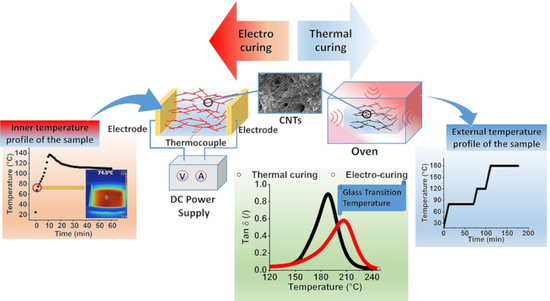
Design of Multifunctional Composites: New Strategy to Save Energy and Improve Mechanical Performance
Abstract
:1. Introduction
2. Materials and Methods
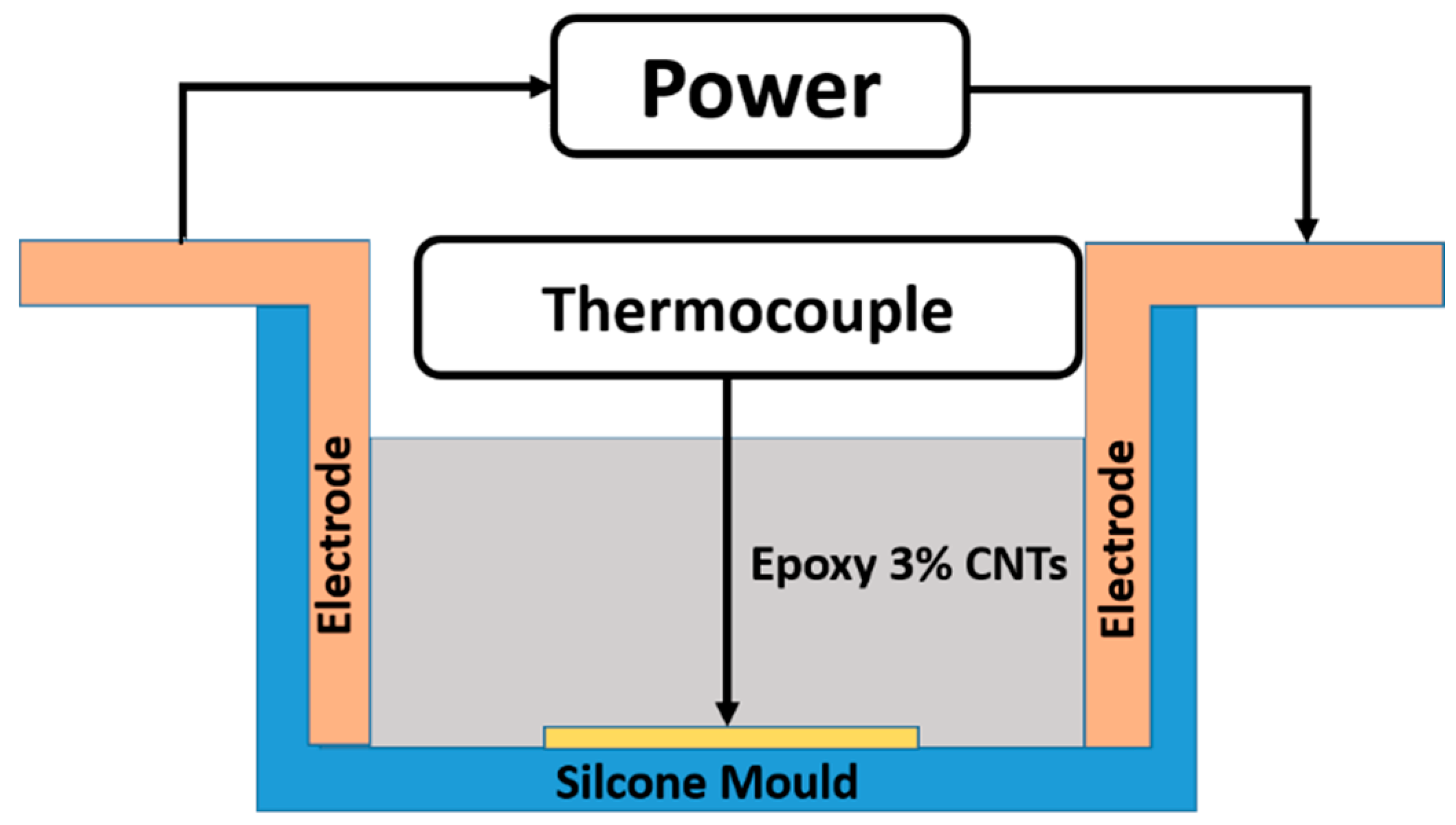
2.1. Materials and Sample Preparation
2.2. Characterization Methods
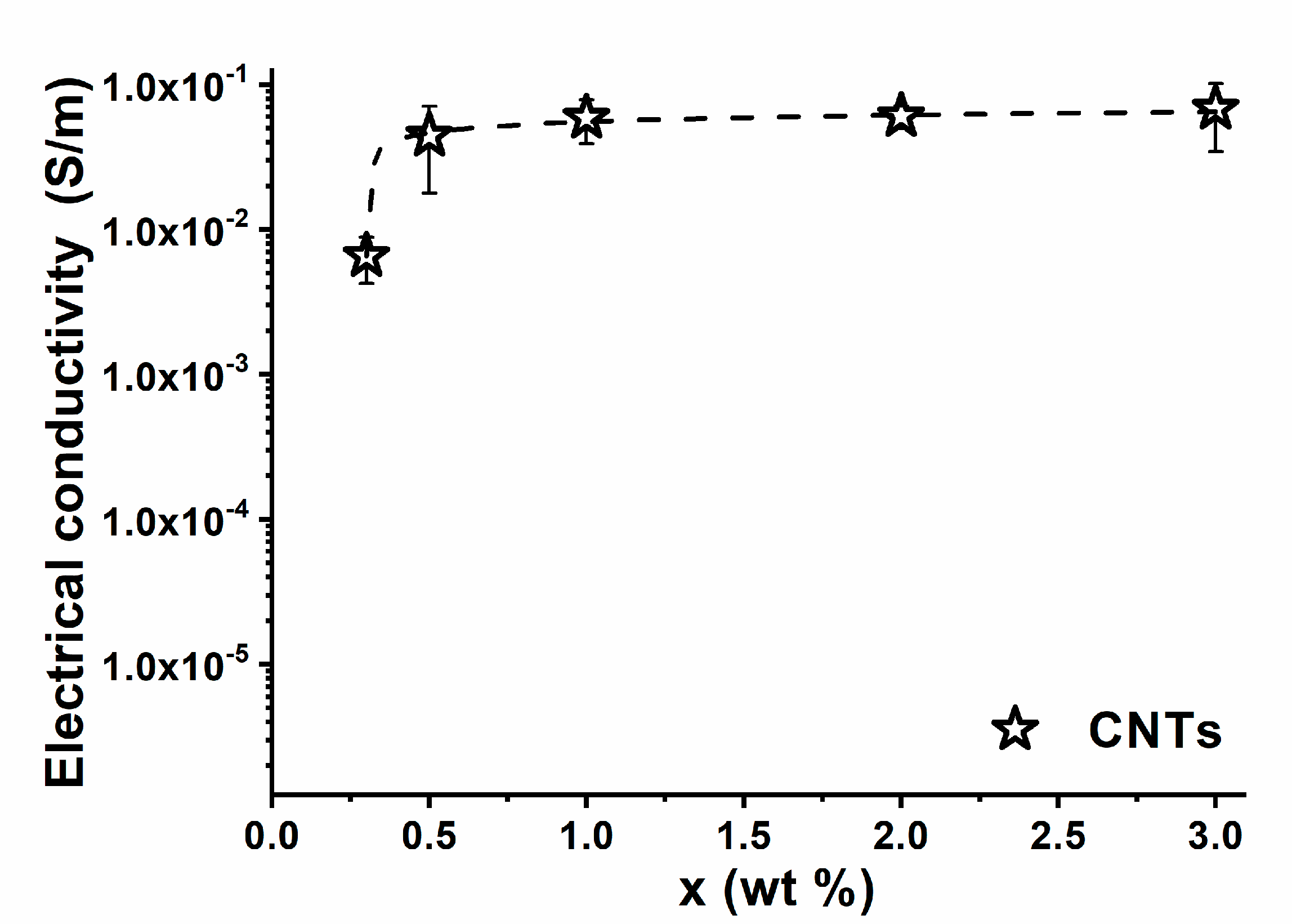
3. Results and Discussion
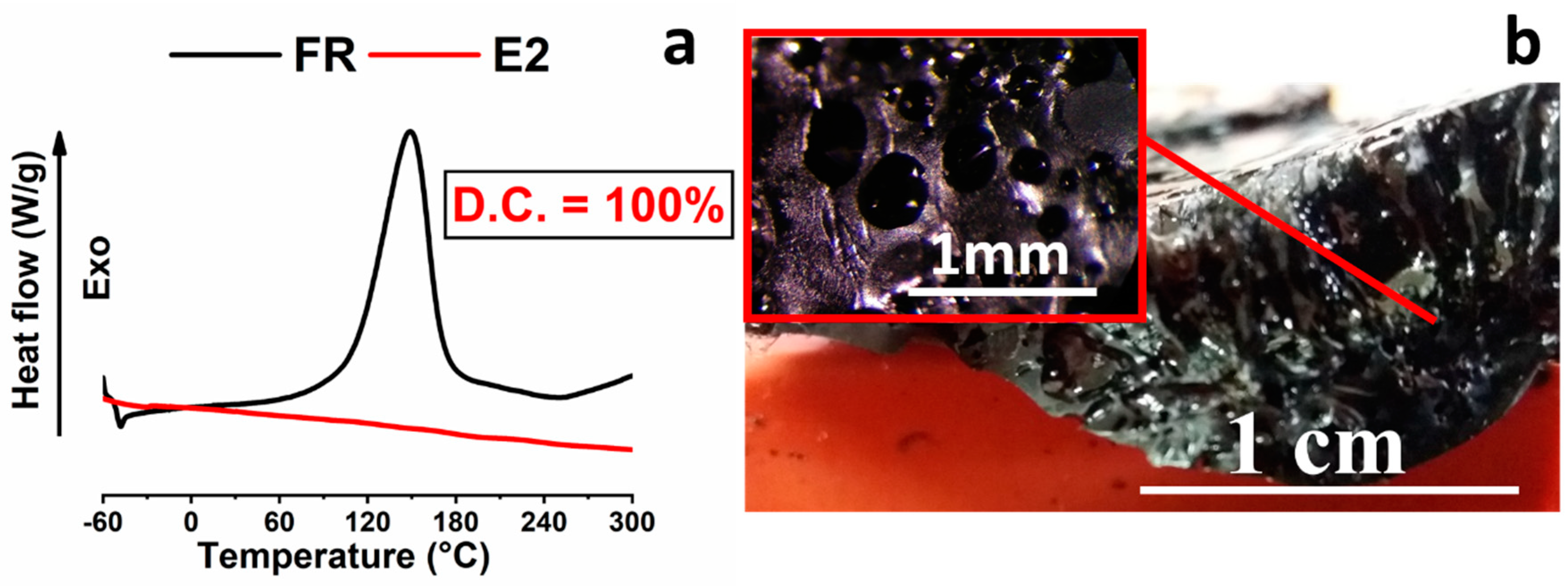
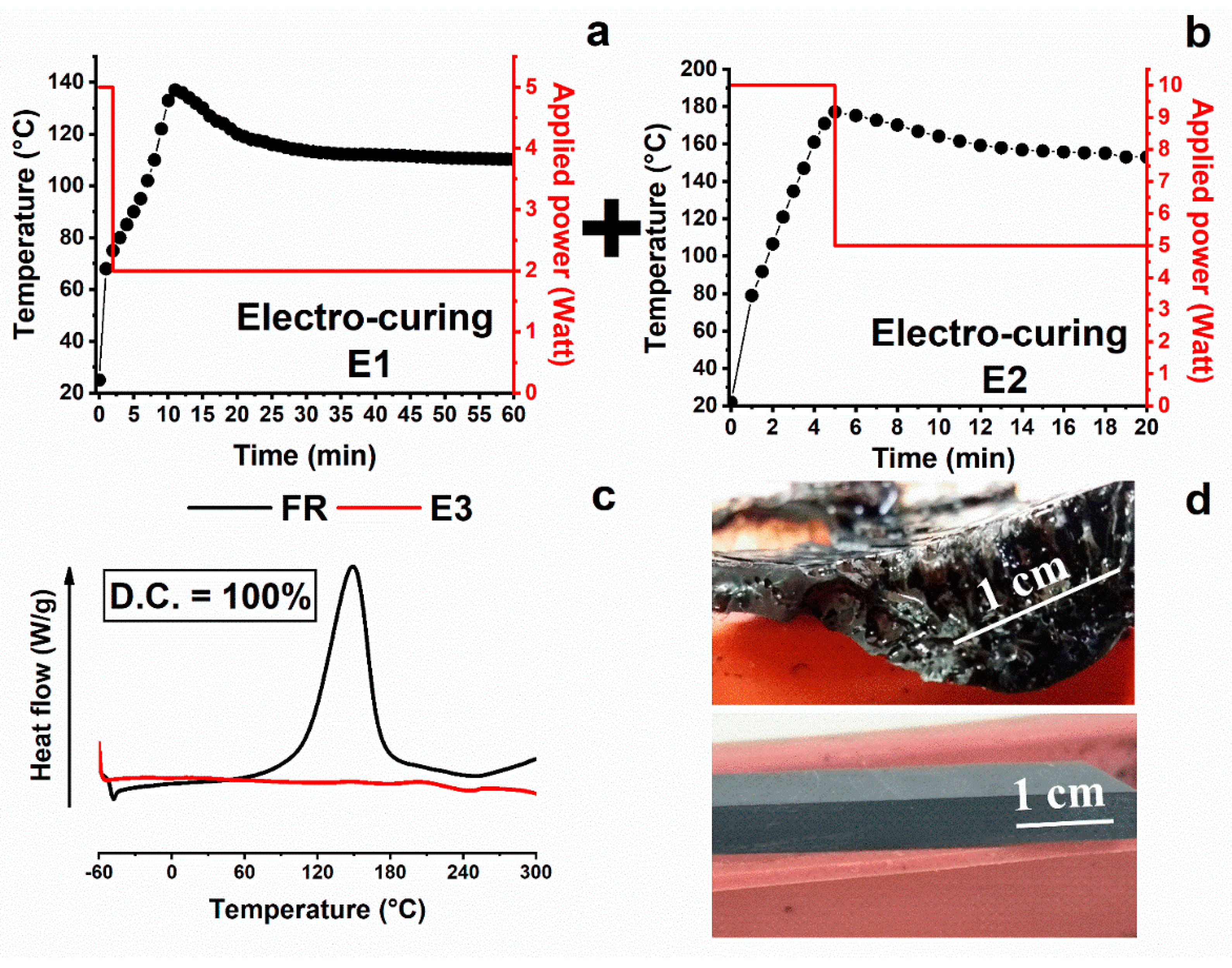
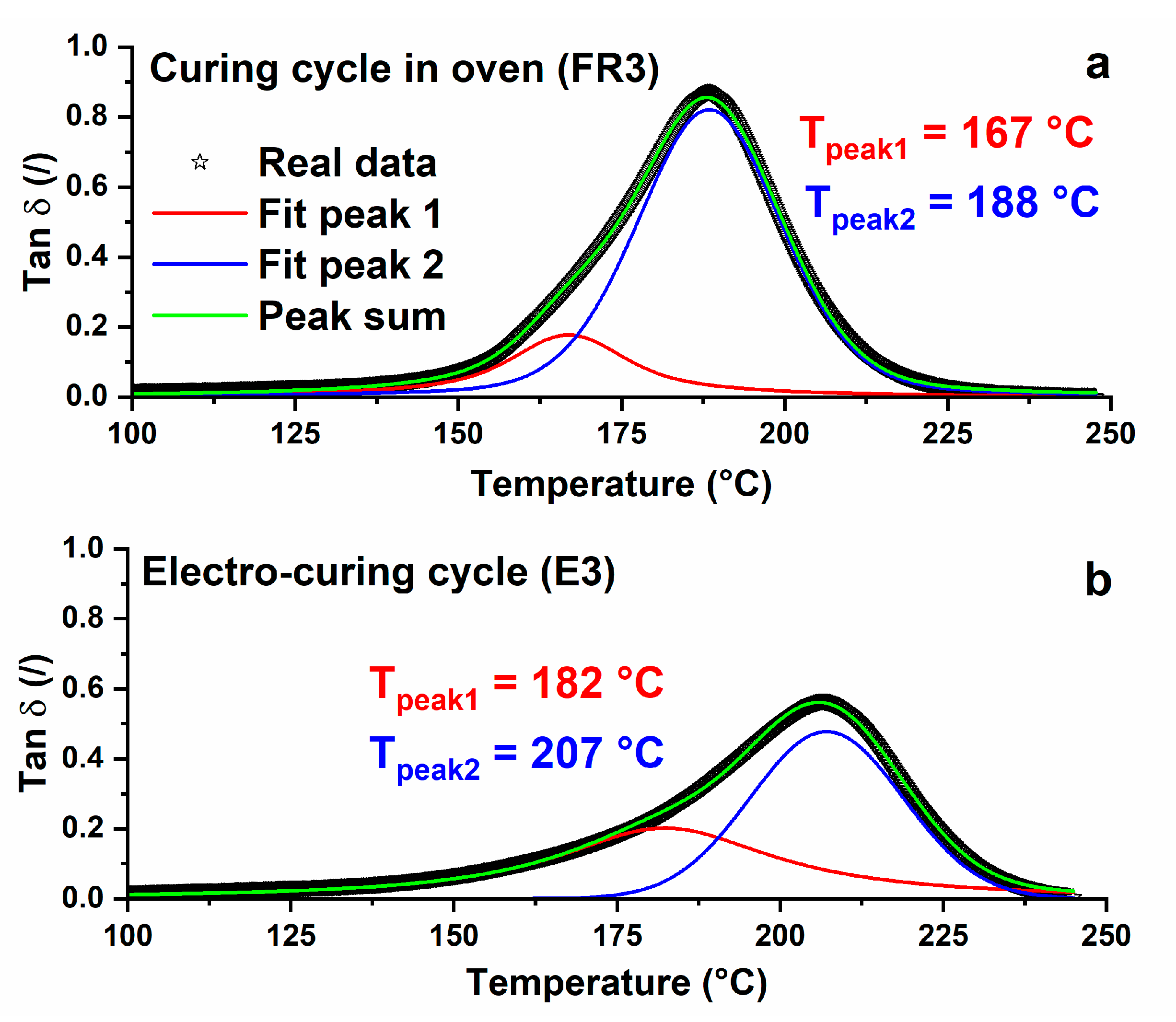
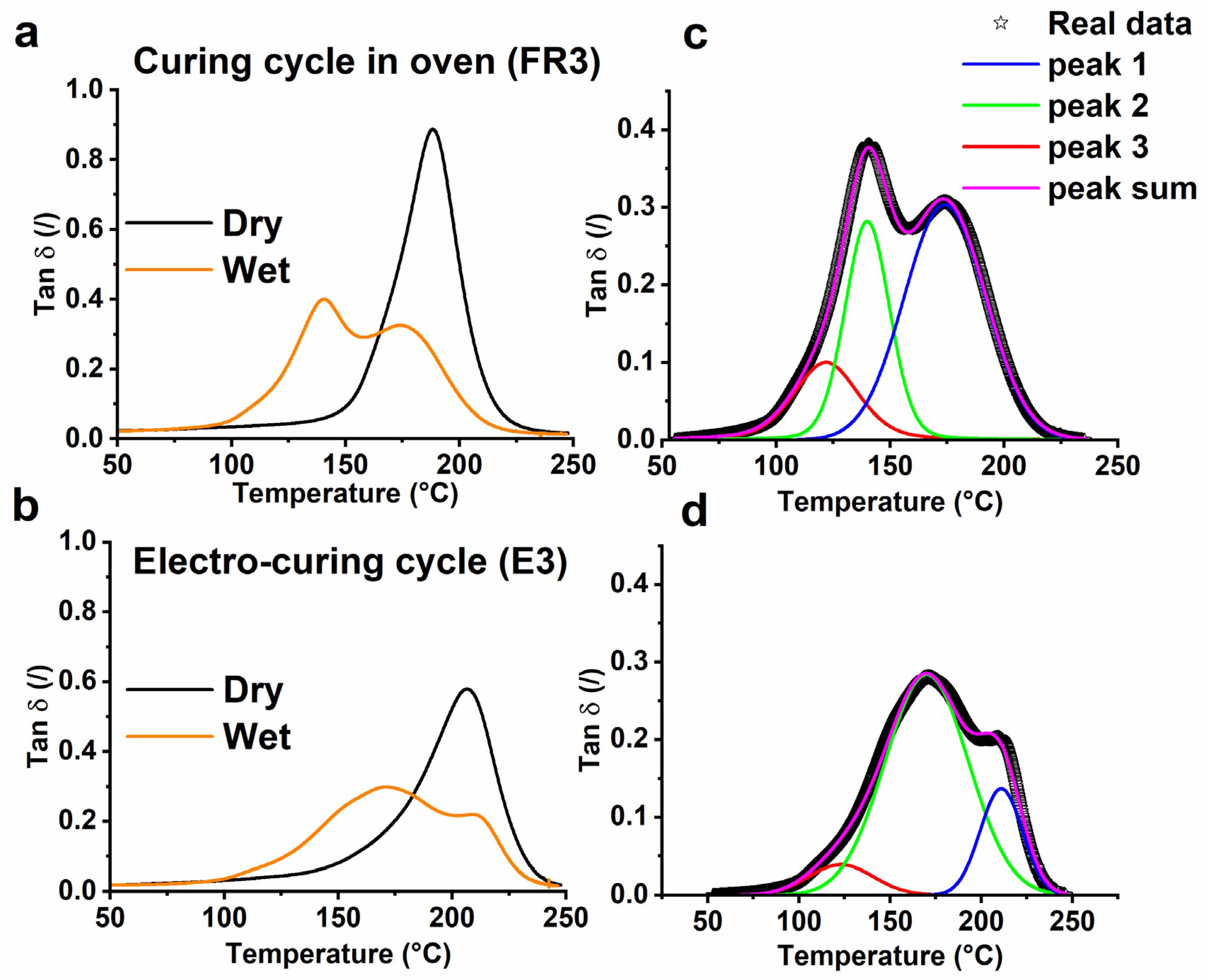
3.1. Thermal Analysis
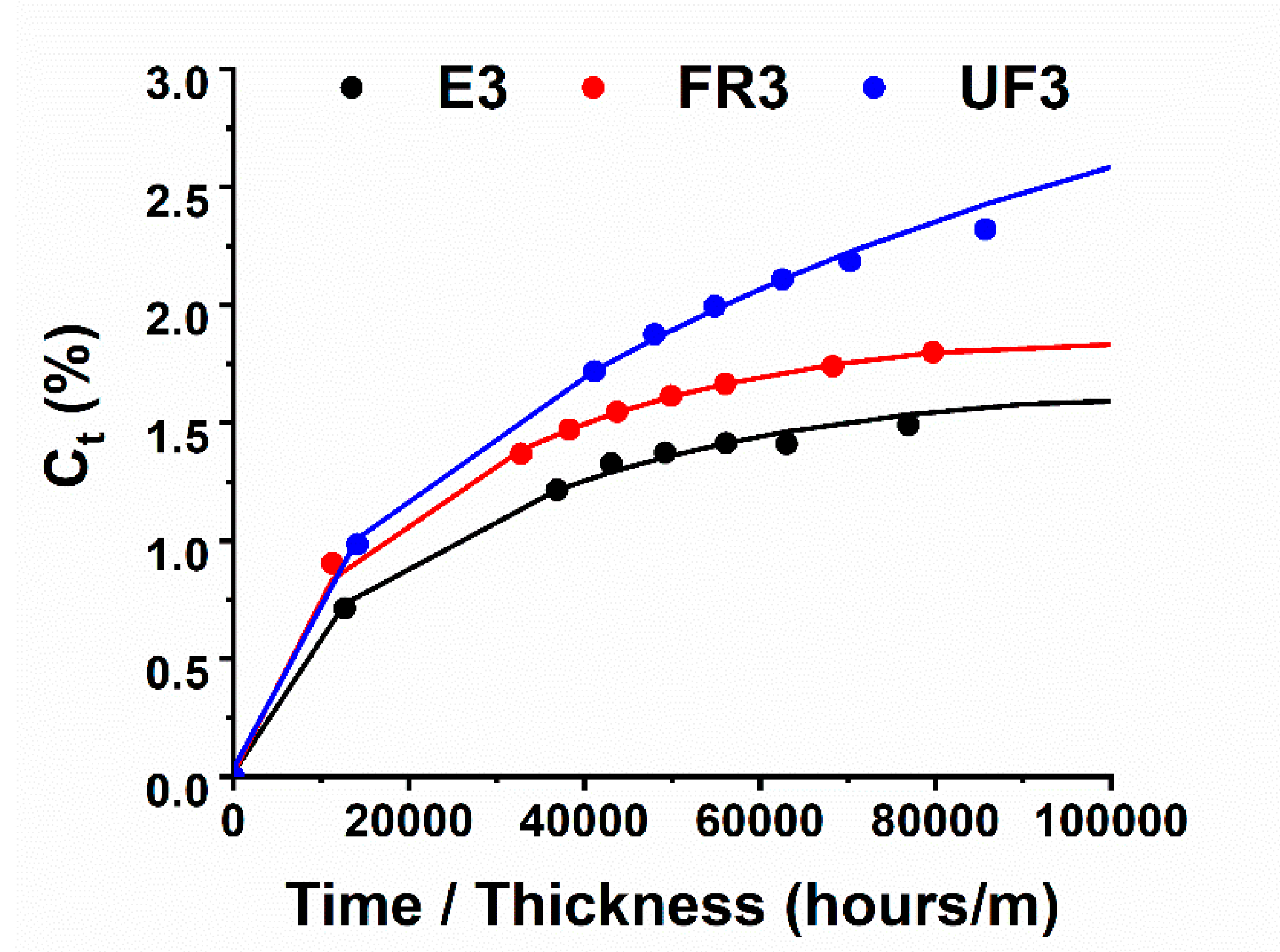
3.2. Sorption and Mechanical Analysis
3.3. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, Q.; Wang, J.; Wen, J.; Liu, C.; Jiang, K.; Li, Q.; Fan, S. Carbon nanotube/epoxy composites fabricated by resin transfer molding. Carbon 2010, 48, 260–266. [Google Scholar] [CrossRef]
- Guadagno, L.; De Vivo, B.; Di Bartolomeo, A.; Lamberti, P.; Sorrentino, A.; Tucci, V.; Vertuccio, L.; Vittoria, V. Effect of functionalization on the thermo-mechanical and electrical behavior of multi-wall carbon nanotube/epoxy composites. Carbon 2011, 49, 1919–1930. [Google Scholar] [CrossRef]
- Hadden, C.M.; Klimek-McDonald, D.R.; Pineda, E.J.; King, J.A.; Reichanadter, A.M.; Miskioglu, I.; Gowtham, S.; Odegard, G.M. Mechanical properties of graphene nanoplatelet/carbon fiber/epoxy hybrid composites: Multiscale modeling and experiments. Carbon 2015, 95, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Pascault, J.-P.; Sautereau, H.; Verdu, J.; Williams, R.J. Thermosetting Polymers; CRC Press: Boca Raton, FL, USA, 2002; Volume 64. [Google Scholar]
- Falzon, B.G.; Robinson, P.; Frenz, S.; Gilbert, B. Development and evaluation of a novel integrated anti-icing/de-icing technology for carbon fibre composite aerostructures using an electro-conductive textile. Compos. Part A Appl. Sci. Manuf. 2015, 68, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Gorrasi, G.; Vittoria, V. Effect of carbon nanotubes on the photo-oxidative durability of syndiotactic polypropylene. Polym. Degrad. Stab. 2010, 95, 1614–1626. