Aminodextran Coated CoFe2O4 Nanoparticles for Combined Magnetic Resonance Imaging and Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CoFe2O4 Nanoparticles Preparation
2.3. Synthesis of Aminodextran (AMD)
2.4. Adsorption of Aminodextran (AMD) to Magnetic Nanoparticles
2.5. Characterization Techniques
2.5.1. X-ray Diffraction (XRD) of Co2Fe2O4
2.5.2. Conductometric Titration of Aminodextran (AMD)
2.5.3. Elemental Analysis of Aminodextran (AMD)
2.5.4. FTIR Spectroscopy
2.5.5. Zeta Potential Measurements
2.5.6. Measurements of Particle Size
2.5.7. Particle Size and Morphology Analysis
2.5.8. Magnetization Measurements
2.5.9. Magnetic Resonance Imaging (MRI) Analysis
2.5.10. Hyperthermia Study
3. Results and Discussion
3.1. XRD Study of CoFe2O4 Nanoparticles
3.2. Conductometric Analysis of Aminodextran (AMD)
3.3. Elemental Analysis of Aminodextran (AMD)
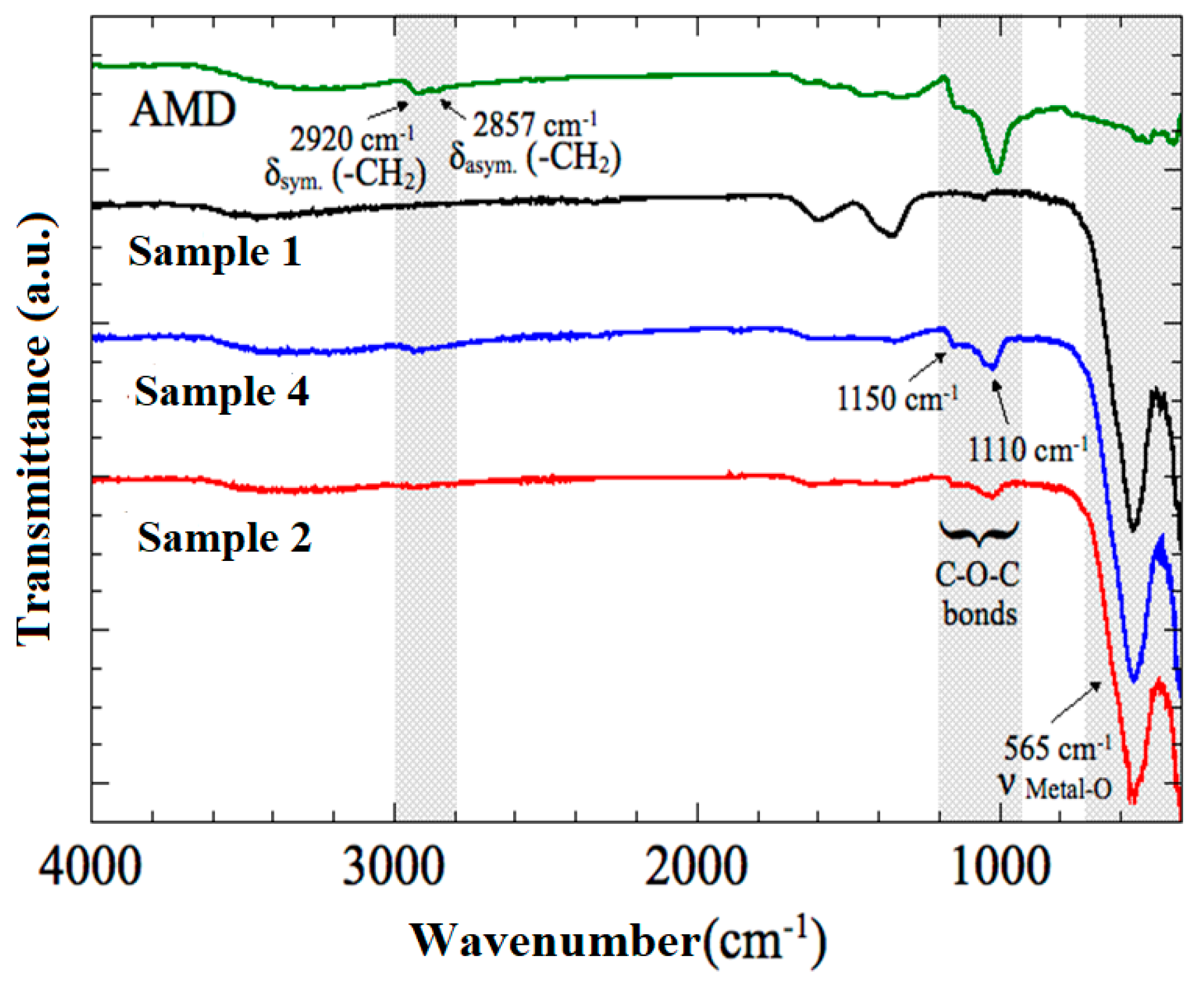
3.4. FTIR Analysis
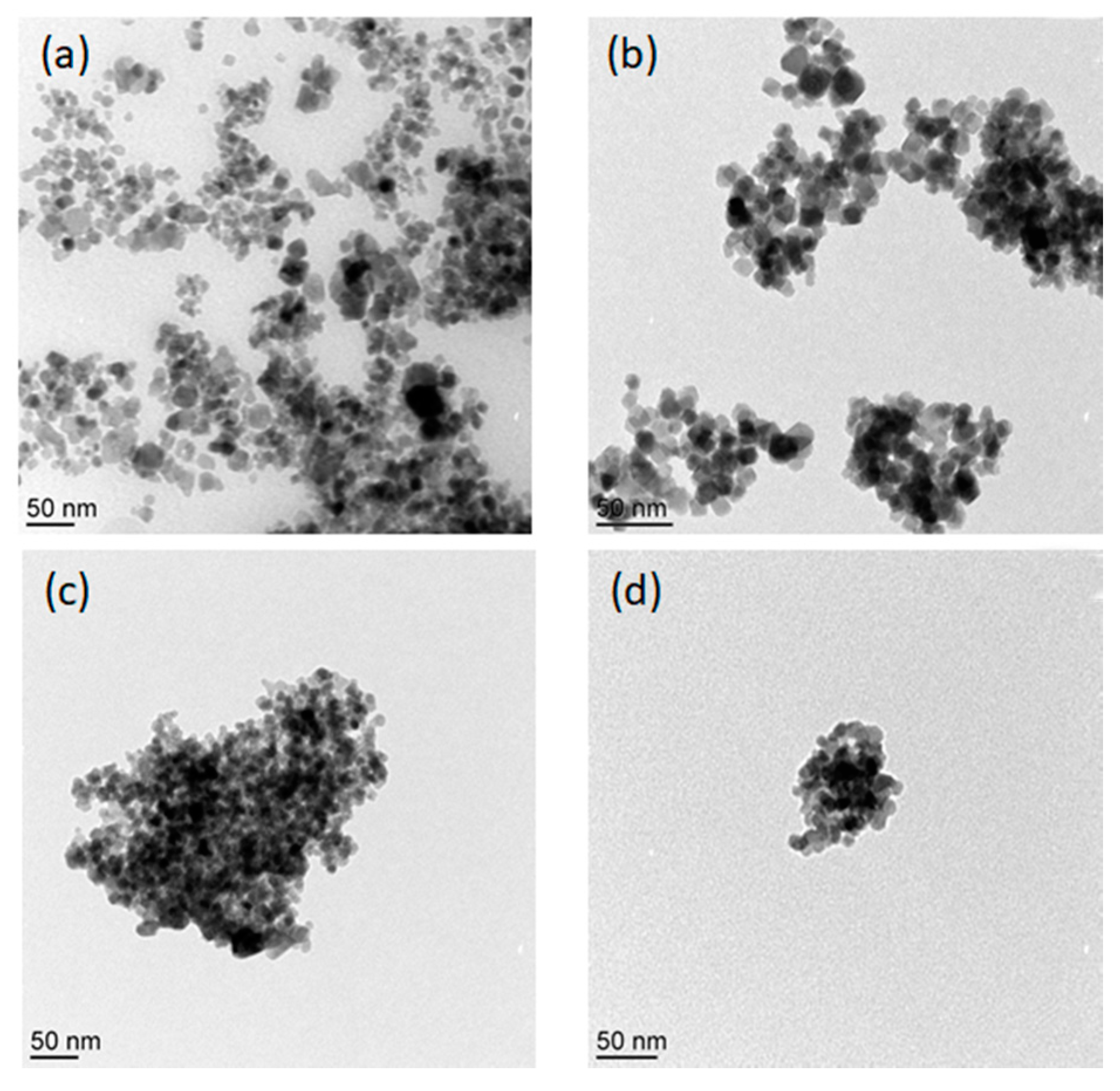
3.5. Size and Surface Morphology
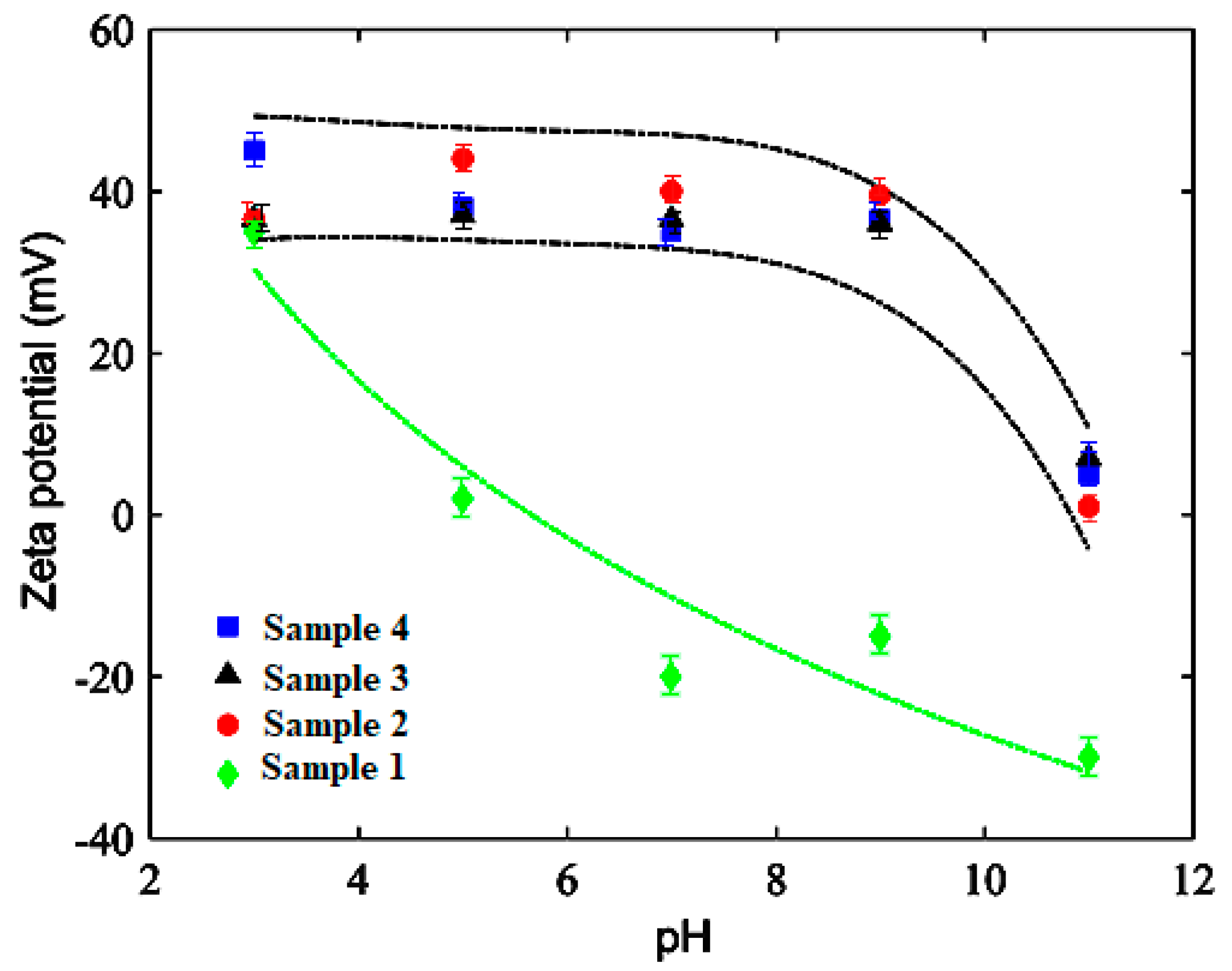
3.6. Electrokinetic Study
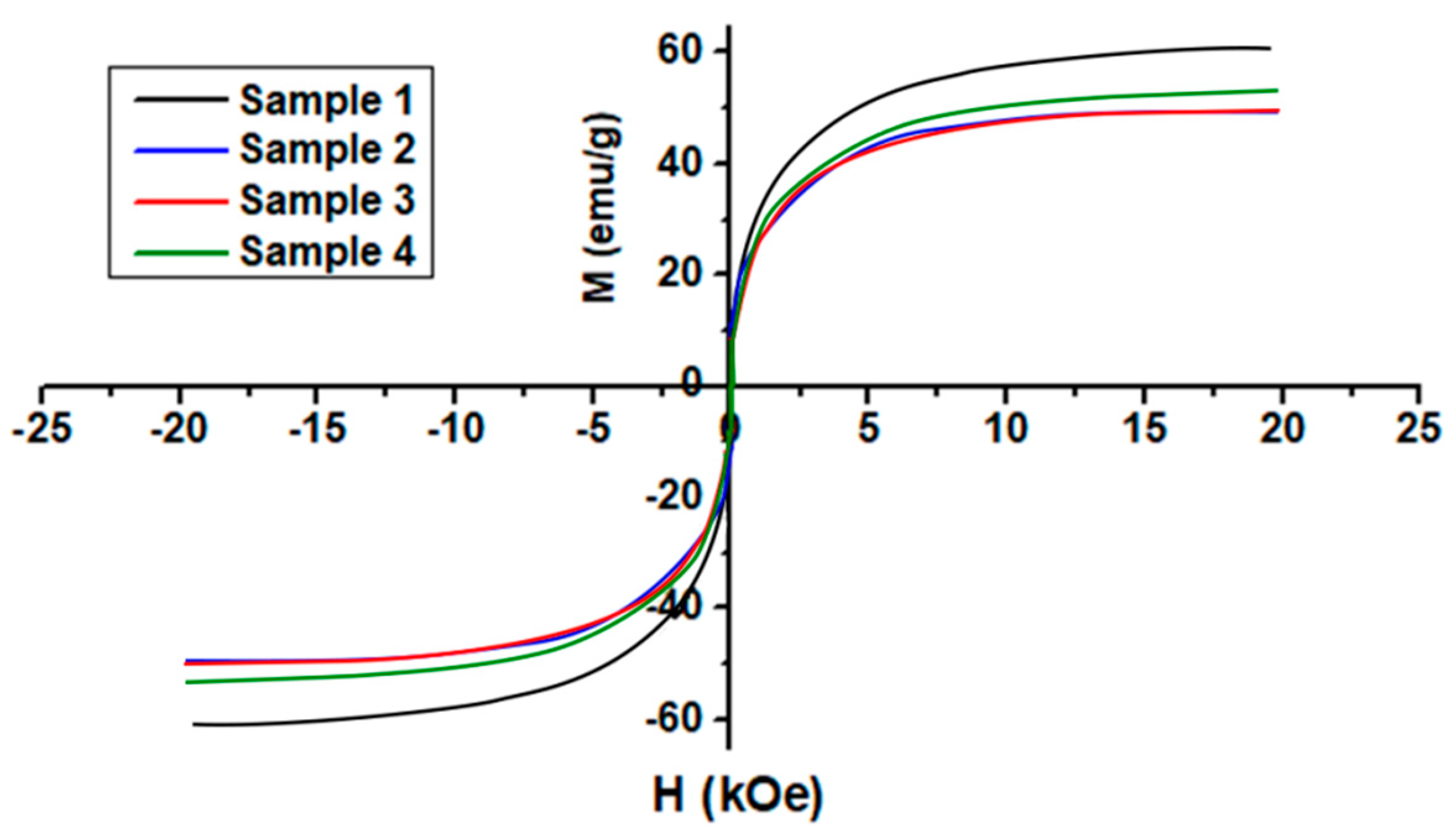
3.7. Magnetic Properties
3.8. MRI Diagnostics via Contrast Enhancement
3.9. Hyperthermia Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sagayaraj, R.; Jegadheeswari, M.; Aravazhi, S.; Chandrasekaran, G.; Dhanalakshmi, A. Structural, Spectroscopic and Magnetic Study of Nanocrystalline Terbium–Nickel Ferrite by Oxalate Co-Precipitation Method. Chem. Afr. 2020, 1–9. [Google Scholar] [CrossRef]
