Atomic Layer Deposition of Mixed-Layered Aurivillius Phase on TiO2 Nanotubes: Synthesis, Characterization and Photoelectrocatalytic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis Procedure
2.2. Characterizations
2.3. Photoelectrochemical Measurement
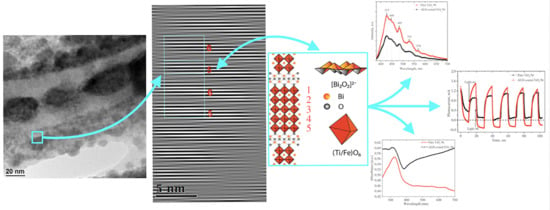
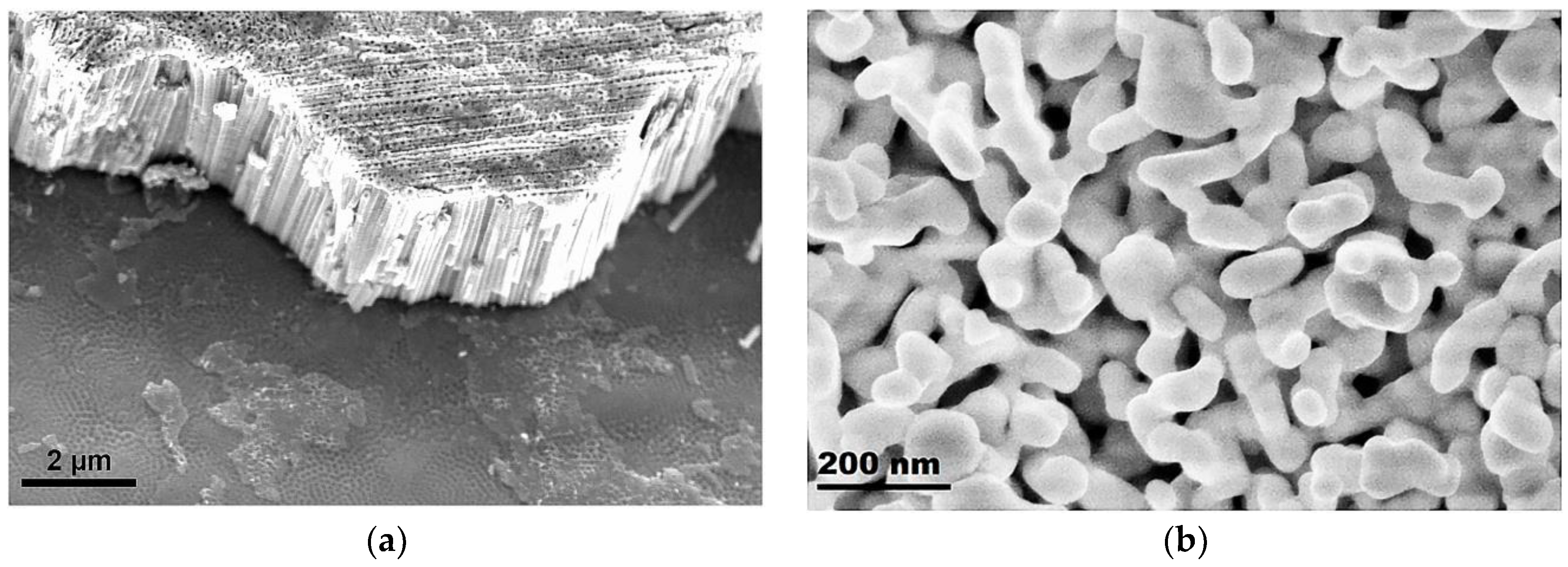
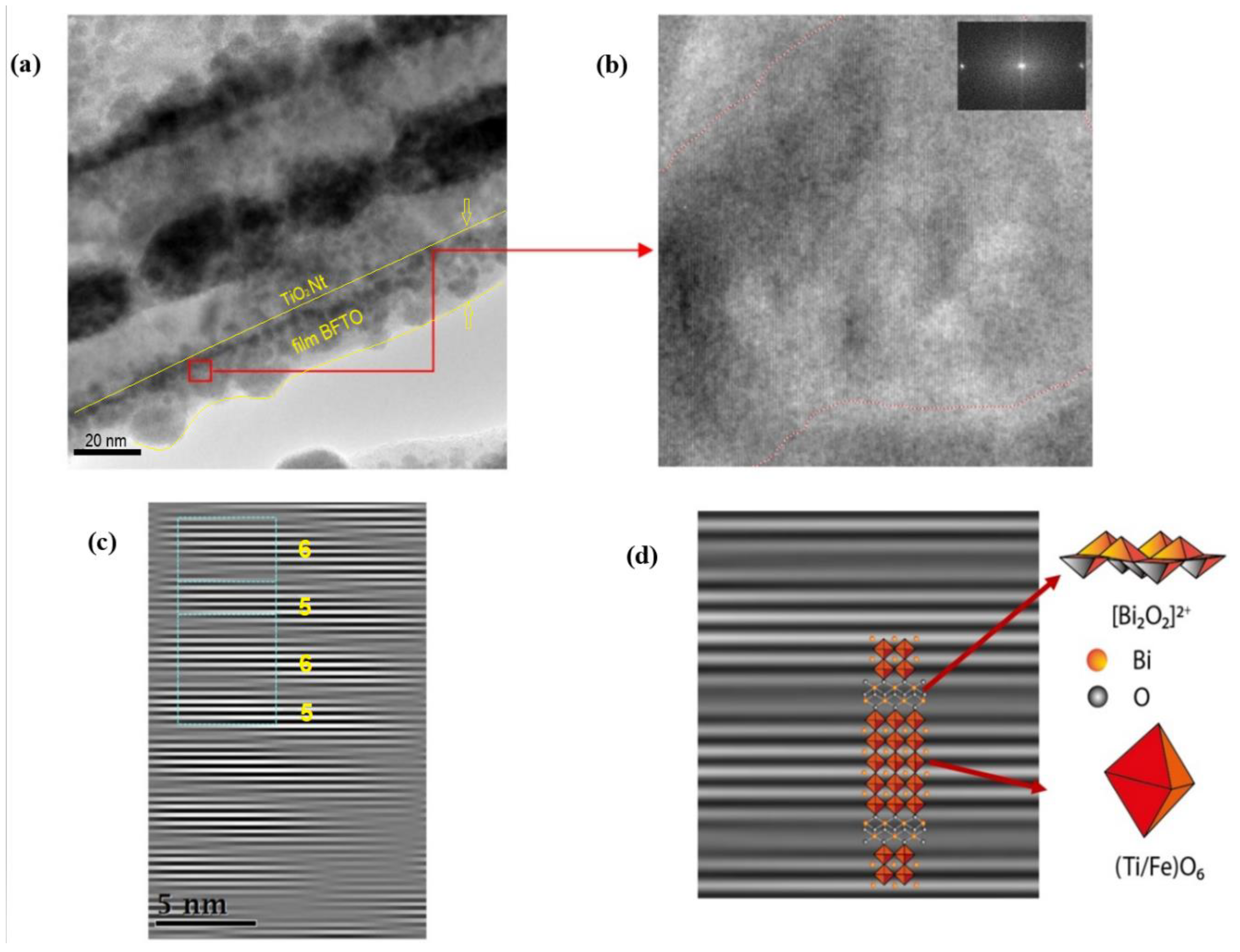
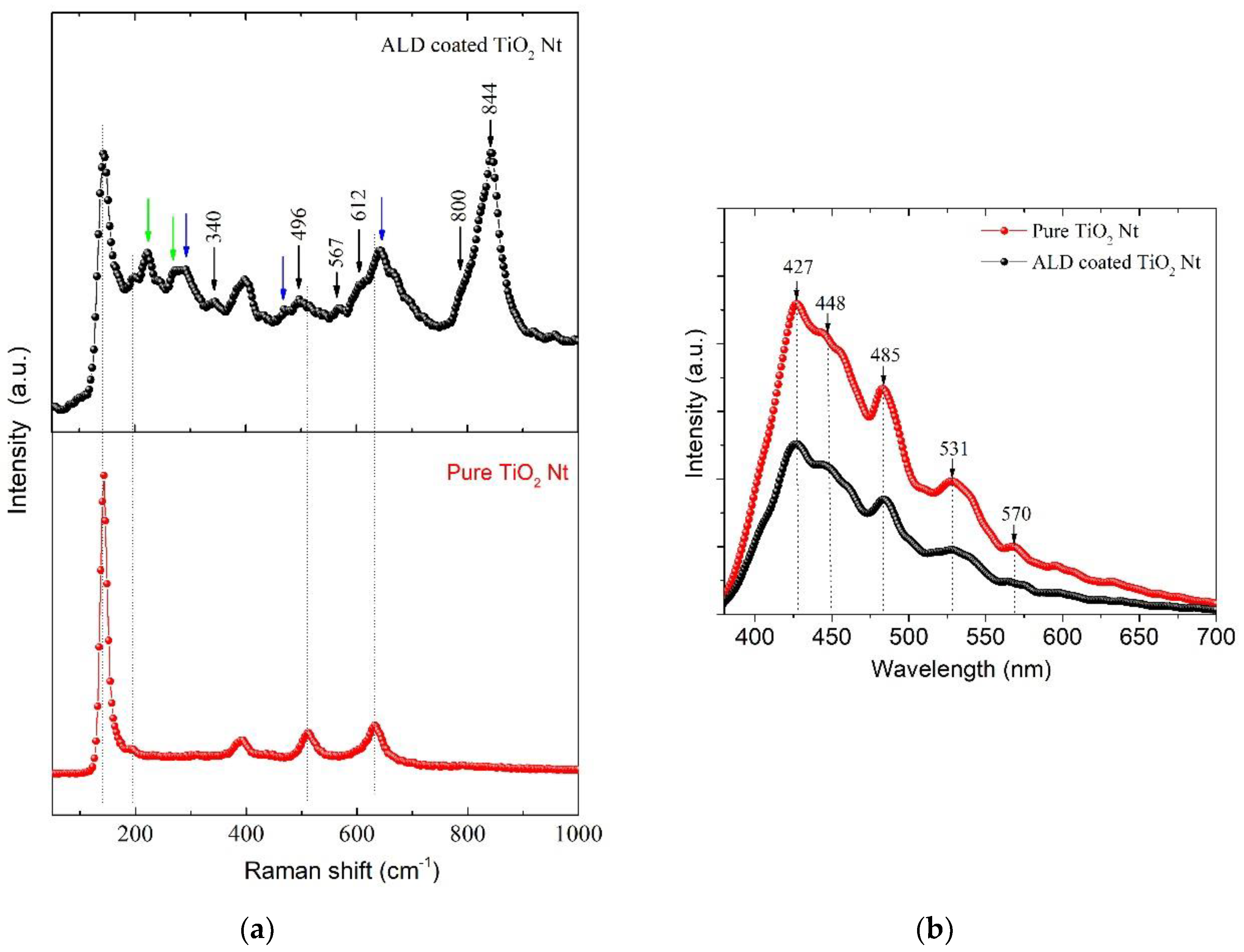
