An OMV-Based Nanovaccine Confers Safety and Protection against Pathogenic Escherichia coli via Both Humoral and Predominantly Th1 Immune Responses in Poultry
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Preparation of APEC_OMVs
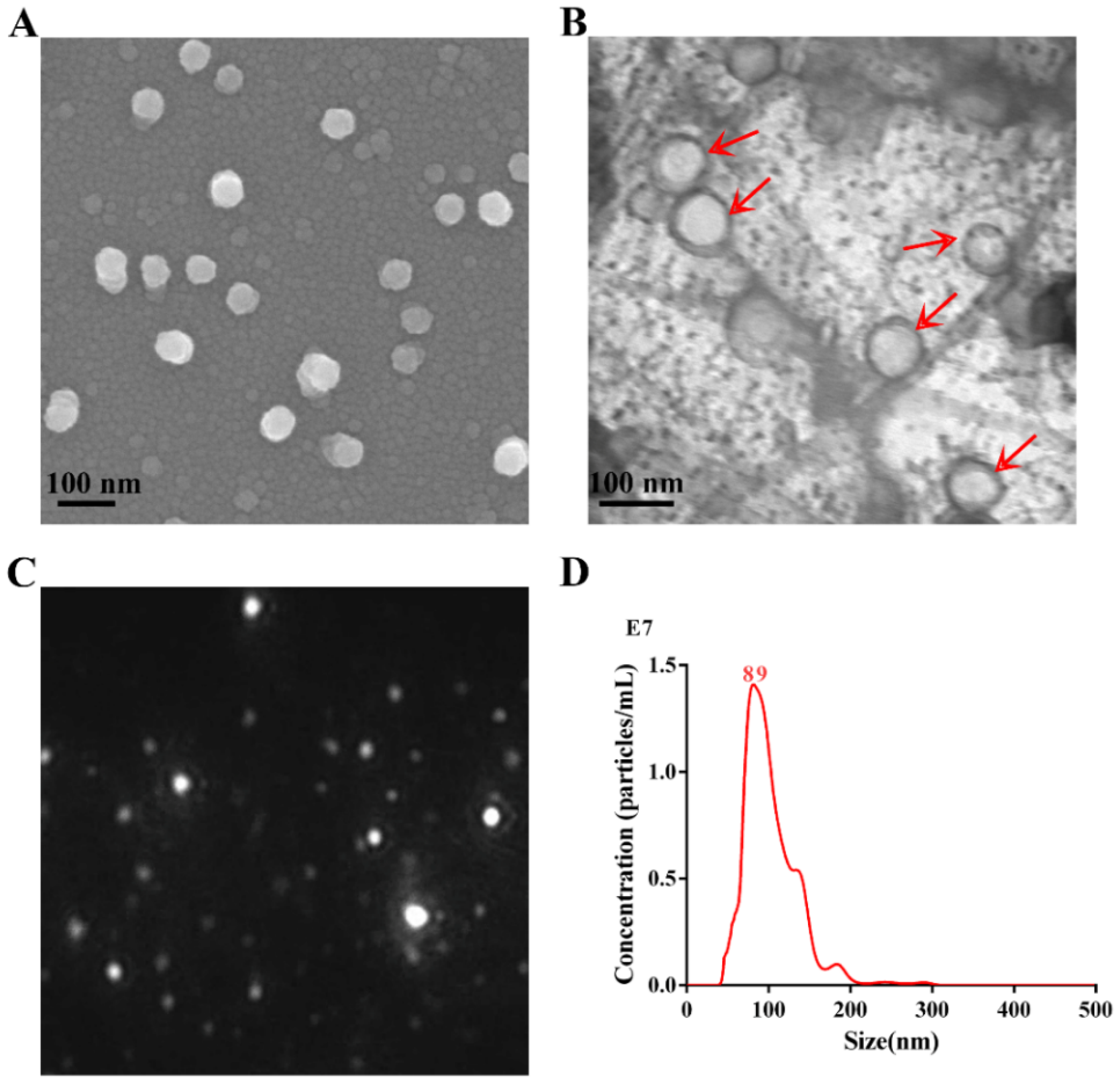
2.2. Electron Microscopy Analysis
2.3. Nanoparticle Tracking Analysis
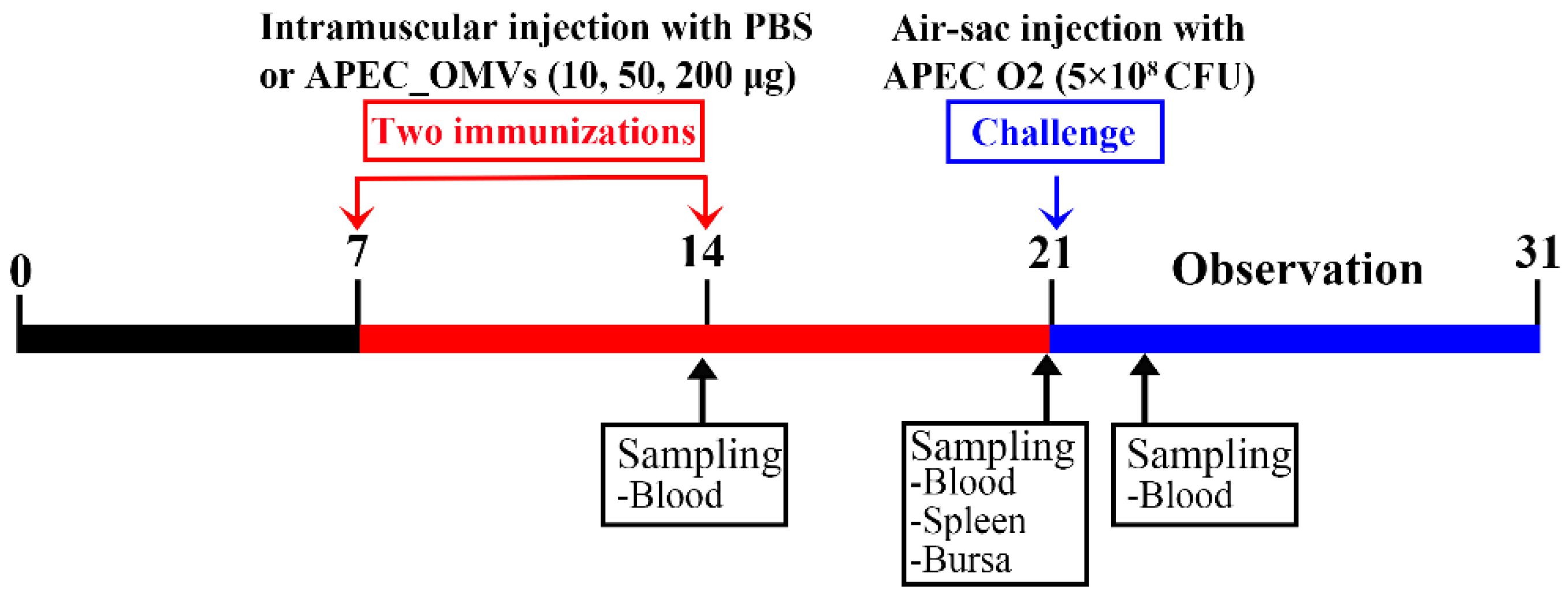
2.4. Animals and Housing
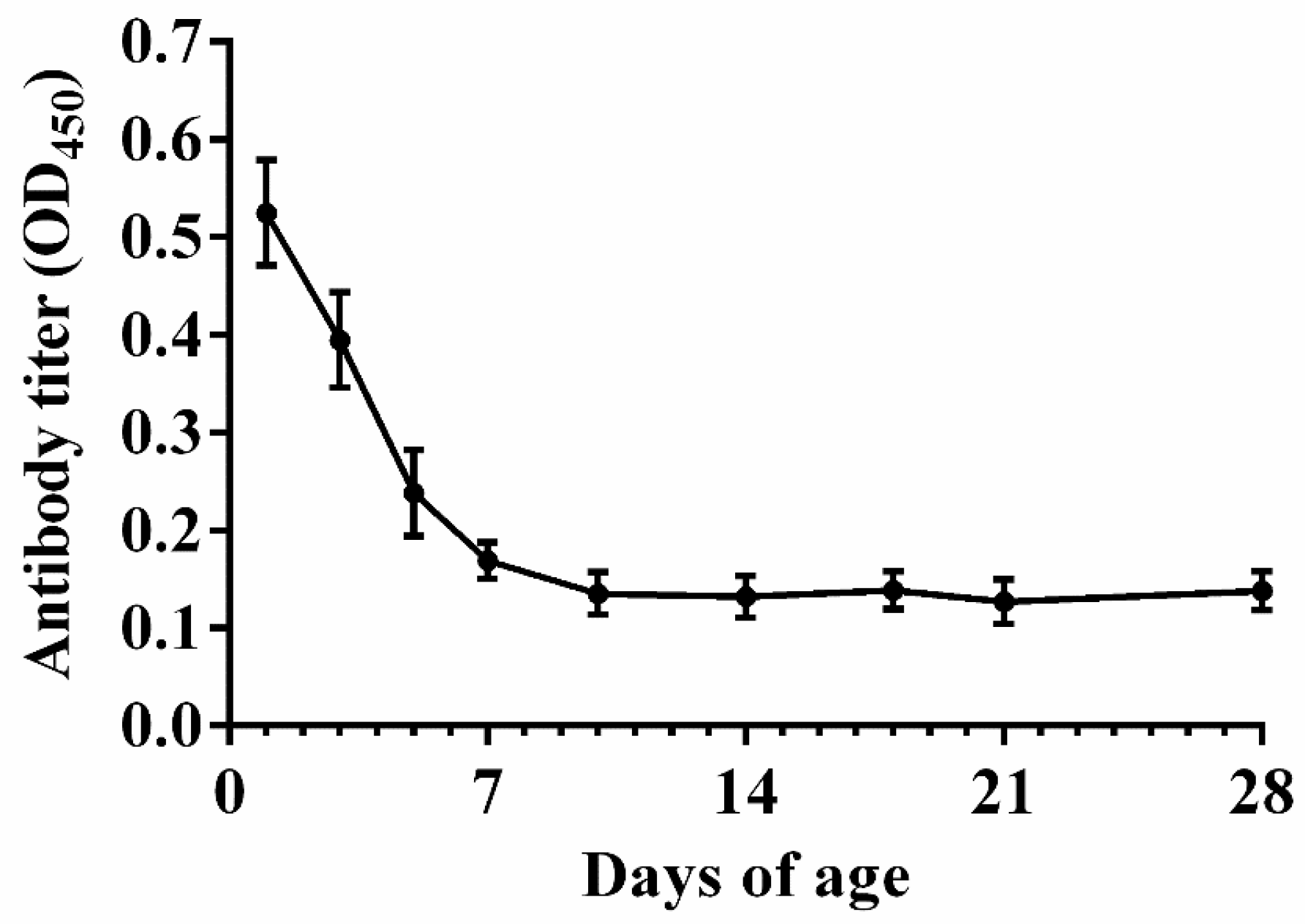
2.5. Maternal Anti-APEC Antibody Levels in Broiler Chicks
2.6. Effect of APEC_OMV Vaccination on the Growth Performance, Immune Organ Index and Blood Cell Counts
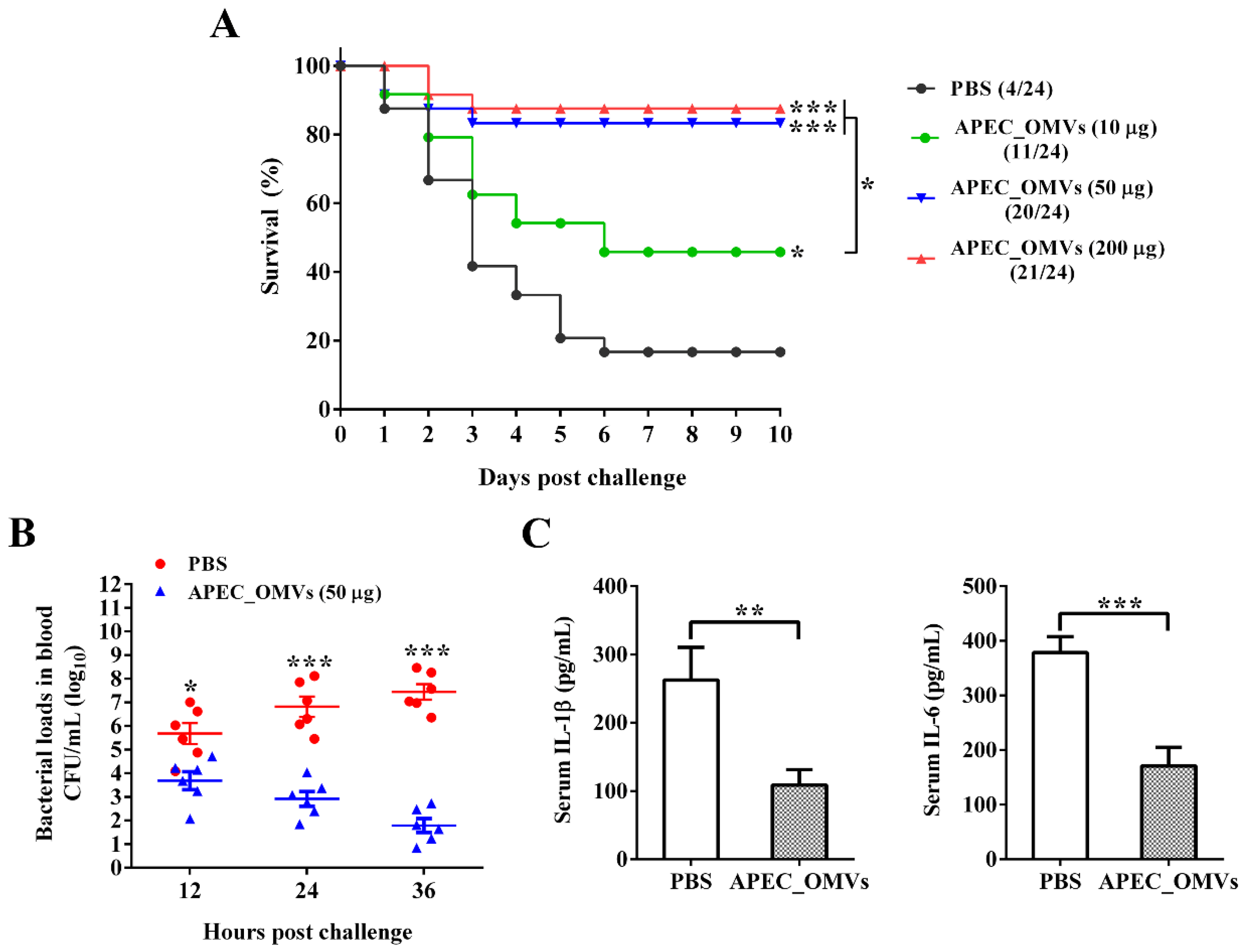
2.7. Effect of APEC_OMV Vaccination on the Protective Efficacy against Homologous Infection in Broiler Chicks
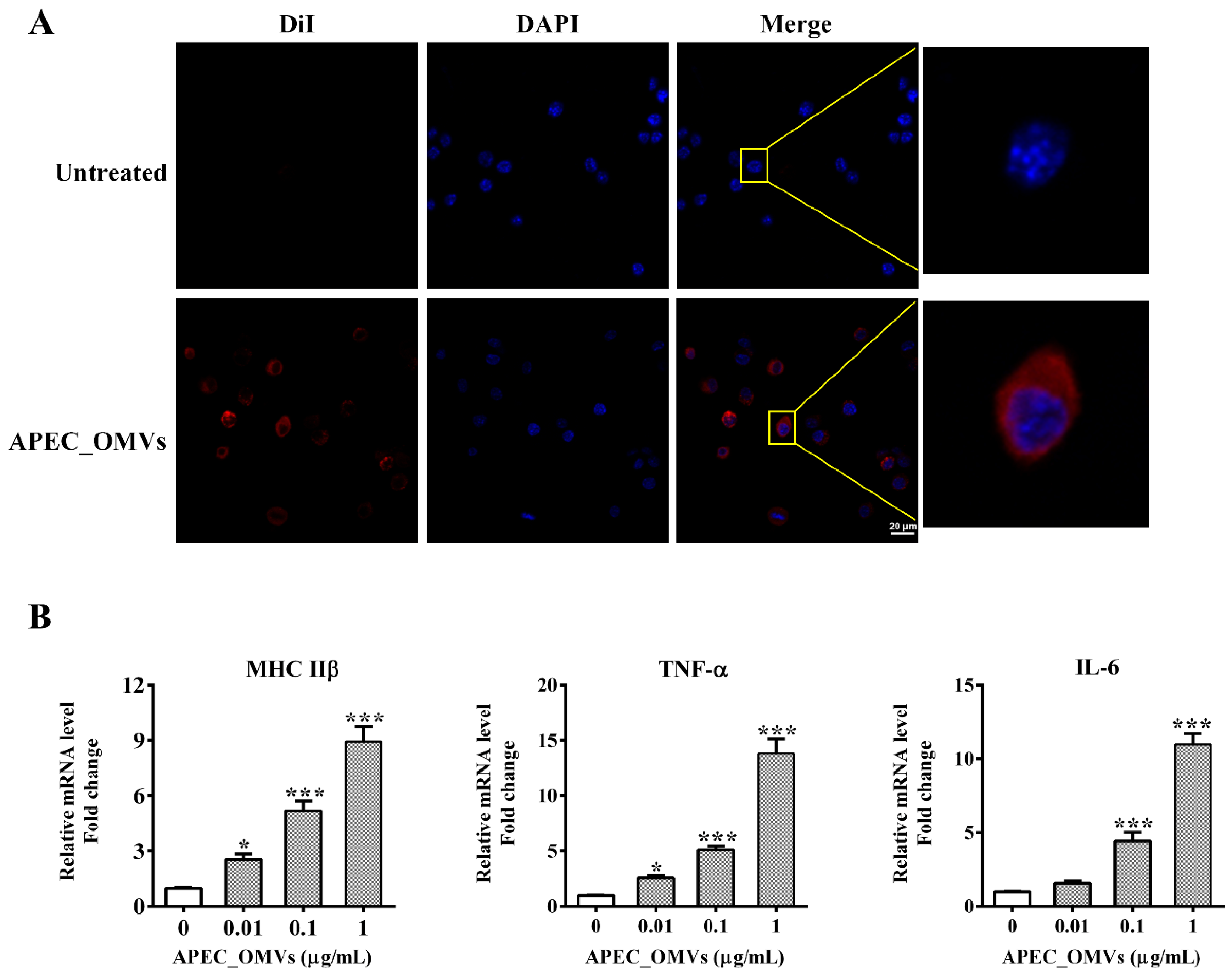
2.8. In Vitro Chicken Macrophage Assays
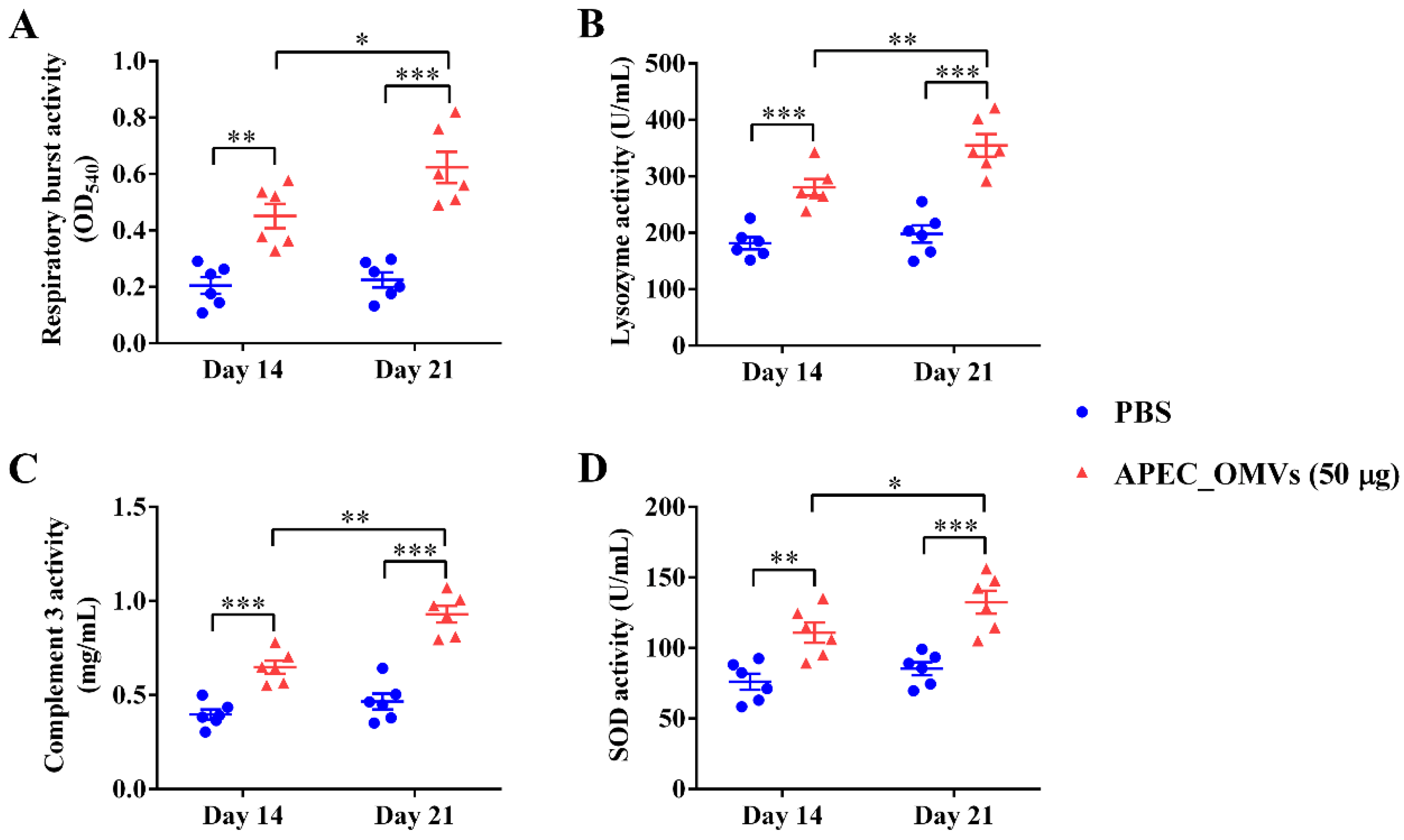
2.9. Serum Non-Specific Immune Factor Activities
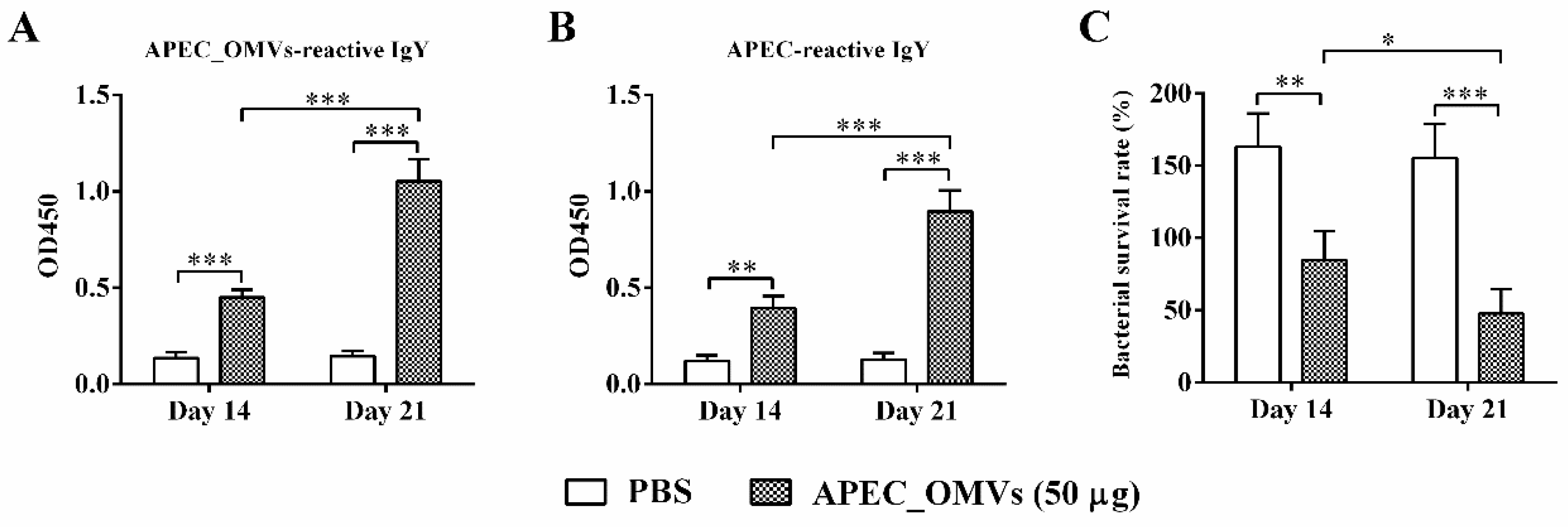
2.10. Determination of Specific Antibody Titer and Bactericidal Activity in Serum
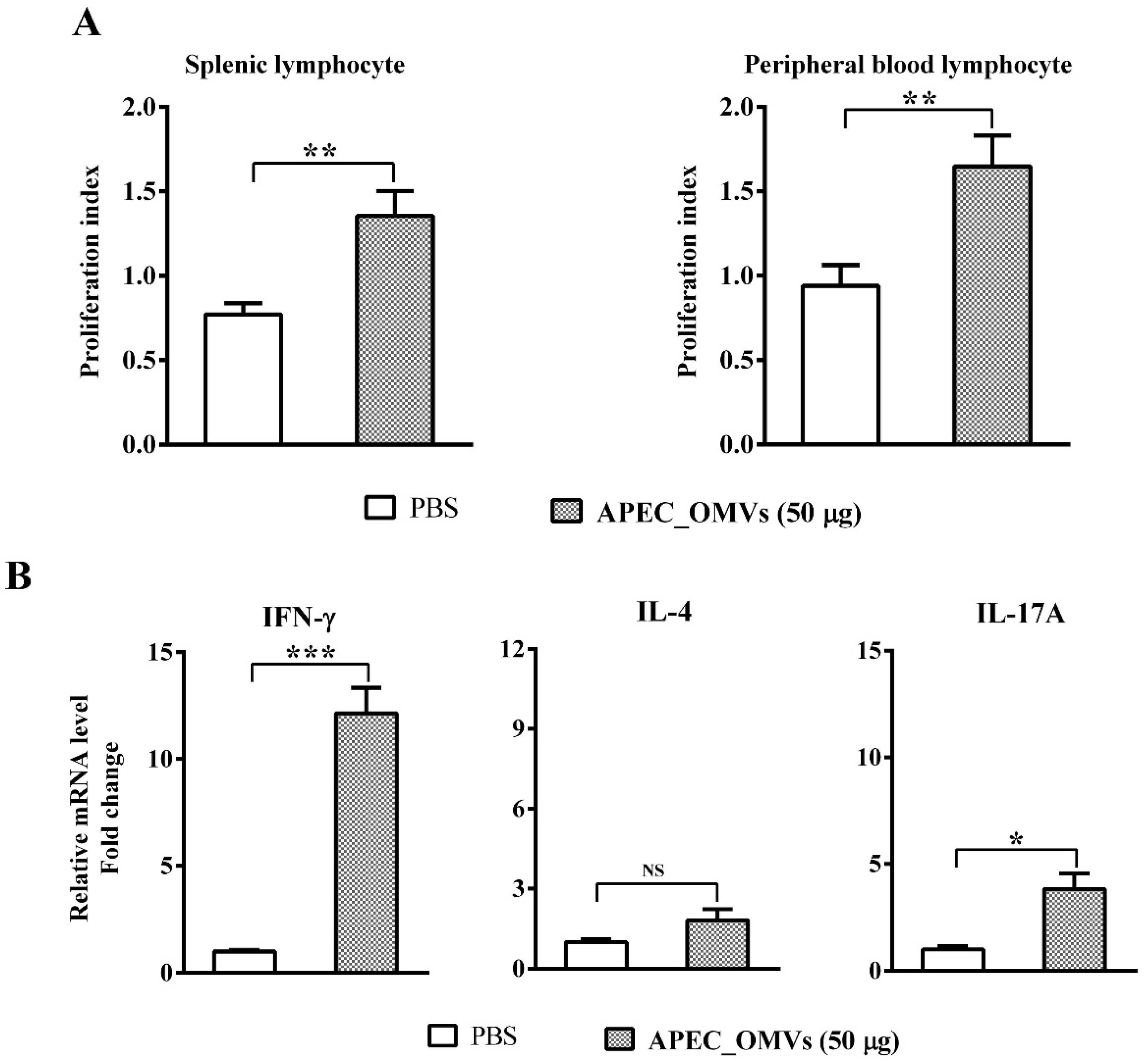
2.11. Lymphocyte Proliferation Assays
2.12. Re-Stimulation Assay of Splenic Lymphocyte
2.13. Quantitative Real-Time PCR (qRT-PCR) for mRNA Quantification
2.14. Statistical Analysis
3. Results
3.1. Characterization of APEC_OMVs
3.2. Natural Antibody Levels in Nonimmunized Chicks
3.3. Effect of APEC_OMVs Vaccination on the Growth Performance, Immune Organ Index and Blood Cell Counts
3.4. Vaccination with APEC_OMVs Was Protective against Homologous Infection in Broiler Chicks
3.5. APEC_OMVs Activated Innate Immune Responses In Vitro
3.6. Vaccination with APEC_OMVs Improved Serum Non-Specific Immune Factor Activities
3.7. APEC_OMV-Induced Protection Was Associated with Elevated Antibody Responses
3.8. Vaccination with APEC_OMVs Induced Lymphocyte Proliferation and a Predominant Th1 Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antao, E.M.; Glodde, S.; Li, G.; Sharifi, R.; Homeier, T.; Laturnus, C.; Diehl, I.; Bethe, A.; Philipp, H.C.; Preisinger, R.; et al. The chicken as a natural model for extraintestinal infections caused by avian pathogenic Escherichia coli (APEC). Microb. Pathog. 2008, 45, 361–369. [Google Scholar] [PubMed]