- Jamshaid, T.; Tenorio-Neto, E.T.; Baraket, A.; Lebaz, N.; Elaissari, A.; Sanchis, A.; Salvador, J.P.; Marco, M.-P.; Bausells, J.; Errachid, A.; et al. Development of novel magneto-biosensor for sulfapyridine detection. Biosensors 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarhini, M.; Vega-Chacón, J.; Jafelicci, M.; Zine, N.; Errachid, A.; Fessi, H.; Elaissari, A. Structured Magnetic Core/Silica Internal Shell Layer and Protein Out Layer Shell (BSA@SiO2@SME): Preparation and Characterization. Chem. Afr. 2020, 3, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Badri, W.; Tarhini, M.; Lgourna, Z.; Lebaz, N.; Saadaoui, H.; Zine, N.; Errachid, A.; Elaissari, A. Preparation and Characterization of Glued Corn Flakes-Like Protein-Based Magnetic Particles. Chem. Afr. 2020, 3, 803–811. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile Bioactive Polymers with Potential for Biotechnological Applications. Chem. Afr. 2019, 2, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Pakdel, S.; Akhlaghinia, B.; Mohammadinezhad, A. Fe3O4@Boehmite-NH2-CoII NPs: An Environment Friendly Nanocatalyst for Solvent Free Synthesis of Coumarin Derivatives through Pechmann Condensation Reaction. Chem. Afr. 2019, 2, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Vega-Chacón, J.; Tarhini, M.; Lebaz, N.; Jafelicci, M.; Zine, N.; Errachid, A.; Elaissari, A. Protein-Silica Hybrid Submicron Particles: Preparation and Characterization. Chem. Afr. 2020, 3, 793–801. [Google Scholar] [CrossRef]
- Mosayebi, J.; Kiyasatfar, M.; Laurent, S. Synthesis, Functionalization, and Design of Magnetic Nanoparticles for Theranostic Applications. Adv. Healthc. Mater. 2017, 6, 1700306. [Google Scholar] [CrossRef]