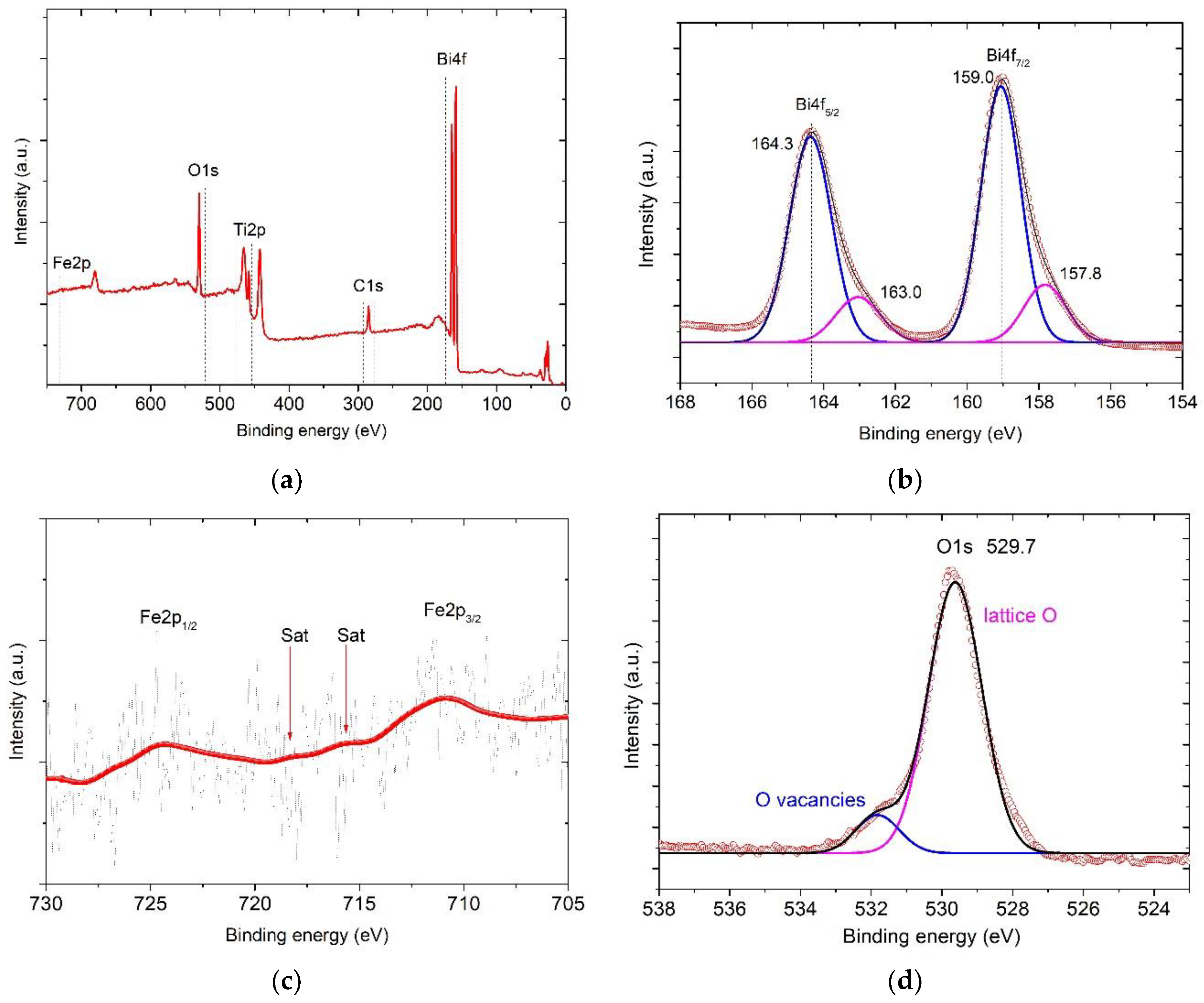
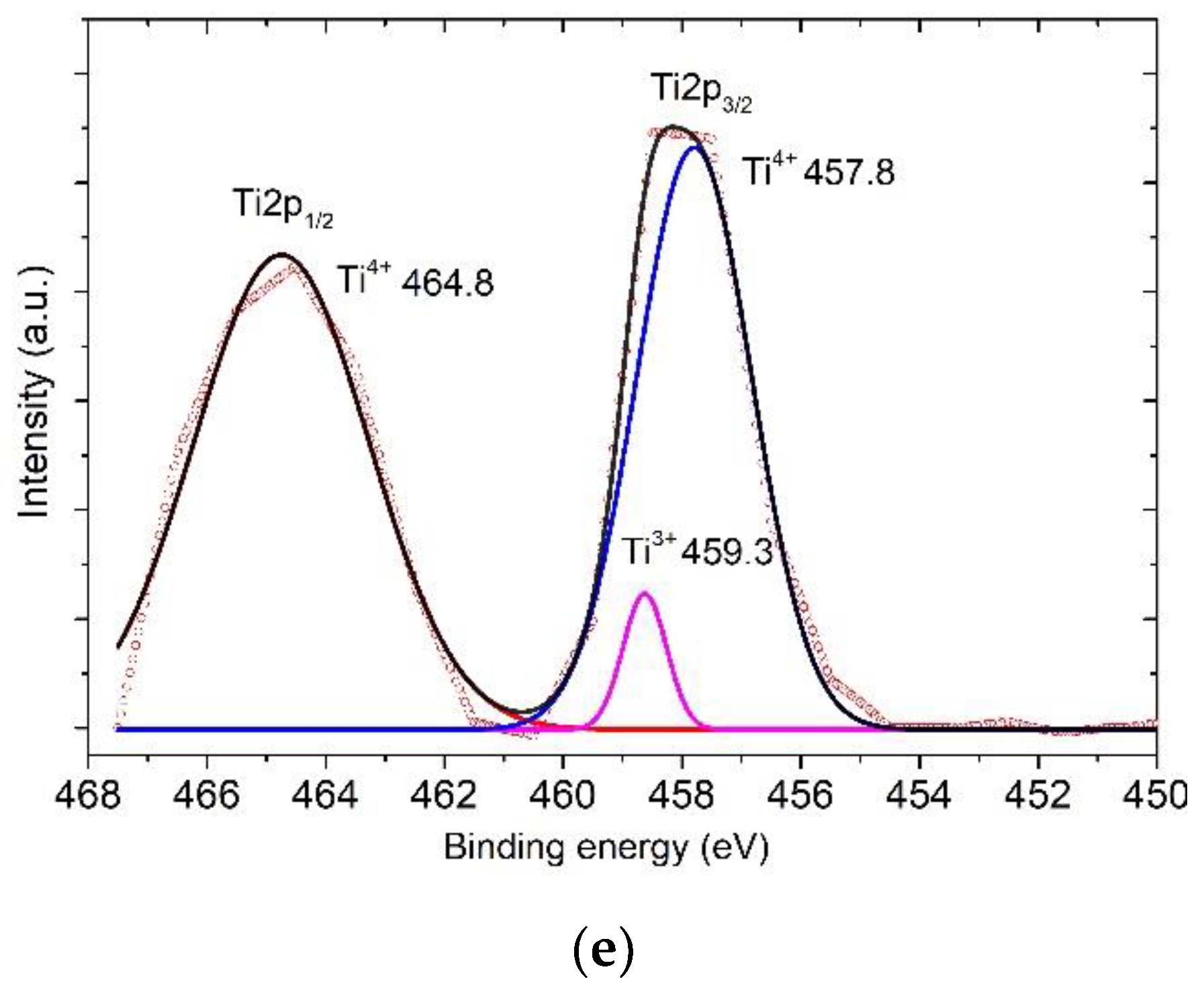
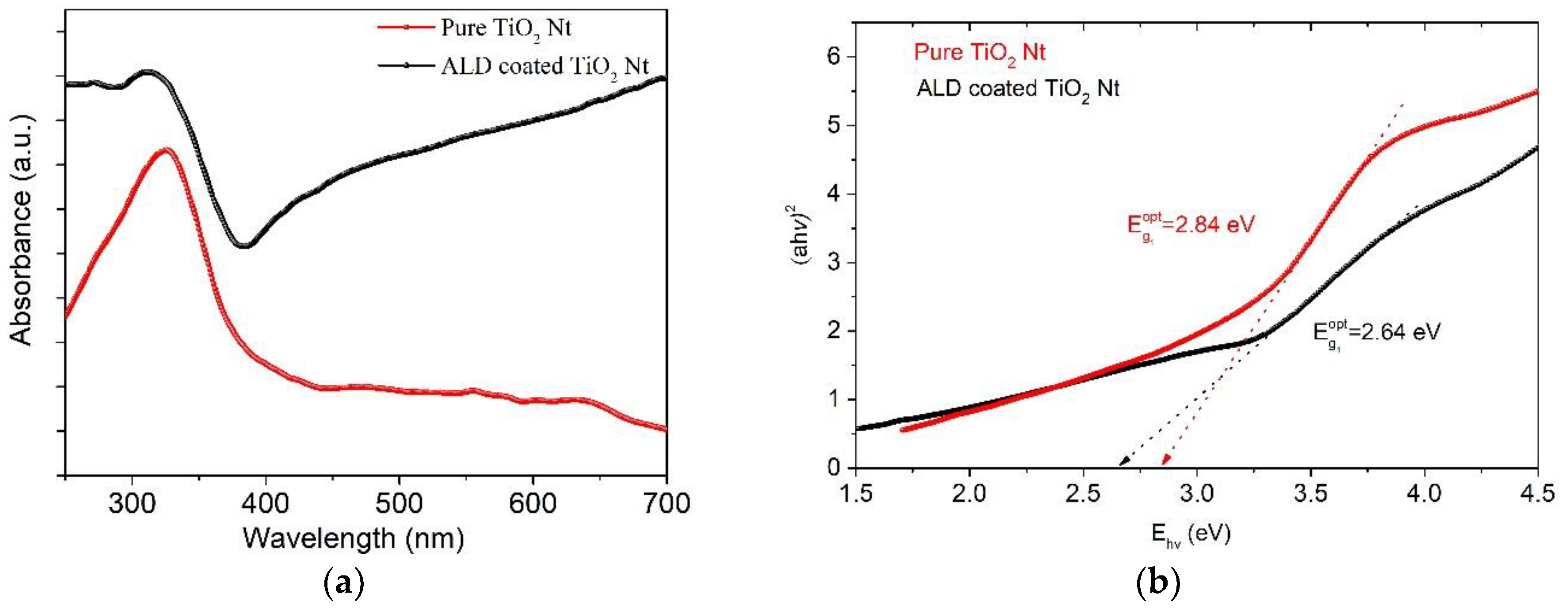
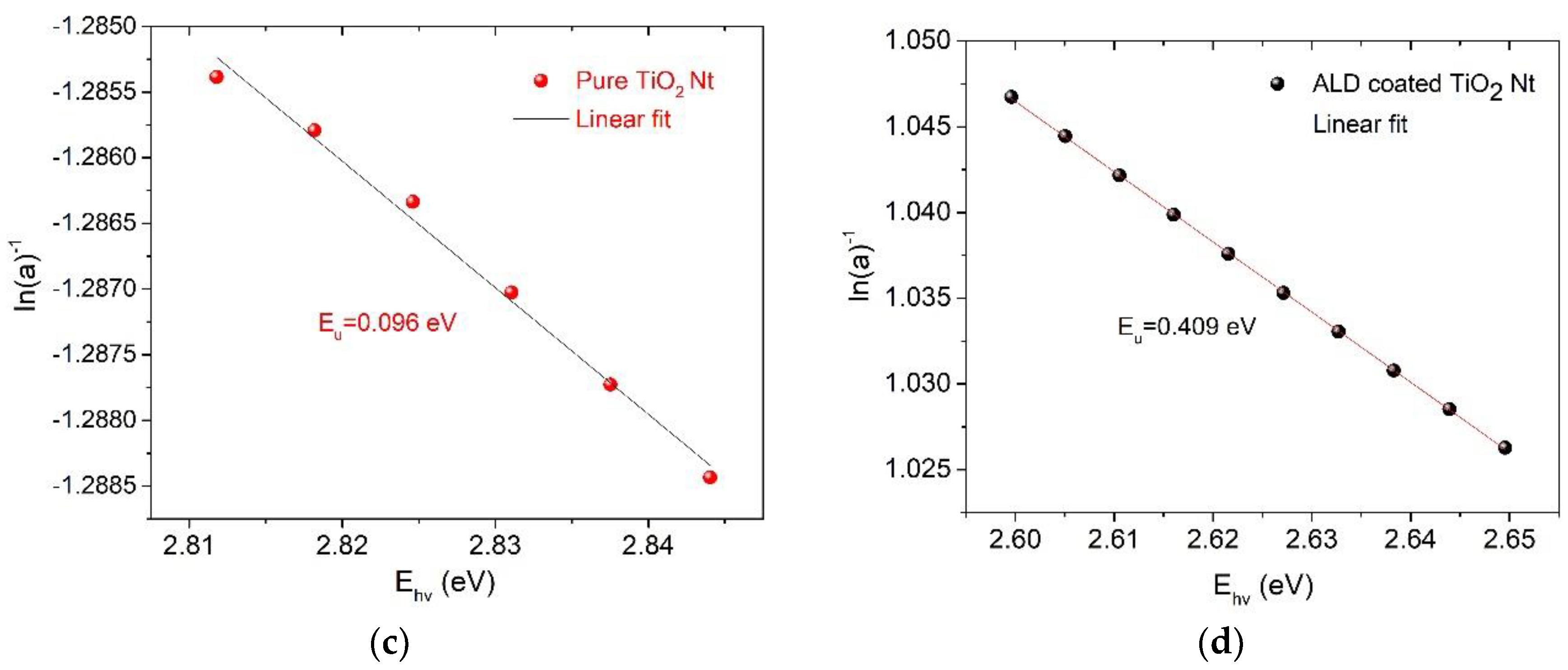
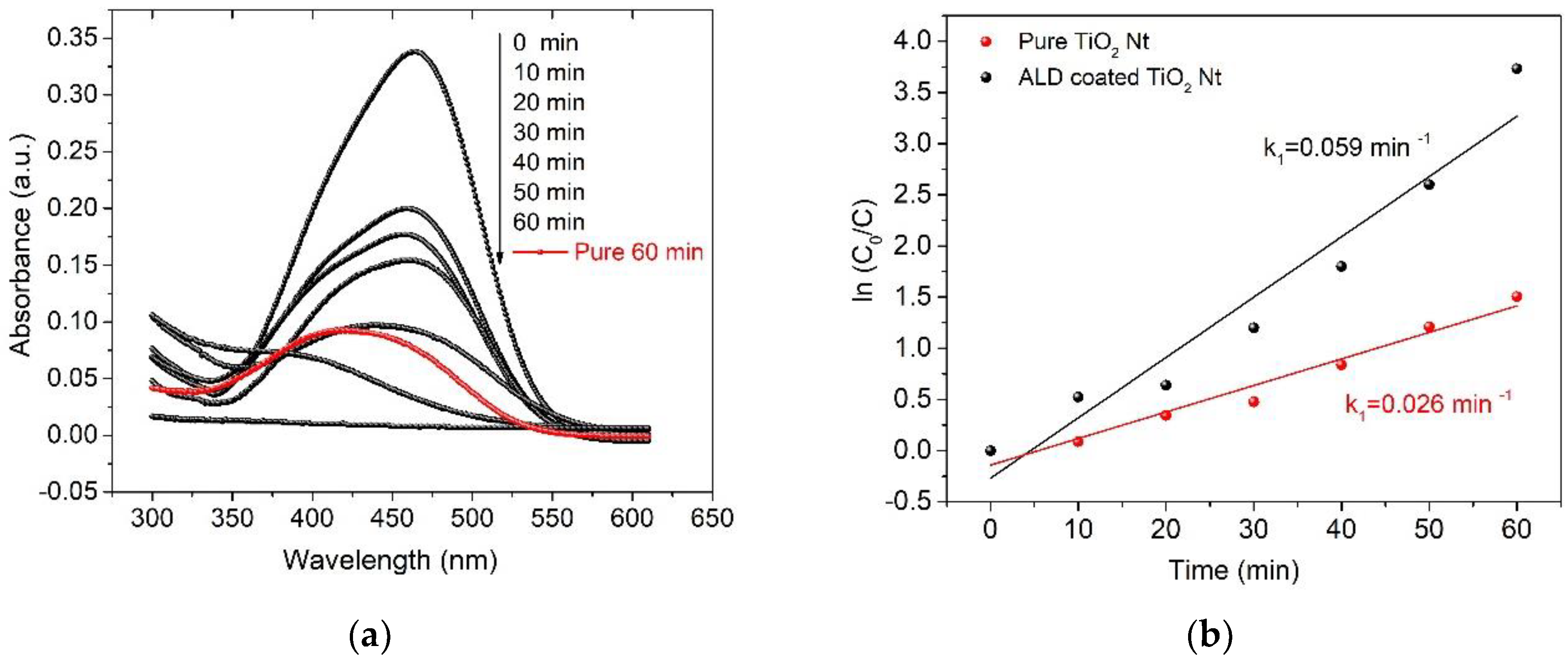
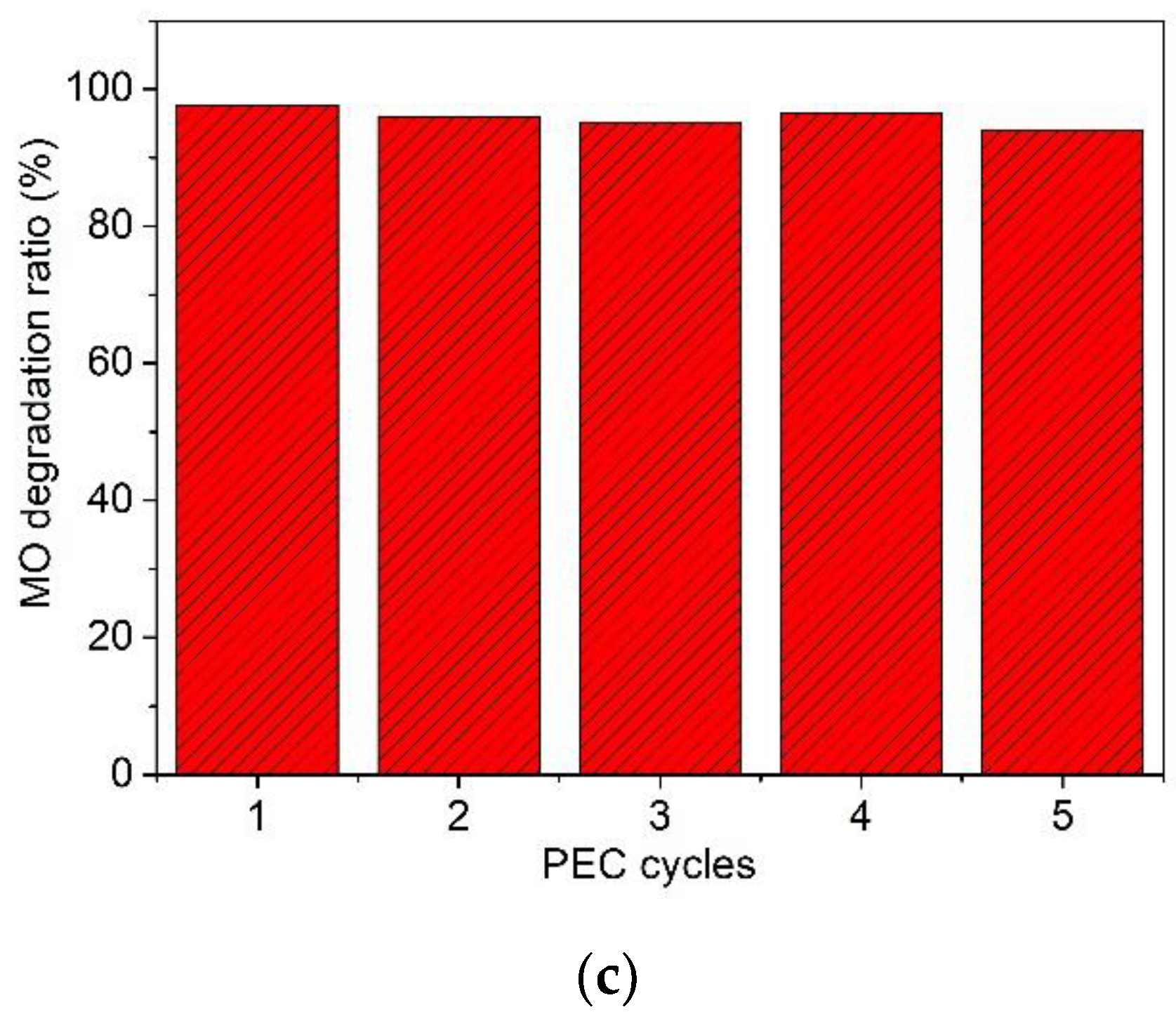
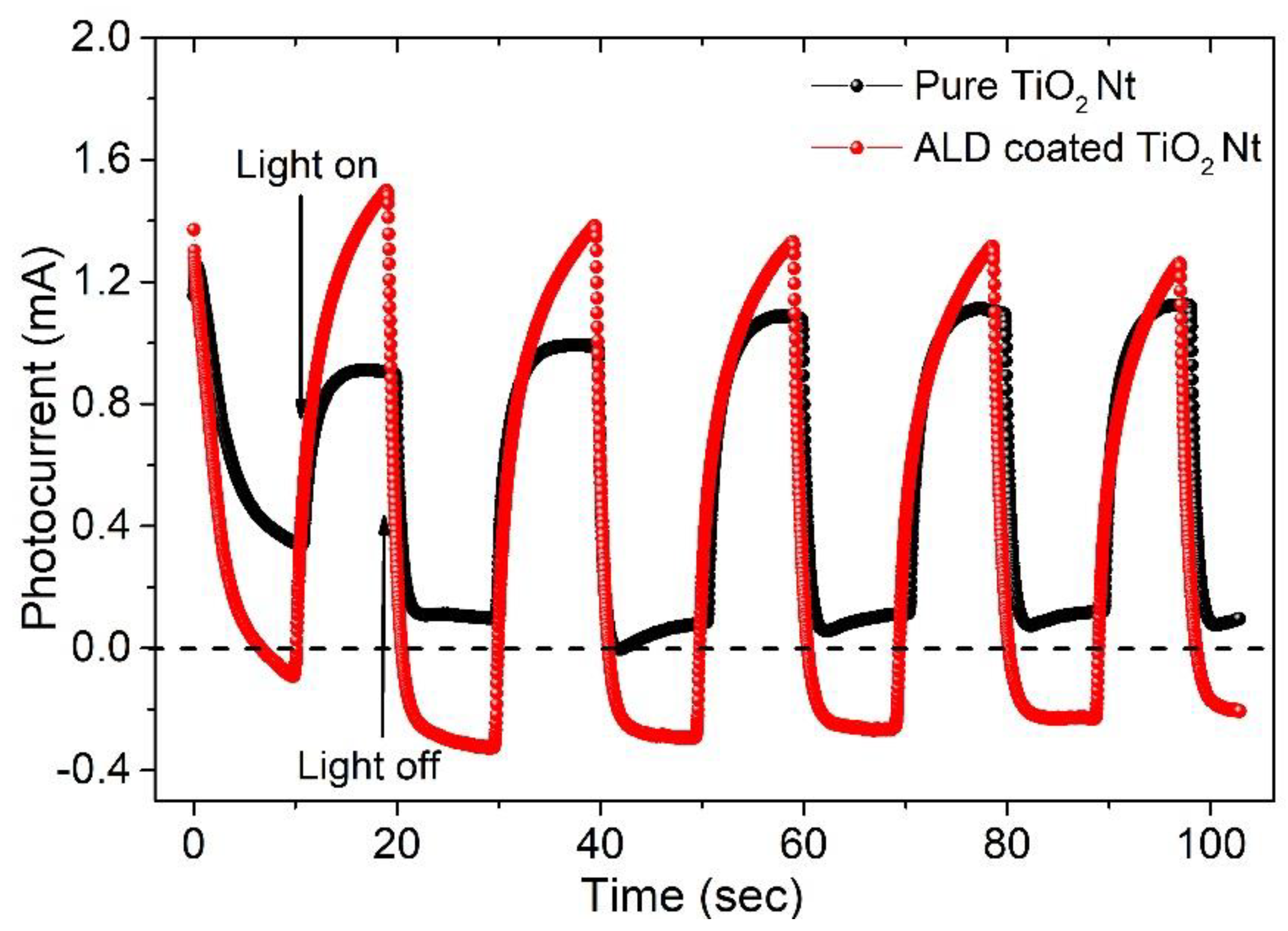
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krzhizhanovskaya, M.; Filatov, S.; Gusarov, V.; Paufler, P.; Bubnova, R.; Morozov, M.; Meyer, D.C. Aurivillius