- Sadeyen, J.-R.; Kaiser, P.; Stevens, M.P.; Dziva, F. Analysis of immune responses induced by avian pathogenic Escherichia coli infection in turkeys and their association with resistance to homologous re-challenge. Vet. Res. 2014, 45, 19. [Google Scholar] [PubMed]
- Ievy, S.; Islam, M.S.; Sobur, M.A.; Talukder, M.; Rahman, M.B.; Khan, M.F.R.; Rahman, M.T. Molecular detection of avian pathogenic Escherichia coli (APEC) for the first time in layer farms in Bangladesh and their antibiotic resistance patterns. Microorganisms 2020, 8, 1021. [Google Scholar]
- Sadeyen, J.-R.; Wu, Z.; Davies, H.; van Diemen, P.M.; Milicic, A.; La Ragione, R.M.; Kaiser, P.; Stevens, M.P.; Dziva, F. Immune responses associated with homologous protection conferred by commercial vaccines for control of avian pathogenic Escherichia coli in turkeys. Vet. Res. 2015, 46, 5. [Google Scholar] [PubMed]
- Wang, S.; Peng, Q.; Jia, H.M.; Zeng, X.F.; Zhu, J.L.; Hou, C.L.; Liu, X.T.; Yang, F.J.; Qiao, S.Y. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci. 2017, 96, 2576–2586. [Google Scholar]
- Redweik, G.A.J.; Stromberg, Z.R.; Van Goor, A.; Mellata, M. Protection against avian pathogenic Escherichia coli and Salmonella Kentucky exhibited in chickens given both probiotics and live Salmonella vaccine. Poult. Sci. 2020, 99, 752–762. [Google Scholar]
- Bélanger, L.; Garenaux, A.; Harel, J.; Boulianne, M.; Nadeau, E.; Dozois, C.M. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol. Med. Microbiol. 2011, 62, 1–10. [Google Scholar]
- Ghunaim, H.; Abdelhamid, M.A.; Kariyawasam, S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: Potentials and limitations. Vet. Microbiol. 2014, 172, 13–22. [Google Scholar]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 2: New approaches and potential solutions. Vet. Res. 2018, 49, 70. [Google Scholar]
- Han, Y.; Liu, Q.; Willias, S.; Liang, K.; Li, P.; Cheng, A.C.; Kong, Q.K. A bivalent vaccine derived from attenuated Salmonella expressing O-antigen polysaccharide provides protection against avian pathogenic Escherichia coli O1 and O2 infection. Vaccine 2018, 36, 1038–1046. [Google Scholar]