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.; Michael, P.; Rana, S. Self-healing epoxy nanocomposites via reversible hydrogen bonding. Compos. Part B Eng. 2019, 157, 1–13. [Google Scholar] [CrossRef]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Guadagno, L.; Vertuccio, L. Damage Monitoring of Structural Resins Loaded with Carbon Fillers: Experimental and Theoretical Study. Nanomaterials 2020, 10, 434. [Google Scholar] [CrossRef] [Green Version]
- Vertuccio, L.; De Santis, F.; Pantani, R.; Lafdi, K.; Guadagno, L. Effective de-icing skin using graphene-based flexible heater. Compos. Part B Eng. 2019, 162, 600–610. [Google Scholar] [CrossRef]
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Russo, S.; Iannuzzo, G. Effect of carbon nanotube and functionalized liquid rubber on mechanical and electrical properties of epoxy adhesives for aircraft structures. Compos. Part B Eng. 2017, 129, 1–10. [Google Scholar] [CrossRef]
- Vertuccio, L.; Vittoria, V.; Guadagno, L.; De Santis, F. Strain and damage monitoring in carbon-nanotube-based composite under cyclic strain. Compos. Part A Appl. Sci. Manuf. 2015, 71, 9–16. [Google Scholar] [CrossRef]
- Zhai, T.; Li, D.; Fei, G.; Xia, H. Piezoresistive and compression resistance relaxation behavior of water blown carbon nanotube/polyurethane composite foam. Compos. Part A Appl. Sci. Manuf. 2015, 72, 108–114. [Google Scholar] [CrossRef]
- Hamerton, I.; Mooring, L. The use of thermosets in aerospace applications. In Thermosets; Elsevier: Amsterdam, The Netherlands, 2012; pp. 189–227. [Google Scholar]
- Song, D.; Gupta, R. The use of thermosets in the building and construction industry. In Thermosets; Elsevier: Amsterdam, The Netherlands, 2012; pp. 165–188. [Google Scholar]
- Abliz, D.; Duan, Y.; Steuernagel, L.; Xie, L.; Li, D.; Ziegmann, G. Curing methods for advanced polymer composites-a review. Polym. Polym. Compos. 2013, 21, 341–348. [Google Scholar] [CrossRef]
- Vergnaud, J.-M.; Bouzon, J. Cure of Thermosetting Resins: Modelling and Experiments; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Romano, V.; Naddeo, C.; Vertuccio, L.; Lafdi, K.; Guadagno, L. Experimental evaluation and modeling of thermal conductivity of tetrafunctional epoxy resin containing different carbon nanostructures. Polym. Eng. Sci. 2017, 57, 779–786. [Google Scholar] [CrossRef]
