High Sensitivity and High Stability QCM Humidity Sensors Based on Polydopamine Coated Cellulose Nanocrystals/Graphene Oxide Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
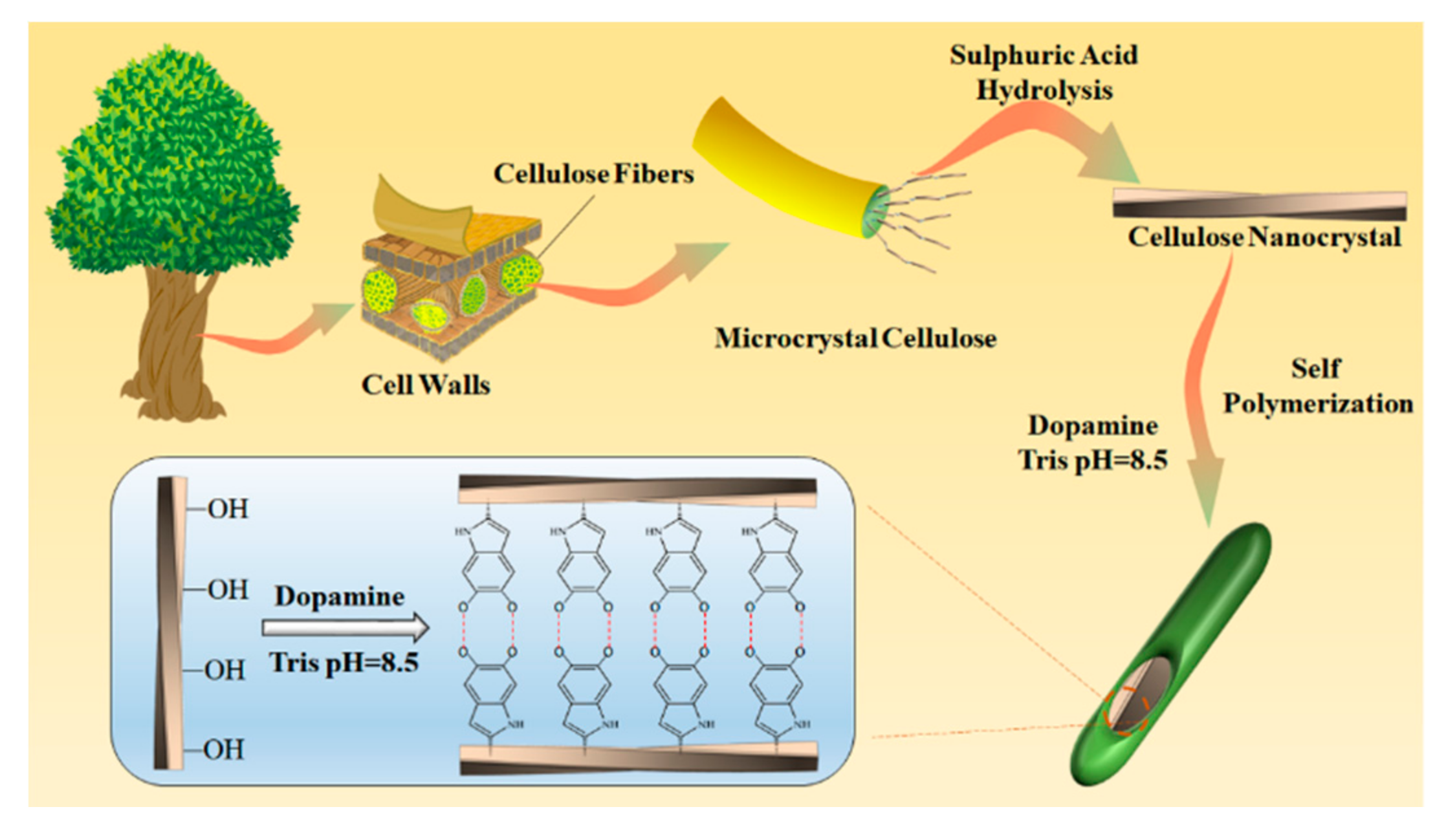
2.1. Synthesis of Sensitive Material
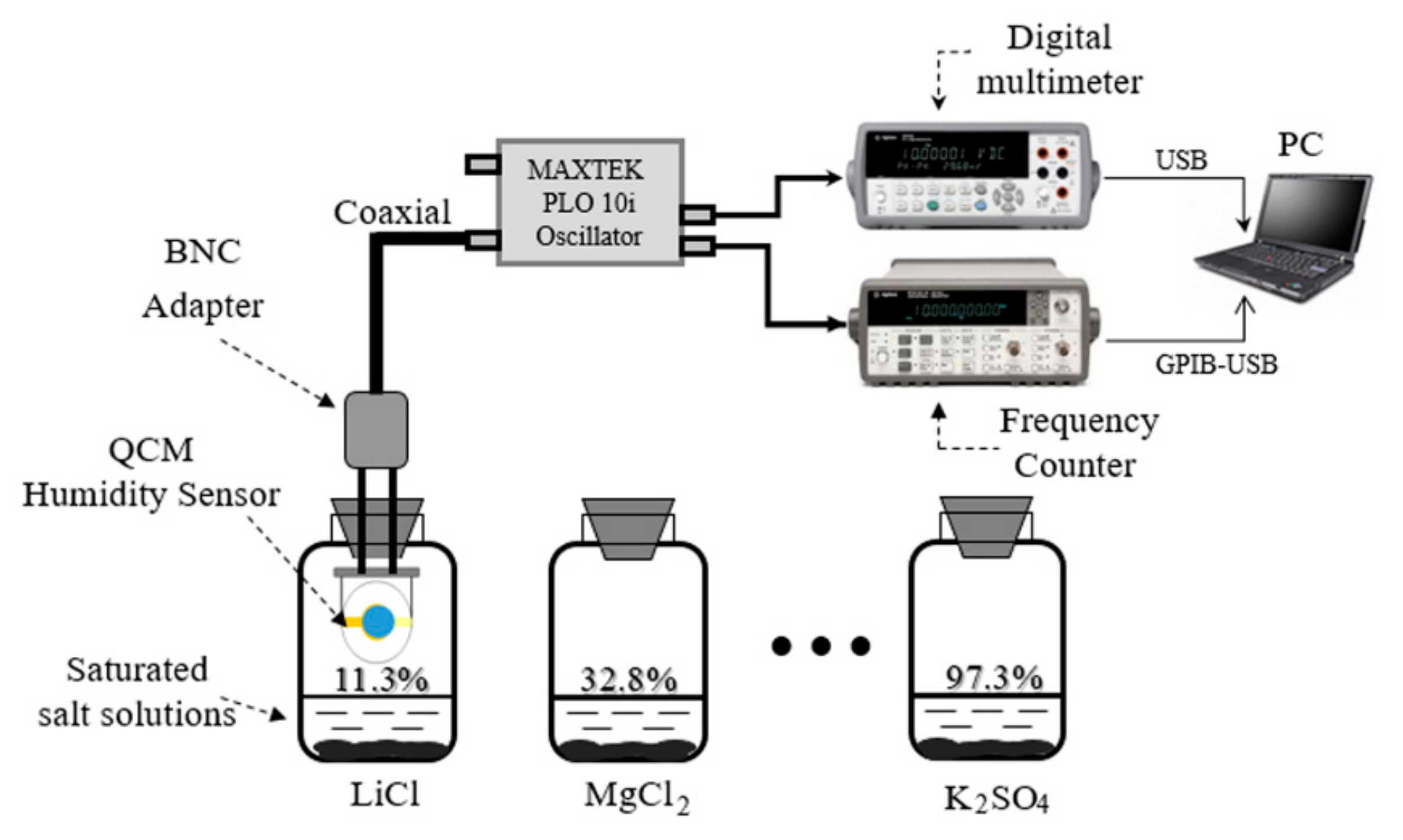
2.2. Sensor Fabrication
2.3. Analysis Instrument
3. Results and Discussions
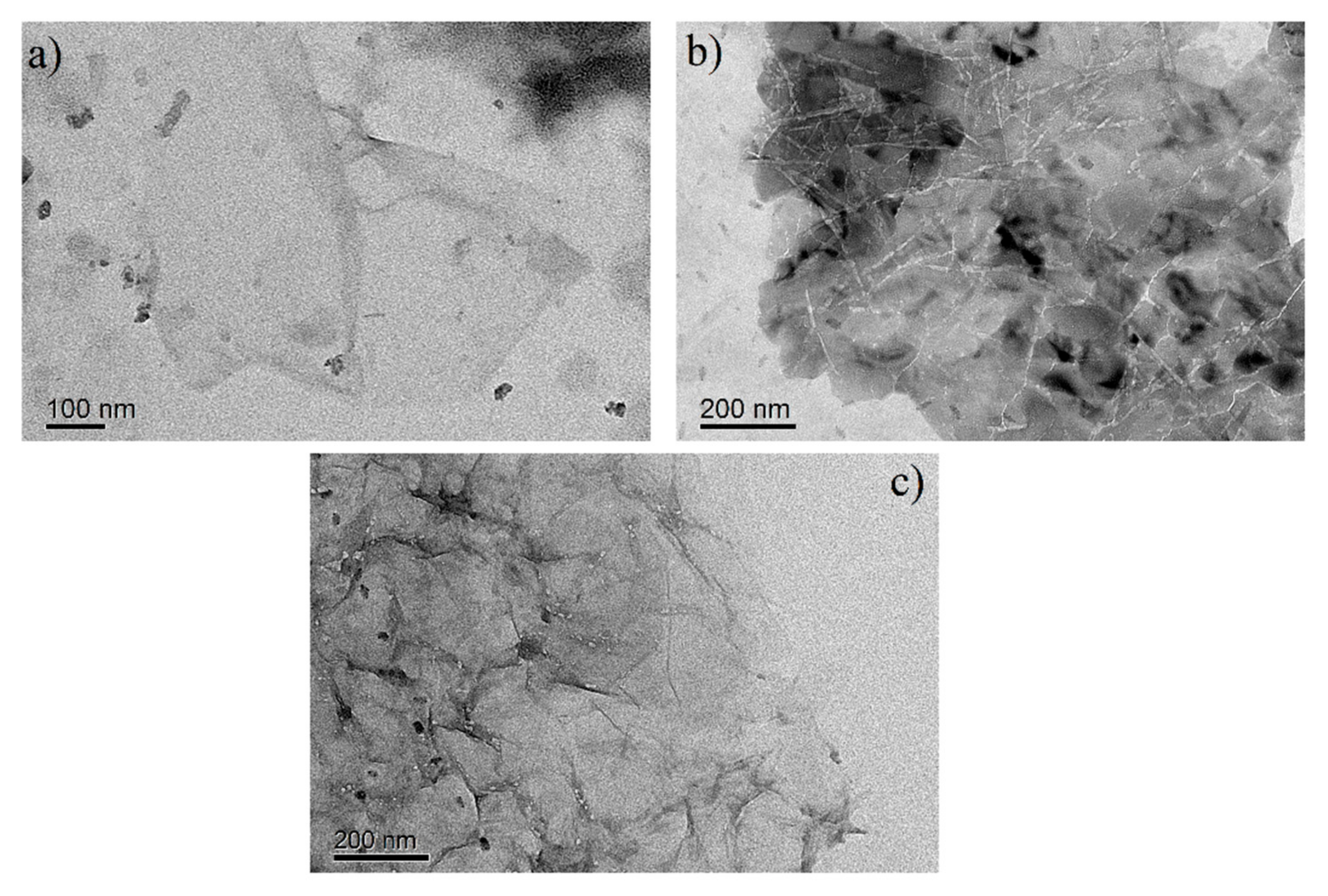
3.1. Material Characterization
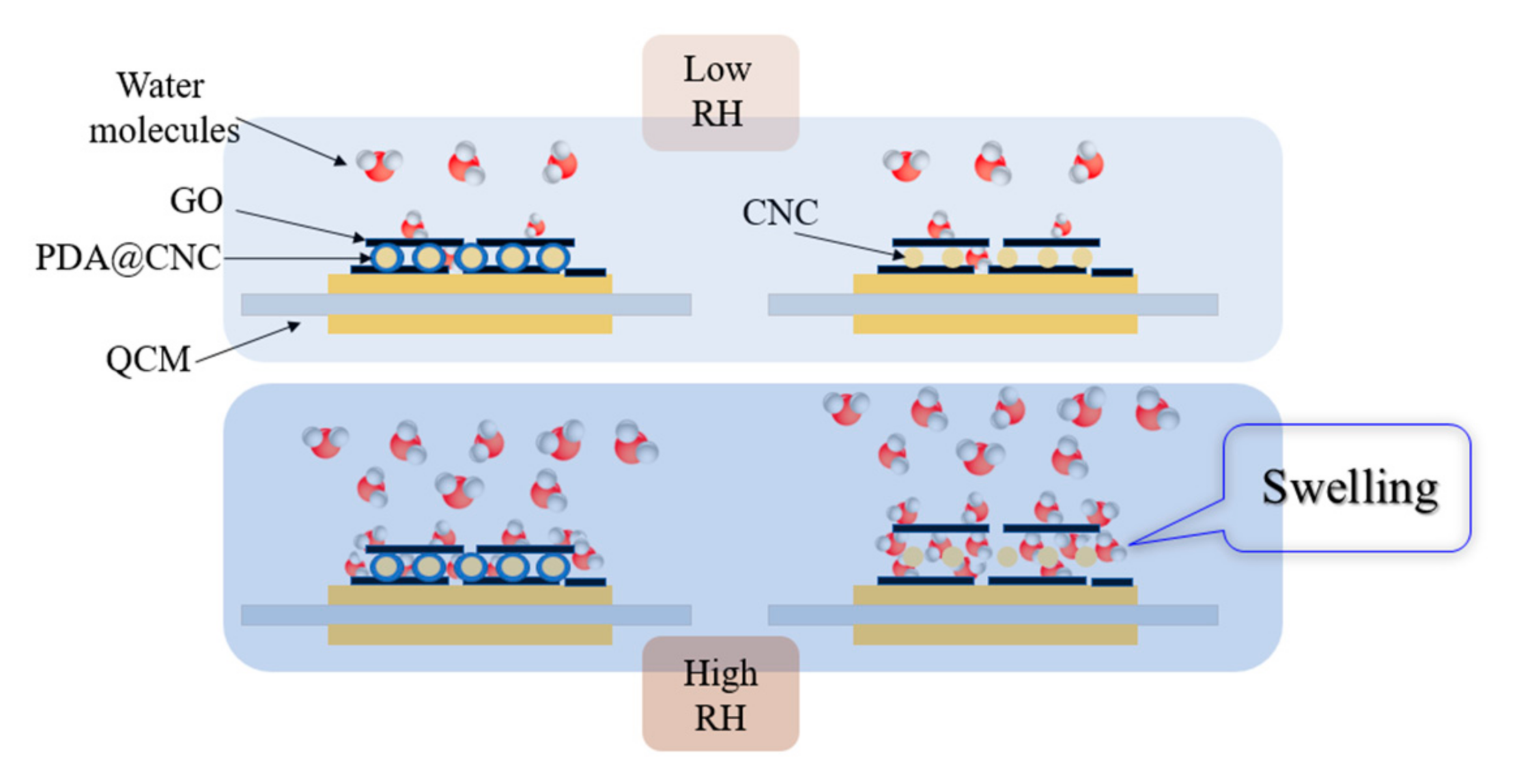
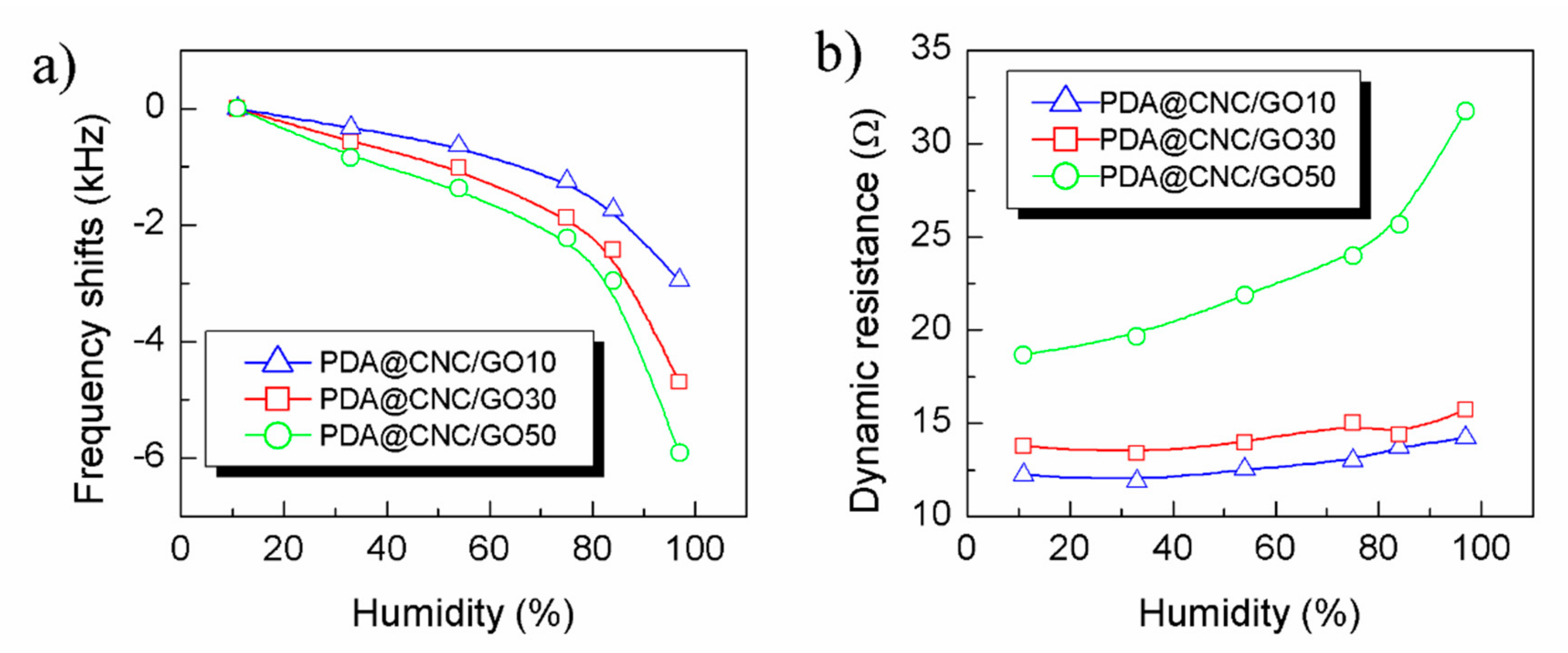
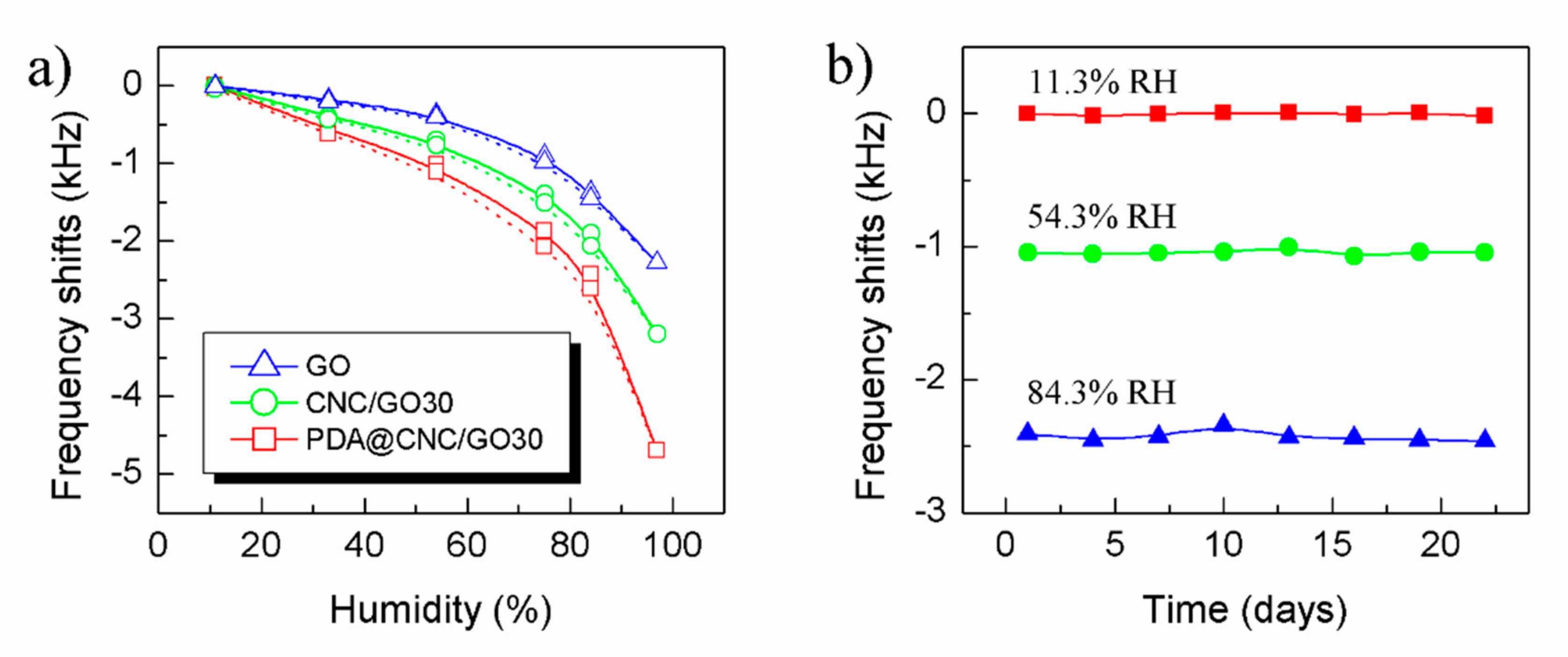
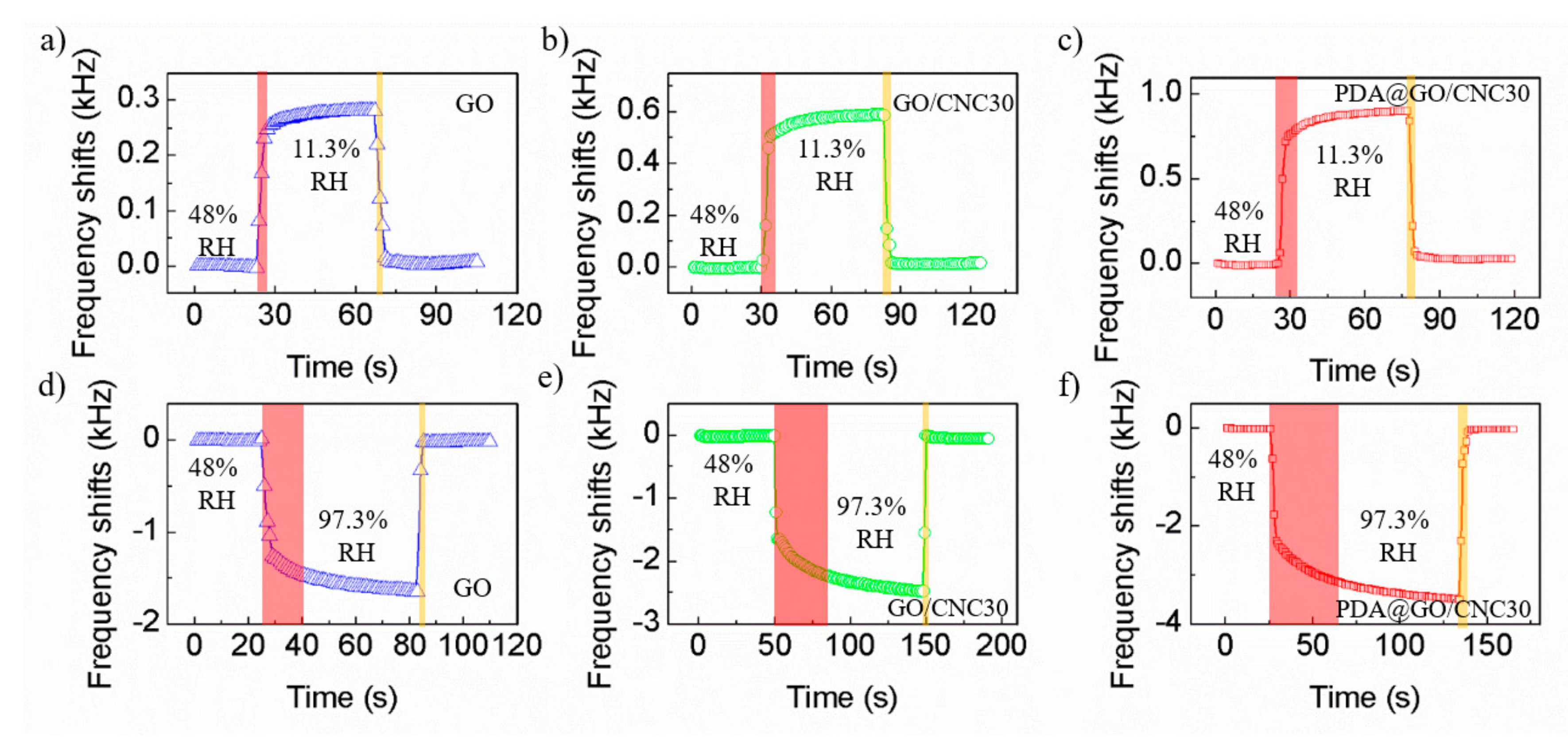
3.2. Humidity Sensing Properties of the Sensors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leong, A.; Kashan, M.A.M.; Swamy, V.; Ramakrishnan, N. 2D material attached quartz crystal microbalance for sensing SF6 gas flow under humid condition. Electron. Lett. 2020, 56, 5–7. [Google Scholar] [CrossRef]
- Kano, S.; Kim, K.; Fujii, M. Fast-response and flexible nanocrystal-based humidity sensor for monitoring human respiration and water evaporation on skin. ACS Sens. 2017, 2, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, Flexible, Cost-Saving, and Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Liu, J.; Qu, W.; Xiao, F.; Liu, A.X.; Cao, J.; Liu, J. Fast and accurate detection of unknown tags for rfid systems-hash collisions are desirable. IEEE/ACM Trans. Netw. 2020, 28, 126–139. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Park, S.Y.; Kang, J.H.; Kim, S.J.; Choi, Y.B.; Shin, U.S. Carbon nanotubes immobilized on gold electrode as an electrochemical humidity sensor. Sens. Actuators B Chem. 2019, 300, 127049. [Google Scholar] [CrossRef]
- Li, B.; Tian, Q.; Su, H.; Wang, X.; Wang, T.; Zhang, D. High sensitivity protable capacitive humidity sensor based on In2O3 nanocubes-decorated GO nanosheets and its wearable application in respiration detection. Sens. Actuators B Chem. 2019, 299, 126973. [Google Scholar] [CrossRef]
- Xu, J.; Bertke, M.; Wasisto, H.S.; Peiner, E. Piezoresistive microcantilevers for humidity sensing. J. Micromech. Microeng. 2019, 29, 053003. [Google Scholar] [CrossRef]
- Zheng, Z.; Yao, Y.; Sun, Y.; Yeow, J.T.W. Development of a highly sensitive humidity sensor based on the capacitive micromachined ultrasonic transducer. Sens. Actuators B Chem. 2019, 286, 39–45. [Google Scholar] [CrossRef]
- Yu, H.; Wang, C.; Meng, F.Y.; Liang, J.G.; Kashan, H.S.; Adhikari, K.K.; Wang, L.; Kim, E.S.; Kim, N.Y. Design and analysis of ultrafast and high-sensitivity microwave transduction humidity sensor based on belt-shaped MoO3 nanomaterial. Sens. Actuators B Chem. 2020, 304, 127138. [Google Scholar] [CrossRef]