- Ebrahimi-Nik, H.; Bassami, M.R.; Mohri, M.; Rad, M.; Khan, M.I. Bacterial ghost of avian pathogenic E. coli (APEC) serotype O78: K80 as a homologous vaccine against avian colibacillosis. PLoS ONE 2018, 13, e0194888. [Google Scholar]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [PubMed]
- Lee, J.; Kim, O.Y.; Gho, Y.S. Proteomic profiling of Gram-negative bacterial outer membrane vesicles: Current perspectives. Proteom. Clin. Appl. 2016, 10, 897–909. [Google Scholar]
- Wai, S.N.; Lindmark, B.; Soderblom, T.; Takade, A.; Westermark, M.; Oscarsson, J.; Jass, J.; Richter-Dahlfors, A.; Mizunoe, Y.; Uhlin, B.E. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 2003, 115, 25–35. [Google Scholar] [PubMed]
- Lusta, K.A. Bacterial outer membrane nanovesicles: Structure, biogenesis, functions, and application in biotechnology and medicine. Appl. Biochem. Microbiol. 2015, 51, 485–493. [Google Scholar]
- Avalos-Gómez, C.; Reyes-López, M.; Ramírez-Rico, G.; Díaz-Aparicio, E.; Zenteno, E.; González-Ruiz, C.; de la Garza, M. Effect of apo-lactoferrin on leukotoxin and outer membrane vesicles of Mannheimia haemolytica A2. Vet. Res. 2020, 51, 36. [Google Scholar]
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104. [Google Scholar]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar]
- Collins, B.S. Gram-negative outer membrane vesicles in vaccine development. Discov. Med. 2011, 12, 7–15. [Google Scholar]
- Huang, W.W.; Wang, S.J.; Yao, Y.F.; Xia, Y.; Yang, X.; Li, K.; Sun, P.Y.; Liu, C.B.; Sun, W.J.; Bai, H.M.; et al. Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection. Sci. Rep. 2016, 6, 37242. [Google Scholar] [PubMed]
- Jain, S.; Pillai, J. Bacterial membrane vesicles as novel nanosystems for drug delivery. Int. J. Nanomed. 2017, 12, 6329–6341. [Google Scholar]
- Liu, Y.; Smid, E.J.; Abee, T.; Notebaart, R.A. Delivery of genome editing tools by bacterial extracellular vesicles. Microb. Biotechnol. 2019, 12, 71–73. [Google Scholar] [PubMed]
- Vogel, U.; Claus, H. Vaccine development against Neisseria meningitidis. Microb. Biotechnol. 2011, 4, 20–31. [Google Scholar] [PubMed]
- Lee, W.H.; Choi, H.I.; Hong, S.W.; Kim, K.S.; Gho, Y.S.; Jeon, S.G. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp. Mol. Med. 2015, 47, e183. [Google Scholar] [PubMed]