- Jung, K.; Kang, T.J.; Kim, J.-H.; Park, J.K.; Lee, B.; Kim, Y. Prediction of deformation of asymmetric hybrid composites from cure monitoring results of non-hybrid composites. Polym. Polym. Compos. 2007, 15, 65–73. [Google Scholar] [CrossRef]
- Endruweit, A.; Ruijter, W.; Johnson, M.S.; Long, A.C. Transmission of ultraviolet light through reinforcement fabrics and its effect on ultraviolet curing of composite laminates. Polym. Compos. 2008, 29, 818–829. [Google Scholar] [CrossRef]
- Gupta, A.; Ogale, A. Dual curing of carbon fiber reinforced photoresins for rapid prototyping. Polym. Compos. 2002, 23, 1162–1170. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Tang, Y.; Li, D.; Lu, B. Fabrication and mechanical properties of UV-curable glass fiber-reinforced polymer—Matrix composite. J. Compos. Mater. 2011, 45, 565–572. [Google Scholar] [CrossRef]
- Berejka, A.J.; Cleland, M.; Galloway, R.; Gregoire, O. X-ray curing of composite materials. Nucl. Instrum. Methods Phys. Res. 2005, 241, 847–849. [Google Scholar] [CrossRef]
- Dispenza, C.; Alessi, S.; Spadaro, G. Carbon fiber composites cured by γ-radiation-induced polymerization of an epoxy resin matrix. Adv. Polym. Technol. 2008, 27, 163–171. [Google Scholar] [CrossRef]
- Athanasopoulos, N.; Kostopoulos, V. Resistive heating of multidirectional and unidirectional dry carbon fibre preforms. Compos. Sci. Technol. 2012, 72, 1273–1282. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Sorrentino, A.; Binder, W.H.; Kadlec, M. Development of self-healing multifunctional materials. Compos. Part B Eng. 2017, 128, 30–38. [Google Scholar] [CrossRef]
- Sarles, S.A.; Leo, D.J. Consolidation of U-Nyte® epoxy-coated carbon-fiber composites via temperature-controlled resistive heating. J. Compos. Mater. 2008, 42, 2551–2566. [Google Scholar] [CrossRef]
- Dombovari, A.; Halonen, N.; Sapi, A.; Szabo, M.; Toth, G.; Mäklin, J.; Kordas, K.; Juuti, J.; Jantunen, H.; Kukovecz, A. Moderate anisotropy in the electrical conductivity of bulk MWCNT/epoxy composites. Carbon 2010, 48, 1918–1925. [Google Scholar] [CrossRef]
