Nanoplasmonic Paper-Based Platform for General Screening of Biomacromolecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Synthesis and Processing of Gold Nanoparticles
2.3. UV-Vis Spectroscopy and Fluorimetry
2.4. Ferricyanide Dissociation Activity
2.5. Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
2.6. Dynamic Light Scattering and Zeta Potential
2.7. Plasmonic Paper Fabrication
2.8. Colour Analysis
3. Results and Discussion
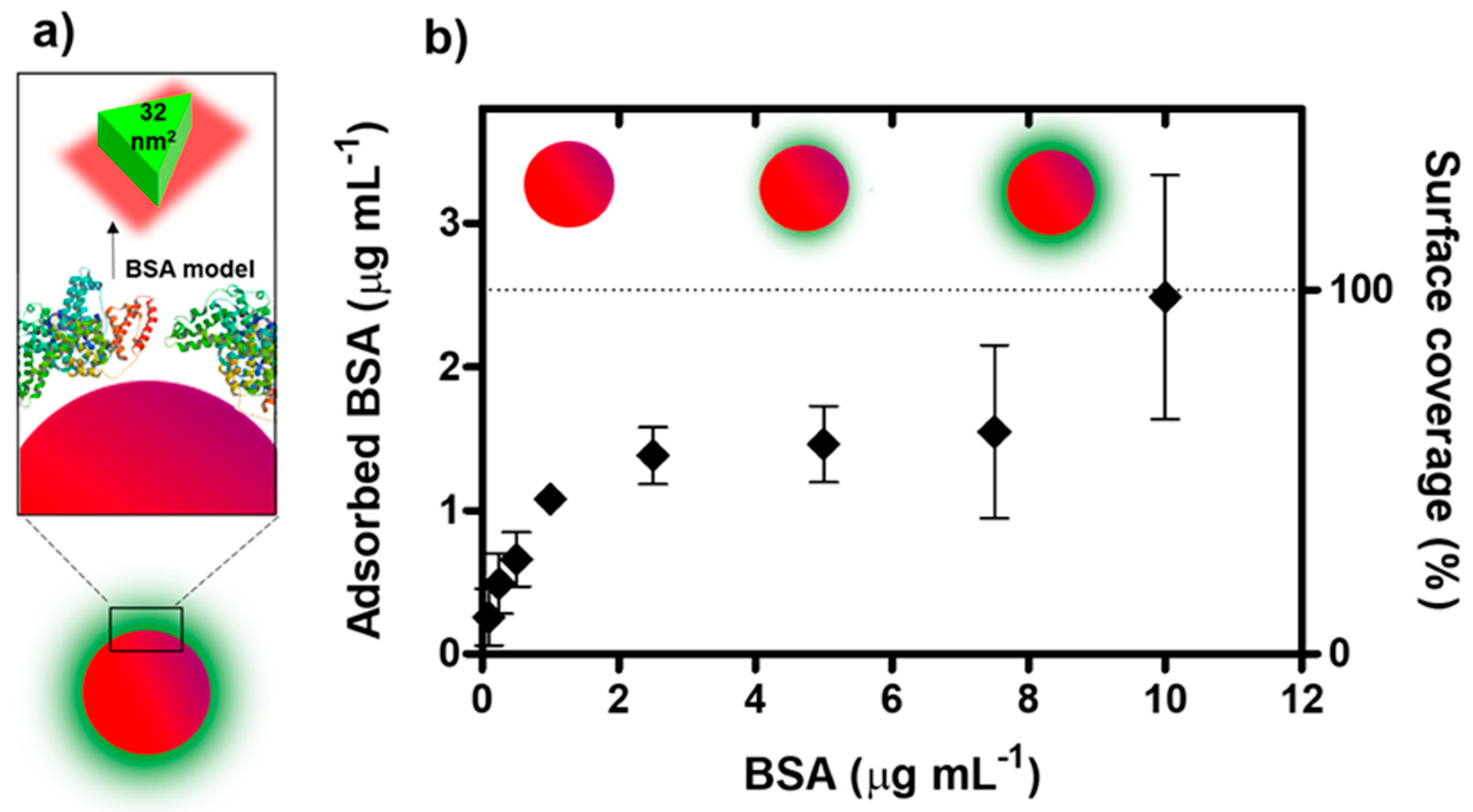
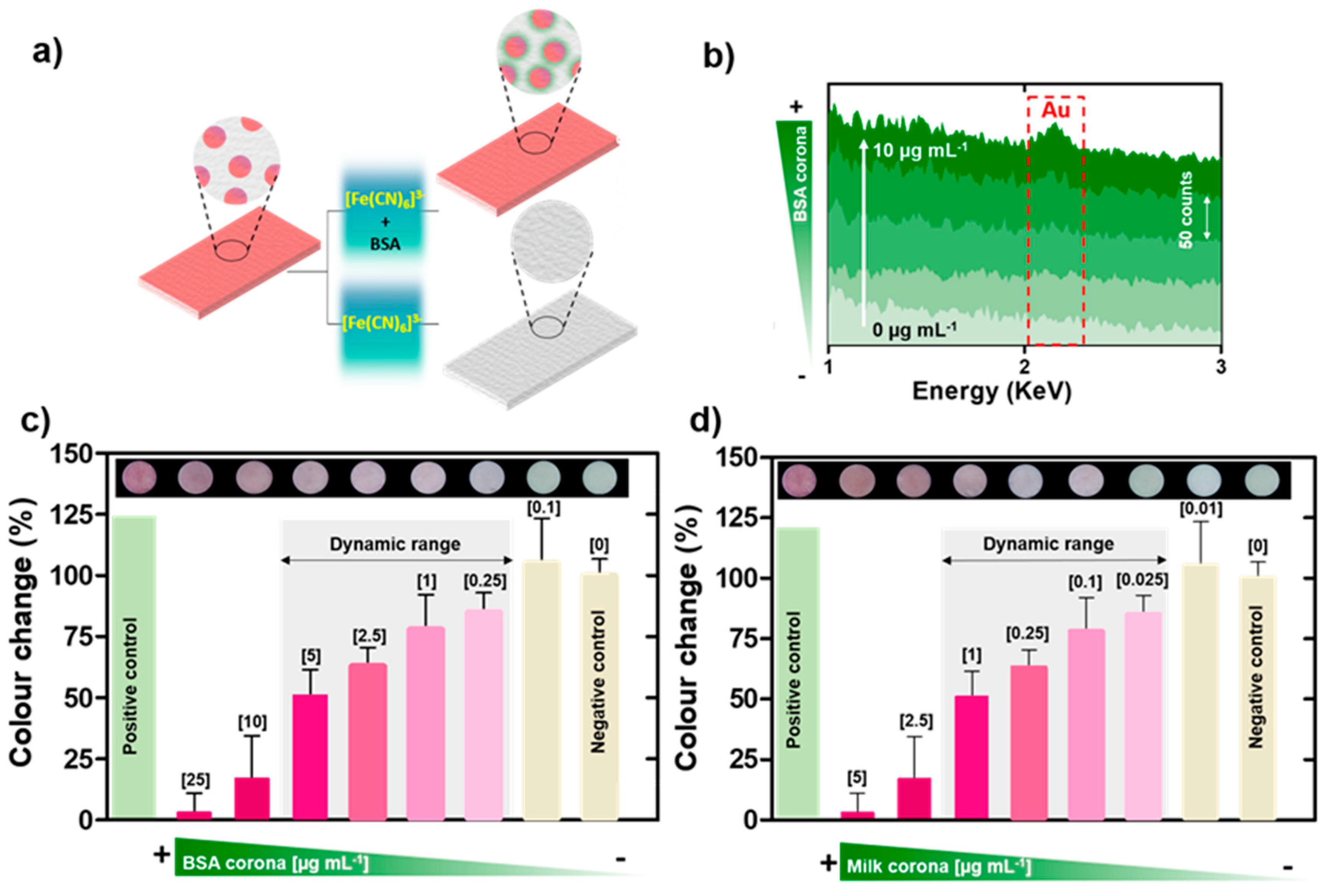
3.1. Biocorona Formation and Characterization
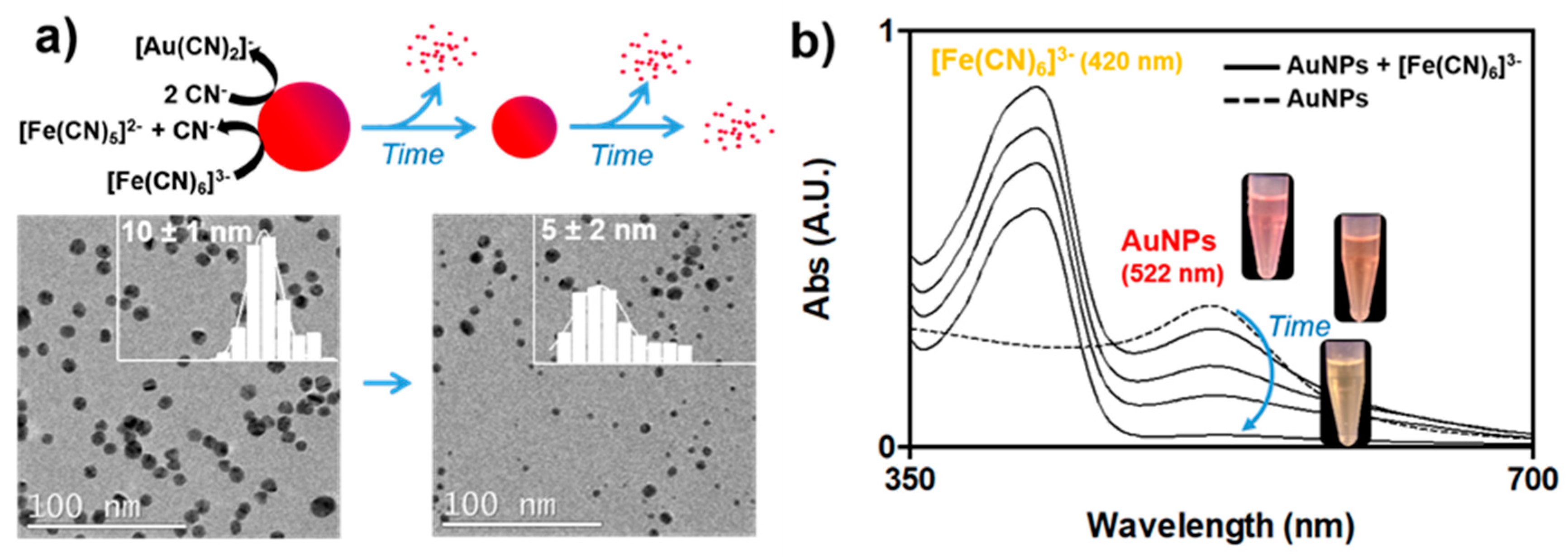
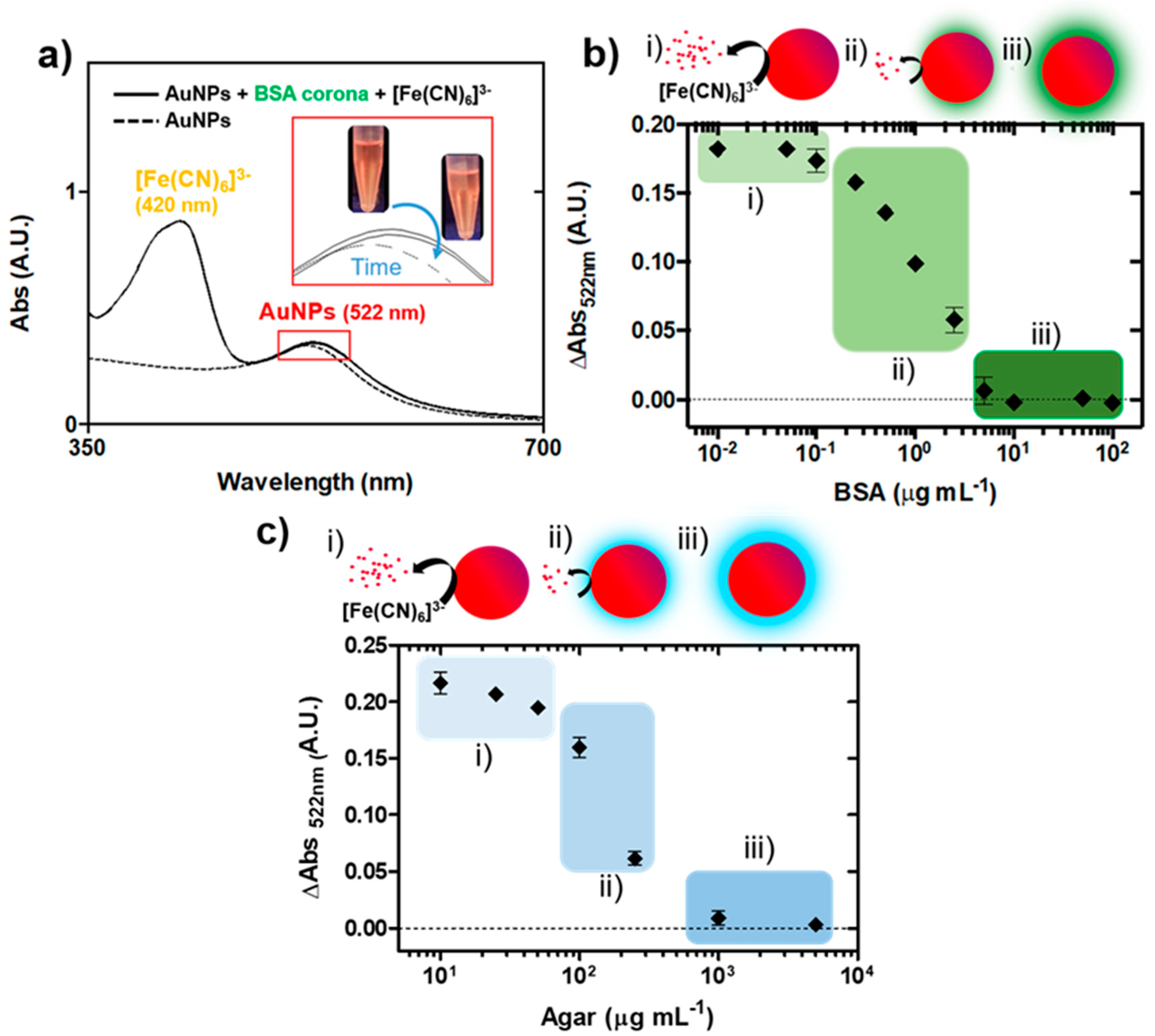
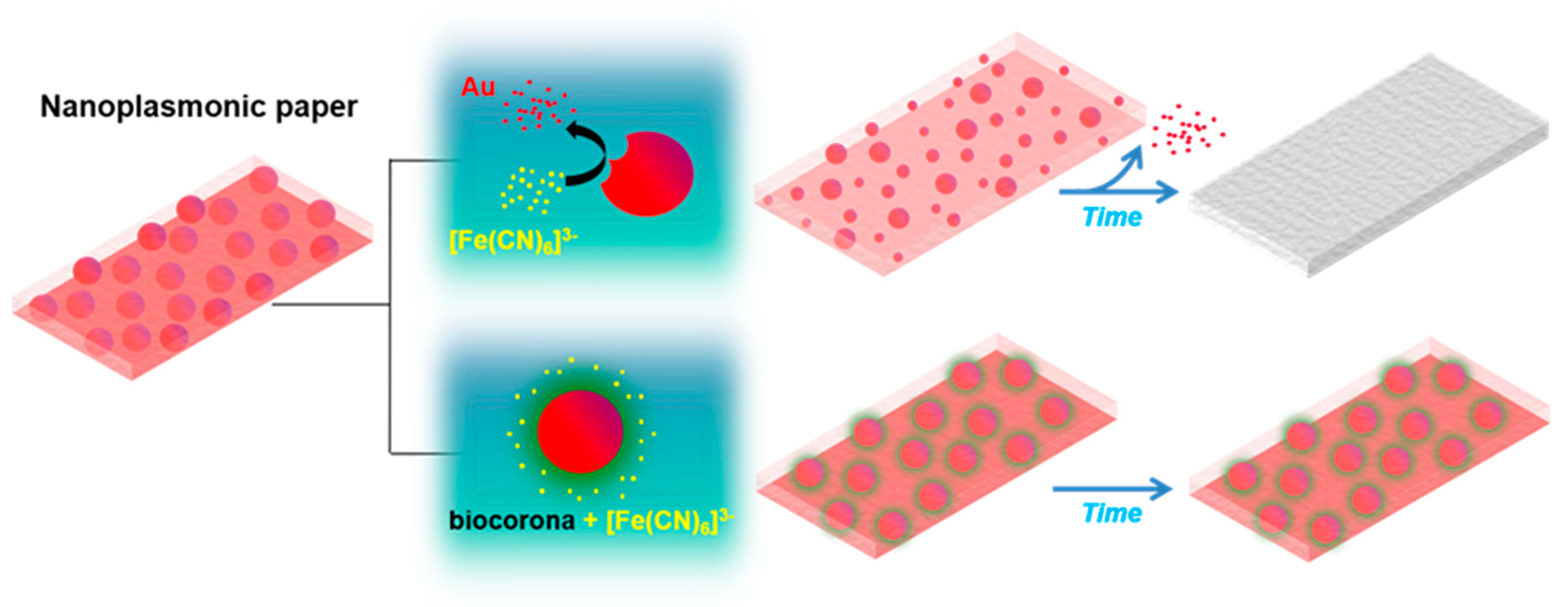
3.2. Impact of the Biocorona on Ferricyanide Dissociation by AuNPs
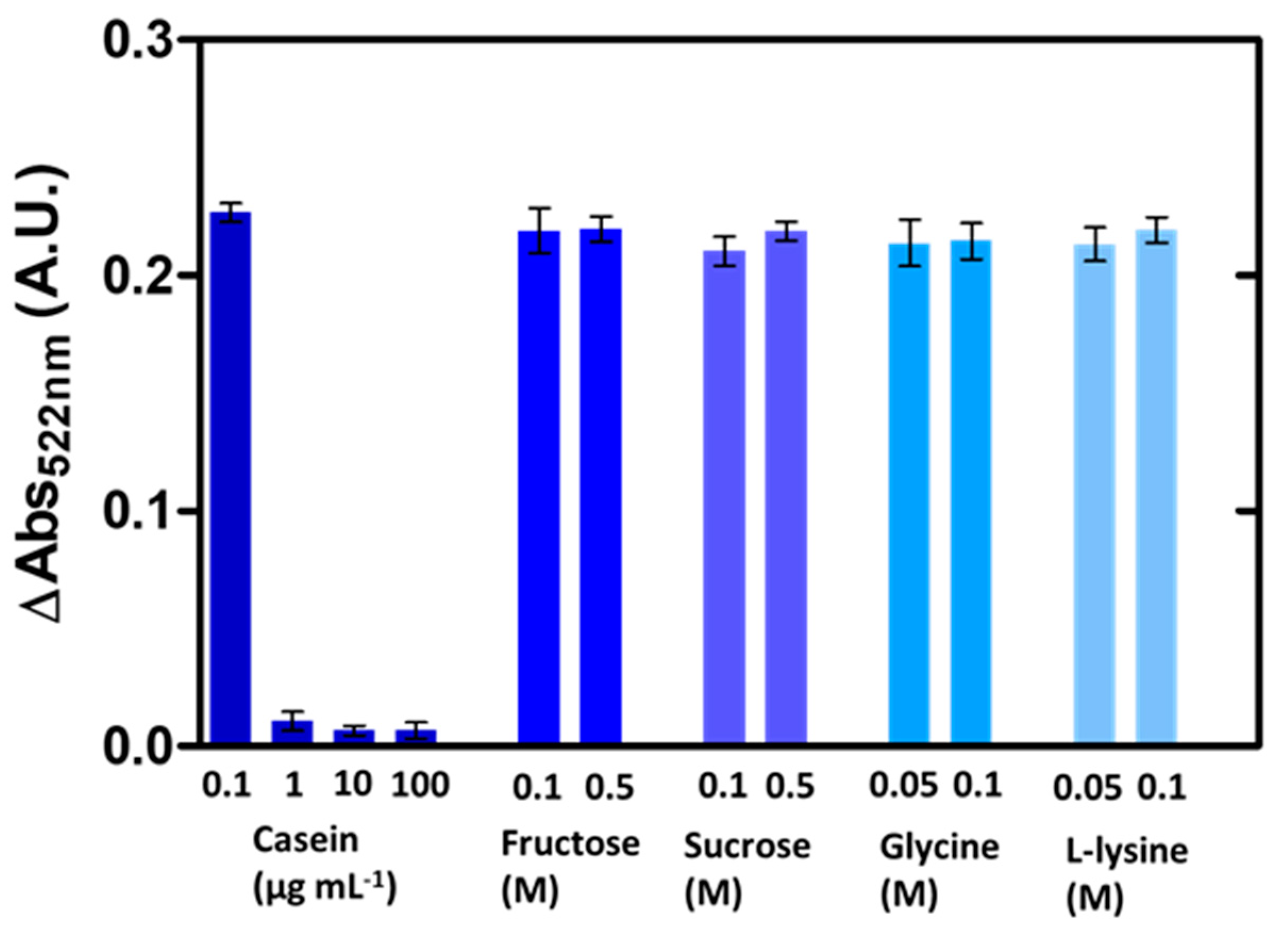
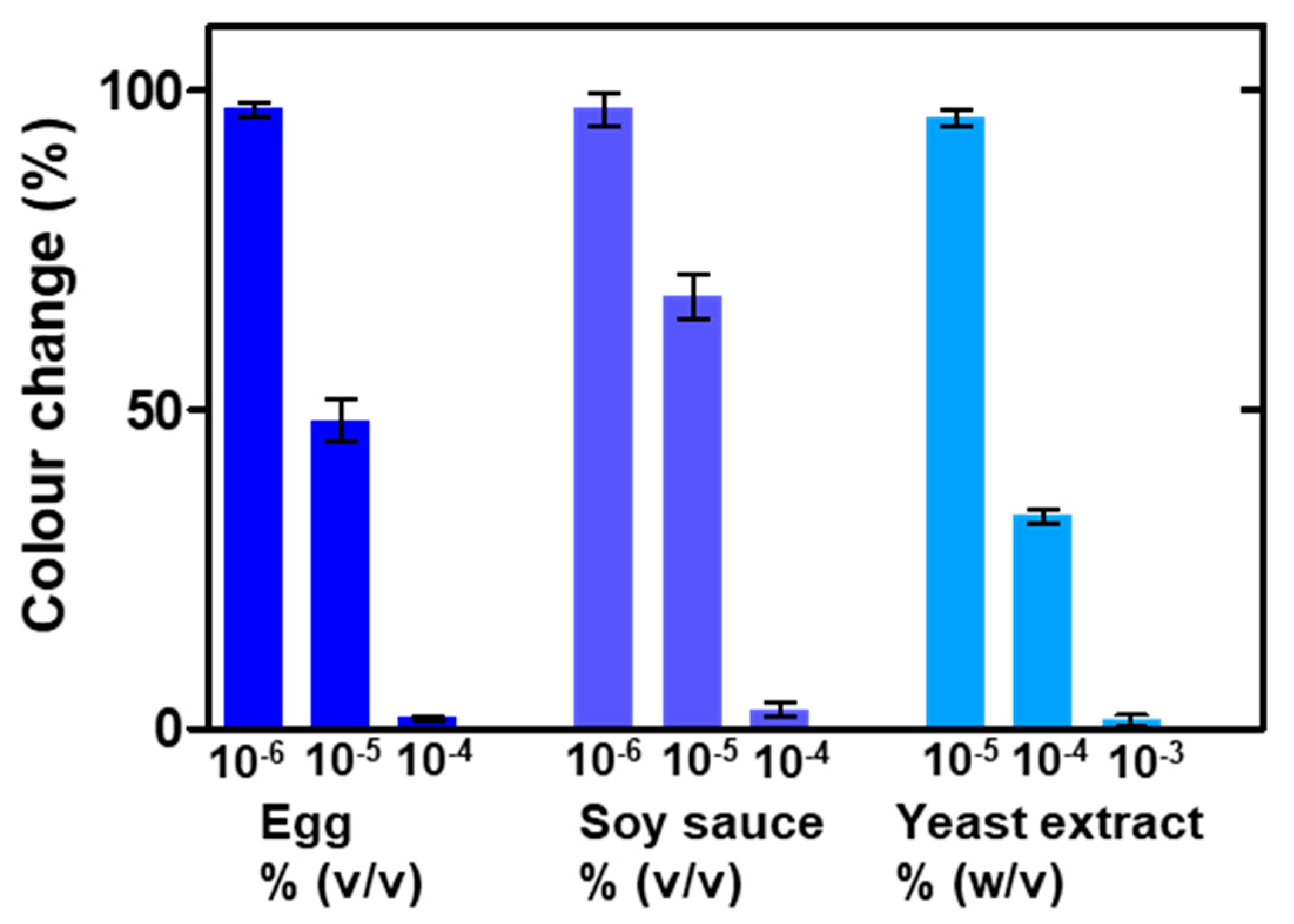
3.3. Nanoplasmonic Paper Platform for General Screening of Biomacromolecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Messina, G.; Ceriale, E.; Nante, N.; Manzi, P. Effectiveness of ATP bioluminescence to assess hospital cleaning: A review. Eur. J. Public Health 2014, 24. [Google Scholar] [CrossRef][Green Version]
