, resulting in the formation of a dense Al2O3 film on the surface, which improves the corrosion resistance of the sample [31].
- After HCPEB irradiation, the Al grains were significantly refined. Wang et al. [27] found that for Al and its alloys, when the grains are refined, the number of active atoms on the sample surface increases, which can promote the generation of a passivation film on the sample surface and thus improve the corrosion resistance of the material. Therefore, HCPEB alloying treatment is very helpful in improving the corrosion resistance of the sample surface.
- After 30-pulsed irradiation, a large number of diffuse and uniformly distributed Ll2-type Al3Zr phases was formed on the sample surface. This helped to improve the corrosion resistance of the sample surface [11].
4. Conclusions
- The HCPEB surface alloying treatment promoted the solid solution of Zr elements in the Al matrix and achieved Zr alloying on the surface of the Al matrix, forming a Zr–Al alloying layer of several micrometers. Among them, 30-pulsed irradiation can produce an alloying layer with a relatively good surface condition on the sample surface.
- After the HCPEB irradiation, the surface of the samples showed obvious grain refinement and uniform composition distribution. In addition, a large number of Ll2-type Al3Zr phases with small size and diffuse uniform distribution appeared in the samples after 30-pulsed irradiation.
- Surface microhardness of samples treated by HCPEB irradiation improved significantly, which was mainly the result of fine crystal strengthening, solid solution strengthening, dispersion strengthening, and dislocation strengthening. Among them, 30-pulsed irradiation of the sample surface hardness was the highest.
- After HCPEB surface alloying, the corrosion resistance improved dramatically due to the crystal defects, significant refinement of Al grains, and generation of a diffusely distributed Al3Zr phase on the top of the surface. The corrosion resistance of the samples after 30-pulsed irradiation treatment was the best.
Author Contributions
Funding
Conflicts of Interest
References
- Karunakaran, M.; Pugazh Vadivu, M. Magnetic and micro-mechanical behavior of Cu-Ni-P-W-TiO2 hybrid composite electroplating on Al alloy substrate. J. Magn. Magn. Mater. 2019, 475, 359–367. [Google Scholar] [CrossRef]
- Kang, R.; Peng, Z.; Liu, B.; Wang, D.; Liang, J. A protocol for fast electroless Ni-P on Al alloy at medium-low temperature accelerated by hierarchically structured Cu immersion layer. Surf. Coatings Technol. 2017, 309, 67–74. [Google Scholar] [CrossRef]