- Gungor, S.; Bakis, C.E. Anisotropic networking of carbon black in glass/epoxy composites using electric field. J. Compos. Mater. 2015, 49, 535–544. [Google Scholar] [CrossRef]
- De Vivo, B.; Guadagno, L.; Lamberti, P.; Raimo, R.; Sarto, M.; Tamburrano, A.; Tucci, V.; Vertuccio, L. Electromagnetic properties of Carbon NanoTube/epoxy nanocomposites. In Proceedings of the 2009 International Symposium on Electromagnetic Compatibility-EMC Europe, Athens, Greece, 11–12 June 2009; pp. 1–4. [Google Scholar]
- Neitzert, H.C.; Vertuccio, L.; Sorrentino, A. Epoxy/MWCNT composite as temperature sensor and electrical heating element. IEEE Trans. Nanotechnol. 2010, 10, 688–693. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Russo, S.; Lafdi, K.; Tucci, V.; Spinelli, G.; Lamberti, P. Influence of carbon nanoparticles/epoxy matrix interaction on mechanical, electrical and transport properties of structural advanced materials. Nanotechnology 2017, 28, 094001. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Vertuccio, L.; Guadagno, L. Experimental and theoretical study on piezoresistive properties of a structural resin reinforced with carbon nanotubes for strain sensing and damage monitoring. Compos. Part B Eng. 2018, 145, 90–99. [Google Scholar] [CrossRef]
- Vertuccio, L.; Russo, S.; Raimondo, M.; Lafdi, K.; Guadagno, L. Influence of carbon nanofillers on the curing kinetics of epoxy-amine resin. RSC Adv. 2015, 5, 90437–90450. [Google Scholar] [CrossRef]
- Ling, X.; Pritzker, M.; Burns, C.; Byerley, J. A mechanism for electropolymerization of 2-vinylpyridine coatings on metal surfaces. Macromolecules 1998, 31, 9134–9140. [Google Scholar] [CrossRef]
- Plamper, F.A. Polymerizations under electrochemical control. Colloid Polym. Sci. 2014, 292, 777–783. [Google Scholar] [CrossRef]
- Lai, C.; Guo, W.; Tang, X.; Zhang, G.; Pan, Q.; Pei, M. Cross-linking conducting polythiophene with yellow-green light-emitting properties and good thermal stability via free radical polymerization and electropolymerization. Synth. Met. 2011, 161, 1886–1891. [Google Scholar] [CrossRef]