- Sanna, T.; Dallolio, L.; Raggi, A.; Mazzetti, M.; Lorusso, G.; Zanni, A.; Farruggia, P.; Leoni, E. ATP bioluminescence assay for evaluating cleaning practices in operating theatres: Applicability and limitations. BMC Infect Dis. 2018, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
- Rudkin, J.K.; McLoughlin, R.M.; Preston, A.; Massey, R.C. Bacterial toxins: Offensive, defensive, or something else altogether? PLoS Pathog. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Griffith, C.; Fielding, L. A Comparison of Traditional and Recently Developed Methods for Monitoring Surface Hygiene within the Food Industry: A Laboratory Study. Int. J. Environ. Health Res. 2002, 12, 317–329. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Comotti, M.; Pina, C.D.; Matarrese, R.; Rossi, M. The catalytic activity of “naked” gold particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef]
- Zhai, J.; Zhai, Y.; Dong, S. Direct dissolution of Au nanoparticles induced by potassium ferricyanide. Colloids Surf. A Physicochem. Eng. Asp. 2009, 335, 207–210. [Google Scholar] [CrossRef]
- Lang, N.J.; Liu, B.; Liu, J. Characterization of glucose oxidation by gold nanoparticles using nanoceria. J. Colloid Interface Sci. 2014, 428, 78–83. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef]
- Pamies, R.; Cifre, J.G.H.; Espín, V.F.; Collado-González, M.; Baños, F.G.D.; de la Torre, J.G. Aggregation behaviour of gold nanoparticles in saline aqueous media. J. Nanopart Res. 2014, 16, 2376. [Google Scholar] [CrossRef]
- Ho, Y.T.; Poinard, B.; Yeo, E.L.L.; Kah, J.C.Y. An instantaneous colorimetric protein assay based on spontaneous formation of a protein corona on gold nanoparticles. Analyst 2015, 140, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- García Vence, M.; Chantada-Vázquez, M.D.P.; Vázquez-Estévez, S.; Manuel Cameselle-Teijeiro, J.; Bravo, S.B.; Núñez, C. Potential clinical applications of the personalized, disease-specific protein corona on nanoparticles. Clin. Chim. Acta 2020, 501, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Chen, C. The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine. Adv. Mater. Weinh. 