- Malik, A.; Arooj, M.; Butt, T.T.; Zahid, S.; Zahid, F.; Jafar, T.H.; Waquar, S.; Gan, S.H.; Ahmad, S.; Mirza, M.U. In silico and in vivo characterization of cabralealactone, solasodin and salvadorin in a rat model: Potential anti-inflammatory agents. Drug Des. Dev. 2018, 12, 1431–1443. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Rinaldi, C. Magnetization Dynamics and Energy Dissipation of Interacting Magnetic Nanoparticles in Alternating Magnetic Fields with and without a Static Bias Field. J. Phys. Chem. C 2018, 122, 21018–21030. [Google Scholar] [CrossRef]
- Williams, H.M. The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases. Biosci. Horiz. Int. J. Stud. Res. 2017, 10, hzx009. [Google Scholar] [CrossRef] [Green Version]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine 2018, 13, 1221–1238. [Google Scholar] [CrossRef]
- Mushtaq, M.W.; Kanwal, F.; Islam, A.; Ahmed, K.; Haq, Z.-U.; Jamil, T.; Imran, M.; Abbas, S.M.; Huang, Q. Synthesis and characterisation of doxorubicin-loaded functionalised cobalt ferrite nanoparticles and their in vitro anti-tumour activity under an AC-magnetic field. Trop. J. Pharm. Res. 2017, 16, 1663. [Google Scholar] [CrossRef] [Green Version]
- Sha, A.L.; Hassan, R.; Alharbi, A.A.; Alomayri, T.S.; Alamri, H. Magnetic Hyperthermia using Cobalt Ferrite Nanoparticles: The Influence of Particle Size. Int. J. Adv. Technol. 2017, 8, 1000196–1000201. [Google Scholar] [CrossRef]
- Fantechi, E.; Innocenti, C.; Albino, M.; Lottini, E.; Sangregorio, C. Influence of cobalt doping on the hyperthermic efficiency of magnetite nanoparticles. J. Magn. Magn. Mater. 2015, 380, 365–371. [Google Scholar] [CrossRef]
- Sathya, A.; Guardia, P.; Brescia, R.; Silvestri, N.; Pugliese, G.; Nitti, S.; Manna, L.; Pellegrino, T. CoxFe3–xO4Nanocubes for Theranostic Applications: Effect of Cobalt Content and Particle Size. Chem. Mater. 2016, 28, 1769–1780. [Google Scholar] [CrossRef]