- Kadirsoy, S.; Atar, N.; Yola, M.L. Molecularly imprinted QCM sensor based on delaminated MXene for chlorpyrifos detection and QCM sensor validation. New J. Chem. 2020, 44, 6524–6532. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; McCarter, K.S.; Vaughan, S.; Warner, I.M. QCM virtual sensor array: Vapor identification and molecular weight approximation. Sens. Actuators B Chem. 2017, 246, 952–960. [Google Scholar] [CrossRef]
- Su, P.G.; Chuang, T.Y. Simple and rapid differentiation of toxic gases using a quartz crystal microbalance sensor array coupled with principal component analysis. Sens. Actuators A Phys. 2017, 263, 1–7. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Muckley, E.S.; Lynch, J.; Kumar, R.; Sumpter, B.; Ivanov, I.N. PEDOT:PSS/QCM-based multimodal humidity and pressure sensor. Sens. Actuators B Chem. 2016, 236, 91–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, K.; Xu, R.; Jiang, D.; Luo, L.; Zhu, Z. Quartz crystal microbalance coated with carbon nanotube films used as humidity sensor. Sens. Actuators A Phys. 2005, 120, 142–146. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Ma, W.; Ling, W. Quartz crystal microbalance humidity sensors based on nanodiamond sensing films. IEEE Trans. Nanotechnol. 2014, 13, 386–393. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Highly sensitive humidity sensors based on electro-spinning/netting a polyamide 6 nano-fiber/net modified by polyethyleneimine. J. Mater. Chem. 2011, 21, 16231–16238. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Wang, X.; Cheng, Z.; Xu, J. Facile preparation of N-rich functional polymer with porous framework as QCM sensing material for rapid humidity detection. Sens. Actuators B Chem. 2019, 288, 289–297. [Google Scholar] [CrossRef]