- Wang, Z.; Lazinski, D.W.; Camilli, A. Immunity provided by an outer membrane vesicle cholera vaccine is due to O-antigen-specific antibodies inhibiting bacterial motility. Infect. Immun. 2017, 85, e00626-16. [Google Scholar]
- Bottero, D.; Gaillard, M.E.; Zurita, E.; Moreno, G.; Martinez, D.S.; Bartel, E.; Bravo, S.; Carriquiriborde, F.; Errea, A.; Castuma, C.; et al. Characterization of the immune response induced by pertussis OMVs-based vaccine. Vaccine 2016, 34, 3303–3309. [Google Scholar]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Necchi, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P.; et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, 10428–10433. [Google Scholar]
- Irene, C.; Fantappiè, L.; Caproni, E.; Zerbini, F.; Anesi, A.; Tomasi, M.; Zanella, I.; Stupia, S.; Prete, S.; Valensin, S.; et al. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine. Proc. Natl. Acad. Sci. USA 2019, 116, 21780–21788. [Google Scholar]
- Baker, S.M.; Davitt, C.J.; Motyka, N.; Kikendall, N.L.; Russell-Lodrigue, K.; Roy, C.J.; Morici, L.A. A Burkholderia pseudomallei outer membrane vesicle vaccine provides cross protection against inhalational glanders in mice and non-human primates. Vaccines 2017, 5, 49. [Google Scholar]
- Bae, E.-H.; Seo, S.H.; Kim, C.-U.; Jang, M.S.; Song, M.-S.; Lee, T.-Y.; Jeong, Y.-J.; Lee, M.-S.; Park, J.-H.; Lee, P. Bacterial outer membrane vesicles provide broad-spectrum protection against Influenza virus infection via recruitment and activation of macrophages. J. Innate Immun. 2019, 11, 316–329. [Google Scholar] [PubMed]
- Raeven, R.H.M.; Rockx-Brouwer, D.; Kanojia, G.; Van Der Maas, L.; Bindels, T.H.E.; Have, R.T.; Van Riet, E.; Metz, B.; Kersten, G.F.A. Intranasal immunization with outer membrane vesicle pertussis vaccine confers broad protection through mucosal IgA and Th17 responses. Sci. Rep. 2020, 10, 7396. [Google Scholar] [PubMed]
- Kim, O.Y.; Hong, B.S.; Park, K.-S.; Yoon, Y.J.; Choi, S.J.; Lee, W.H.; Roh, T.-Y.; Lotvall, J.; Kim, Y.-K.; Gho, Y.S. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J. Immunol. 2013, 190, 4092–4102. [Google Scholar] [PubMed]
