Compositionally Disordered Crystalline Compounds for Next Generation of Radiation Detectors
Abstract
1. Introduction
2. Next Step: Compositional Disordering of the Materials
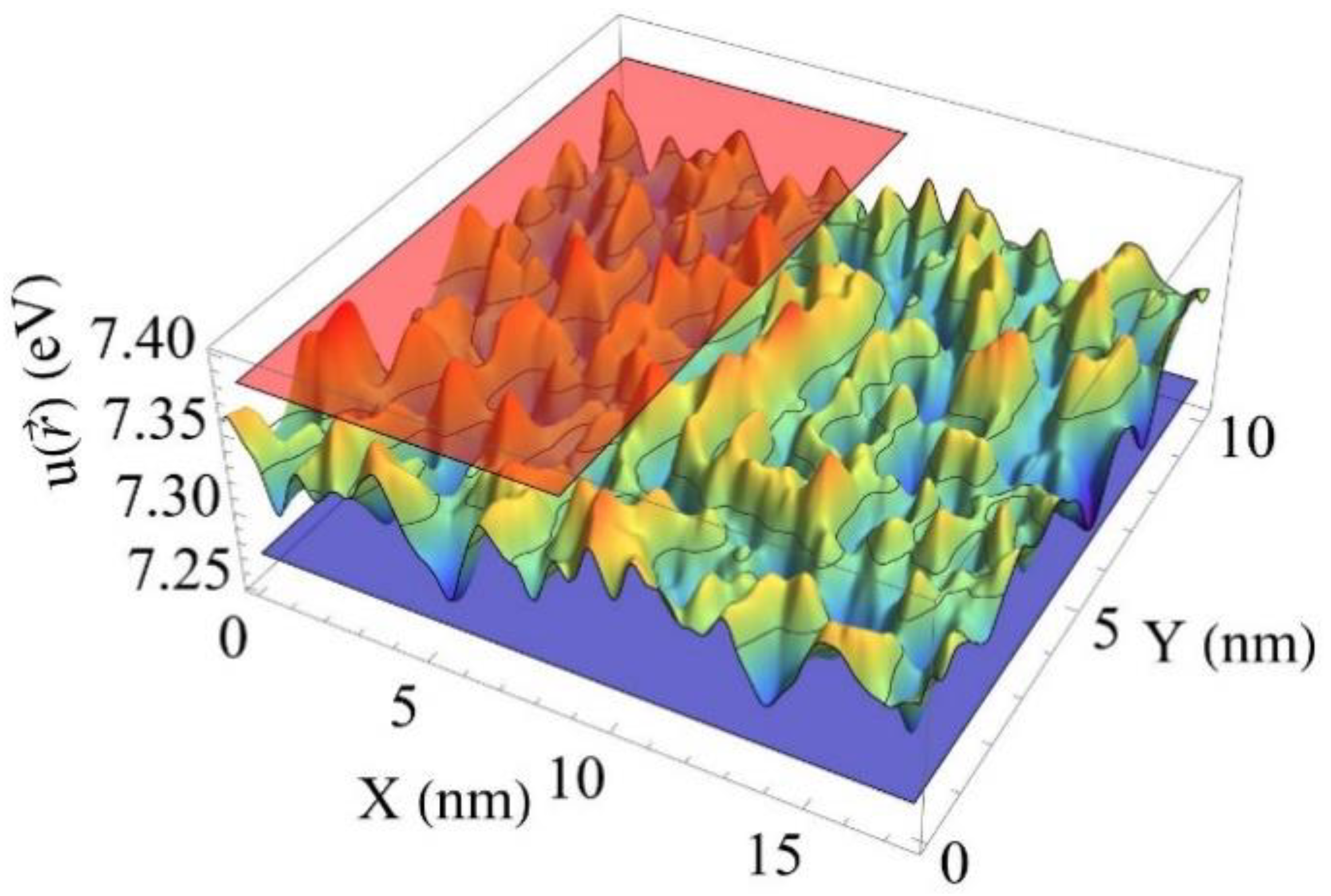
2.1. Conduction Band Bottom Landscape Modulation
2.2. Changing the Parameters of the Local Crystal Field
2.3. Modulation of Local Charge Distribution
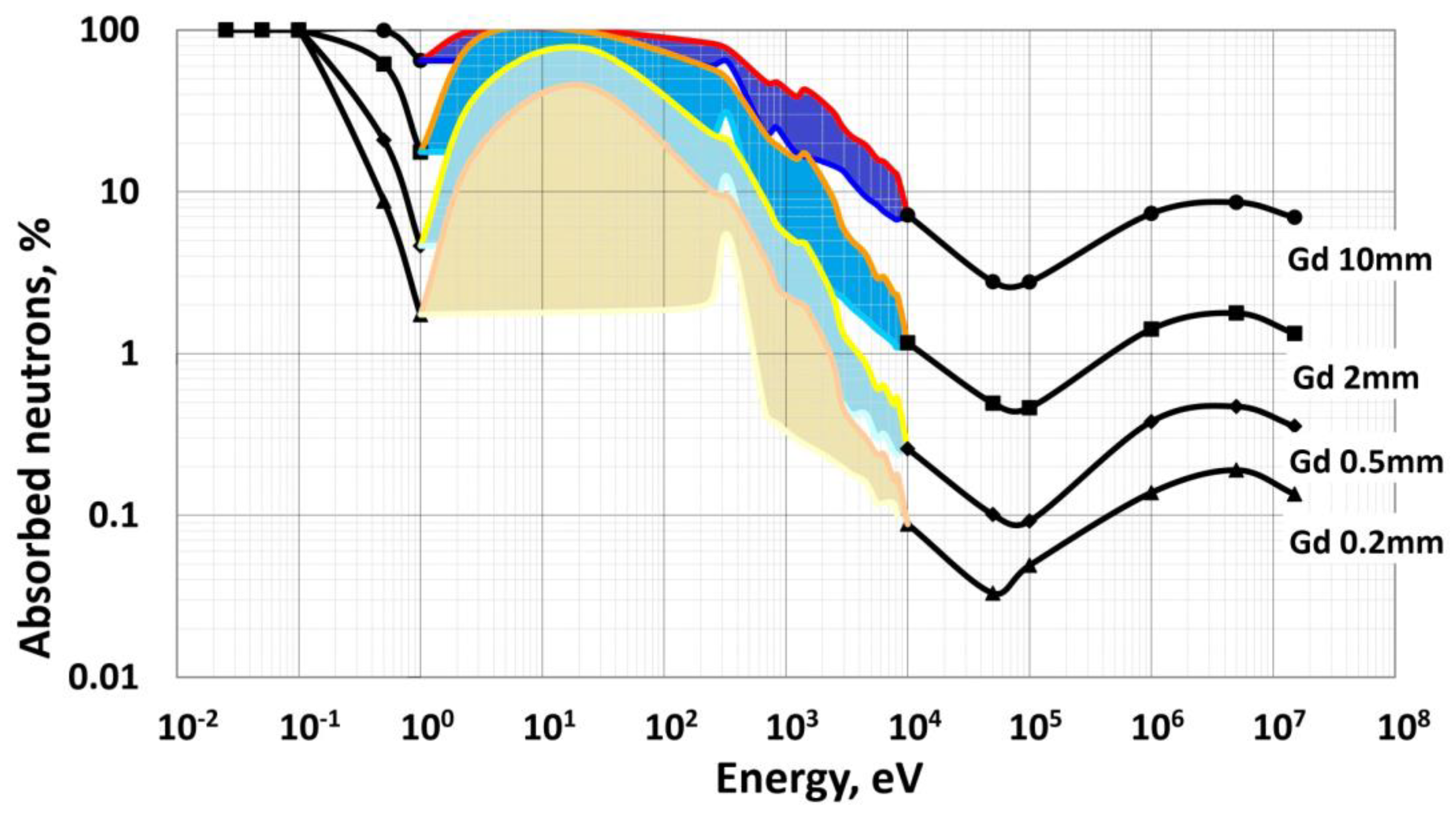
2.4. Variation in Stopping Power and Sensitivity to Various Types of Ionizing Radiation
3. Producing and Utilizing of the Precursors
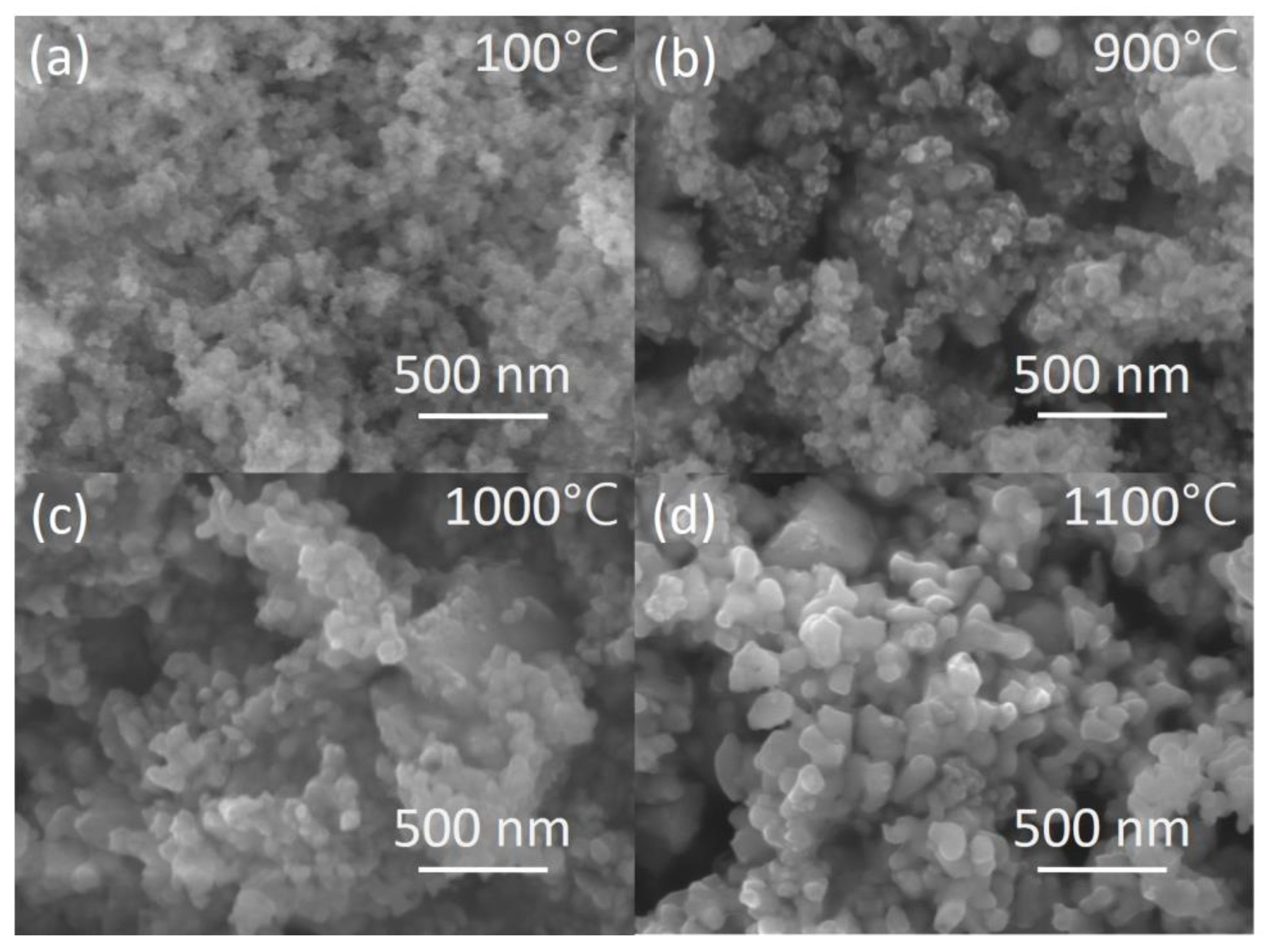
3.1. Development of Nanosized Powders
3.2. 3D Printing Advances in Precursors Compacting
3.3. Sintering Process and Polycrystalline Structure Formation
4. Disordered Doped Scintillation Materials
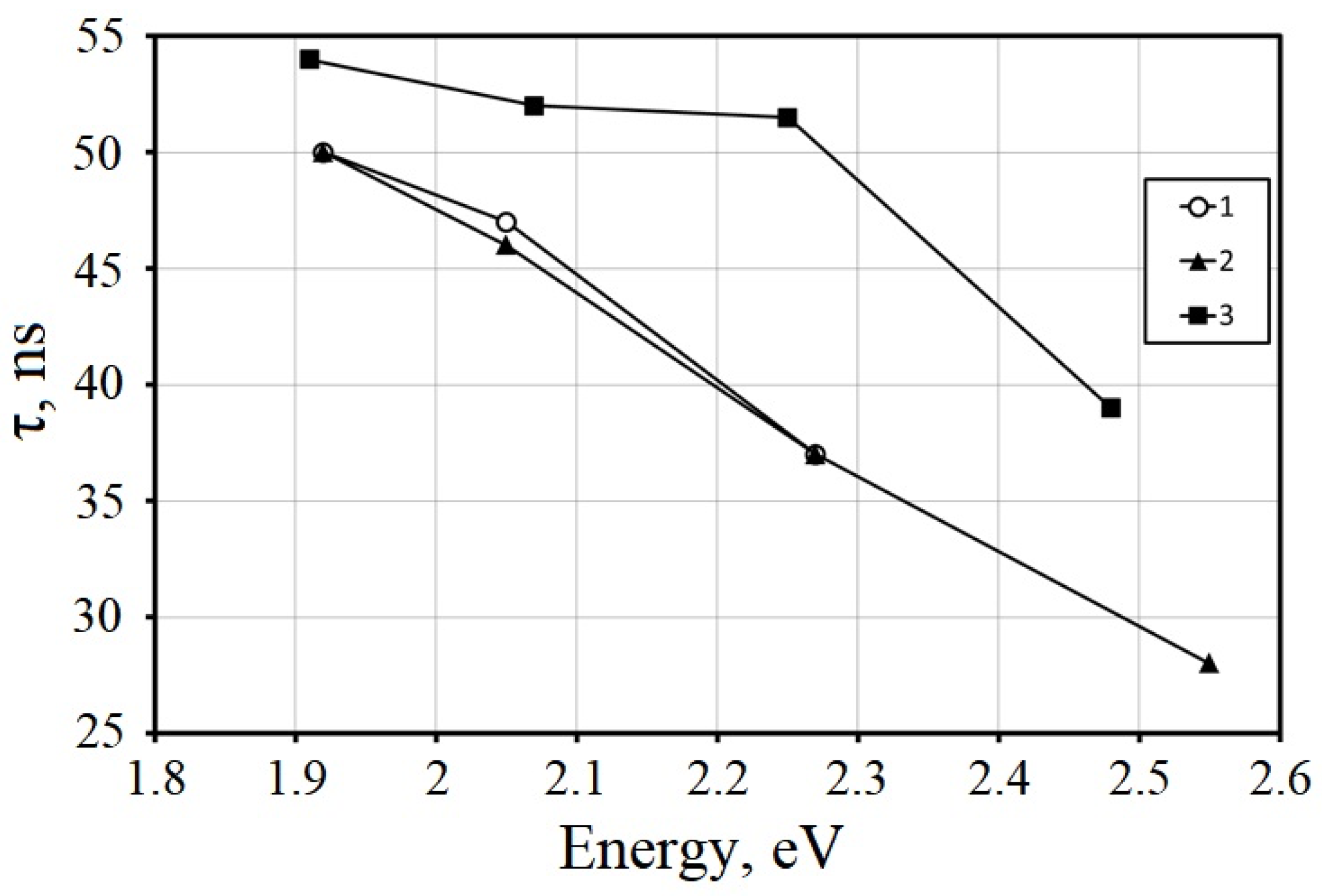
4.1. Heavy and Light Eu-Doped Scintillation Materials
4.2. Ce-Doped Materials
4.3. Tb Doped Materials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, M.J. Inorganic scintillators: Today and tomorrow. J. Lumin. 2002, 100, 35–45. [Google Scholar] [CrossRef]
- Yao, N.; Wang, Z.L. Handbook of Microscopy for Nanotechnology; Kluwer Academic Publishers: Boston, MA, USA, 2005; p. 745. [Google Scholar]
- Courtland, R. The microscope revolution that’s sweeping through materials science. Nature 2018, 563, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Chiang, Y.W.; Santos, R. X-Ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2021, 12, 205. [Google Scholar] [CrossRef]
- Tamulaitis, G.; Nargelas, S.; Korjik, M.; Mechinsky, V.; Talochka, Y.; Vaitkevičius, A.; Vasil’ev, A. Transient optical absorption as a powerful tool for engineering of lead tungstate scintillators towards faster response. J. Mater. Chem. C 2022, 10, 9521–9529. [Google Scholar] [CrossRef]
- Nargelas, S.; Korjik, M.; Vengris, M.; Tamulaitis, G. Study of electronic excitation relaxation at cerium ions in Gd3Al2Ga3O12 matrix using multipulse transient absorption technique. J. Appl. Phys. 2020, 128, 103104. [Google Scholar] [CrossRef]
- Gektin, A.V.; Belsky, A.N.; Vasil’ev, A.N. Scintillation Efficiency Improvement by Mixed Crystal Use. IEEE Trans. Nucl. Sci. 2014, 61, 262–270. [Google Scholar] [CrossRef]
- Korzhik, M.; Tamulaitis, G.; Vasil’ev, A.N. Physics of Fast Processes in Scintillators; Particle Acceleration and Detection; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-21965-9. [Google Scholar]
- Dorenbos, P. Fundamental Limitations in the Performance of Ce3+, Pr3+, and Eu2+ Activated Scintillators. IEEE Trans. Nucl. Sci. 2010, 57, 1162–1167. [Google Scholar] [CrossRef]
- Dorenbos, P. Energy of the First 4f7→4f65d Transition of Eu2+ in Inorganic Compounds. J. Lumin. 2003, 104, 239–260. [Google Scholar] [CrossRef]
- Dorenbos, P. The 5d Level Positions of the Trivalent Lanthanides in Inorganic Compounds. J. Lumin. 2000, 91, 155–176. [Google Scholar] [CrossRef]
- Dorenbos, P. The 4fn↔4fn−15d Transitions of the Trivalent Lanthanides in Halogenides and Chalcogenides. J. Lumin. 2000, 91, 91–106. [Google Scholar] [CrossRef]
- Dorenbos, P. 5d-Level Energies of Ce3+ and the Crystalline Environment. I. Fluoride Compounds. Phys. Rev. B 2000, 62, 15640–15649. [Google Scholar] [CrossRef]
- Dorenbos, P. 5d-level Energies of Ce3+ and the Crystalline Environment. II. Chloride, Bromide, and Iodide Compounds. Phys. Rev. B 2000, 62, 15650–15659. [Google Scholar] [CrossRef]
- Dorenbos, P. 5d-Level Energies of Ce3+ and the Crystalline Environment. III. Oxides Containing Ionic Complexes. Phys. Rev. B 2001, 64, 125117. [Google Scholar] [CrossRef]
- Zhuo, Y.; Hariyani, S.; You, S.; Dorenbos, P.; Brgoch, J. Machine Learning 5d-Level Centroid Shift of Ce3+ Inorganic Phosphors. J. Appl. Phys. 2020, 128, 013104. [Google Scholar] [CrossRef]
- Lecoq, P.; Gektin, A.; Korzhik, M. Inorganic Scintillators for Detector Systems; Particle Acceleration and Detection; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-45521-1. [Google Scholar]
- Yoshikawa, A.; Kamada, K.; Kurosawa, S.; Yokota, Y.; Yamaji, A.; Chani, V.I.; Ohashi, Y.; Yoshino, M. Growth and Characterization of Directionally Solidified Eutectic Systems for Scintillator Applications. J. Cryst. Growth 2018, 498, 170–178. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kamada, K.; Yoshikawa, A. Ultrahigh Resolution Radiation Imaging System Using an Optical Fiber Structure Scintillator Plate. Sci. Rep. 2018, 8, 3194. [Google Scholar] [CrossRef]
- Kim, K.J.; Kamada, K.; Murakami, R.; Yoshino, M.; Kurosawa, S.; Yamaji, A.; Shoji, Y.; Kochurikhin, V.V.; Sato, H.; Toyoda, S.; et al. Growth and Scintillation Properties of Directionally Solidified Ce:LaCl3/AECl2 (AE = Mg, Ca, Sr) Eutectic Scintillators. J. Cryst. Growth 2022, 584, 126549. [Google Scholar] [CrossRef]
- Takizawa, Y.; Kamada, K.; Kim, K.J.; Yoshino, M.; Yamaji, A.; Kurosawa, S.; Yokota, Y.; Sato, H.; Toyoda, S.; Ohashi, Y.; et al. Large Size Growth of Terbium Doped BaCl2/NaCl/KCl Eutectic for Radiation Imaging. Jpn. J. Appl. Phys. 2022, 61, SC1009. [Google Scholar] [CrossRef]
- Lin, Z.; Lv, S.; Yang, Z.; Qiu, J.; Zhou, S. Structured Scintillators for Efficient Radiation Detection. Adv. Sci. 2022, 9, 2102439. [Google Scholar] [CrossRef]
- Vaněček, V.; Děcká, K.; Mihóková, E.; Čuba, V.; Král, R.; Nikl, M. Advanced Halide Scintillators: From the Bulk to Nano. Adv. Photonics Res. 2022, 3, 2200011. [Google Scholar] [CrossRef]
- Sidletskiy, O.; Gorbenko, V.; Zorenko, T.; Syrotych, Y.; Witkiwicz-Łukaszek, S.; Mares, J.A.; Kucerkova, R.; Nikl, M.; Gerasymov, I.; Kurtsev, D.; et al. Composition Engineering of (Lu,Gd,Tb)3(Al,Ga)5O12:Ce Film/Gd3(Al,Ga)5O12:Ce Substrate Scintillators. Crystals 2022, 12, 1366. [Google Scholar] [CrossRef]
- Alekhin, M.S.; de Haas, J.T.M.; Khodyuk, I.V.; Krämer, K.W.; Menge, P.R.; Ouspenski, V.; Dorenbos, P. Improvement of γ-Ray Energy Resolution of LaBr3:Ce3+ Scintillation Detectors by Sr2+ and Ca2+ Co-Doping. Appl. Phys. Lett. 2013, 102, 161915. [Google Scholar] [CrossRef]