- Lu, L.T.; Dung, N.T.; Tung, L.D.; Thanh, C.T.; Quy, O.K.; Chuc, N.V.; Maenosono, S.; Thanh, N.T.K. Synthesis of magnetic cobalt ferrite nanoparticles with controlled morphology, monodispersity and composition: The influence of solvent, surfactant, reductant and synthetic conditions. Nanoscale 2015, 7, 19596–19610. [Google Scholar] [CrossRef] [Green Version]
- Parhizkar, J.; Habibi, M.H.; Mosavian, S.Y. Synthesis and Characterization of Nano CoFe2O4 Prepared by Sol-Gel Auto-Combustion with Ultrasonic Irradiation and Evaluation of Photocatalytic Removal and Degradation Kinetic of Reactive Red 195. Silicon 2018, 11, 1119–1129. [Google Scholar] [CrossRef]
- Mohapatra, S.; Rout, S.R.; Maiti, S.; Maiti, T.K.; Panda, A.B. Monodisperse mesoporous cobalt ferrite nanoparticles: Synthesis and application in targeted delivery of antitumor drugs. J. Mater. Chem. 2011, 21, 9185. [Google Scholar] [CrossRef]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.V.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of Shape and Size of Cobalt Ferrite Nanostructures on Their MRI Contrast and Thermal Activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef] [Green Version]
- Piché, D.; Tavernaro, I.; Fleddermann, J.; Lozano, J.G.; Varambhia, A.; Maguire, M.L.; Koch, M.; Ukai, T.; Rodríguez, A.J.H.; Jones, L.; et al. Targeted T1 Magnetic Resonance Imaging Contrast Enhancement with Extraordinarily Small CoFe2O4 Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 6724–6740. [Google Scholar] [CrossRef] [Green Version]
- Venturini, J.; Zampiva, R.Y.S.; Arcaro, S.; Bergmann, C.P. Sol-gel synthesis of substoichiometric cobalt ferrite (CoFe2O4) spinels: Influence of additives on their stoichiometry and magnetic properties. Ceram. Int. 2018, 44, 12381–12388. [Google Scholar] [CrossRef]