- Qi, P.; Xu, Z.; Zhang, T.; Fei, T.; Wang, R. Chitosan wrapped multiwalled carbon nanotubes as quartz crystal microbalance sensing material for humidity detection. J. Colloid Interface Sci. 2020, 560, 284–292. [Google Scholar] [CrossRef]
- Erol, A.; Okur, S.; Yaǧmurcukardeş, N.; Arikan, M.Ç. Humidity-sensing properties of a ZnO nanowire film as measured with a QCM. Sens. Actuators B Chem. 2011, 152, 115–120. [Google Scholar] [CrossRef]
- Üzar, N.; Okur, S.; Arikan, M.Ç. Investigation of humidity sensing properties of ZnS nanowires synthesized by vapor liquid solid (VLS) technique. Sens. Actuators A Phys. 2011, 167, 188–193. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Guo, H.; Wu, Z. Graphene oxide thin film coated quartz crystal microbalance for humidity detection. Appl. Surf. Sci. 2011, 257, 7778–7782. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene oxide. Origin of acidity, its instability in water, and a new dynamic structural model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Li, X.; Chen, X.; Li, N. Investigation of the stability of QCM humidity sensor using graphene oxide as sensing films. Sens. Actuators B Chem. 2014, 191, 779–783. [Google Scholar] [CrossRef]