- Yang, K.; Menge, P.R. Improving γ-Ray Energy Resolution, Non-Proportionality, and Decay Time of NaI:Tl+ with Sr2+ and Ca2+ Co-Doping. J. Appl. Phys. 2015, 118, 213106. [Google Scholar] [CrossRef]
- Khodyuk, I.V.; Messina, S.A.; Hayden, T.J.; Bourret, E.D.; Bizarri, G.A. Optimization of Scintillation Performance via a Combinatorial Multi-Element Co-Doping Strategy: Application to NaI:Tl. J. Appl. Phys. 2015, 118, 084901. [Google Scholar] [CrossRef]
- Brecher, C.; Lempicki, A.; Miller, S.R.; Glodo, J.; Ovechkina, E.E.; Gaysinskiy, V.; Nagarkar, V.V.; Bartram, R.H. Suppression of Afterglow in CsI:Tl by Codoping with Eu2+—I: Experimental. Nucl. Instrum. Methods A 2006, 558, 450–457. [Google Scholar] [CrossRef]
- Novotny, R.W.; Bremer, D.; Dormenev, V. The PANDA electro- magnetic calorimeter–a high-resolution detector based on PWO-II. In Proceedings of the IEEE 10th International Conference Inorganic Scintillators and Their Applications, Jeju, Republic of Korea, 14 June 2009; pp. 7–12. [Google Scholar]
- van Loef, E.V.D.; Dorenbos, P.; van Eijk, C.W.E.; Krämer, K.; Güdel, H.U. High-Energy-Resolution Scintillator: Ce3+ Activated LaCl3. Appl. Phys. Lett. 2000, 77, 1467–1468. [Google Scholar] [CrossRef]
- van Loef, E.V.D.; Dorenbos, P.; van Eijk, C.W.E.; Krämer, K.; Güdel, H.U. High-Energy-Resolution Scintillator: Ce3+ Activated LaBr3. Appl. Phys. Lett. 2001, 79, 1573–1575. [Google Scholar] [CrossRef]
- Derenzo, S.E.; Moses, W.W. Experimental efforts and results in finding new heavy scintillators. In Proceedings of the Crystal 2000 International Workshop on Heavy Scintillators for Scientific and Industrial Applications, Chamonix, France, 22–26 September 1992. [Google Scholar]
- Melcher, C.L.; Schweitzer, J.S. Cerium-doped lutetium oxyorthosilicate: A fast, efficient new scintillator. IEEE Trans. Nucl. Sci. 1992, 39, 502–505. [Google Scholar] [CrossRef]
- Nikl, M.; Mihoková, E.; Mareš, J.A.; Vedda, A.; Martini, M.; Nejezchleb, K.; Blažek, K. Traps and Timing Characteristics of LuAG:Ce3+ Scintillator. Phys. Status Solidi A 2000, 181, R10–R12. [Google Scholar] [CrossRef]
- Nikl, M.; Ogino, H.; Krasnikov, A.; Beitlerova, A.; Yoshikawa, A.; Fukuda, T. Photo- and Radioluminescence of Pr-Doped Lu3Al5O12 Single Crystal. Phys. Status Solidi A 2005, 202, R4–R6. [Google Scholar] [CrossRef]
- Ogino, H.; Yoshikawa, A.; Nikl, M.; Kamada, K.; Fukuda, T. Scintillation Characteristics of Pr-Doped Lu3Al5O12 Single Crystals. J. Cryst. Growth 2006, 292, 239–242. [Google Scholar] [CrossRef]
- Shi, Y.; Nikl, M.; Feng, X.; Mares, J.A.; Shen, Y.; Beitlerova, A.; Kucerkova, R.; Pan, Y.; Liu, Q. Microstructure, Optical, and Scintillation Characteristics of Pr3+ Doped Lu3Al5O12 Optical Ceramics. J. Appl. Phys. 2011, 109, 013522. [Google Scholar] [CrossRef]
- Baryshevskij, V.G.; Zuevskij, R.F.; Korzhik, M.V.; Lobko, A.S.; Moroz, V.I.; Smirnova, S.A.; Pavlenko, V.B.; Fedorov, A.A. YAlO3: Pr fast-response scintillation crystals. Pis’ma V Zhurnal Tekhnicheskoj Fiz. 1991, 17, 82–85. [Google Scholar]
- Moses, W.W.; Fyodorov, A.A.; Korzhik, M.V.; Aslanov, V.; Minkov, B.; Derenzo, S.E.; Gektin, A.V. LuAlO3:Ce-A high density, high speed scintillator for gamma detection. IEEE Nucl. Sci. Symp. 1995, 42, 597–600. [Google Scholar] [CrossRef][Green Version]
- Moszyński, M.; Wolski, D.; Ludziejewski, T.; Kapusta, M.; Lempicki, A.; Brecher, C.; Wiśniewski, D.; Wojtowicz, A.J. Properties of the New LuAP:Ce Scintillator. Nucl. Instrum. Methods A 1997, 385, 123–131. [Google Scholar] [CrossRef]
- Euler, F.; Bruce, J.A. Oxygen Coordinates of Compounds with Garnet Structure. Acta Crystallogr. 1965, 19, 971–978. [Google Scholar] [CrossRef]
- Autrata, R.; Schauer, P.; Kuapil, J.; Kuapil, J. A Single Crystal of YAG-New Fast Scintillator in SEM. J. Phys. E 1978, 11, 707. [Google Scholar] [CrossRef]
- Yanagida, T.; Takahashi, H.; Ito, T.; Kasama, D.; Enoto, T.; Sato, M.; Hirakuri, S.; Kokubun, M.; Makishima, K.; Yanagitani, T.; et al. Evaluation of Properties of YAG (Ce) Ceramic Scintillators. IEEE Trans. Nucl. Sci. 2005, 52, 1836–1841. [Google Scholar] [CrossRef]
- Ludziejewski, T.; Moszyński, M.; Kapusta, M.; Wolski, D.; Klamra, W.; Moszyńska, K. Investigation of Some Scintillation Properties of YAG:Ce Crystals. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 1997, 398, 287–294. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Zhang, Z.; Su, L.; Kou, H.; Wang, Y.; Wu, A. Optical and Scintillation Properties of Ce:Y3Al5O12 Single Crystal Fibers Grown by Laser Heated Pedestal Growth Method. J. Rare Earths 2021, 39, 1533–1539. [Google Scholar] [CrossRef]
- Hawthorne, F.C. Some Systematics of the Garnet Structure. J. Solid State Chem. 1981, 37, 157–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, X.; Xi, Q. Temperature Effects on Photoluminescence of YAG:Ce3+ Phosphor and Performance in White Light-Emitting Diodes. J. Rare Earths 2008, 26, 446–449. [Google Scholar] [CrossRef]
- Zhong, J.; Zhuang, W.; Xing, X.; Liu, R.; Li, Y.; Liu, Y.; Hu, Y. Synthesis, Crystal Structures, and Photoluminescence Properties of Ce3+-Doped Ca2LaZr2Ga3O12: New Garnet Green-Emitting Phosphors for White LEDs. J. Phys. Chem. C 2015, 119, 5562–5569. [Google Scholar] [CrossRef]
- Swiderski, L.; Moszynski, M.; Nassalski, A.; Syntfeld-Kazuch, A.; Szczesniak, T.; Kamada, K.; Tsutsumi, K.; Usuki, Y.; Yanagida, T.; Yoshikawa, A.; et al. Scintillation Properties of Praseodymium Doped LuAG Scintillator Compared to Cerium Doped LuAG, LSO and LaBr3. IEEE Trans. Nucl. Sci. 2009, 56, 2499–2505. [Google Scholar] [CrossRef]
- Nikl, M.; Mares, J.A.; Solovieva, N.; Li, H.-L.; Liu, X.-J.; Huang, L.-P.; Fontana, I.; Fasoli, M.; Vedda, A.; D’Ambrosio, C. Scintillation Characteristics of Lu3Al5O12:Ce Optical Ceramics. J. Appl. Phys. 2007, 101, 033515. [Google Scholar] [CrossRef]