- Reuber, J.; Reinhardt, H.; Johannsmann, D. Formation of surface-attached responsive gel layers via electrochemically induced free-radical polymerization. Langmuir 2006, 22, 3362–3367. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Lei, Y.; Wang, S.; Shiu, K.K. Electrochemically Induced Free-Radical Polymerization for the Fabrication of Amperometric Glucose Biosensors. Electroanalysis 2010, 22, 2366–2375. [Google Scholar] [CrossRef]
- Ping, J.; Gao, F.; Chen, J.L.; Webster, R.D.; Steele, T.W. Adhesive curing through low-voltage activation. Nat. Commun. 2015, 6, 8050. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Ansell, M.P.; Smedley, D. Effect of nano-and micro-particle additions on moisture absorption in thixotropic room temperature cure epoxy-based adhesives for bonded-in timber connections. Int. J. Adhes. Adhes. 2010, 30, 448–455. [Google Scholar] [CrossRef]
- Tucker, S.J.; Fu, B.; Kar, S.; Heinz, S.; Wiggins, J.S. Ambient cure POSS–epoxy matrices for marine composites. Compos. Part A Applil. Sci. Manuf. 2010, 41, 1441–1446. [Google Scholar] [CrossRef]
- Ku-Herrera, J.; Aviles, F. Cyclic tension and compression piezoresistivity of carbon nanotube/vinyl ester composites in the elastic and plastic regimes. Carbon 2012, 50, 2592–2598. [Google Scholar] [CrossRef]
- Oliva-Avilés, A.; Avilés, F.; Sosa, V. Electrical and piezoresistive properties of multi-walled carbon nanotube/polymer composite films aligned by an electric field. Carbon 2011, 49, 2989–2997. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Naddeo, C.; Barra, G.; Longo, P.; Lamberti, P.; Spinelli, G.; Nobile, M. Morphological, rheological and electrical properties of composites filled with carbon nanotubes functionalized with 1-pyrenebutyric acid. Compos. Part B Eng. 2018, 147, 12–21. [Google Scholar] [CrossRef]
- Guadagno, L.; Longo, P.; Raimondo, M.; Naddeo, C.; Mariconda, A.; Sorrentino, A.; Vittoria, V.; Iannuzzo, G.; Russo, S. Cure behavior and mechanical properties of structural self-healing epoxy resins. J. Polym. Sci. B Polym. Phys. 2010, 48, 2413–2423. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Huang, P. Effect of cure cycle on curing process and hardness for epoxy resin. Express Polym. Lett. 2009, 3, 534–541. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, T.S.; Do Choi, H.; Kwon, J.H.; Chung, Y.-C.; Yoon, H.G. Electrical conductivity of chemically modified multiwalled carbon nanotube/epoxy composites. Carbon 2005, 43, 23–30. [Google Scholar] [CrossRef]