- Bartůněk, V.; Sedmidubský, D.; Huber, Š.; Švecová, M.; Ulbrich, P.; Jankovský, O. Synthesis and Properties of Nanosized Stoichiometric Cobalt Ferrite Spinel. Materials 2018, 11, 1241. [Google Scholar] [CrossRef] [Green Version]
- Mouaziz, H.; Veyret, R.; Theretz, A.; Ginot, F.; Elaissari, A. Aminodextran Containing Magnetite Nanoparticles for Molecular Biology Applications: Preparation and Evaluation. J. Biomed. Nanotechnol. 2009, 5, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, F.; Delgado, Á.; Schneider, E.; Checa Fernández, B.; Iglesias, G. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymers 2017, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Magnetic properties of cobalt ferrite synthesized by hydrothermal method. Int. Nano Lett. 2015, 5, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Tamhankar, P.M.; Kulkarni, A.M.; Watawe, S.C. Functionalization of Cobalt Ferrite Nanoparticles with Alginate Coating for Biocompatible Applications. Mater. Sci. Appl. 2011, 2, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of Aminodextran-Coated Fe3O4 Nanoparticles and Graphene Oxide for Cellular Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.W.; Kanwal, F.; Batool, A.; Jamil, T.; Zia-ul-Haq, M.; Ijaz, B.; Huang, Q.; Ullah, Z. Polymer-coated CoFe2O4 nanoassemblies as biocompatible magnetic nanocarriers for anticancer drug delivery. J. Mater. Sci. 2017, 52, 9282–9293. [Google Scholar] [CrossRef]
- Khizar, S.; Ahmad, N.M.; Saleem, H.; Hamayun, M.A.; Manzoor, S.; Lebaz, N.; Elaissari, A. Magnetic Colloidal Particles in Combinatorial Thin-Film Gradients for Magnetic Resonance Imaging and Hyperthermia. Adv. Polym. Technol. 2020, 2020, 7163985. [Google Scholar] [CrossRef]