- Hu, C.; Liu, S.; Shi, Y.; Kou, H.; Li, J.; Pan, Y.; Feng, X.; Liu, Q. Antisite Defects in Nonstoichiometric Lu3Al5O12:Ce Ceramic Scintillators. Phys. Status Solidi B 2015, 252, 1993–1999. [Google Scholar] [CrossRef]
- Takahashi, H.; Yanagida, T.; Kasama, D.; Ito, T.; Kokubun, M.; Makishima, K.; Yanagitani, T.; Yagi, H.; Shigeta, T.; Ito, T. The Temperature Dependence of Gamma-Ray Responses of YAG:Ce Ceramic Scintillators. IEEE Trans. Nucl. Sci. 2006, 53, 2404–2408. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Yokota, Y.; Kamada, K.; Yanagida, S.; Yoshikawa, A.; Yagi, H.; Yanagitani, T. Comparative Study of Transparent Ceramic and Single Crystal Ce Doped LuAG Scintillators. Radiat. Meas. 2011, 46, 1503–1505. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Kamada, K.; Totsuka, D.; Yagi, H.; Yanagitani, T.; Futami, Y.; Yanagida, S.; Kurosawa, S.; Yokota, Y.; et al. Scintillation Properties of Transparent Ceramic Pr:LuAG for Different Pr Concentration. IEEE Trans. Nucl. Sci. 2012, 59, 2146–2151. [Google Scholar] [CrossRef]
- Liu, S.; Feng, X.; Zhou, Z.; Nikl, M.; Shi, Y.; Pan, Y. Effect of Mg2+ Co-Doping on the Scintillation Performance of LuAG:Ce Ceramics. Phys. Status Solidi RRL–Rapid Res. Lett. 2014, 8, 105–109. [Google Scholar] [CrossRef]
- Liu, S.; Feng, X.; Mares, J.A.; Babin, V.; Nikl, M.; Beitlerova, A.; Shi, Y.; Zeng, Y.; Pan, Y.; D’Ambrosio, C.; et al. Effect of Li+ ions co-doping on luminescence, scintillation properties and defects characteristics of LuAG:Ce ceramics. Opt. Mater. 2017, 64, 245–249. [Google Scholar] [CrossRef]
- Liu, S.; Feng, X.; Mares, J.A.; Babin, V.; Nikl, M.; Beitlerova, A.; Shi, Y.; Zeng, Y.; Pan, Y.; D’Ambrosio, C.; et al. Optical, Luminescence and Scintillation Characteristics of Non-Stoichiometric LuAG:Ce Ceramics. J. Lumin. 2016, 169, 72–77. [Google Scholar] [CrossRef]
- Liu, S.; Mares, J.A.; Babin, V.; Hu, C.; Kou, H.; D’Ambrosio, C.; Pan, Y.; Nikl, M. Effect of Reducing Lu3+ Content on the Fabrication and Scintillation Properties of Non-Stoichiometric Lu3−xAl5O12:Ce Ceramics. Opt. Mater. 2017, 63, 179–184. [Google Scholar] [CrossRef]
- Dexter, D.L. Possibility of Luminescent Quantum Yields Greater than Unity. Phys. Rev. 1957, 108, 630–633. [Google Scholar] [CrossRef]
- van der Kolk, E.; Dorenbos, P.; Vink, A.P.; Perego, R.C.; van Eijk, C.W.E.; Lakshmanan, A.R. Vacuum Ultraviolet Excitation and Emission Properties of Pr3+ and Ce3+ in MSO4 (MA=Ba, Sr, and Ca) and Predicting Quantum Splitting by Pr3+ in Oxides and Fluorides. Phys. Rev. B 2001, 64, 195129. [Google Scholar] [CrossRef]
- Lakshmanan, A.R.; Kim, S.-B.; Jang, H.M.; Kum, B.G.; Kang, B.K.; Heo, S.; Seo, D. A Quantum-Splitting Phosphor Exploiting the Energy Transfer from Anion Excitons to Tb3+ in CaSO4:Tb,Na. Adv. Funct. Mater. 2007, 17, 212–218. [Google Scholar] [CrossRef]
- Kudryavtseva, I.; Lushchik, A.; Lushchik, C.; Maaroos, A.; Nagirnyi, V.; Pazylbek, S.; Tussupbekova, A.; Vasil’chenko, E. Complex Terbium Luminescence Centers in Spectral Transformers Based on CaSO4. Phys. Solid State 2015, 57, 2191–2201. [Google Scholar] [CrossRef]
- Lushchik, A.; Lushchik, C.; Kudryavtseva, I.; Maaroos, A.; Nagirnyi, V.; Savikhin, F. Resonant Processes Causing Photon Multiplication in CaSO4:Tb3+. Radiat. Meas. 2013, 56, 139–142. [Google Scholar] [CrossRef][Green Version]
- Oses, C.; Toher, C.; Curtarolo, S. High-Entropy Ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Reece, M.J. Review of High Entropy Ceramics: Design, Synthesis, Structure and Properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Nundy, S.; Tatar, D.; Kojčinović, J.; Ullah, H.; Ghosh, A.; Mallick, T.K.; Meinusch, R.; Smarsly, B.M.; Tahir, A.A.; Djerdj, I. Bandgap Engineering in Novel Fluorite-Type Rare Earth High-Entropy Oxides (RE-HEOs) with Computational and Experimental Validation for Photocatalytic Water Splitting Applications. Adv. Sustain. Syst. 2022, 6, 2200067. [Google Scholar] [CrossRef]
- Corey, Z.J.; Lu, P.; Zhang, G.; Sharma, Y.; Rutherford, B.X.; Dhole, S.; Roy, P.; Wang, Z.; Wu, Y.; Wang, H.; et al. Structural and Optical Properties of High Entropy (La,Lu,Y,Gd,Ce)AlO3 Perovskite Thin Films. Adv. Sci. 2022, 9, 2202671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, Y. High-Entropy Transparent Ceramics: Review of Potential Candidates and Recently Studied Cases. Int. J. Appl. Ceram. Technol. 2022, 19, 644–672. [Google Scholar] [CrossRef]
- Rabatin, J.G. Luminescence of Rare Earth Activated Lutetium Oxyhalide Phosphors. J. Electrochem. Soc. 1982, 129, 1552–1555. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakagawa, M.; Fujii, H.; Kawaguchi, F.; Yamada, H.; Ito, Y.; Takeuchi, H.; Hayakawa, T.; Tsukuda, Y. Application of Gd2O2S Ceramic Scintillator for X-Ray Solid State Detector in X-Ray CT. Jpn. J. Appl. Phys. 1988, 27, L1572. [Google Scholar] [CrossRef]
- Korzhik, M.V. Physics of Scintillators on a Base of Oxide Single Crystals; BSU: Minsk, Belarus, 2003; p. 263. ISBN 985-485-061-7. [Google Scholar]
- Chai, B.H.T.; Chai, D.Y.; Randall, A. Method of Enhancing Performance of Doped Scintillation Crystals. U.S. Patent 7,397,034 B2, 8 July 2008. [Google Scholar]
- Belsky, A.N.; Auffray, E.; Lecoq, P.; Dujardin, C.; Garnier, N.; Canibano, H.; Pedrini, C.; Petrosyan, A.G. Progress in the Development of LuAlO3-Based Scintillators. IEEE Trans. Nucl. Sci. 2001, 48, 1095–1100. [Google Scholar] [CrossRef]
- Simonov, A.; Goodwin, A.L. Designing Disorder into Crystalline Materials. Nat. Rev. Chem. 2020, 4, 657–673. [Google Scholar] [CrossRef]
- Talochka, Y.; Vasil’ev, A.; Korzhik, M.; Tamulaitis, G. Impact of Compositional Disorder on Electron Migration in Lutetium–Yttrium Oxyorthosilicate Scintillator. J. Appl. Phys. 2022, 132, 053101. [Google Scholar] [CrossRef]
- Mp-3050: Y3Al5O12 (Cubic, Ia-3d, 230). Available online: https://materialsproject.org/materials/mp-3050/ (accessed on 24 October 2022).