- Wang, L.S.; Bu, Z.X.; Lu, M.; Geng, Y.; Chen, M.H.; Sun, L. Thick oxide coatings formed by spark anodizing of Mg-Al alloy in alkaline phosphate-silicate electrolytes. J. Alloys Compd. 2017, 710, 121–129. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Yang, Z.; Li, S.; Wu, T.; Liu, G. Fabrication of polydimethylsiloxane-derived superhydrophobic surface on aluminium via chemical vapour deposition technique for corrosion protection. Corros. Sci. 2017, 128, 176–185. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Zhang, M.-X.; Niu, W.-J.; Tang, C.-B.; Wang, K.-S.; Rui, X.; Zhai, L.; Wang, L. The influence of cold and detonation thermal spraying processes on the microstructure and properties of Al-based composite coatings on Mg alloy. Surf. Coat. Technol. 2018, 352, 627–633. [Google Scholar] [CrossRef]
- Phanikumar, G.; Dutta, P.; Galun, R.; Chattopadhyay, K. Microstructural evolution during remelting of laser surface alloyed hyper-monotectic Al–Bi alloy. Mater. Sci. Eng. A 2004, 371, 91–102. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, J. Solidification of monotectic alloy under laser surface treatment conditions. Acta Metall. Sin. 2013, 49, 537. [Google Scholar] [CrossRef]
- Li, H.; Liang, X.; Li, F.; Guo, F.; Li, Z.; Zhang, X. Effect of Y content on microstructure and mechanical properties of 2519 aluminum alloy. Trans. Nonferr. Met. Soc. China 2007, 17, 1194–1198. [Google Scholar] [CrossRef]
- Nayak, S.S.; Murty, B.S. Synthesis of nanocrystalline L12-Al3Zr and Al3Ti by mechanical alloying. Trans. Indian Inst. Met. 2003, 56, 457–463. [Google Scholar]
- Fang, L.; Zhang, Z.; Fang, H.; Huang, L.; Chen, K. Effects of Si additions on the precipitation evolution of dilute Al-Zr-Yb alloys. Mater. Charact. 2019, 152, 130–133. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Robson, J.D. Heterogeneous Zr solute segregation and Al3Zr dispersoid distributions in Al–Cu–Li alloys. Acta Mater. 2015, 93, 73–86. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, M.; Wu, X.; Jia, Z.; Wang, R.; Li, W.; Liu, Q. The structural stability, mechanical properties and stacking fault energy of Al3Zr precipitates in Al-Cu-Zr alloys: HRTEM observations and first-principles calculations. J. Alloys Compd. 2016, 681, 96–108. [Google Scholar] [CrossRef]
- Jiao, L.; Zhao, Y.-T.; Chen, J.-C.; Chen, L. Microstructure and properties of Al3Zr/2024Al in situ composites after forging. Rare Met. 2016, 35, 920–925. [Google Scholar] [CrossRef]
- Luzin, V.; Spencer, K.; Zhang, M.X. Residual stress and thermo-mechanical properties of cold spray metal coatings. Acta Mater. 2011, 59, 1259–1270. [Google Scholar] [CrossRef]
- Hao, L.; Lu, Y.; Sato, H. Influence of Metal Properties on the Formation and Evolution of Metal Coatings during Mechanical Coating. Metall. Mater. Trans. A 2013, 44, 2717–2724. [Google Scholar] [CrossRef]