phases in the Bi4Ti3O12/ BiFeO3 system: Thermal behaviour and crystal structure. Z. Anorg. Allg. Chem. 2005, 631, 1603–1608. [Google Scholar] [CrossRef]
- Lomanova, N.A.; Morozov, M.I.; Ugolkov, V.L.; Gusarov, V.V. Properties of Aurivillius phases in the Bi4Ti3O12-BiFeO3 system. Inorg. Mater. 2006, 42, 189–195. [Google Scholar] [CrossRef]
- Lomanova, N.A.; Semenov, V.G.; Panchuk, V.V.; Gusarov, V.V. Structural changes in the homologous series of the Aurivillius phases Bin+1Fen-3Ti3O3n+3. J. Alloys Compd. 2012, 528, 103–108. [Google Scholar] [CrossRef]
- Deepak, N.; Carolan, P.; Keeney, L.; Pemble, M.E.; Whatmore, R.W. Tunable nanoscale structural disorder in Aurivillius phase, n = 3 Bi4Ti3O12 thin films and their role in the transformation to n = 4, Bi5Ti3FeO15 phase. J. Mater. Chem. C 2015, 3, 5727–5732. [Google Scholar] [CrossRef]
- García-Guaderrama, M.; Fuentes, L.; Márquez-Lucero, A.; Blanco, O. Structural stability and cation disorder in Aurivillius phases. Mater. Res. Bull. 2012, 47, 3850–3854. [Google Scholar] [CrossRef]
- Wu, M.; Tian, Z.; Yuan, S.; Huang, Z. Magnetic and optical properties of the Aurivillius phase Bi5Ti3FeO15. Mater. Lett. 2012, 68, 190–192. [Google Scholar] [CrossRef]