- Mp-5444: Y3Ga5O12 (Cubic, Ia-3d, 230). Available online: https://materialsproject.org/materials/mp-5444#electronic_structure (accessed on 24 October 2022).
- Mp-14132: Lu3Al5O12 (Cubic, Ia-3d, 230). Available online: https://materialsproject.org/materials/mp-14132/ (accessed on 24 October 2022).
- Mp-14134: Lu3Ga5O12 (Cubic, Ia-3d, 230). Available online: https://materialsproject.org/materials/mp-14134/ (accessed on 24 October 2022).
- Mp-16969: Lu2SiO5 (Monoclinic, C2/c, 15). Available online: https://materialsproject.org/materials/mp-16969/ (accessed on 24 October 2022).
- Mp-3520: Y2SiO5 (Monoclinic, C2/c, 15). Available online: https://materialsproject.org/materials/mp-3520/#electronic_structure (accessed on 24 October 2022).
- Yonezawa, F.; Morigaki, K. Coherent Potential Approximation. Basic Concepts and Applications. Prog. Theor. Phys. Suppl. 1973, 53, 1–76. [Google Scholar] [CrossRef]
- Sidletskiy, O. Trends in Search for Bright Mixed Scintillators. Phys. Status Solidi A 2018, 215, 1701034. [Google Scholar] [CrossRef]
- Dorenbos, P. Electronic Structure and Optical Properties of the Lanthanide Activated RE3(Al1−xGax)5O12 (RE=Gd, Y, Lu) Garnet Compounds. J. Lumin. 2013, 134, 310–318. [Google Scholar] [CrossRef]
- Yadav, S.K.; Uberuaga, B.P.; Nikl, M.; Jiang, C.; Stanek, C.R. Band-Gap and Band-Edge Engineering of Multicomponent Garnet Scintillators from First Principles. Phys. Rev. Appl. 2015, 4, 054012. [Google Scholar] [CrossRef]
- Kanai, T.; Satoh, M.; Miura, I. Characteristics of a Nonstoichiometric Gd3+δ(Al,Ga)5−δO12:Ce Garnet Scintillator. J. Am. Ceram. Soc. 2008, 91, 456–462. [Google Scholar] [CrossRef]
- Sifat, R.; Grosvenor, A.P. Examination of the Site Preference in Garnet Type (X3A2B3O12; X = Y, A/B = Al, Ga, Fe) Materials. Solid State Sci. 2018, 83, 56–64. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S. (INVITED) Review of Luminescent Properties of Ce3+-Doped Garnet Phosphors: New Insight into the Effect of Crystal and Electronic Structure. Opt. Mater. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Ogino, H.; Yoshikawa, A.; Nikl, M.; Kucerkova, R.; Shimoyama, J.; Kishio, K. Suppression of Defect Related Host Luminescence in LuAG Single Crystals. Phys. Procedia 2009, 2, 191–205. [Google Scholar] [CrossRef][Green Version]
- Fasoli, M.; Vedda, A.; Nikl, M.; Jiang, C.; Uberuaga, B.P.; Andersson, D.A.; McClellan, K.J.; Stanek, C.R. Band-Gap Engineering for Removing Shallow Traps in Rare-Earth Lu3Al5O12 Garnet Scintillators Using Ga3+ Doping. Phys. Rev. B 2011, 84, 081102. [Google Scholar] [CrossRef]
- Ogino, H.; Yoshikawa, A.; Nikl, M.; Pejchal, J.; Fukuda, T. Growth and Luminescence Properties of Pr-Doped Lu3(Ga,Al)5O12 Single Crystals. Jpn. J. Appl. Phys. 2007, 46, 3514–3517. [Google Scholar] [CrossRef]
- Hu, C.; Liu, S.; Fasoli, M.; Vedda, A.; Nikl, M.; Feng, X.; Pan, Y. O– Centers in LuAG:Ce,Mg Ceramics. Phys. Status Solidi RRL–Rapid Res. Lett. 2015, 9, 245–249. [Google Scholar] [CrossRef]
- Nikl, M.; Kamada, K.; Babin, V.; Pejchal, J.; Pilarova, K.; Mihokova, E.; Beitlerova, A.; Bartosiewicz, K.; Kurosawa, S.; Yoshikawa, A. Defect Engineering in Ce-Doped Aluminum Garnet Single Crystal Scintillators. Cryst. Growth Des. 2014, 14, 4827–4833. [Google Scholar] [CrossRef]
- Auffray, E.; Augulis, R.; Fedorov, A.; Dosovitskiy, G.; Grigorjeva, L.; Gulbinas, V.; Koschan, M.; Lucchini, M.; Melcher, C.; Nargelas, S.; et al. Excitation Transfer Engineering in Ce-Doped Oxide Crystalline Scintillators by Codoping with Alkali-Earth Ions. Phys. Status Solidi A 2018, 215, 1700798. [Google Scholar] [CrossRef]
- Feofilov, S.P.; Kaplyanskii, A.A.; Kulinkin, A.B.; Zakharchenya, R.I.; Ovanesyan, K.; Petrosyan, A.; Dujardin, C. Inhomogeneous Broadening: Symmetry of Centers and Disorder in Solid Solutions and Nanocrystals. EPJ Web Conf. 2015, 103, 01003. [Google Scholar] [CrossRef]
- Nargelas, S.; Talochka, Y.; Vaitkevičius, A.; Dosovitskiy, G.; Buzanov, O.; Vasil’ev, A.; Malinauskas, T.; Korzhik, M.; Tamulaitis, G. Influence of Matrix Composition and Its Fluctuations on Excitation Relaxation and Emission Spectrum of Ce Ions in (GdxY1-x)3Al2Ga3O12:Ce Scintillators. J. Lumin. 2022, 242, 118590. [Google Scholar] [CrossRef]
- Shakhno, A.; Markovskyi, A.; Zorenko, T.; Witkiewicz-Łukaszek, S.; Vlasyuk, Y.; Osvet, A.; Elia, J.; Brabec, C.J.; Batentschuk, M.; Zorenko, Y. Micropowder Ca2YMgScSi3O12:Ce Silicate Garnet as an Efficient Light Converter for White LEDs. Materials 2022, 15, 3942. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, J.-G.; Zhu, Q.; Li, X.; Sun, X. The Effects of Mg2+/Si4+ Substitution on Crystal Structure, Local Coordination and Photoluminescence of (Gd,Lu)3Al5O12:Ce Garnet Phosphor. J. Alloys Compd. 2019, 797, 477–485. [Google Scholar] [CrossRef]
- Tratsiak, Y.; Bokshits, Y.; Borisevich, A.; Korjik, M.; Vaitkevičius, A.; Tamulaitis, G. Y2CaAlGe(AlO4)3:Ce and Y2MgAlGe(AlO4)3:Ce Garnet Phosphors for White LEDs. Opt. Mater. 2017, 67, 108–112. [Google Scholar] [CrossRef]
- Chyzh, A.; Baramsai, B.; Becker, J.A.; Bečvář, F.; Bredeweg, T.A.; Couture, A.; Dashdorj, D.; Haight, R.C.; Jandel, M.; Kroll, J.; et al. Measurement of the 157Gd(n,γ) Reaction with the DANCE γ Calorimeter Array. Phys. Rev. C 2011, 84, 014306. [Google Scholar] [CrossRef]
- Hagiwara, K.; Yano, T.; Tanaka, T.; Reen, M.S.; Das, P.K.; Lorenz, S.; Ou, I.; Sudo, T.; Yamada, Y.; Mori, T.; et al. Gamma-Ray Spectrum from Thermal Neutron Capture on Gadolinium-157. Prog. Theor. Exp. Phys. 2019, 2019, 023D01. [Google Scholar] [CrossRef]
- Menge, P.R.; Yang, K.; Ouspenski, V. Large Format Li Co-Doped Nai:Tl (NailTM) Scintillation Detector for Gamma-Ray and Neutron Dual Detection. In Proceedings of the 12th Pacific Rim Conference on Ceramic and Glass Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 201–208, ISBN 978-1-119-49409-6. [Google Scholar]
- Takizawa, Y.; Kamada, K.; Yoshino, M.; Kim, K.J.; Yamaji, A.; Kurosawa, S.; Yokota, Y.; Sato, H.; Toyoda, S.; Ohashi, Y.; et al. Growth and Scintillation Properties of Ce Doped 6LiBr/LaBr3 Eutectic Scintillator for Neutron Detection. Nucl. Instrum. Methods A 2022, 1028, 166384. [Google Scholar] [CrossRef]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white lightemitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. R. 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Kanai, T.; Satoh, M.; Miura, I. Hot-Pressing Method to Consolidate Gd3(Al,Ga)5O12:Ce Garnet Scintillator Powder for use in an X-ray CT Detector. Int. J. Appl. Ceram. Technol. 2013, 10, E1–E10. [Google Scholar] [CrossRef]
- Li, J.G.; Ikegami, T.; Lee, J.H.; Mori, T.; Yajima, Y. Reactive yttrium aluminate garnet powder via coprecipitation using ammonium hydrogen carbonate as the precipitant. J. Mater. Res. 2000, 15, 1864–1867. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, H.; Jiang, J. Synthesis of Cerium-Doped Gd3(Al,Ga)5O12 Powder for Ceramic Scintillators with Ultrasonic-Assisted Chemical Coprecipitation Method. J. Am. Ceram. Soc. 2013, 96, 3038–3041. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A.; Taheri-Nassaj, E.; Sarpoolaky, H. Synthesis of an alumina–YAG nanopowder via sol–gel method. J. Alloys Compd. 2008, 456, 282–285. [Google Scholar] [CrossRef]
- Drozdowski, W.; Witkowski, M.E.; Solarz, P.; Głuchowski, P.; Głowacki, M.; Brylew, K. Scintillation properties of Gd3Al2Ga3O12:Ce (GAGG:Ce): A comparison between monocrystalline and nanoceramic samples. Opt. Mater. 2018, 79, 227–231. [Google Scholar] [CrossRef]
- Marchal, J.; John, T.; Baranwal, R.; Hinklin, T.; Laine, R.M. Yttrium aluminum garnet nanopowders produced by liquid-feed flame spray pyrolysis (LF-FSP) of metalloorganic precursors. Chem. Mater. 2004, 16, 822–831. [Google Scholar] [CrossRef]
- Seeley, Z.M.; Cherepy, N.J.; Payne, S.A. Expanded phase stability of Gd-based garnet transparent ceramic scintillators. J. Mater. Res. 2014, 29, 2332–2337. [Google Scholar] [CrossRef]
- Lamoreaux, R.H.; Hildenbrand, D.L.; Brewer, L. High-Temperature Vaporization Behavior of Oxides II. Oxides of Be, Mg, Ca, Sr, Ba, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, Zn, Cd, and Hg. J. Phys. Chem. Ref. Data 1987, 16, 419–443. [Google Scholar] [CrossRef]
- Kurosawa, S.; Shoji, Y.; Yokota, Y.; Kamada, K.; Chani, V.I.; Yoshikawa, A. Czochralski growth of Gd3(Al5−xGax)O12 (GAGG) single crystals and their scintillation properties. J. Cryst. Growth 2014, 393, 134–137. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Luo, Z.H.; Jiang, H.C.; Jiang, J.; Gui, Z.Z.; Chen, C.H.; Liu, Y.F.; Liu, F.; Ci, M.M. Sintering of GGAG: Ce3+, xY3+ transparent ceramics in oxygen atmosphere. Ceram. Int. 2017, 43, 16036–16041. [Google Scholar] [CrossRef]
- Korzhik, M.; Alenkov, V.; Buzanov, O.; Fedorov, A.; Dosovitskiy, G.; Grigorjeva, L.; Mechinsky, V.; Sokolov, P.; Tratsiak, Y.; Zolotarjovs, A.; et al. Nanoengineered Gd3Al2Ga3O12 Scintillation Materials with Disordered Garnet Structure for Novel Detectors of Ionizing Radiation. Cryst. Res. Technol. 2019, 54, 1800172. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, X.J.; Huang, L.P. Fabrication of transparent cerium-doped lutetium aluminum garnet (LuAG:Ce) ceramics by a solid-state reaction method. J. Am. Ceram. Soc. 2005, 88, 3226–3228. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, X.J.; Huang, L.P. Fabrication of transparent Ce:LuAG ceramics by a solid-state reaction method. J. Inorg. Mater. 2006, 21, 1161–1166. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, X.J.; Xie, R.J.; Zeng, Y.; Huang, L.P. Fabrication of transparent cerium-doped lutetium aluminum garnet ceramics by co-precipitation routes. J. Am. Ceram. Soc. 2006, 89, 2356–2358. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, X.J.; Xie, R.J.; Zhou, G.H.; Hirosaki, N.; Pu, X.P.; Huang, L.P. Cerium-doped lutetium aluminum garnet phosphors and optically transparent ceramics prepared from powder precursors by a urea homogeneous precipitation method. Jpn. J. Appl. Phys. 2008, 47, 1657–1661. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Payne, S.A.; Asztalos, S.J.; Hull, G.; Kuntz, J.D.; Niedermayr, T.; Pimputkar, S.; Roberts, J.J.; Sanner, R.D.; Tillotson, T.M.; et al. Scintillators with potential to supersede lanthanum bromide. IEEE Trans. Nucl. Sci. 2009, 56, 873–880. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Kuntz, J.D.; Roberts, J.J.; Hurst, T.A.; Drury, O.B.; Sanner, R.D.; Tillotson, T.M.; Payne, S.A. Transparent ceramic scintillator fabrication, properties, and applications. In Proceedings of the SPIE 7079, Hard X-ray, Gamma-Ray, and Neutron Detector Physics X, San Diego, CA, USA, 4 September 2008. [Google Scholar] [CrossRef]