- Fogagnolo, J.; Velasco, F.; Robert, M.; Torralba, J. Effect of mechanical alloying on the morphology, microstructure and properties of aluminium matrix composite powders. Mater. Sci. Eng. A 2003, 342, 131–143. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V. The science and technology of mechanical alloying. Mater. Sci. Eng. A 2001, 304–306, 151–158. [Google Scholar] [CrossRef]
- Lyu, P.; Chen, Y.; Liu, Z.; Peng, C.T.; Cai, J.; Zhang, C.; Jin, Y.; Guan, Q. The effect of high current pulsed electron beam irradiation on microstructure and properties Cu-Fe powder metallurgical alloys. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, P.; Xia, H.; Yang, Z.; Konovalov, S.; Chen, X.; Guan, Q. The microstructure and properties of nanostructured Cr-Al alloying layer fabricated by high-current pulsed electron beam. Vacuum 2019, 167, 263–270. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, C.T.; Guan, J.; Lv, P.; Guan, Q.; Lu, R. Nanocrystalline Cr-Ni alloying layer induced by high-current pulsed electron beam. Nanomaterials 2019, 9, 74. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, C.; Zhang, L.; Cai, J.; Lv, P.; Jin, Y.; Guan, Q. Microstructure and properties of Cu-Cr powder metallurgical alloy induced by high-current pulsed electron beam. J. Alloys Compd. 2018, 755, 251–256. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, J.; Lv, P.; Zhang, Y.; Xia, H.; Guan, Q. Surface microstructure and properties of Cu-C powder metallurgical alloy induced by high-current pulsed electron beam. J. Alloys Compd. 2017, 697, 96–103. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, C.; Lv, P.; Cai, J.; Jin, Y.; Guan, Q. Surface alloying of aluminum with molybdenum by high-current pulsed electron beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2018, 416, 9–15. [Google Scholar] [CrossRef]
- Chernov, I.P.; Ivanova, S.V.; Krening, M.K.; Koval, N.; Larionov, V.V.; Lider, A.M.; Pushilina, N.S.; Stepanova, E.N.; Stepanova, O.M.; Cherdantsev, Y.P. Properties and structural state of the surface layer in a zirconium alloy modified by a pulsed electron beam and saturated by hydrogen. Tech. Phys. 2012, 57, 392–398. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, P.; Cai, J.; Zhang, Y.; Xia, H.; Guan, Q. Enhanced corrosion property of W-Al coatings fabricated on aluminum using surface alloying under high-current pulsed electron beam. J. Alloys Compd. 2017, 723, 258–265. [Google Scholar] [CrossRef]
- Moon, K.I.; Chang, K.Y.; Lee, K.S. The effect of ternary addition on the formation and the thermal stability of L12 Al3Zr alloy with nanocrystalline structure by mechanical alloying. J. Alloys Compd. 2000, 312, 273–283. [Google Scholar] [CrossRef]
