Highly Conductive PEDOT:PSS Thin Films with Two-Dimensional Lamellar Stacked Multi-Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PEDOT:PSS Thin Films
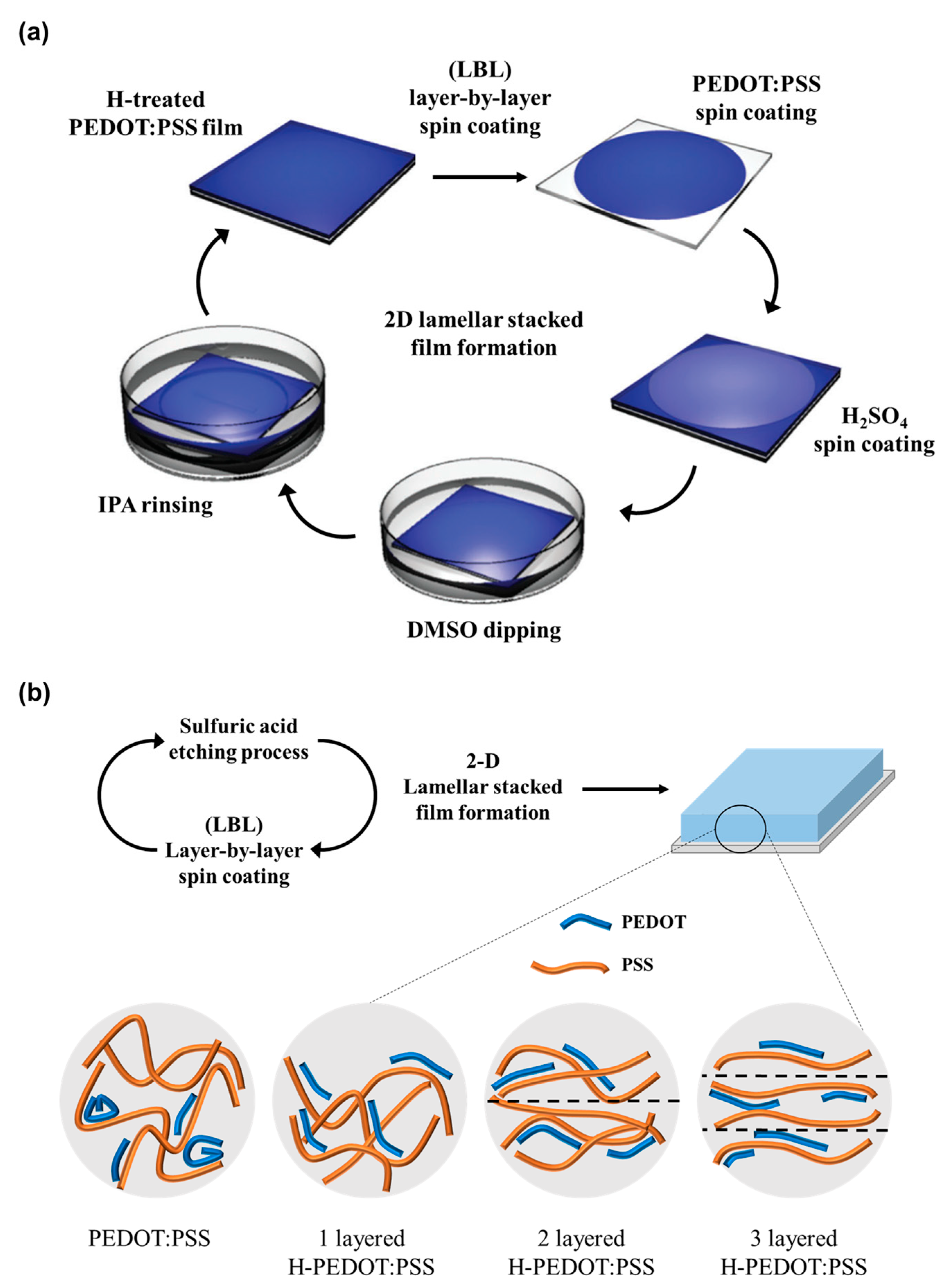
2.3. Fabrication of Two-Dimensional Lamellar Stacked PEDOT:PSS Thin Films
2.4. Characterization
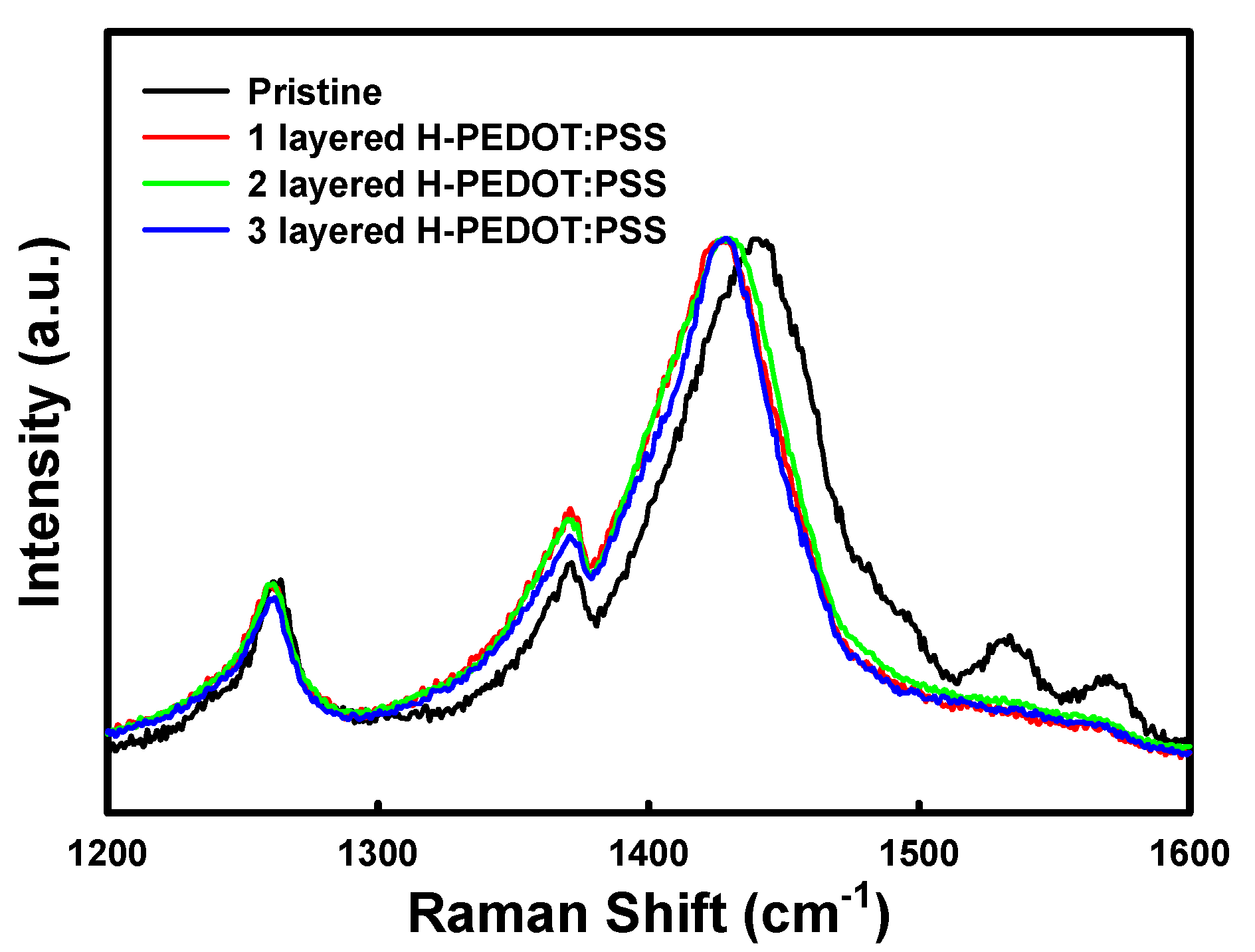
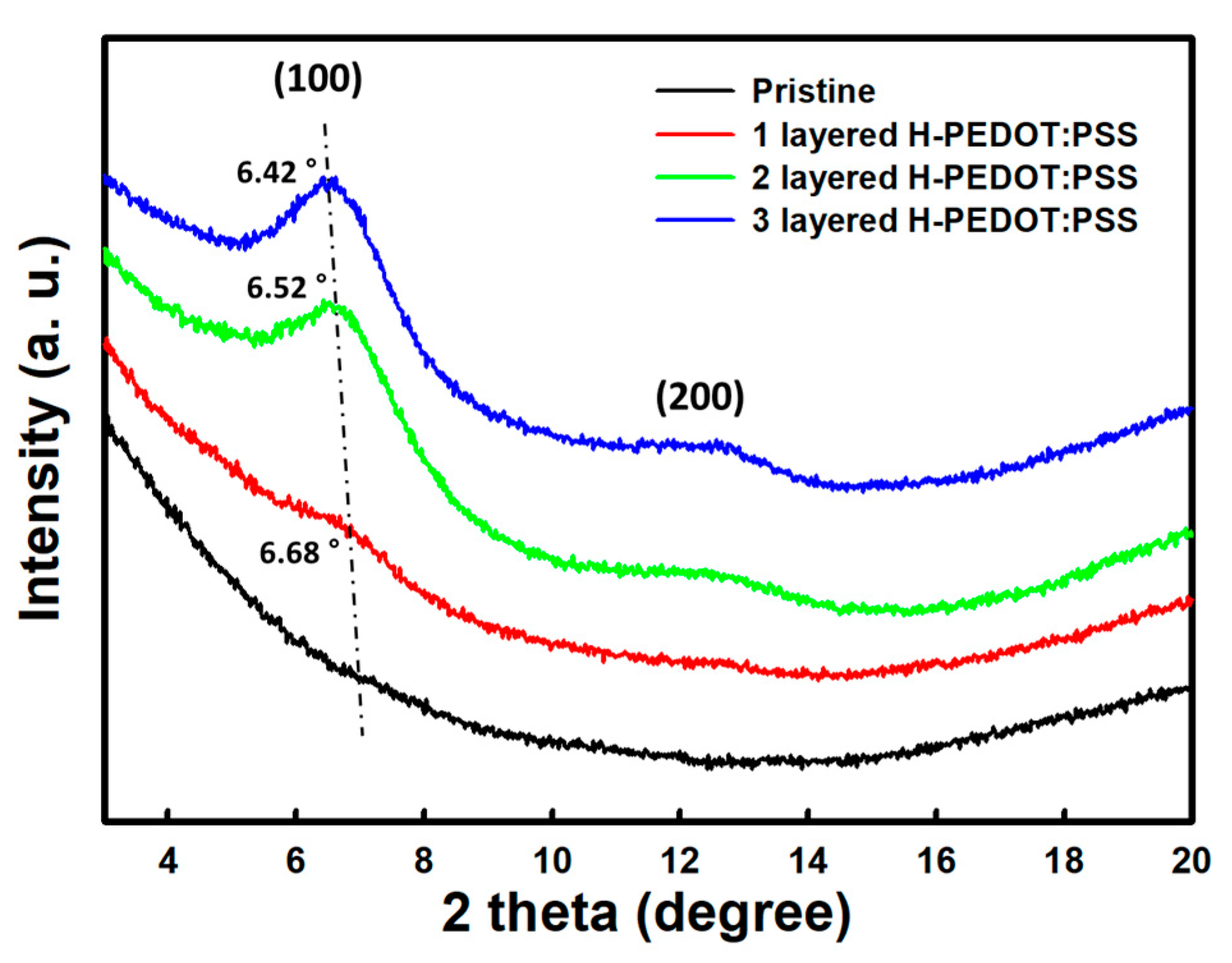
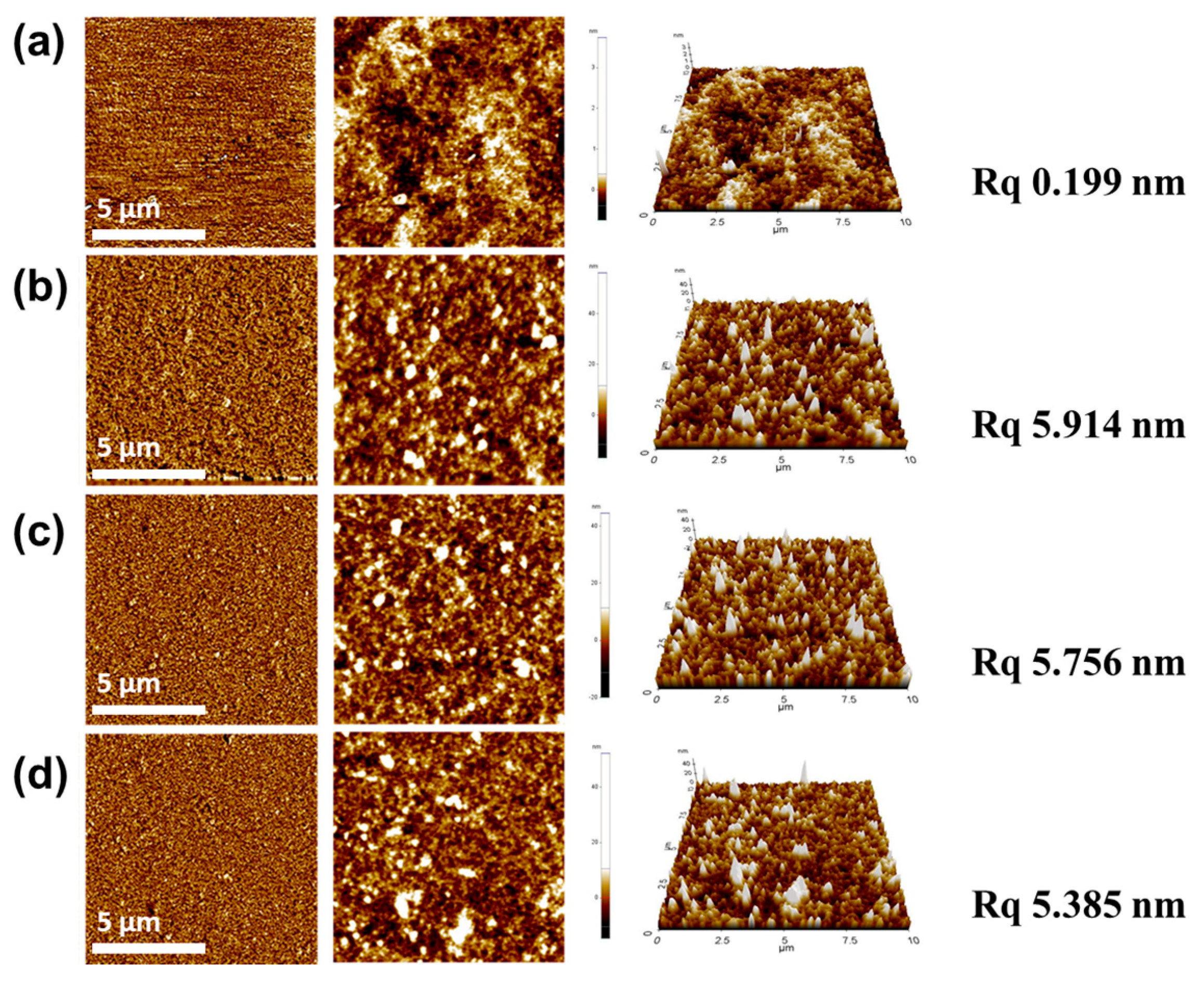
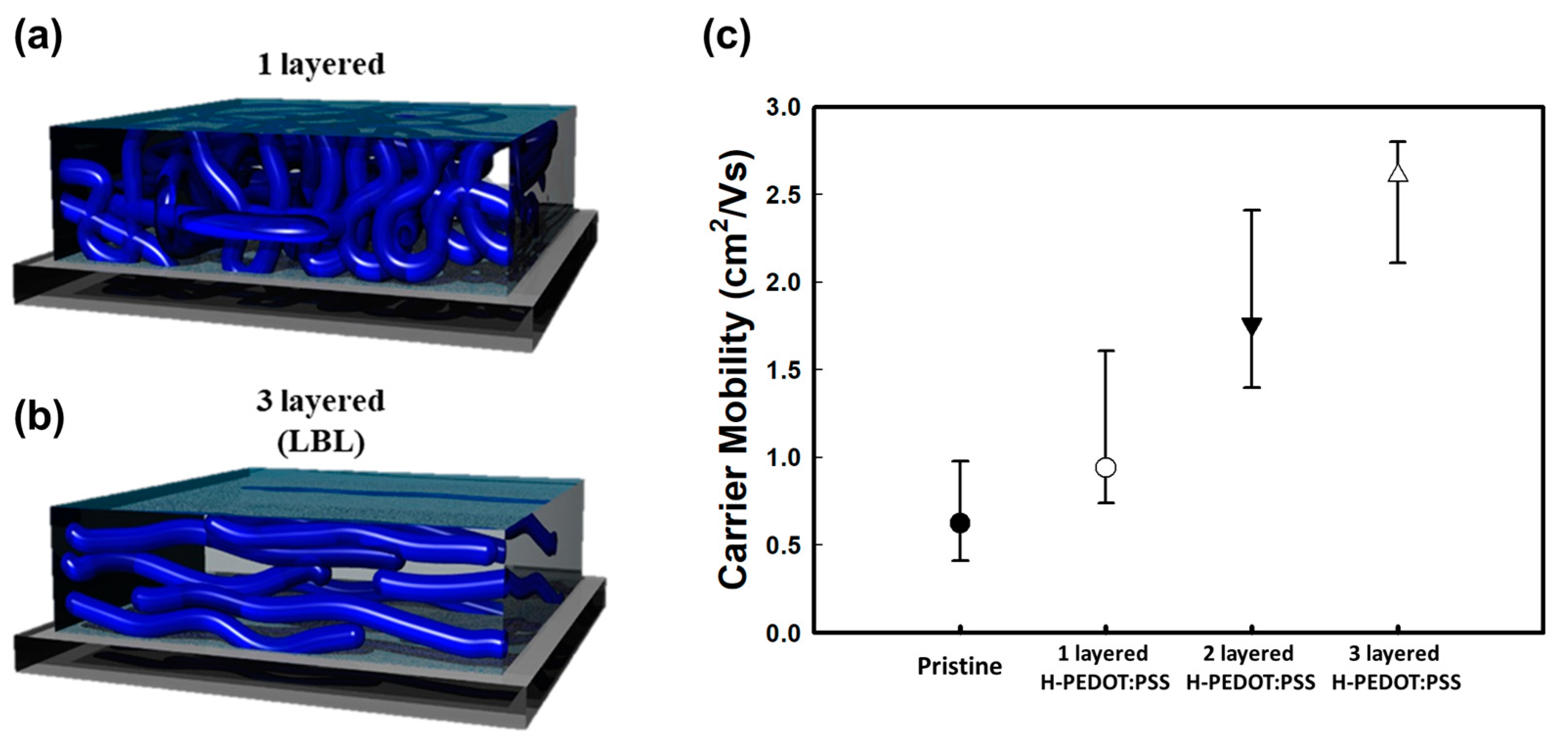
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.; Park, C.; Im, S.; Kim, J.H. Design of intrinsically stretchable and highly conductive polymers for fully stretchable electrochromic devices. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; Lee, H.; Park, C.; Im, S.; Kim, J.H. Synthesis of stretchable, environmentally stable, conducting polymer PEDOT using a modified acid template random copolymer. Macromol. Chem. Phys. 2020, 221, 1900465. [Google Scholar] [CrossRef]