- Seely, Z.M.; Cherepy, N.J.; Payne, S.A. Homogenity of Gd-based garnet transparent ceramic scintillators for gamma spectroscopy. J. Cryst. Growth 2013, 379, 79–83. [Google Scholar] [CrossRef]
- Yang, S.H.; Sun, Y.S.; Chen, X.Q.; Zhang, Y.; Luo, Z.; Jiang, J.; Jiang, H. The effects of cation concentration in the salt solution on the cerium doped gadolinium gallium aluminum oxide nanopowders prepared by co-precipitation method. IEEE Trans. Nucl. Sci. 2014, 61, 301–305. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, S.; Zhang, Y.; Jiang, J.; Jiang, H. Coprecipitation synthesis of gadolinium aluminum gallium oxide (GAGG) via different precipitants. IEEE Trans. Nucl. Sci. 2014, 61, 306–311. [Google Scholar] [CrossRef]
- Luo, Z.H.; Liu, Y.F.; Zhang, C.H.; Zhang, J.X.; Qin, H.M.; Jiang, H.C.; Jiang, J. Effect of Yb3+ on the Crystal Structural Modification and Photoluminescence Properties of GGAG:Ce3+. Inorg. Chem. 2016, 55, 3040–3046. [Google Scholar] [CrossRef]
- Chen, J.; Ji, H.; Wang, S.; Liu, Y. Fabrication of YAG ceramic tube by UV-assisted direct ink writing. Ceram. Int. 2022, 48, 19703–19708. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, J.; Chen, J.; Shimai, S.; Chen, H.; Zhou, G.; Liu, Y.; Zhang, J.; Wang, S.; Yang, D. Direct ink writing of cellulose-plasticized aqueous ceramic slurry for YAG transparent ceramics. MRS Commun. 2022, 12, 206–212. [Google Scholar] [CrossRef]
- Zhang, G.; Carloni, D.; Wu, Y. 3D printing of transparent YAG ceramics using copolymer-assisted slurry. Ceram. Int. 2020, 46, 17130–17134. [Google Scholar] [CrossRef]
- Seeley, Z.; Yee, T.; Cherepy, N.; Drobshoff, A.; Herrara, O.; Ryerson, R.; Payne, S.A. 3D printed transparent ceramic YAG laser rods: Matching the core-clad refractive index. Opt. Mater. 2020, 107, 110121. [Google Scholar] [CrossRef]
- Jones, I.K.; Seeley, Z.M.; Cherepy, N.J.; Duoss, E.B.; Payne, S.A. Direct ink write fabrication of transparent ceramic gain media. Opt.Mater. 2018, 75, 19. [Google Scholar] [CrossRef]
- Carloni, D.; Zhang, G.; Wu, Y. Transparent alumina ceramics fabricated by 3D printing and vacuum sintering. J. Eur. Ceram. Soc. 2021, 41, 781–791. [Google Scholar] [CrossRef]
- Dosovitskiy, G.A.; Karpyuk, P.V.; Evdokimov, P.V.; Kuznetsova, D.E.; Mechinsky, V.A.; Borisevich, A.E.; Fedorov, A.A.; Putlayev, V.I.; Dosovitskiy, A.E.; Korjik, M.V. First 3D-printed complex inorganic polycrystalline scintillator. CrystEngComm 2017, 19, 4260–4264. [Google Scholar] [CrossRef]
- Hostasa, J.; Schwentenwein, M.; Toci, G.; Esposito, L.; Brouczek, D.; Piancastelli, A.; Pirri, A.; Patrizi, B.; Vannini, M.; Biasini, V. Transparent laser ceramics by stereolithography. Scr. Mater. 2020, 187, 194–196. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, Y.; Jin, B.; Li, M.; Xing, B.; Zhao, Z. Effect of debinding and sintering profile on the optical properties of DLP-3D printed YAG transparent ceramic. Ceram. Int. 2022, 48, 21134–21140. [Google Scholar] [CrossRef]
- Hu, S.; Liu, Y.; Zhang, Y.; Xue, Z.; Wang, Z.; Zhou, G.; Lu, C.; Li, H.; Wang, S. 3D printed ceramic phosphor and the photoluminescence property under blue laser excitation. J. Eur. Ceram. Soc. 2019, 39, 2731–2738. [Google Scholar] [CrossRef]
- Cooperstein, I.; Indukuri, S.R.K.C.; Bouketov, A.; Levy, U.; Magdassi, S. 3D printing of micrometer-sized transparent ceramics with on-demand optical-gain properties. Adv. Mater. 2020, 32, 2001675. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, M.; Jiang, Y.; Xing, B.; Shen, M.; Cao, C.; Wang, C.; Zhao, Z. High-quality translucent alumina ceramic through digital light processing stereolithography method. Adv. Eng. Mater. 2021, 23, 2001475. [Google Scholar] [CrossRef]
- Duaud, O.; Marchal, P.; Corbel, S. Rheological properties of PZT suspensions for stereolithography. J. Eur. Ceram. Soc. 2002, 22, 2081–2092. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Guo, B.; Chen, X.; Ananth, P.; Bai, J. Fabrication of piezoelectric nano-ceramics via stereolithography of low viscous and non-aqueous suspensions. J. Eur. Ceram. Soc. 2020, 40, 682–688. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, P.; Li, J.; Lu, Z. High-resolution multiceramic additive manufacturing based on digital light processing. Addit. Manuf. 2022, 54, 102732. [Google Scholar] [CrossRef]
- Schlacher, J.; Hofer, A.-K.; Geier, S.; Kraleva, I.; Papsik, R.; Schwentenwein, M.; Bermejo, R. Additive manufacturing of high-strength alumina through a multi-material approach. Open Ceram. 2021, 5, 100082. [Google Scholar] [CrossRef]
- Fedorov, A.A.; Dubov, V.V.; Ermakova, L.V.; Bondarev, A.G.; Karpyuk, P.V.; Korzhik, M.V.; Kuznetsova, D.E.; Mechinsky, V.A.; Smyslova, V.G.; Dosovitskiy, G.A.; et al. Gd3Al2Ga3O12:Ce: Scintillation ceramic elements for measuring ionising radiation in gases and liquids. Instrum. Exp. Tech. 2023, 2. in press. [Google Scholar]
- Parker, G. Encyclopedia of materials: Science and technology. In Guide-Wave Optical Communications: Materials; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Li, J.; Wu, Y.S.; Pan, Y.B.; Liu, W.B.; Huang, L.P.; Guo, J.K. Fabrication, microstructure and properties of highly transparent Nd:YAG laser ceramics. Opt. Mater. 2008, 31, 6–17. [Google Scholar] [CrossRef]
- Nishiura, S.; Tanabe, S.; Fujioka, K.; Fujimoto, Y. Properties of transparent Ce: YAG ceramic phosphors for white LED. Opt. Mater. 2011, 33, 688–691. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, H.; Jiang, J.; Mao, R. Microstructure and optical characteristics of Ce:Gd3(Ga,Al)5O12 ceramic for scintillator application. Ceram. Int. 2015, 41, 873–876. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Zhang, Y.; Jiang, J.; Jiang, H. Highly transparent ZrO2-doped (Ce,Gd)3Al3Ga2O12 ceramics prepared via oxygen sintering. J. Eur. Ceram. Soc. 2015, 35, 3879–3883. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Sun, P.; Du, Y.; Lin, S.; Xie, R.J.; Jiang, H. Transparent Ceramics Enabling High Luminous Flux and Efficacy for the Next-Generation High-Power LED Light. ACS Appl. Mater. Interfaces 2019, 24, 21697–21701. [Google Scholar] [CrossRef] [PubMed]
- Karpyuk, P.; Shurkina, A.; Kuznetsova, D.; Smyslova, V.; Dubov, V.; Dosovitskiy, G.; Korzhik, M.; Retiviv, V.; Bondarev, A. Effect of Sintering Additives on the Sintering and Spectral-Luminescent Characteristics of Quaternary GYAGG:Ce Scintillation Ceramics. J. Electron. Mater. 2022, 51, 6481–6491. [Google Scholar] [CrossRef]
- Hofstadter, R. Europium Activated Strontium Iodide Scintillators. U.S. Patent 3,373,279, 2 March 1968. [Google Scholar]
- Wilson, C.M.; van Loef, E.V.; Glodo, J.; Cherepy, N.; Hull, G.; Payne, S.; Choong, W.-S.; Moses, W.; Shah, K.S. Strontium Iodide Scintillators for High Energy Resolution Gamma Ray Spectroscopy. In Proceedings of the Hard X-Ray, Gamma-Ray, and Neutron Detector Physics X, San Diego, CA, USA, 5 September 2008; pp. 334–340. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Hull, G.; Drobshoff, A.D.; Payne, S.A.; van Loef, E.; Wilson, C.M.; Shah, K.S.; Roy, U.N.; Burger, A.; Boatner, L.A.; et al. Strontium and Barium Iodide High Light Yield Scintillators. Appl. Phys. Lett. 2008, 92, 083508. [Google Scholar] [CrossRef]
- Bourret-Courchesne, E.D.; Bizarri, G.A.; Borade, R.; Gundiah, G.; Samulon, E.C.; Yan, Z.; Derenzo, S.E. Crystal Growth and Characterization of Alkali-Earth Halide Scintillators. J. Cryst. Growth 2012, 352, 78–83. [Google Scholar] [CrossRef]
- Bourret-Courchesne, E.D.; Bizarri, G.; Hanrahan, S.M.; Gundiah, G.; Yan, Z.; Derenzo, S.E. BaBrI:Eu2+, a New Bright Scintillator. Nucl. Instrum. Methods A 2010, 613, 95–97. [Google Scholar] [CrossRef]
- Gundiah, G.; Bizarri, G.; Hanrahan, S.M.; Weber, M.J.; Bourret-Courchesne, E.D.; Derenzo, S.E. Structure and Scintillation of Eu2+-Activated Solid Solutions in the BaBr2–BaI2 System. Nucl. Instrum. Methods A 2011, 652, 234–237. [Google Scholar] [CrossRef]
- Bizarri, G.; Bourret-Courchesne, E.D.; Yan, Z.; Derenzo, S.E. Scintillation and Optical Properties of BaBrI:Eu2+ and CsBa2I5Eu2+. IEEE Trans. Nucl. Sci. 2011, 58, 3403–3410. [Google Scholar] [CrossRef]
- Yan, Z.; Shalapska, T.; Bourret, E.D. Czochralski Growth of the Mixed Halides BaBrCl and BaBrCl:Eu. J. Cryst. Growth 2016, 435, 42–45. [Google Scholar] [CrossRef]
- Gundiah, G.; Yan, Z.; Bizarri, G.; Derenzo, S.E.; Bourret-Courchesne, E.D. Structure and Scintillation of Eu2+-Activated BaBrCl and Solid Solutions in the BaCl2–BaBr2 System. J. Lumin. 2013, 138, 143–149. [Google Scholar] [CrossRef]
- Shalapska, T.; Moretti, F.; Bourret, E.; Bizarri, G. Effect of Au Codoping on the Scintillation Properties of BaBrCl:Eu Single Crystals. J. Lumin. 2018, 202, 497–501. [Google Scholar] [CrossRef]
- Li, P.; Gridin, S.; Ucer, K.B.; Williams, R.T.; Del Ben, M.; Canning, A.; Moretti, F.; Bourret, E. Picosecond Absorption Spectroscopy of Excited States in BaBrCl with and without Eu Dopant and Au Codopant. Phys. Rev. Appl. 2019, 12, 014035. [Google Scholar] [CrossRef]
- Mp-23260: BaI2 (Orthorhombic, Pnma, 62). Available online: https://materialsproject.org/materials/mp-23260/ (accessed on 21 October 2022).