- Yuan, Z.; Tai, H.; Ye, Z.; Liu, C.; Xie, G.; Du, X.; Jiang, Y. Novel highly sensitive QCM humidity sensor with low hysteresis based on graphene oxide (GO)/poly(ethyleneimine) layered film. Sens. Actuators B Chem. 2016, 234, 145–154. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Li, P.; Zhou, X.; Zong, X.; Dong, G. Facile fabrication of high-performance QCM humidity sensor based on layer-by-layer self-assembled polyaniline/graphene oxide nanocomposite film. Sens. Actuators B Chem. 2018, 255, 1869–1877. [Google Scholar] [CrossRef]
- Yuan, Z.; Tai, H.; Bao, X.; Liu, C.; Ye, Z.; Jiang, Y. Enhanced humidity-sensing properties of novel graphene oxide/zinc oxide nanoparticles layered thin film QCM sensor. Mater. Lett. 2016, 174, 28–31. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Zong, X.; Dong, G.; Zhang, Y. High-performance QCM humidity sensor based on graphene oxide/tin oxide/polyaniline ternary nanocomposite prepared by in-situ oxidative polymerization method. Sens. Actuators B Chem. 2018, 262, 531–541. [Google Scholar] [CrossRef]
- Yao, Y.; Xue, Y. Impedance analysis of quartz crystal microbalance humidity sensors based on nanodiamond/graphene oxide nanocomposite film. Sens. Actuators B Chem. 2015, 211, 52–58. [Google Scholar] [CrossRef]
- Ding, X.; Chen, X.; Chen, X.; Zhao, X.; Li, N. A QCM humidity sensor based on fullerene/graphene oxide nanocomposites with high quality factor. Sens. Actuators B Chem. 2018, 266, 534–542. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, J.; Yu, J. Surface acetylation of cellulose nanocrystal and its reinforcing function in poly(lactic acid). Carbohydr. Polym. 2011, 83, 1834–1842. [Google Scholar] [CrossRef]