- Kovacs, J.Z.; Velagala, B.S.; Schulte, K.; Bauhofer, W. Two percolation thresholds in carbon nanotube epoxy composites. Compos. Sci. Technol. 2007, 67, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Moisala, A.; Li, Q.; Kinloch, I.; Windle, A. Thermal and electrical conductivity of single-and multi-walled carbon nanotube-epoxy composites. Compos. Sci. Technol. 2006, 66, 1285–1288. [Google Scholar] [CrossRef]
- Romano, V.; Naddeo, C.; Guadagno, L.; Vertuccio, L. Thermal conductivity of epoxy resins filled with MWCNT and hydrotalcite clay: Experimental data and theoretical predictive modeling. Polym. Compos. 2015, 36, 1118–1123. [Google Scholar] [CrossRef]
- De Vivo, B.; Lamberti, P.; Tucci, V.; Guadagno, L.; Vertuccio, L.; Vittoria, V.; Sorrentino, A. Comparison of the physical properties of epoxy-based composites filled with different types of carbon nanotubes for aeronautic applications. Adv. Polym. Technol. 2012, 31, 205–218. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Vittoria, V.; Sorrentino, A.; Vertuccio, L.; Raimondo, M.; Tucci, V.; De Vivo, B.; Lamberti, P.; Iannuzzo, G. Cure behavior and physical properties of epoxy resin—filled with multiwalled carbon nanotubes. J. Nanosci. Nanotechnol. 2010, 10, 2686–2693. [Google Scholar] [CrossRef]
- Stimoniaris, A.; Stergiou, C.; Delides, C. A detailed study of α-relaxation in epoxy/carbon nanoparticles composites using computational analysis. eXPRESS Polym. Lett. 2012, 6, 120–128. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Fragiadakis, D.; Pissis, P. Reinforcement effect of carbon nanofillers in an epoxy resin system: Rheology, molecular dynamics, and mechanical studies. J. Polym. Sci. B Polym. Phys. 2005, 43, 522–533. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.-H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Moon, K.S.; Wong, C. Glass transition and relaxation behavior of epoxy nanocomposites. J. Polym. Sci. B Polym. Phys. 2004, 42, 3849–3858. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Maddams, W. The scope and limitations of curve fitting. Appl. Spectrosc. 1980, 34, 245–267. [Google Scholar] [CrossRef]