- Park, K.-S.; Choi, K.-H.; Kim, Y.-S.; Hong, B.S.; Kim, O.Y.; Kim, J.H.; Yoon, C.M.; Koh, G.-Y.; Kim, Y.-K.; Gho, Y.S. Outer membrane vesicles derived from Escherichia coli induce systemic inflammatory response syndrome. PLoS ONE 2010, 5, e11334. [Google Scholar]
- Wang, H.; Liang, K.; Kong, Q.; Liu, Q. Immunization with outer membrane vesicles of avian pathogenic Escherichia coli O78 induces protective immunity in chickens. Vet. Microbiol. 2019, 236, 108367. [Google Scholar]
- Hu, R.; Lin, H.; Li, J.; Zhao, Y.; Wang, M.; Sun, X.; Min, Y.; Gao, Y.; Yang, M. Probiotic Escherichia coli Nissle 1917-derived outer membrane vesicles enhance immunomodulation and antimicrobial activity in RAW264.7 macrophages. BMC Microbiol. 2020, 20, 268. [Google Scholar]
- Hu, R.; Li, J.; Zhao, Y.; Lin, H.; Liang, L.; Wang, M.; Liu, H.; Min, Y.; Gao, Y.; Yang, M. Exploiting bacterial outer membrane vesicles as a cross-protective vaccine candidate against avian pathogenic Escherichia coli (APEC). Microb. Cell Factories 2020, 19, 119. [Google Scholar]
- Prados-Rosales, R.; Brown, L.; Casadevall, A.; Montalvo-Quirós, S.; Luque-Garcia, J.L. Isolation and identification of membrane vesicle-associated proteins in Gram-positive bacteria and mycobacteria. MethodsX 2014, 1, 124–129. [Google Scholar]
- Natt, M.P.; Herrick, C.A. A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poult. Sci. 1952, 31, 735–738. [Google Scholar]
- Beug, H.; von Kirchbach, A.; Döderlein, G.; Conscience, J.-F.; Graf, T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 1979, 18, 375–390. [Google Scholar]
- Nicola, A.M.; Frases, S.; Casadevall, A. Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryot. Cell 2009, 8, 1373–1380. [Google Scholar] [PubMed]
- Dan, X.-M.; Zhang, T.-W.; Li, Y.-W.; Li, A.-X. Immune responses and immune-related gene expression profile in orange-spotted grouper after immunization with Cryptocaryon irritans vaccine. Fish Shellfish Immunol. 2013, 34, 885–891. [Google Scholar] [PubMed]
- Verma, A.; Prasad, K.N.; Singh, A.K.; Nyati, K.K.; Gupta, R.K.; Paliwal, V.K. Evaluation of the MTT lymphocyte proliferation assay for the diagnosis of neurocysticercosis. J. Microbiol. Methods 2010, 81, 175–178. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar]