- Bitinis, N.; Fortunati, E.; Verdejo, R.; Bras, J.; Kenny, J.M.; Torre, L.; López-Manchado, M.A. Poly(lactic acid)/natural rubber/cellulose nanocrystal bionanocomposites. Part II: Properties evaluation. Carbohydr. Polym. 2013, 96, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Y.; Liu, C.; Gan, L.; Ma, X.; Huang, J. Polydopamine-coated cellulose nanocrystals as an active ingredient in poly(vinyl alcohol) films towards intensifying packaging application potential. Cellulose 2019, 26, 9599–9612. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Vig, J.R.; Walls, F.L. A review of sensor sensitivity and stability. Proc. Annu. IEEE Int. Freq. Control Symp. 2000, 30–33. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yao, Y.; Li, N.; Chen, X.; Bi, X. Multi-walled carbon nanotubes/graphene oxide composites for humidity sensing. IEEE Sens. J. 2013, 13, 4749–4756. [Google Scholar] [CrossRef]
- Addabbo, T.; Fort, A.; Mugnaini, M.; Vignoli, V.; Baldi, A.; Bruzzi, M. Quartz-Crystal Microbalance Gas Sensors Based on TiO2 Nanoparticles. IEEE Trans. Instrum. Meas. 2018, 67, 722–730. [Google Scholar] [CrossRef]
- Mittal, U.; Islam, T.; Nimal, A.T.; Sharma, M.U. A Novel Sol–Gel γ-Al2O3 Thin-Film-Based Rapid SAW Humidity Sensor. IEEE Trans. Electron. Devices 2015, 62, 4242–4250. [Google Scholar] [CrossRef]