- Masoudi, A.; Hosseini, H.R.M.; Shokrgozar, M.A.; Ahmadi, R.; Oghabian, M.A. The effect of poly(ethylene glycol) coating on colloidal stability of superparamagnetic iron oxide nanoparticles as potential MRI contrast agent. Int. J. Pharm. 2012, 433, 129–141. [Google Scholar] [CrossRef]
- Perales-Pérez, O.; Cedeño-Mattei, Y. Optimizing Processing Conditions to Produce Cobalt Ferrite Nanoparticles of Desired Size and Magnetic Properties. In Magnetic Spinels–Synthesis, Properties and Applications; InTech: London, UK, 2017. [Google Scholar]
- Palomec-Garfias, A.F.; Jardim, K.V.; Sousa, M.H.; Márquez-Beltrán, C. Influence of polyelectrolyte chains on surface charge and magnetization of iron oxide nanostructures. Colloids Surf. Phys. Eng. Asp. 2018, 549, 13–24. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Grodzinski, P.; Cai, W.; Liu, C.H. Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics. ACS Nano 2018, 12, 2106–2121. [Google Scholar] [CrossRef]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef]
- Unterweger, H.; Janko, C.; Schwarz, M.; Dézsi, L.; Urbanics, R.; Matuszak, J.; Őrfi, E.; Fülöp, T.; Bäuerle, T.; Szebeni, J.; et al. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: A biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int. J. Nanomed. 2017, 12, 5223–5238. [Google Scholar] [CrossRef] [Green Version]
- Gan, S.; Lin, Y.; Feng, Y.; Shui, L.; Li, H.; Zhou, G. Magnetic polymeric nanoassemblies for magnetic resonance imaging-combined cancer theranostics. Int. J. Nanomed. 2018, 13, 4263–4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamvakidis, K.; Mourdikoudis, S.; Makridis, A.; Paulidou, E.; Angelakeris, M.; Dendrinou-Samara, C. Magnetic hyperthermia efficiency and MRI contrast sensitivity of colloidal soft/hard ferrite nanoclusters. J. Colloid Interface Sci. 2018, 511, 101–109. [Google Scholar] [CrossRef]