2019, 31, e1805740. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Riviere, J.E.; Monteiro-Riviere, N.A. Gold and silver nanoparticle interactions with human proteins: Impact and implications in biocorona formation. J. Mater. Chem. B 2015, 3, 2075–2082. [Google Scholar] [CrossRef]
- Shemetov, A.A.; Nabiev, I.; Sukhanova, A. Molecular Interaction of Proteins and Peptides with Nanoparticles. ACS Nano 2012, 6, 4585–4602. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Q.; Jing, C.; Li, Y.; Li, D.; Luo, W.; Wen, Y.; He, Y.; Huang, Q.; Long, Y.-T.; et al. Catalytic Gold Nanoparticles for Nanoplasmonic Detection of DNA Hybridization. Angew. Chem. Int. Ed. 2011, 50, 11994–11998. [Google Scholar] [CrossRef]
- Zhou, P.; Jia, S.; Pan, D.; Wang, L.; Gao, J.; Lu, J.; Shi, J.; Tang, Z.; Liu, H. Reversible Regulation of Catalytic Activity of Gold Nanoparticles with DNA Nanomachines. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ozcan, S. Green nanomaterials: On track for a sustainable future. Nano Today 2015, 10, 417–420. [Google Scholar] [CrossRef]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H.; Volckens, J.; Henry, C.S. Paper-based analytical devices for environmental analysis. Analyst 2016, 141, 1874–1887. [Google Scholar] [CrossRef]
- Sanahuja, D.; Giménez-Gómez, P.; Vigués, N.; Ackermann, T.N.; Guerrero-Navarro, A.E.; Pujol-Vila, F.; Sacristán, J.; Santamaria, N.; Sánchez-Contreras, M.; Díaz-González, M.; et al. Microbial trench-based optofluidic system for reagentless determination of phenolic compounds. Lab Chip 2015, 15, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus Ostwald ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Pujol-Vila, F.; Vigués, N.; Guerrero-Navarro, A.; Jiménez, S.; Gómez, D.; Fernández, M.; Bori, J.; Vallès, B.; Riva, M.C.; Muñoz-Berbel, X.; et al. Paper-based chromatic toxicity bioassay by analysis of bacterial ferricyanide reduction. Anal. Chim. Acta 2016, 910, 60–67. [Google Scholar] [CrossRef]