- Rhee, R.; Im, S.; Lee, H.; Lee, J.H.; Kim, Y.; Chun, D.H.; Park, C.; Lee, S.; Kim, J.H.; Park, J.H. Stretchable hole extraction layer for improved stability in perovskite solar cells. ACS Sustain. Chem. Eng. 2020, 8, 8004–8010. [Google Scholar] [CrossRef]
- Choi, S.; Yoon, K.; Lee, S.; Lee, H.J.; Lee, J.; Kim, D.W.; Kim, M.S.; Lee, T.; Pang, C. Conductive hierarchical hairy fibers for highly sensitive, stretchable, and water-resistant multimodal gesture-distinguishable sensor, VR applications. Adv. Funt. Mater. 2019, 29, 1905808. [Google Scholar] [CrossRef]
- Nardes, A.M. On the conductivity of PEDOT:PSS thin films. Tech. Univ. Eindh. 2007, 132. [Google Scholar] [CrossRef]
- Cho, H.; Cho, W.; Kim, Y.; Lee, J.-G.; Kim, J.H. Influence of residual sodium ions on the structure and properties of poly (3, 4-ethylenedioxythiophene): Poly (styrenesulfonate). RSC Adv. 2018, 8, 29044–29050. [Google Scholar] [CrossRef]
- Deetuam, C.; Weise, D.; Samthong, C.; Praserthdam, P.; Baumann, R.R.; Somwangthanaroj, A. Electrical conductivity enhancement of spin-coated PEDOT:PSS thin film via dipping method in low concentration aqueous DMSO. J. Appl. Polym. Sci. 2015, 132, 42108. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ito-free organic solar cells. Adv. Funt. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Andrei, V.; Bethke, K.; Madzharova, F.; Beeg, S.; Knop-Gericke, A.; Kneipp, J.; Rademann, K. Size dependence of electrical conductivity and thermoelectric enhancements in spin-coated PEDOT:PSS single and multiple layers. Adv. Electron. Mater. 2017, 3, 1600473. [Google Scholar] [CrossRef]
- Lee, H.J.; Anoop, G.; Lee, H.J.; Kim, C.; Park, J.-W.; Choi, J.; Kim, H.; Kim, Y.-J.; Lee, E.; Lee, S.-G.; et al. Enhanced thermoelectric performance of PEDOT:PSS/PANI–CSA polymer multilayer structures. Energy Environ. Sci. 2016, 9, 2806–2811. [Google Scholar] [CrossRef]
- Rutledge, S.; Helmy, A. Carrier mobility enhancement in poly (3, 4-ethylenedioxythiophene)-poly (styrenesulfonate) having undergone rapid thermal annealing. J. Appl. Phys. 2013, 114, 133708. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, W.; Kim, Y.; Cho, H.; Kim, J.H. Electrical characteristics of heterogeneous polymer layers in PEDOT:PSS films. J. Mater. Chem. C 2018, 6, 8906–8913. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.H.; Kim, F.S.; Choi, H.H.; Cheong, I.W.; Kim, J.H. Enhanced thermoelectric properties of PEDOT:PSS nanofilms by a chemical dedoping process. J. Mater. Chem. A 2014, 2, 6532–6539. [Google Scholar] [CrossRef]
- Kim, S.; Sanyoto, B.; Park, W.T.; Kim, S.; Mandal, S.; Lim, J.C.; Noh, Y.Y.; Kim, J.H. Purification of PEDOT:PSS by ultrafiltration for highly conductive transparent electrode of all-printed organic devices. Adv. Mater. 2016, 28, 10149–10154. [Google Scholar] [CrossRef]
- Ouyang, L.; Musumeci, C.; Jafari, M.J.; Ederth, T.; Inganäs, O. Imaging the phase separation between PEDOT and polyelectrolytes during processing of highly conductive PEDOT:PSS films. ACS Appl. Mater. Interfaces 2015, 7, 19764–19773. [Google Scholar] [CrossRef]