- Mp-27456: BaBr2 (Orthorhombic, Pnma, 62). Available online: https://materialsproject.org/materials/mp-27456/ (accessed on 21 October 2022).
- Mp-23199: BaCl2 (Orthorhombic, Pnma, 62). Available online: https://materialsproject.org/materials/mp-23199/ (accessed on 21 October 2022).
- Hussey, D.S.; LaManna, J.M.; Baltic, E.; Jacobson, D.L. Neutron Imaging Detector with 2 µm Spatial Resolution Based on Event Reconstruction of Neutron Capture in Gadolinium Oxysulfide Scintillators. Nucl. Instrum. Methods A 2017, 866, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xiu, Q.; Zhou, J.; Yang, J.; Tan, J.; Yang, W.; Zhang, L.; Xia, Y.; Zhou, X.; Zhou, J.; et al. Study on the Neutron Imaging Detector with High Spatial Resolution at China Spallation Neutron Source. Nucl. Eng. Technol. 2021, 53, 1942–1946. [Google Scholar] [CrossRef]
- Fedorov, A.; Komendo, I.; Amelina, A.; Gordienko, E.; Gurinovich, V.; Guzov, V.; Dosovitskiy, G.; Kozhemyakin, V.; Kozlov, D.; Lopatik, A.; et al. GYAGG/6LiF Composite Scintillation Screen for Neutron Detection. Nucl. Eng. Technol. 2022, 54, 1024–1029. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Kamada, K.; Yanagida, T.; Kawaguchi, N.; Kurosawa, S.; Totsuka, D.; Fukuda, K.; Watanabe, K.; Yamazaki, A.; Yokota, Y.; et al. Lithium Aluminate Crystals as Scintillator for Thermal Neutron Detection. IEEE Trans. Nucl. Sci. 2012, 59, 2252–2255. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Koshimizu, M.; Kawano, N.; Okada, G.; Kawaguchi, N. Comparative Studies of Optical and Scintillation Properties between LiGaO2 and LiAlO2 Crystals. J. Phys. Soc. Jpn. 2017, 86, 094201. [Google Scholar] [CrossRef]
- Kamada, K.; Takizawa, Y.; Yoshino, M.; Kutsuzawa, N.; Kim, K.J.; Murakami, R.; Kochurikhin, V.; Yoshikawa, A. Growth and Scintillation Properties of Eu Doped Li2SrCl4/LiSr2Cl5 Eutectic. J. Cryst. Growth 2022, 581, 126500. [Google Scholar] [CrossRef]
- Takizawa, Y.; Kamada, K.; Yoshino, M.; Yajima, R.; Kim, K.J.; Kochurikhin, V.V.; Yoshikawa, A. Growth of 6Li-Enriched LiCl/BaCl2 Eutectic as a Novel Neutron Scintillator. Jpn. J. Appl. Phys. 2022, 61, SC1038. [Google Scholar] [CrossRef]
- Gektin, A.; Shiran, N.; Neicheva, S.; Gavrilyuk, V.; Bensalah, A.; Fukuda, T.; Shimamura, K. LiCaAlF6:Ce Crystal: A New Scintillator. Nucl. Instrum. Methods A 2002, 486, 274–277. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Yanagida, T.; Novoselov, A.; Kim, K.J.; Fukuda, K.; Yoshikawa, A.; Miyake, M.; Baba, M. Neutron Responses of Eu2+ Activated LiCaAlF6 Scintillator. In Proceedings of the 2008 IEEE Nuclear Science Symposium Conference Record, Dresden, Germany, 19–25 October 2008; pp. 1174–1176. [Google Scholar]
- Zhou, W.; Hou, D.; Pan, F.; Zhang, B.; Dorenbos, P.; Huang, Y.; Tao, Y.; Liang, H. VUV-Vis Photoluminescence, X-Ray Radioluminescence and Energy Transfer Dynamics of Ce3+ and Pr3+ Doped LiCaBO3. J. Mater. Chem. C 2015, 3, 9161–9169. [Google Scholar] [CrossRef]
- Pierron, L.; Kahn-Harari, A.; Viana, B.; Dorenbos, P.; van Eijk, C.W.E. X-Ray Excited Luminescence of Ce:Li2CaSiO4, Ce:CaBPO5 and Ce:LiCaPO4. J. Phys. Chem. Solids 2003, 64, 1743–1747. [Google Scholar] [CrossRef]
- Xie, M.; Liang, H.; Su, Q.; Huang, Y.; Gao, Z.; Tao, Y. Intense Cyan-Emitting of Li2CaSiO4:Eu2+ Under Low-Voltage Cathode Ray Excitation. Electrochem. Solid-State Lett. 2011, 14, J69. [Google Scholar] [CrossRef]
- Komendo, I.; Bondarev, A.; Fedorov, A.; Dosovitskiy, G.; Gurinovich, V.; Kazlou, D.; Kozhemyakin, V.; Mechinsky, V.; Mikhlin, A.; Retivov, V.; et al. New scintillator 6Li2CaSiO4:Eu2+for neutron sensitive screens. Nucl. Instrum. Methods A 2022, 1045, 167637. [Google Scholar] [CrossRef]
- Komendo, I.; Mechinsky, V.; Fedorov, A.; Dosovitskiy, G.; Schukin, V.; Kuznetsova, D.; Zykova, M.; Velikodny, Y.; Korzhik, M. Effect of the Synthesis Conditions on the Morphology, Luminescence and Scintillation Properties of a New Light Scintillation Material Li2CaSiO4:Eu2+ for Neutron and Charged Particle Detection. Inorganics 2022, 10, 127. [Google Scholar] [CrossRef]
- Bessiere, A.; Dorenbos, P.; van Eijk, C.; Kramer, K.; Gudel, H.U. New Thermal Neutron Scintillators: Cs2LiYCl6: Ce3+ and Cs2LiYBr6:Ce3+. IEEE Trans. Nucl. Sci. 2004, 51, 2970–2972. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, W.; Lan, L.; Wang, J.; Chen, J.; Wang, N. Enhanced Emission from Li2CaSiO4:Eu2+ Phosphors by Doping with Y3+. J. Alloys Compd. 2014, 592, 213–219. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, W.; Lan, L.; Wang, J. Strong Luminescence Enhancement of Li2CaSiO4:Eu2+ Phosphors by Codoping with La3+. J. Mater. Sci. Mater. Electron. 2014, 25, 736–741. [Google Scholar] [CrossRef]
- Yin, L.-J.; Ji, W.-W.; Liu, S.-Y.; He, W.-D.; Zhao, L.; Xu, X.; Fabre, A.; Dierre, B.; Lee, M.-H.; van Ommen, J.R.; et al. Intriguing Luminescence Properties of (Ba, Sr)3Si6O9N4:Eu2+ Phosphors via Modifying Synthesis Method and Cation Substitution. J. Alloys Compd. 2016, 682, 481–488. [Google Scholar] [CrossRef]
- Birowosuto, M.D.; Dorenbos, P.; Krämer, K.W.; Güdel, H.U. Ce3+ Activated LaBr3−xIx: High-Light-Yield and Fast-Response Mixed Halide Scintillators. J. Appl. Phys. 2008, 103, 103517. [Google Scholar] [CrossRef]
- Dorenbos, P.; Birowosuto, M.D.; Kraemer, K.W.; Guedel, H.-U. Scintillator Based on Lanthanum Iodide and Lanthanum Bromide. U.S. Patent 20100224798A1, 09 September 2010. [Google Scholar]
- Glodo, J.; van Loef, E.V.D.; Higgins, W.M.; Shah, K.S. Mixed Lutetium Iodide Compounds. IEEE Trans. Nucl. Sci. 2008, 55, 1496–1500. [Google Scholar] [CrossRef]