- Xu, J.; Bertke, M.; Li, X.; Mu, H.; Zhou, H.; Yu, F.; Hamdana, G.; Schmidt, A.; Bremers, H.; Peiner, E. Fabrication of ZnO nanorods and Chitosan@ZnO nanorods on MEMS piezoresistive self-actuating silicon microcantilever for humidity sensing. Sens. Actuators B Chem. 2018, 273, 276–287. [Google Scholar] [CrossRef]


| Mark Number | Sensing Material | Before the Deposition of Thin Film | After the Deposition of Thin Film | ||
|---|---|---|---|---|---|
| Frequency (Hz) | Dynamic Resistance (Ω) | Frequency (Hz) | Dynamic Resistance (Ω) | ||
| GO-coated QCM | GO | 9,984,827 | 11.0945 | 9,978,865 | 11.1042 |
| CNC/GO30-coated QCM | CNC/GO30 | 9,984,811 | 10.349 | 9,975,052 | 11.6756 |
| PDA@CNC/GO30-coated QCM | PDA@CNC/GO30 | 9,984,821 | 11.0945 | 9,972,163 | 13.7241 |
| Sensing Material | Sensor Type | Sensitivity (ppm/% RH)/Range | Response and Recovery Times (s) | Dynamic Resistance (Ω) | Hysteresis (% RH) | Ref |
|---|---|---|---|---|---|---|
| GO | CMUT | 241.67/22.5–43.2% RH | 10/4 s | Not given | Not given | [8] |
| MWCNTs-CS | QCM | 4.67/11–95% RH | 75/34 s | 51.92@11% RH 151.32@95% RH | 1.1 | [20] |
| GO-PEI | QCM | 2.73/11–97% RH | 53/18 s | Not given | 0.54 | [26] |
| GO/SnO2/PANI | QCM | 3.64/0–97% RH | 7/2 s | Not given | Not given | [29] |
| MWCNTs-GO | QCM | 0.98/10–95% RH | 12/6 s | 42.8164@10% RH 96.2581@95% RH | Not given | [37] |
| TiO2 nano-particles | QCM | 0.75/0–20% RH | 2/4 min | Not given | <2 | [38] |