- Alber, A.; Morris, K.M.; Bryson, K.J.; Sutton, K.M.; Monson, M.S.; Chintoan-Uta, C.; Borowska, D.; Lamont, S.J.; Schouler, C.; Kaiser, P.; et al. Avian pathogenic Escherichia coli (APEC) strain-dependent immunomodulation of respiratory granulocytes and mononuclear phagocytes in CSF1R-reporter transgenic chickens. Front. Immunol. 2020, 10, 3055. [Google Scholar] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 2016, 165, 1106–1119. [Google Scholar]
- Fransen, F.; Boog, C.J.; van Putten, J.P.; van der Ley, P. Agonists of toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infect. Immun. 2007, 75, 5939–5946. [Google Scholar]
- Chen, D.J.; Osterrieder, N.; Metzger, S.M.; Buckles, E.; Doody, A.M.; DeLisa, M.P.; Putnam, D. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 3099–3104. [Google Scholar]
- Kim, O.Y.; Choi, S.J.; Jang, S.C.; Park, K.S.; Kim, S.R.; Choi, J.P.; Lim, J.H.; Lee, S.W.; Park, J.; Di Vizio, D.; et al. Bacterial protoplast-derived nanovesicles as vaccine delivery system against bacterial infection. Nano Lett. 2015, 15, 266–274. [Google Scholar]
- Varin, A.; Gordon, S. Alternative activation of macrophages: Immune function and cellular biology. Immunobiology 2009, 214, 630–641. [Google Scholar] [PubMed]
- Juul-Madsen, H.R.; Nielsen, O.L.; Krogh-Maibom, T.; Rontved, C.M.; Dalgaard, T.S.; Bumstead, N.; Jorgensen, P.H. Major histocompatibility complex-linked immune response of young chickens vaccinated with an attenuated live infectious bursal disease virus vaccine followed by an infection. Poult. Sci. 2002, 81, 649–656. [Google Scholar] [PubMed]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar]
- Franciosini, M.P.; Bietta, A.; Moscati, L.; Battistacci, L.; Pela, M.; Tacconi, G.; Davidson, I.; Proietti, P.C. Influence of different rearing systems on natural immune parameters in broiler turkeys. Poult. Sci. 2011, 90, 1462–1466. [Google Scholar] [PubMed]
- Genovese, K.J.; He, H.; Swaggerty, C.L.; Kogut, M.H. The avian heterophil. Dev. Comp. Immunol. 2013, 41, 334–340. [Google Scholar] [PubMed]
- Ishfaq, M.; Chen, C.; Bao, J.; Zhang, W.; Wu, Z.; Wang, J.; Liu, Y.; Tian, E.; Hamid, S.; Li, R.; et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-κB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult. Sci. 2019, 98, 6296–6310. [Google Scholar]