- Sun, X.; Sun, T.; Lu, X.; Wang, L.; Jiang, W. Simultaneously improving thermopower and electrical conductivity via polar organic solvents aided layer-by-layer technique. Mat. Sci. Semicon. Proc. 2020, 108, 104909. [Google Scholar] [CrossRef]
- Bae, E.J.; Kang, Y.H.; Jang, K.-S.; Cho, S.Y. Enhancement of thermoelectric properties of PEDOT:PSS and tellurium-PEDOT:PSS hybrid composites by simple chemical treatment. Sci. Rep. 2016, 6, 18805. [Google Scholar]
- Ling, H.; Lu, J.; Phua, S.; Liu, H.; Liu, L.; Huang, Y.; Mandler, D.; Lee, P.S.; Lu, X. One-pot sequential electrochemical deposition of multilayer poly (3, 4-ethylenedioxythiophene): Poly (4-styrenesulfonic acid)/tungsten trioxide hybrid films and their enhanced electrochromic properties. J. Mater. Chem. A 2014, 2, 2708–2717. [Google Scholar] [CrossRef]
- Sze, P.-W.; Lee, K.-W.; Huang, P.-C.; Chou, D.-W.; Kao, B.-S.; Huang, C.-J. The investigation of high quality PEDOT:PSS film by multilayer-processing and acid treatment. Energies 2017, 10, 716. [Google Scholar] [CrossRef]