- Eagleman, Y.D.; Bourret-Courchesne, E.; Derenzo, S.E. Room-Temperature Scintillation Properties of Cerium-Doped REOX (RE=Y, La, Gd, and Lu; X = F, Cl, Br, and I). J. Lumin. 2011, 131, 669–675. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Szupryczynski, P.; Glodo, J.; Drozdowski, W.; Wisniewski, D. Radioluminescence and recombination process in BaF2:Ce. J. Phys. Condens. Matter 2000, 12, 4097. [Google Scholar] [CrossRef]
- Luo, J.; Sahi, S.; Groza, M.; Wang, Z.; Ma, L.; Chen, W.; Burger, A.; Kenarangui, R.; Sham, T.-K.; Selim, F.A. Luminescence and scintillation properties of BaF2Ce transparent ceramics. Opt. Mater. 2016, 58, 353–356. [Google Scholar] [CrossRef]
- Rodnyĭ, P.A.; Gain, S.D.; Mironov, I.A.; Garibin, E.A.; Demidenko, A.A.; Seliverstov, D.M.; Kuznetsov, S.V. Spectral-kinetic characteristics of crystals and nanoceramics based on BaF2 and BaF2: Ce. Phys. Solid State 2010, 52, 1910–1914. [Google Scholar] [CrossRef]
- Auffray, E.; Baccaro, S.; Beckers, T.; Benhammou, Y.; Belsky, A.; Borgia, B.; Boutet, D.; Chipaux, R.; Dafinei, I.; de Notaristefani, F.; et al. Extensive studies on CeF3 crystals, a good candidate for electromagnetic calorimetry at future accelerators. Nucl. Instrum. Methods Phys. Res. Sect. A 1996, 388, 367–390. [Google Scholar] [CrossRef][Green Version]
- Burger, A.; Rowe, E.; Groza, M.; Figueroa, K.M.; Cherepy, N.J.; Beck, P.R.; Hunter, S.; Payne, S.A. Cesium hafnium chloride: A high light yield, non-hygroscopic cubic crystal scintillator for gamma spectroscopy. Appl. Phys. Lett. 2015, 107, 143505. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Buliga, V.; Matei, L.; Motakef, S.; Burger, A. Advanced high-performance large diameter Cs2HfCl6 (CHC) and mixed halides scintillator. J. Cryst. Growth 2020, 533, 125473. [Google Scholar] [CrossRef]
- Nikl, M.; Yoshikawa, A.; Kamada, K.; Nejezchleb, K.; Stanek, C.R.; Mares, J.A.; Blazek, K. Development of LuAG-Based Scintillator Crystals–A Review. Prog. Cryst. Growth Charact. Mater. 2013, 59, 47–72. [Google Scholar] [CrossRef]
- Kamada, K.; Endo, T.; Tsutumi, K.; Yanagida, T.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A.; Pejchal, J.; Nikl, M. Composition Engineering in Cerium-Doped (Lu,Gd)3(Ga,Al)5O12 Single-Crystal Scintillators. Cryst. Growth Des. 2011, 11, 4484–4490. [Google Scholar] [CrossRef]
- Kamada, K.; Yanagida, T.; Endo, T.; Tsutumi, K.; Usuki, Y.; Nikl, M.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A. 2inch Diameter Single Crystal Growth and Scintillation Properties of Ce:Gd3Al2Ga3O12. J. Cryst. Growth 2012, 352, 88–90. [Google Scholar] [CrossRef]
- Nikl, M.; Yoshikawa, A. Recent R&D Trends in Inorganic Single-Crystal Scintillator Materials for Radiation Detection. Adv. Opt. Mater. 2015, 3, 463–481. [Google Scholar] [CrossRef]
- Kuwano, Y.; Suda, K.; Ishizawa, N.; Yamada, T. Crystal Growth and Properties of (Lu,Y)3Al5O12. J. Cryst. Growth 2004, 260, 159–165. [Google Scholar] [CrossRef]
- Yanagida, T.; Itoh, T.; Takahashi, H.; Hirakuri, S.; Kokubun, M.; Makishima, K.; Sato, M.; Enoto, T.; Yanagitani, T.; Yagi, H.; et al. Improvement of Ceramic YAG(Ce) Scintillators to (YGd)3Al5O12(Ce) for Gamma-Ray Detectors. Nucl. Instrum. Methods A 2007, 579, 23–26. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Wantong, K.; Chewpraditkul, W.; Yawai, N.; Kamada, K.; Yoshikawa, A.; Witkowski, M.E.; Makowski, M.; Drozdowski, W.; Nikl, M. Scintillation Yield and Temperature Dependence of Radioluminescence of (Lu,Gd)3Al5O12:Ce Garnet Crystals. Opt. Mater. 2021, 120, 111471. [Google Scholar] [CrossRef]
- Korzhik, M.; Borisevich, A.; Fedorov, A.; Gordienko, E.; Karpyuk, P.; Dubov, V.; Sokolov, P.; Mikhlin, A.; Dosovitskiy, G.; Mechninsky, V.; et al. The Scintillation Mechanisms in Ce and Tb Doped (GdxY1-x)Al2Ga3O12 Quaternary Garnet Structure Crystalline Ceramics. J. Lumin. 2021, 234, 117933. [Google Scholar] [CrossRef]
- Wegh, R.T.; Meijerink, A.; Lamminmäki, R.-J. Jorma Hölsä Extending Dieke’s Diagram. J. Lumin. 2000, 87–89, 1002–1004. [Google Scholar] [CrossRef]
- Retivov, V.; Dubov, V.; Kuznetsova, D.; Ismagulov, A.; Korzhik, M. Gd3+ Content Optimization for Mastering High Light Yield and Fast GdxAl2Ga3O12:Ce3+ Scintillation Ceramics. J. Rare Earths 2022, in press. [Google Scholar] [CrossRef]
- Dosovitskiy, G.; Dubov, V.; Karpyuk, P.; Volkov, P.; Tamulaitis, G.; Borisevich, A.; Vaitkevičius, A.; Prikhodko, K.; Kutuzov, L.; Svetogorov, R.; et al. Activator Segregation and Micro-Luminescence Properties in GAGG:Ce Ceramics. J. Lumin. 2021, 236, 118140. [Google Scholar] [CrossRef]
- Korzhik, M.; Alenkov, V.; Buzanov, O.; Dosovitskiy, G.; Fedorov, A.; Kozlov, D.; Mechinsky, V.; Nargelas, S.; Tamulaitis, G.; Vaitkevičius, A. Engineering of a New Single-Crystal Multi-Ionic Fast and High-Light-Yield Scintillation Material (Gd0.5–Y0.5)3Al2Ga3O12:Ce,Mg. CrystEngComm 2020, 22, 2502–2506. [Google Scholar] [CrossRef]
- Kamada, K.; Yoshikawa, A.; Endo, T.; Tsutsumi, K.; Shoji, Y.; Kurosawa, S.; Yokota, Y.; Prusa, P.; Nikl, M. Growth of 2-Inch Size Ce:Doped Lu2Gd1Al2Ga3O12 Single Crystal by the Czochralski Method and Their Scintillation Properties. J. Cryst. Growth 2015, 410, 14–17. [Google Scholar] [CrossRef]
- Witkiewicz-Lukaszek, S.; Gorbenko, V.; Zorenko, T.; Sidletskiy, O.; Gerasymov, I.; Fedorov, A.; Yoshikawa, A.; Mares, J.A.; Nikl, M.; Zorenko, Y. Development of Composite Scintillators Based on Single Crystalline Films and Crystals of Ce3+-Doped (Lu,Gd)3(Al,Ga)5O12 Mixed Garnet Compounds. Cryst. Growth Des. 2018, 18, 1834–1842. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Pattanaboonmee, N.; Chewpraditkul, W.; Szczesniak, T.; Moszynski, M.; Kamada, K.; Yoshikawa, A.; Kucerkova, R.; Nikl, M. Optical and Scintillation Properties of LuGd2Al2Ga3O12:Ce, Lu2GdAl2Ga3O12:Ce, and Lu2YAl2Ga3O12:Ce Single Crystals: A Comparative Study. Nucl. Instrum. Methods A 2021, 1004, 165381. [Google Scholar] [CrossRef]