- Zhu, B.; Liu, G.L.; Gong, Y.X.; Ling, F.; Wang, G.X. Protective immunity of grass carp immunized with DNA vaccine encoding the vp7 gene of grass carp reovirus using carbon nanotubes as a carrier molecule. Fish Shellfish Immunol. 2015, 42, 325–334. [Google Scholar]
- Liu, Q.; Liu, Q.; Yi, J.; Liang, K.; Liu, T.; Roland, K.L.; Jiang, Y.L.; Kong, Q.K. Outer membrane vesicles derived from Salmonella Typhimurium mutants with truncated LPS induce cross-protective immune responses against infection of Salmonella enterica serovars in the mouse model. Int. J. Med. Microbiol. 2016, 306, 697–706. [Google Scholar]
- Roberts, L.M.; Davies, J.S.; Sempowski, G.D.; Frelinger, J.A. IFN-γ, but not IL-17A, is required for survival during secondary pulmonary Francisella tularensis live vaccine stain infection. Vaccine 2014, 32, 3595–3603. [Google Scholar]
- Ross, P.J.; Sutton, C.E.; Higgins, S.; Allen, A.C.; Walsh, K.; Misiak, A.; Lavelle, E.C.; McLoughlin, R.M.; Mills, K.H. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: Towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013, 9, 1003264. [Google Scholar]
- Jan, A.T. Outer membrane vesicles (OMVs) of gram-negative bacteria: A perspective update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [PubMed]
- Scorza, F.B.; Doro, F.; Rodriguez-Ortega, M.J.; Stella, M.; Liberatori, S.; Taddei, A.R.; Serino, L.; Moriel, D.G.; Nesta, B.; Fontana, M.R.; et al. Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli ΔtolR IHE3034 mutant. Mol. Cell Proteom. 2008, 7, 473–485. [Google Scholar]
- Shahin, R.; Brennan, M.; Li, Z.; Meade, B.; Manclark, C. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J. Exp. Med. 1990, 171, 63–73. [Google Scholar] [PubMed]
- Pillai, S.; Howell, A.; Alexander, K.; Bentley, B.E.; Jiang, H.Q.; Ambrose, K.; Zhu, D.Z.; Zlotnick, G. Outer membrane protein (OMP) based vaccine for Neisseria meningitidis serogroup B. Vaccine 2005, 23, 2206–2209. [Google Scholar]
- Lee, E.-Y.; Bang, J.Y.; Park, G.W.; Choi, D.-S.; Kang, J.S.; Kim, H.-J.; Park, K.-S.; Lee, J.-O.; Kim, Y.-K.; Kwon, K.-H.; et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [Google Scholar] [PubMed]