- Fabretto, M.V.; Evans, D.R.; Mueller, M.; Zuber, K.; Hojati-Talemi, P.; Short, R.D.; Wallace, G.G.; Murphy, P.J. Polymeric matierlas with metal-like conductivity for next generation organic electronic devices. Chem. Mater. 2012, 24, 3998–4003. [Google Scholar] [CrossRef]
- Hu, A.; Tan, L.; Hu, X.; Hu, L.; Ai, Q.; Meng, X.; Chen, L.; Chen, Y. Crystallization and conformation engineering of solution-processed polymer transparent electrodes with high conductivity. J. Mater. Chem. C 2017, 5, 382–389. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.; Cho, H.; Lee, J.-g.; Kim, J.H. Improvement of PEDOT:PSS linearity via controlled addition process. RSC Adv. 2019, 9, 17318–17324. [Google Scholar] [CrossRef]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.R.; Kim, B.J.; Lee, K. Highly conductive PEDOT:PSS nanofibrils induced by solution-processed crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, Y.R.; Lee, G.; Jin, S.W.; Lee, Y.H.; Hong, S.Y.; Park, H.; Kim, J.W.; Lee, S.-S.; Ha, J.S. Highly conductive, stretchable, and transparent PEDOT:PSS electrodes fabricated with triblock copolymer additives and acid treatment. ACS Appl. Mater. Interfaces 2018, 10, 28027–28035. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Mukaida, M.; Naitoh, Y.; Ishida, T. Morphological change and mobility enhancement in PEDOT:PSS by adding co-solvents. Adv. Mater. 2013, 25, 2831–2836. [Google Scholar] [CrossRef]
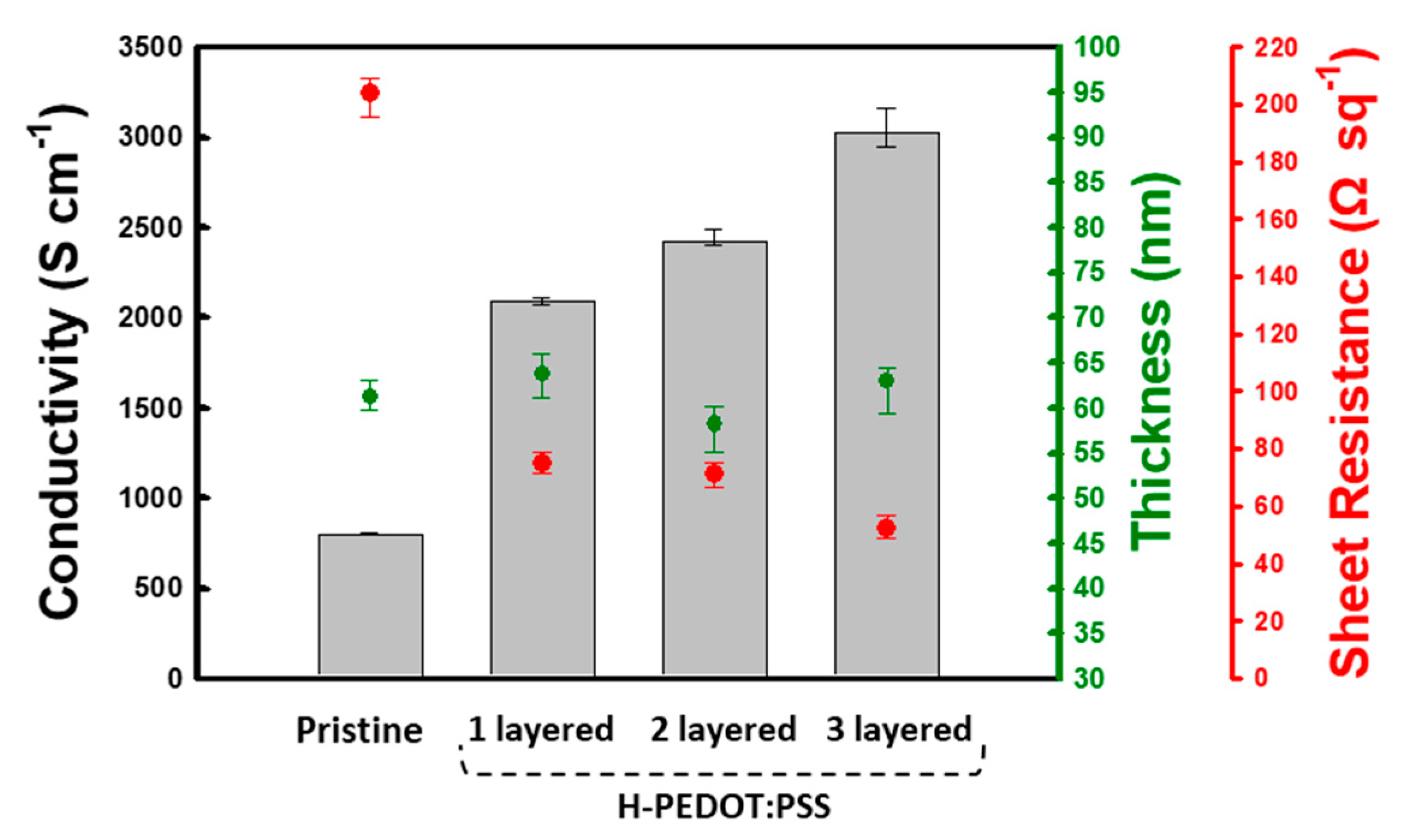

| Sample | Untreated | H | HS | HSH | HSHS | HSHSH | |
|---|---|---|---|---|---|---|---|
| Pristine PEDOT:PSS | Sheet resistance (Ω sq−1) | 204 | - | - | - | - | - |
| Film thickness (nm) | 61 | - | - | - | - | - | |
| Conductivity (S cm−1) | 802 | - | - | - | - | - | |
| 1 layered H-PEDOT:PSS | Sheet resistance (Ω sq−1) | 75 | 75 | - | - | - | - |
| Film thickness (nm) | 170 | 64 | - | - | - | - | |
| Conductivity (S cm−1) | 788 | 2091 | - | - | - | - | |
| 2 layered H-PEDOT:PSS | Sheet resistance (Ω sq−1) | 144 | 155 | 70 | 71 | - | - |
| Film thickness (nm) | 88 | 30 | 90 | 58 | - | - | |
| Conductivity (S cm−1) | 792 | 2125 | 1591 | 2420 | - | - | |