- Banerjee, A.; Blasiak, B.; Pasquier, E.; Tomanek, B.; Trudel, S. Synthesis, characterization, and evaluation of PEGylated first-row transition metal ferrite nanoparticles as T2contrast agents for high-field MRI. RSC Adv. 2017, 7, 38125–38134. [Google Scholar] [CrossRef] [Green Version]
- Nam, P.H.; Lu, L.T.; Linh, P.H.; Manh, D.H.; Tam, L.T.T.; Phuc, N.X.; Phong, P.T.; Lee, I.-J. Polymer-coated cobalt ferrite nanoparticles: Synthesis, characterization, and toxicity for hyperthermia applications. New J. Chem. 2018, 42, 14530–14541. [Google Scholar] [CrossRef]
- Dönmez, Ç.E.D.; Manna, P.K.; Nickel, R.; Aktürk, S.; van Lierop, J. Comparative Heating Efficiency of Cobalt-, Manganese-, and Nickel-Ferrite Nanoparticles for a Hyperthermia Agent in Biomedicines. ACS Appl. Mater. Interfaces 2019, 11, 6858–6866. [Google Scholar] [CrossRef]
- Mdlalose, W.B.; Mokhosi, S.R.; Dlamini, S.; Moyo, T.; Singh, M. Effect of chitosan coating on the structural and magnetic properties of MnFe2O4 and Mn0.5Co0.5Fe2O4 nanoparticles. AIP Adv. 2018, 8, 056726. [Google Scholar] [CrossRef] [Green Version]
- Torres, T.E.; Lima, E.; Calatayud, M.P.; Sanz, B.; Ibarra, A.; Fernández-Pacheco, R.; Mayoral, A.; Marquina, C.; Ibarra, M.R.; Goya, G.F. The relevance of Brownian relaxation as power absorption mechanism in Magnetic Hyperthermia. Sci. Rep. 2019, 9, 3992. [Google Scholar] [CrossRef] [Green Version]
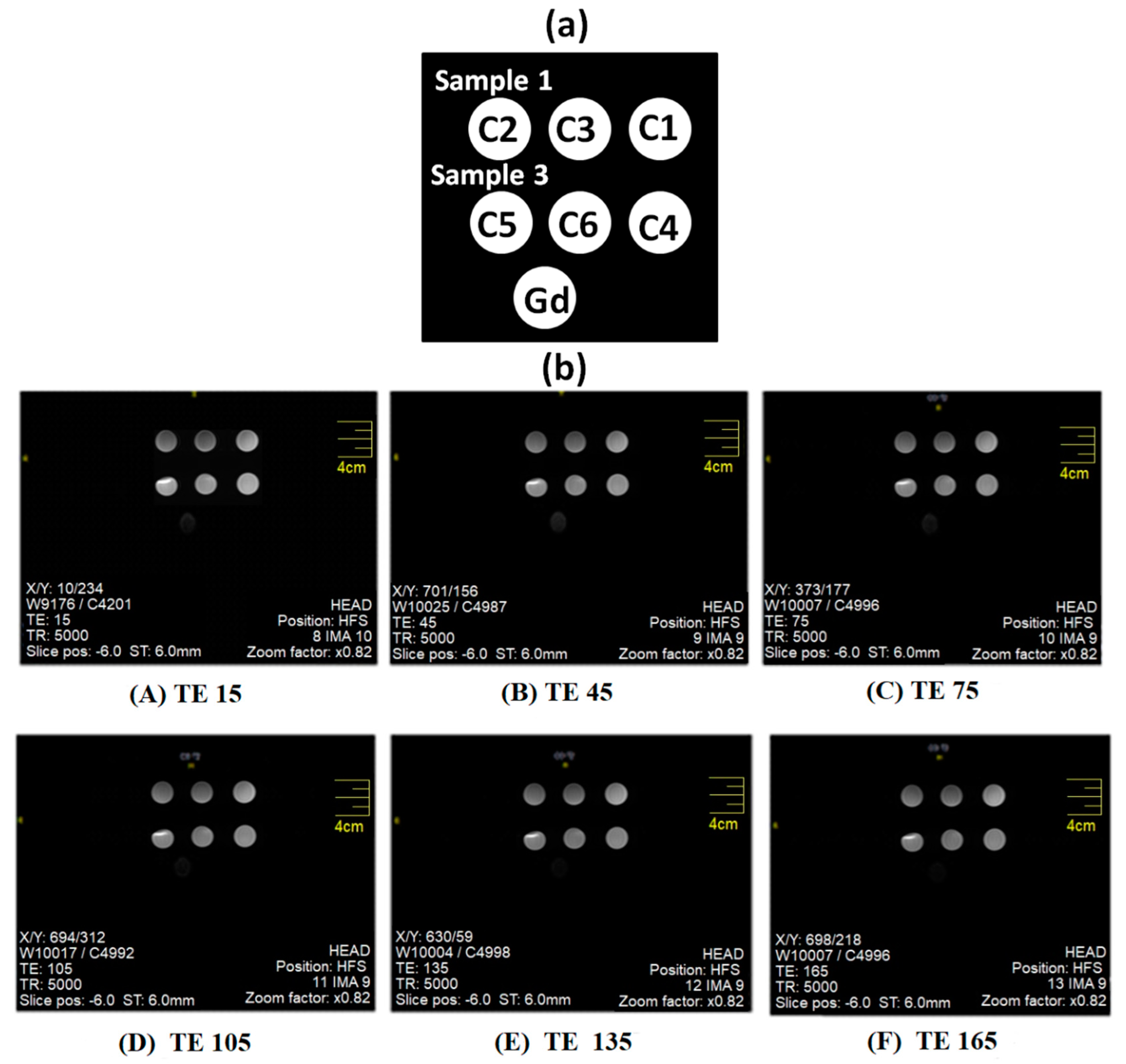


| Sr. No. | Sample Code | Ratio of AMD/Cobalt Ferrite Nanoparticles (mg/g) |
|---|---|---|
| 1 | Sample 1 | - |
| 2 | Sample 2 | 20 mg/g |
| 3 | Sample 3 | 640 mg/g |