- Hvolbæk, B.; Janssens, T.V.W.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Nørskov, J.K. Catalytic activity of Au nanoparticles. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Dependent Protein–Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconjugate Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef]
- Gökelma, M.; Birich, A.; Stopic, S.; Friedrich, B. A Review on Alternative Gold Recovery Re-agents to Cyanide. MSCE 2016, 04, 8–17. [Google Scholar] [CrossRef]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of gold extraction from ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Xie, F.; Dreisinger, D.B. Use of ferricyanide for gold and silver cyanidation. Trans. Nonferrous Met. Soc. China 2009, 19, 714–718. [Google Scholar] [CrossRef]
- Lai, T.-S.; Chang, T.-C.; Wang, S.-C. Gold nanoparticle-based colorimetric methods to determine protein contents in artificial urine using membrane micro-concentrators and mobile phone camera. Sens. Actuators B Chem. 2017, 239, 9–16. [Google Scholar] [CrossRef]
- Noble, J.E.; Knight, A.E.; Reason, A.J.; Di Matola, A.; Bailey, M.J.A. A Comparison of Protein Quantitation Assays for Biopharmaceutical Applications. Mol. Biotechnol. 2007, 37, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Nitinaivinij, K.; Parnklang, T.; Thammacharoen, C.; Ekgasit, S.; Wongravee, K. Colorimetric determination of hydrogen peroxide by morphological decomposition of silver nanoprisms coupled with chromaticity analysis. Anal. Methods 2014, 6, 9816–9824. [Google Scholar] [CrossRef]
- Liang, J.; Yao, C.; Li, X.; Wu, Z.; Huang, C.; Fu, Q.; Lan, C.; Cao, D.; Tang, Y. Silver nanoprism etching-based plasmonic ELISA for the high sensitive detection of prostate-specific antigen. Biosens. Bioelectron. 2015, 69, 128–134. [Google Scholar] [CrossRef]
- Gu, Y.; Kong, S.; Diao, X.; Guo, Y.; Zhang, K.; He, H. Mechanistic study on the facet etching effect of silver nanoprisms in the presence of halide ions and their application in the colorimetric sensing of metformin hydrochloride. New J. Chem. 2016, 40, 7557–7563. [Google Scholar] [CrossRef]