- Gorrasi, G.; Di Lieto, R.; Patimo, G.; De Pasquale, S.; Sorrentino, A. Structure–property relationships on uniaxially oriented carbon nanotube/polyethylene composites. Polymer 2011, 52, 1124–1132. [Google Scholar] [CrossRef]
- Najeeb, C.; Chang, J.; Lee, J.-H.; Kim, J.-H. Fabrication of aligned ultra-thin transparent conductive films of single-walled carbon nanotubes by a compression/sliding method. Scr. Mater. 2011, 64, 126–129. [Google Scholar] [CrossRef]
- Ma, C.; Liu, H.-Y.; Du, X.; Mach, L.; Xu, F.; Mai, Y.-W. Fracture resistance, thermal and electrical properties of epoxy composites containing aligned carbon nanotubes by low magnetic field. Compos. Sci. Technol. 2015, 114, 126–135. [Google Scholar] [CrossRef]
- Arguin, M.; Sirois, F.; Therriault, D. Electric field induced alignment of multiwalled carbon nanotubes in polymers and multiscale composites. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 16–25. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Ma, C.; Zhang, W.; Zhang, R.-P.; Koratkar, N.; Liang, J. Alignment of multiwalled carbon nanotubes in bulk epoxy composites via electric field. J. Appl. Phys. 2009, 105, 054319. [Google Scholar] [CrossRef]
- He, Y.; Cao, Z.; Ma, L. Shear flow induced alignment of carbon nanotubes in natural rubber. Int. J. Polym. Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Ng, B. Directional alignment of carbon nanotubes in polymer matrices: Contemporary approaches and future advances. Compos. Part A Appl. Sci. Manuf. 2014, 56, 103–126. [Google Scholar] [CrossRef]
- Park, C.; Wilkinson, J.; Banda, S.; Ounaies, Z.; Wise, K.E.; Sauti, G.; Lillehei, P.T.; Harrison, J.S. Aligned single-wall carbon nanotube polymer composites using an electric field. J. Polym. Sci. B Polym. Phys. 2006, 44, 1751–1762. [Google Scholar] [CrossRef]
- Sun, W.; Tomita, H.; Hasegawa, S.; Kitamura, Y.; Nakano, M.; Suehiro, J. An array of interdigitated parallel wire electrodes for preparing a large-scale nanocomposite film with aligned carbon nanotubes. J. Phys. D Appl. Phys. 2011, 44, 445303. [Google Scholar] [CrossRef]