- Wang, R.-N.; Tang, B.-Y.; Peng, L.-M.; Ding, W.-J. Ab initio study of the effect of Zr content on elastic and electronic properties of L12–Al3(Sc1−xZrx) alloys. Comput. Mater. Sci. 2012, 59, 87–93. [Google Scholar] [CrossRef]
- Li, G.R.; Zhao, Y.T.; Wang, H.M.; Chen, G.; Dai, Q.X.; Cheng, X.N. Fabrication and properties of in situ (Al 3Zr + Al2O3) p/A356 composites cast by permanent mould and squeeze casting. J. Alloys Compd. 2009, 471, 530–535. [Google Scholar] [CrossRef]
- Kumar Makineni, S.; Sugathan, S.; Meher, S.; Banerjee, R.; Bhattacharya, S.; Kumar, S.; Chattopadhyay, K. Enhancing elevated temperature strength of copper containing aluminium alloys by forming L12 Al3Zr precipitates and nucleating θ″ precipitates on them. Sci. Rep. 2017, 7, 11154. [Google Scholar] [CrossRef]
- Tian, N.; Li, S.; Zhang, C.; Cai, J.; Lyu, P.; Konovalov, S.; Chen, X.; Peng, C.T.; Guan, Q. The surface modification of aluminum by mechanical milling of Pb coating and high current pulsed electron beam irradiation. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Diankun, L.; Bo, G.; Guanglin, Z.; Jike, L.; Liang, H. High-Current Pulsed Electron Treatment of Hypoeutectic Al–10Si Alloy. High Temp. Mater. Process. 2017, 36, 97–100. [Google Scholar] [CrossRef]



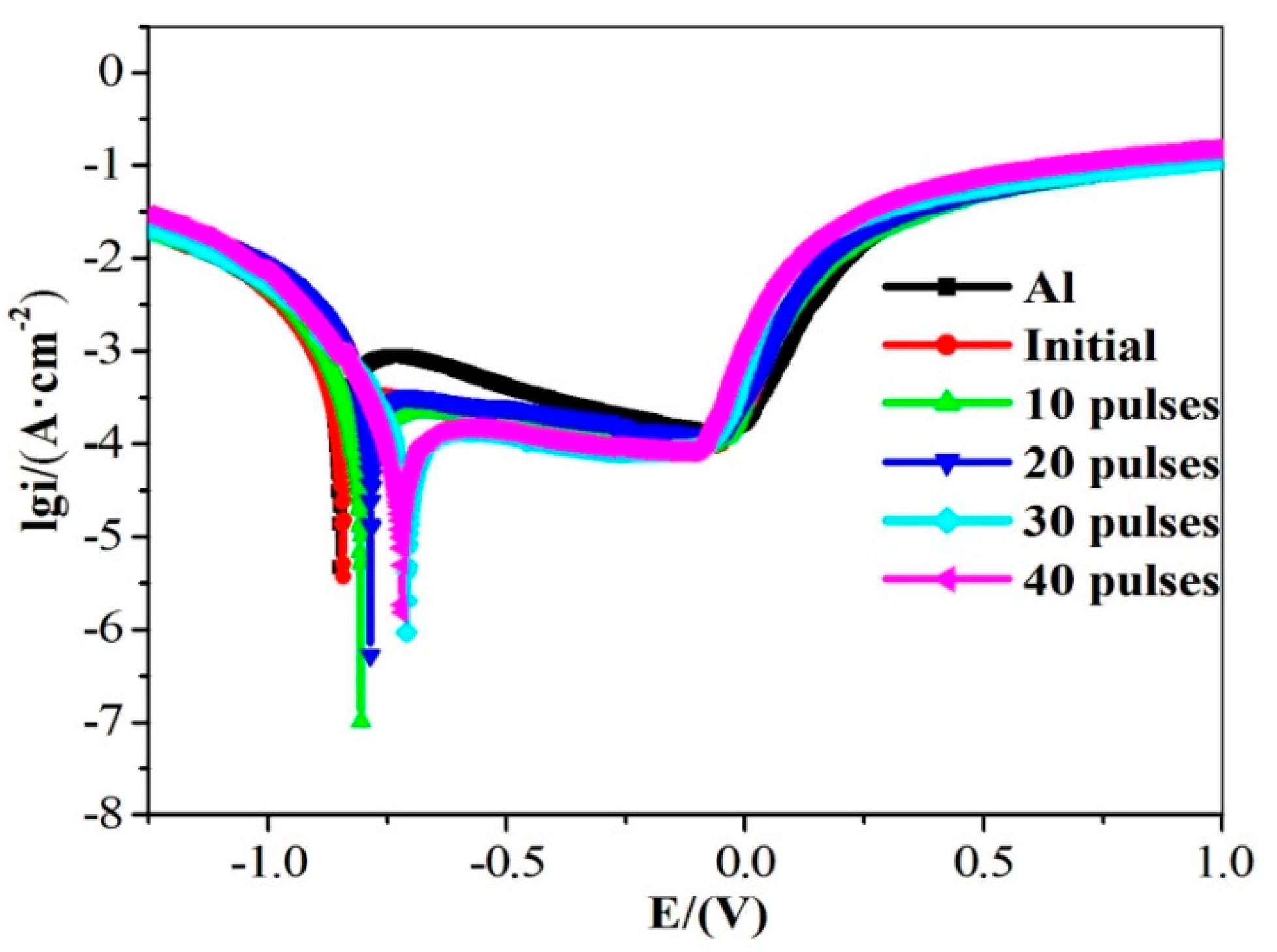
| Sample | Ecorr/V | Icorr/(μA·cm−2) |
|---|---|---|
| Al | −0.873 | 2.786 |
| original sample | −0.860 | 2.710 |
| Al–Zr 10 pulses | −0.623 | 0.821 |
| Al–Zr 20 pulses | −0.605 | 0.622 |
| Al–Zr 30 pulses | −0.591 | 0.443 |
| Al–Zr 40 pulses | −0.584 | 0.450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, H.; Tian, N.; Zhang, C.; Lyu, P.; Guan, Q. Microstructure and Properties of Mechanical Alloying Al-Zr Coating by High Current Pulsed Electron Beam Irradiation. Nanomaterials 2020, 10, 2398. https://doi.org/10.3390/nano10122398
Li X, Liu H, Tian N, Zhang C, Lyu P, Guan Q. Microstructure and Properties of Mechanical Alloying Al-Zr Coating by High Current Pulsed Electron Beam Irradiation. Nanomaterials. 2020; 10(12):2398. https://doi.org/10.3390/nano10122398
Chicago/Turabian StyleLi, Xiangcheng, Huiru Liu, Nana Tian, Conglin Zhang, Peng Lyu, and Qingfeng Guan. 2020. "Microstructure and Properties of Mechanical Alloying Al-Zr Coating by High Current Pulsed Electron Beam Irradiation" Nanomaterials 10, no. 12: 2398. https://doi.org/10.3390/nano10122398
APA StyleLi, X., Liu, H., Tian, N., Zhang, C., Lyu, P., & Guan, Q. (2020). Microstructure and Properties of Mechanical Alloying Al-Zr Coating by High Current Pulsed Electron Beam Irradiation. Nanomaterials, 10(12), 2398. https://doi.org/10.3390/nano10122398