| γ-Al2O3 | SAW | 0.28/3–20% RH | 1/3 s | Not given | 0.3 | [39] |
| Chitosan@ZONRs | Cantilever | 16.9/30–70% RH | 1 s/Not given | Not given | 2.1 | [40] |
| PDA@CNC/GO | QCM | 5.466/11.3–97.3% RH | 11/4 s (from 48% to 11.3% RH) 37/5 s (from 48% to 97.3% RH) | 13.7724@11.3% RH 15.7015@97.3% RH | 4.3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Huang, X.; Chen, Q.; Zhang, Z.; Ling, W. High Sensitivity and High Stability QCM Humidity Sensors Based on Polydopamine Coated Cellulose Nanocrystals/Graphene Oxide Nanocomposite. Nanomaterials 2020, 10, 2210. https://doi.org/10.3390/nano10112210
Yao Y, Huang X, Chen Q, Zhang Z, Ling W. High Sensitivity and High Stability QCM Humidity Sensors Based on Polydopamine Coated Cellulose Nanocrystals/Graphene Oxide Nanocomposite. Nanomaterials. 2020; 10(11):2210. https://doi.org/10.3390/nano10112210
Chicago/Turabian StyleYao, Yao, Xianhe Huang, Qiao Chen, Zhen Zhang, and Weiwei Ling. 2020. "High Sensitivity and High Stability QCM Humidity Sensors Based on Polydopamine Coated Cellulose Nanocrystals/Graphene Oxide Nanocomposite" Nanomaterials 10, no. 11: 2210. https://doi.org/10.3390/nano10112210
APA StyleYao, Y., Huang, X., Chen, Q., Zhang, Z., & Ling, W. (2020). High Sensitivity and High Stability QCM Humidity Sensors Based on Polydopamine Coated Cellulose Nanocrystals/Graphene Oxide Nanocomposite. Nanomaterials, 10(11), 2210. https://doi.org/10.3390/nano10112210