- Gao, J.; He, Y.; Gong, X. Effect of electric field induced alignment and dispersion of functionalized carbon nanotubes on properties of natural rubber. Results Phys. 2018, 9, 493–499. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Tortora, M.; Vittoria, V. Barrier properties of polymer/clay nanocomposites. In Polymer Nanocomposites; Mai, Y.-W., Yu, Z.-Z., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2006; pp. 273–296. [Google Scholar]




| Sample | Approach | Curing Cycle | ΔHdyn (J/g) |
|---|---|---|---|
| UF | DSC | Heating scan from 30 to 260 °C | 375 |
| FR | DSC | Heating scan from 30 to 260 °C | 367 |
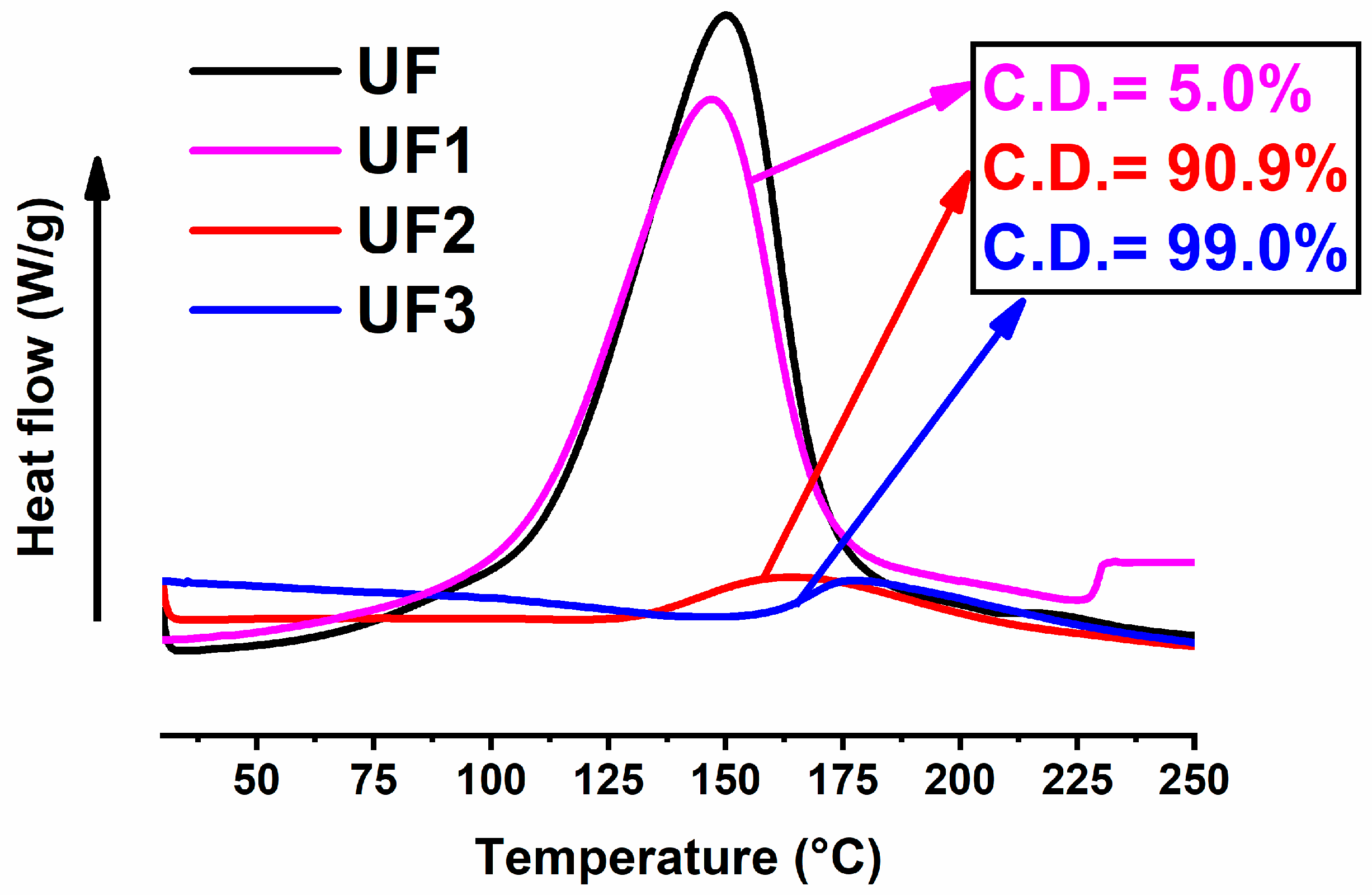
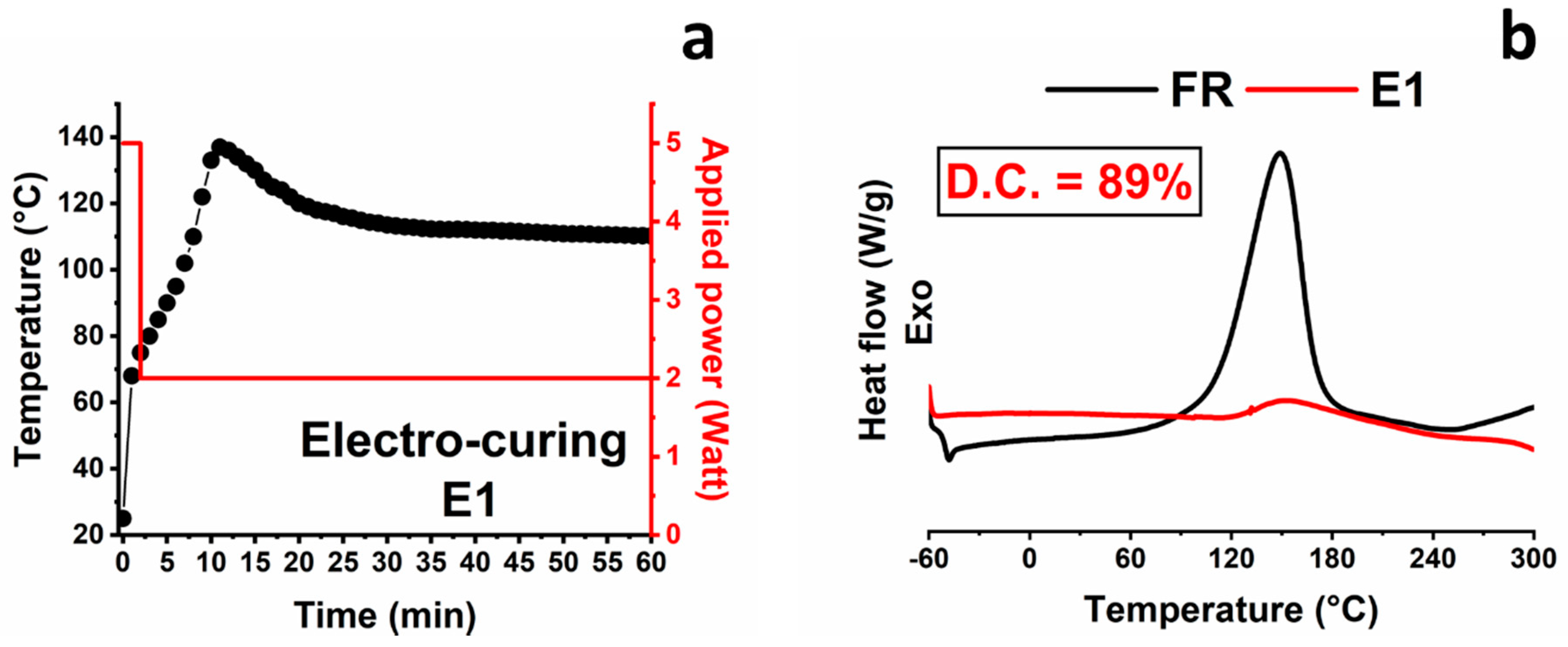
| Sample | Method | Curing Cycle | ΔHresidual (J/g) | Curing Degree (C.D.) (%) |
|---|---|---|---|---|
| UF1 | Oven | (1 h@80 °C) | 356 | 5 |
| UF2 | Oven | (1 h@80 °C + 20 min@120 °C) | 34 | 91 |
| UF3 | Oven | (1 h@80 °C + 20 min@120 °C + 1 h@180 °C) | 4 | 99 |
| FR3 | Oven | (1 h@80 °C + 20 min@120 °C + 1 h@180 °C) | 33 | 91 |
| FR4 | Oven | (3 h@180 °C) | 10 | 95 |
| E1 | Electro-curing | (2 min@5 W + 58 min@2 W) | 42 | 89 |
| E2 | Electro-curing | (5 min@10 W + 15 min@5 W) | 0 | 100 |
| E3 | Electro-curing (steps performed on E1 + steps performed on E2) | (2 min@5 W + 58 min@2 W) + (5 min@10 W + 15 min@5 W) | 0 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadagno, L.; Sorrentino, A.; Delprat, P.; Vertuccio, L. Design of Multifunctional Composites: New Strategy to Save Energy and Improve Mechanical Performance. Nanomaterials 2020, 10, 2285. https://doi.org/10.3390/nano10112285
Guadagno L, Sorrentino A, Delprat P, Vertuccio L. Design of Multifunctional Composites: New Strategy to Save Energy and Improve Mechanical Performance. Nanomaterials. 2020; 10(11):2285. https://doi.org/10.3390/nano10112285
Chicago/Turabian StyleGuadagno, Liberata, Andrea Sorrentino, Patrick Delprat, and Luigi Vertuccio. 2020. "Design of Multifunctional Composites: New Strategy to Save Energy and Improve Mechanical Performance" Nanomaterials 10, no. 11: 2285. https://doi.org/10.3390/nano10112285