- Monaci, L.; Tregoat, V.; van Hengel, A.J.; Anklam, E. Milk allergens, their characteristics and their detection in food: A review. Eur. Food Res. Technol. 2006, 223, 149–179. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujol-Vila, F.; Aveling Jenkins, A.T.; Muñoz-Berbel, X.; Mas Gordi, J. Nanoplasmonic Paper-Based Platform for General Screening of Biomacromolecules. Nanomaterials 2020, 10, 2335. https://doi.org/10.3390/nano10122335
Pujol-Vila F, Aveling Jenkins AT, Muñoz-Berbel X, Mas Gordi J. Nanoplasmonic Paper-Based Platform for General Screening of Biomacromolecules. Nanomaterials. 2020; 10(12):2335. https://doi.org/10.3390/nano10122335
Chicago/Turabian StylePujol-Vila, Ferran, Andrew Tobias Aveling Jenkins, Xavier Muñoz-Berbel, and Jordi Mas Gordi. 2020. "Nanoplasmonic Paper-Based Platform for General Screening of Biomacromolecules" Nanomaterials 10, no. 12: 2335. https://doi.org/10.3390/nano10122335
APA StylePujol-Vila, F., Aveling Jenkins, A. T., Muñoz-Berbel, X., & Mas Gordi, J. (2020). Nanoplasmonic Paper-Based Platform for General Screening of Biomacromolecules. Nanomaterials, 10(12), 2335. https://doi.org/10.3390/nano10122335