- Pore, D.; Mahata, N.; Pal, A.; Chakrabarti, M.K. Outer membrane protein A (OmpA) of Shigella flexneri 2a, induces protective immune response in a mouse model. PLoS ONE 2011, 6, e22663. [Google Scholar]
| Item 2 | APEC_OMVs 1 (μg/bird) | SE 3 | P-Value | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 50 | 200 | |||
| Growth Performance | ||||||
| ADFI (g/d) | 64.5 a | 63.9 a | 62.6 a | 57.2 b | 1.35 | 0.024 |
| ADWG (g/d) | 51.3 a | 49.6 a | 49.2 a | 42.4 b | 1.40 | 0.003 |
| FCR | 1.26 b | 1.29 b | 1.27 b | 1.35 a | 0.013 | 0.031 |
| Immune Organ Index (g/kg body weight) | ||||||
| Thymus index | 2.06 | 2.40 | 2.48 | 2.39 | 0.100 | 0.498 |
| Spleen index | 1.09 | 1.164 | 1.21 | 1.12 | 0.045 | 0.801 |
| Bursa index | 1.60 | 1.86 | 1.83 | 1.74 | 0.069 | 0.339 |
| Blood Cell Counts | ||||||
| WBC (103/μL) | 26.7 b | 28.3 b | 31.5 b | 39.3 a | 3.82 | 0.042 |
| RBC (106/μL) | 2.24 | 1.86 | 1.93 | 1.71 | 0.29 | 0.126 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Liu, H.; Wang, M.; Li, J.; Lin, H.; Liang, M.; Gao, Y.; Yang, M. An OMV-Based Nanovaccine Confers Safety and Protection against Pathogenic Escherichia coli via Both Humoral and Predominantly Th1 Immune Responses in Poultry. Nanomaterials 2020, 10, 2293. https://doi.org/10.3390/nano10112293
Hu R, Liu H, Wang M, Li J, Lin H, Liang M, Gao Y, Yang M. An OMV-Based Nanovaccine Confers Safety and Protection against Pathogenic Escherichia coli via Both Humoral and Predominantly Th1 Immune Responses in Poultry. Nanomaterials. 2020; 10(11):2293. https://doi.org/10.3390/nano10112293
Chicago/Turabian StyleHu, Rujiu, Haojing Liu, Mimi Wang, Jing Li, Hua Lin, Mingyue Liang, Yupeng Gao, and Mingming Yang. 2020. "An OMV-Based Nanovaccine Confers Safety and Protection against Pathogenic Escherichia coli via Both Humoral and Predominantly Th1 Immune Responses in Poultry" Nanomaterials 10, no. 11: 2293. https://doi.org/10.3390/nano10112293
APA StyleHu, R., Liu, H., Wang, M., Li, J., Lin, H., Liang, M., Gao, Y., & Yang, M. (2020). An OMV-Based Nanovaccine Confers Safety and Protection against Pathogenic Escherichia coli via Both Humoral and Predominantly Th1 Immune Responses in Poultry. Nanomaterials, 10(11), 2293. https://doi.org/10.3390/nano10112293