| 4 | Sample 4 | 1280 mg/g |
| Sr. No. | Sample Code | Description | Amount in H2O (g/10 mL) |
|---|---|---|---|
| 1 | Sample 1 | Solid contents = 100% powder Size: 303 nm Zeta Potential: −13.4 mV | 0.1073 (C1) 0.2201 (C2) 0.2042 (C3) |
| 2 | Sample 3 | Solid contents: 0.40% (W/V) Size: 270 nmZeta Potential: +39.4 mV Aminodextran (AMD) coated positively charged colloids | 0.1254 (C4) 0.2214 (C5) 0.2053 (C6) |
| 3 | Gd | Magnevist® Bayer | 0.1331 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khizar, S.; Ahmad, N.M.; Ahmed, N.; Manzoor, S.; Hamayun, M.A.; Naseer, N.; Tenório, M.K.L.; Lebaz, N.; Elaissari, A. Aminodextran Coated CoFe2O4 Nanoparticles for Combined Magnetic Resonance Imaging and Hyperthermia. Nanomaterials 2020, 10, 2182. https://doi.org/10.3390/nano10112182
Khizar S, Ahmad NM, Ahmed N, Manzoor S, Hamayun MA, Naseer N, Tenório MKL, Lebaz N, Elaissari A. Aminodextran Coated CoFe2O4 Nanoparticles for Combined Magnetic Resonance Imaging and Hyperthermia. Nanomaterials. 2020; 10(11):2182. https://doi.org/10.3390/nano10112182
Chicago/Turabian StyleKhizar, Sumera, Nasir M. Ahmad, Naveed Ahmed, Sadia Manzoor, Muhammad A. Hamayun, Nauman Naseer, Michele K. L. Tenório, Noureddine Lebaz, and Abdelhamid Elaissari. 2020. "Aminodextran Coated CoFe2O4 Nanoparticles for Combined Magnetic Resonance Imaging and Hyperthermia" Nanomaterials 10, no. 11: 2182. https://doi.org/10.3390/nano10112182
APA StyleKhizar, S., Ahmad, N. M., Ahmed, N., Manzoor, S., Hamayun, M. A., Naseer, N., Tenório, M. K. L., Lebaz, N., & Elaissari, A. (2020). Aminodextran Coated CoFe2O4 Nanoparticles for Combined Magnetic Resonance Imaging and Hyperthermia. Nanomaterials, 10(11), 2182. https://doi.org/10.3390/nano10112182