- Korzhik, M.; Retivov, V.; Bondarau, A.; Dosovitskiy, G.; Dubov, V.; Kamenskikh, I.; Karpuk, P.; Kuznetsova, D.; Smyslova, V.; Mechinsky, V.; et al. Role of the Dilution of the Gd Sublattice in Forming the Scintillation Properties of Quaternary (Gd,Lu)3Al2Ga3O12: Ce Ceramics. Crystals 2022, 12, 1196. [Google Scholar] [CrossRef]
- Kuznetsova, D.; Dubov, V.; Bondarev, A.; Dosovitskiy, G.; Mechinsky, V.; Retivov, V.; Kucherov, O.; Saifutyarov, R.; Korzhik, M. Tailoring of the Gd-Y-Lu ratio in quintuple (Gd, Lu, Y)3Al2Ga3O12:Ce ceramics for better scintillation properties. J. Appl. Phys. 2022, 132, 203104. [Google Scholar] [CrossRef]
- Tsubota, Y.; Kaneko, J.H.; Higuchi, M.; Minagawa, M.; Ishibashi, H. Crystal Growth and Scintillation Properties of La0.2Ce0.05Gd1.75Si2O7 (La-GPS) Single Crystal with Triclinic Structure. Opt. Mater. 2014, 36, 665–669. [Google Scholar] [CrossRef]
- Murakami, R.; Kurosawa, S.; Yamane, H.; Horiai, T.; Shoji, Y.; Yokota, Y.; Yamaji, A.; Ohashi, Y.; Kamada, K.; Yoshikawa, A. Crystal Structure of Ce-Doped (La,Gd)2Si2O7 Grown by the Czochralski Process. J. Alloys Compd. 2018, 748, 404–410. [Google Scholar] [CrossRef]
- Lu, L.; Sun, M.; Wu, T.; Lu, Q.; Chen, B.; Huang, B. All-Inorganic Perovskite Nanocrystals: Next-Generation Scintillation Materials for High-Resolution X-Ray Imaging. Nanoscale Adv. 2022, 4, 680–696. [Google Scholar] [CrossRef]
- Pokorný, M.; Babin, V.; Beitlerová, A.; Jurek, K.; Polák, J.; Houžvička, J.; Pánek, D.; Parkman, T.; Vaněček, V.; Nikl, M. Gd-Admixed (Lu,Gd)AlO3 Single Crystals: Breakthrough in Heavy Perovskite Scintillators. NPG Asia Mater. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Brixner, L.H. New X-Ray Phosphors. Mater. Chem. Phys. 1987, 16, 253–281. [Google Scholar] [CrossRef]
- Naik, R.; Prashantha, S.; Nagabhushana, H.; Naik, Y.; Girish, K. Green Light Emitting Tb3+ Doped Phosphors-A Review. Mat. Sci. Res. India 2018, 15, 252–255. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Savchyn, V.; Zorenko, T.; Martin, T.; Douissard, P.-A.; Nikl, M.; Mares, J.A. Luminescent Properties and Energy Transfer Processes in Ce–Tb Doped Single Crystalline Film Screens of Lu-Based Silicate, Perovskite and Garnet Compounds. Radiat. Meas. 2013, 56, 415–419. [Google Scholar] [CrossRef]
- Langley, J.; Litz, M.; Russo, J.; Ray, W. Design of Alpha-Voltaic Power Source Using Americium-241 (241Am) and Diamond with a Power Density of 10 mW/cm3; US Army Research Laboratory Adelphi United States: Adelphi, MD, USA, 2017. [Google Scholar]
- Sheshin, E.P.; Kolodyazhnyj, A.Y.; Chadaev, N.N.; Getman, A.O.; Danilkin, M.I.; Ozol, D.I. Prototype of Cathodoluminescent Lamp for General Lighting Using Carbon Fiber Field Emission Cathode. J. Vac. Sci. Technol. B 2019, 37, 031213. [Google Scholar] [CrossRef]
- Korjik, M.; Brinkmann, K.-T.; Dosovitskiy, G.; Dormenev, V.; Fedorov, A.; Kozlov, D.; Mechinsky, V.; Zaunick, H.-G. Compact and Effective Detector of the Fast Neutrons on a Base of Ce-Doped Gd3Al2Ga3O12 Scintillation Crystal. IEEE Trans. Nucl. Sci. 2019, 66, 536–540. [Google Scholar] [CrossRef]
- Dosovitskiy, G.; Karpyuk, P.; Gordienko, E.; Kuznetsova, D.; Vashchenkova, E.; Volkov, P.; Retivov, V.; Dormenev, V.; Brinkmann, K.-T.; Zaunick, H.-G.; et al. Neutron Detection by Gd-Loaded Garnet Ceramic Scintillators. Radiat. Meas. 2019, 126, 106133–106137. [Google Scholar] [CrossRef]
- Korzhik, M.; Abashev, R.; Fedorov, A.; Dosovitskiy, G.; Gordienko, E.; Kamenskikh, I.; Kazlou, D.; Kuznecova, D.; Mechinsky, V.; Pustovarov, V.; et al. Towards Effective Indirect Radioisotope Energy Converters with Bright and Radiation Hard Scintillators of (Gd,Y)3Al2Ga3O12 Family. Nucl. Eng. Technol. 2022, 54, 2579–2585. [Google Scholar] [CrossRef]


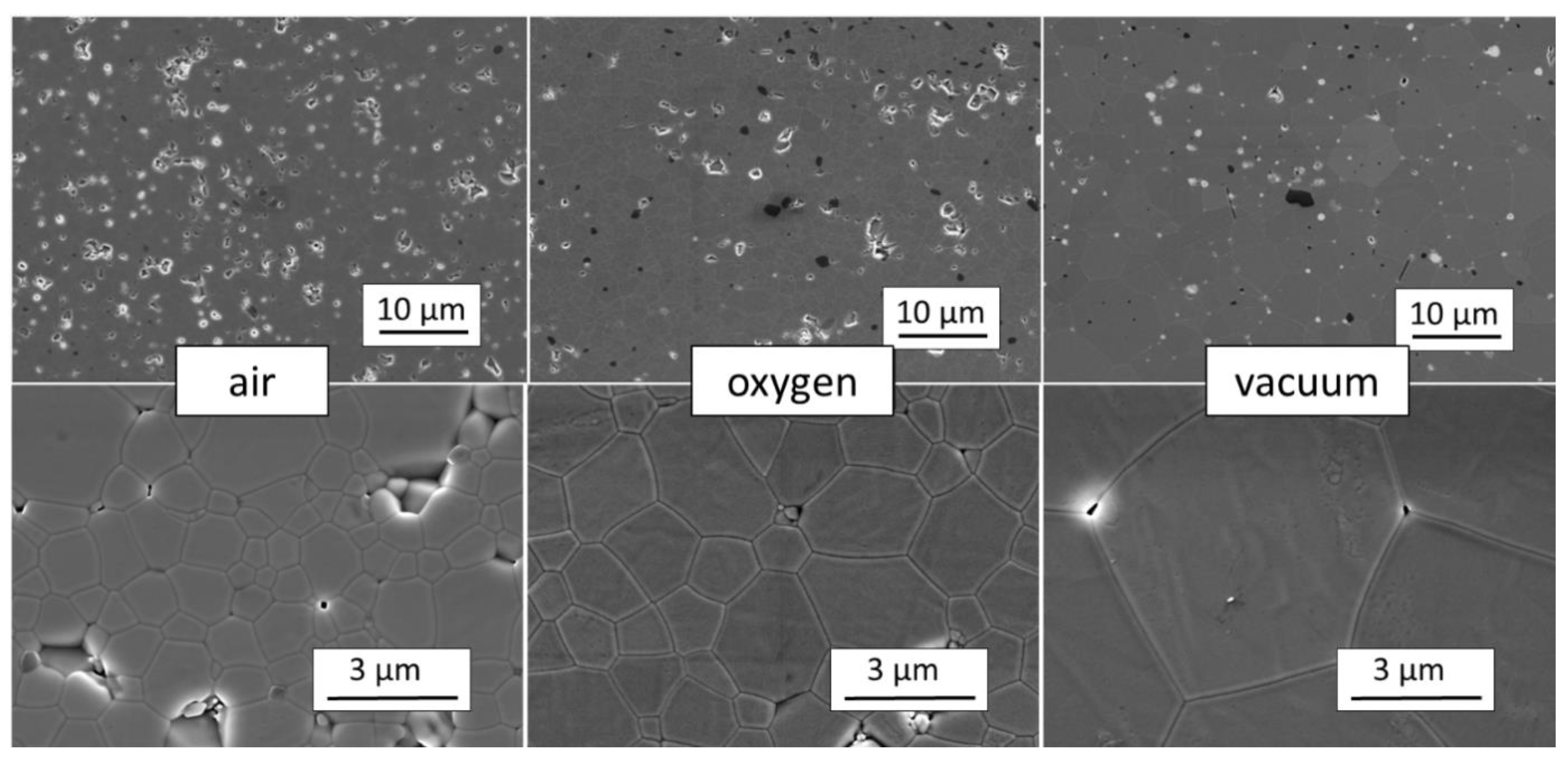

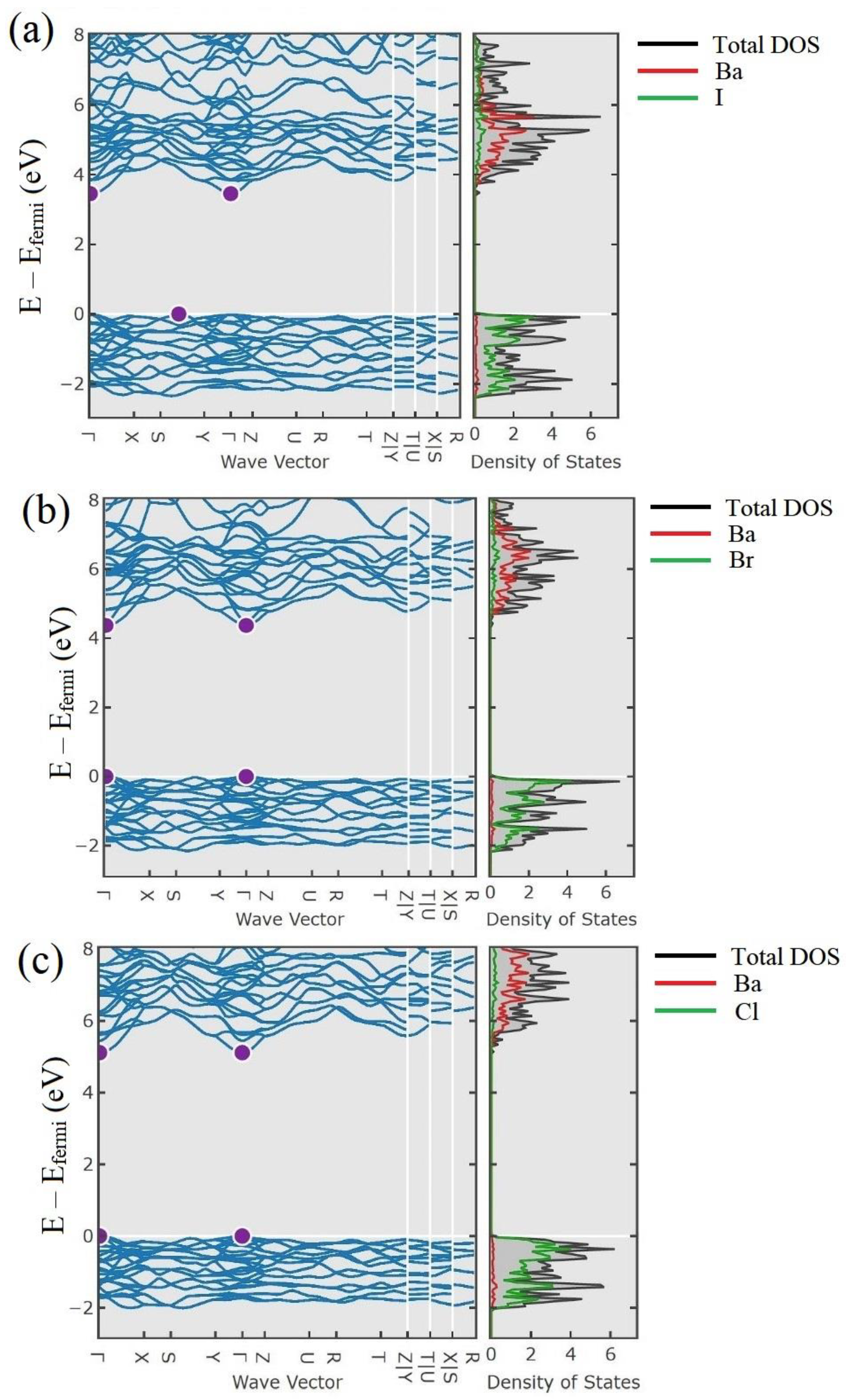
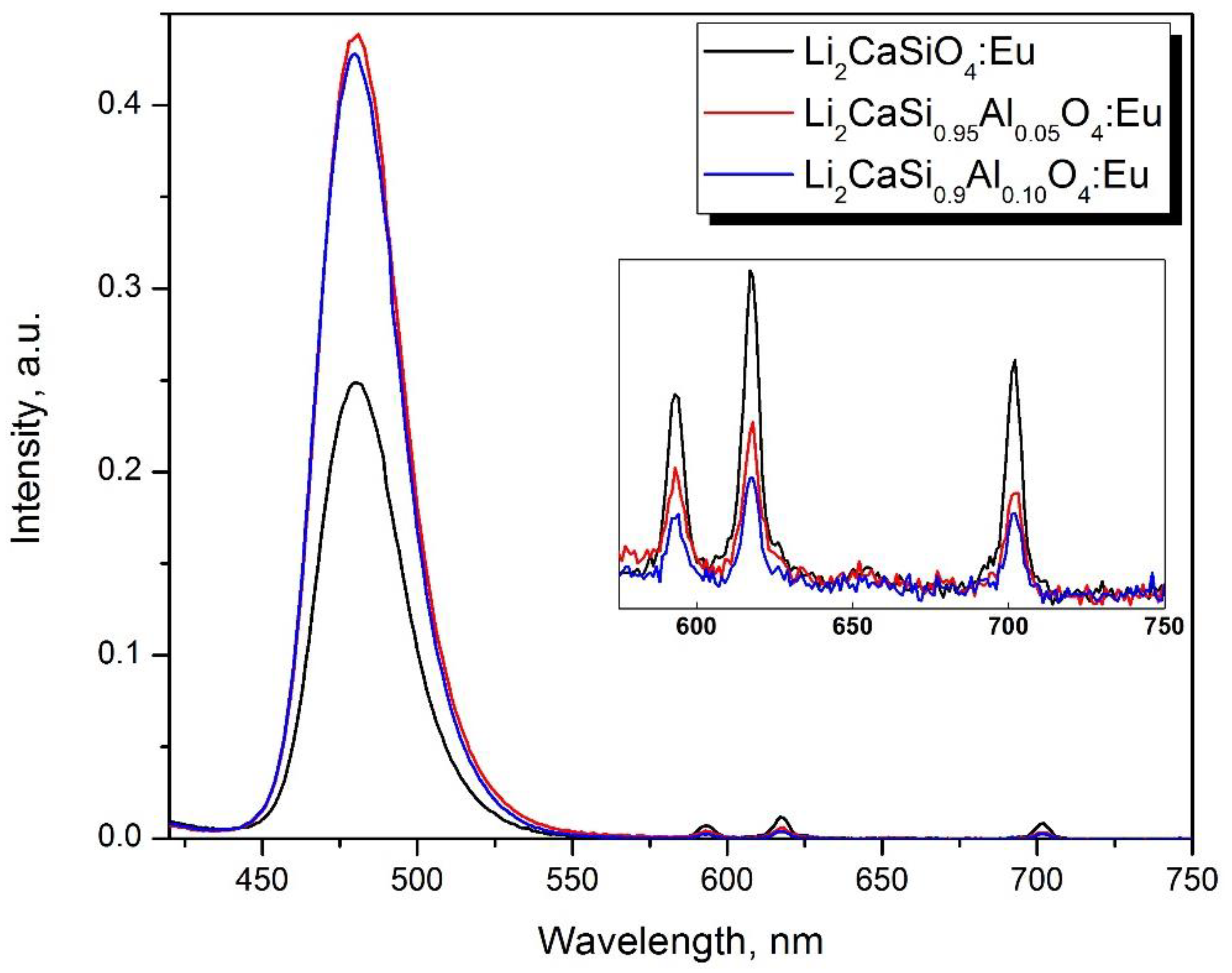
| Composition. | Eu2+% | Eu3+% |
|---|---|---|
| Li2CaSiO4: Eu | 98.7 ± 0.1 | 1.3 ± 0.1 |
| Li2CaSi0.95Al0.05O4: Eu | 99.5 ± 0.1 | 0.5 ± 0.1 |
| Li2CaSi0.90Al0.10O4: Eu | 99.6 ± 0.1 | 0.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retivov, V.; Dubov, V.; Komendo, I.; Karpyuk, P.; Kuznetsova, D.; Sokolov, P.; Talochka, Y.; Korzhik, M. Compositionally Disordered Crystalline Compounds for Next Generation of Radiation Detectors. Nanomaterials 2022, 12, 4295. https://doi.org/10.3390/nano12234295
Retivov V, Dubov V, Komendo I, Karpyuk P, Kuznetsova D, Sokolov P, Talochka Y, Korzhik M. Compositionally Disordered Crystalline Compounds for Next Generation of Radiation Detectors. Nanomaterials. 2022; 12(23):4295. https://doi.org/10.3390/nano12234295
Chicago/Turabian StyleRetivov, Vasili, Valery Dubov, Ilia Komendo, Petr Karpyuk, Daria Kuznetsova, Petr Sokolov, Yauheni Talochka, and Mikhail Korzhik. 2022. "Compositionally Disordered Crystalline Compounds for Next Generation of Radiation Detectors" Nanomaterials 12, no. 23: 4295. https://doi.org/10.3390/nano12234295
APA StyleRetivov, V., Dubov, V., Komendo, I., Karpyuk, P., Kuznetsova, D., Sokolov, P., Talochka, Y., & Korzhik, M. (2022). Compositionally Disordered Crystalline Compounds for Next Generation of Radiation Detectors. Nanomaterials, 12(23), 4295. https://doi.org/10.3390/nano12234295