| 3 layered H-PEDOT:PSS | Sheet resistance (Ω sq−1) | 172 | 175 | 72 | 79 | 50 | 53 |
| Film thickness (nm) | 73 | 26 | 84 | 48 | 110 | 63 | |
| Conductivity (S cm−1) | 800 | 2199 | 1660 | 2645 | 1825 | 3026 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, Y.; Kim, J.H. Highly Conductive PEDOT:PSS Thin Films with Two-Dimensional Lamellar Stacked Multi-Layers. Nanomaterials 2020, 10, 2211. https://doi.org/10.3390/nano10112211
Kim Y, Kim Y, Kim JH. Highly Conductive PEDOT:PSS Thin Films with Two-Dimensional Lamellar Stacked Multi-Layers. Nanomaterials. 2020; 10(11):2211. https://doi.org/10.3390/nano10112211
Chicago/Turabian StyleKim, Youngno, Yunryeol Kim, and Jung Hyun Kim. 2020. "Highly Conductive PEDOT:PSS Thin Films with Two-Dimensional Lamellar Stacked Multi-Layers" Nanomaterials 10, no. 11: 2211. https://doi.org/10.3390/nano10112211
APA StyleKim, Y., Kim, Y., & Kim, J. H. (2020). Highly Conductive PEDOT:PSS Thin Films with Two-Dimensional Lamellar Stacked Multi-Layers. Nanomaterials, 10(11), 2211. https://doi.org/10.3390/nano10112211