- Yan, S.; Feng, Z.; Ma, Z.; Zhang, Y.; Ye, W. Multiferroic properties of Bi5Ti3FeO15 ceramics prepared by hot-pressing methods. Mater. Lett. 2018, 227, 247–249. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Xu, H.; Zhou, L.; Shang, M.; Zhang, L. Bi5FeTi3O15 hierarchical microflowers: Hydrothermal synthesis, growth mechanism, and associated visible-light-driven photocatalysis. J. Phys. Chem. C 2008, 112, 17835–17843. [Google Scholar] [CrossRef]
- Naresh, G.; Malik, J.; Meena, V.; Mandal, T.K. PH-Mediated Collective and Selective Solar Photocatalysis by a Series of Layered Aurivillius Perovskites. ACS Omega 2018, 3, 11104–11116. [Google Scholar] [CrossRef]
- Abbas, W.A.; Abdullah, I.H.; Ali, B.A.; Ahmed, N.; Mohamed, A.M.; Rezk, M.Y.; Ismail, N.; Mohamed, M.A.; Allam, N.K. Recent advances in the use of TiO2 nanotube powder in biological, environmental, and energy applications. Nanoscale Adv. 2019, 1, 2801–2816. [Google Scholar] [CrossRef]
- Dvorak, F.; Zazpe, R.; Krbal, M.; Sopha, H.; Prikryl, J.; Ng, S.; Hromadko, L.; Bures, F.; Macak, J.M. One-dimensional anodic TiO2 nanotubes coated by atomic layer deposition: Towards advanced applications. Appl. Mater. Today 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Orudzhev, F.F.; Aliev, Z.M.; Gasanova, F.G.; Isaev, A.B.; Shabanov, N.S. Photoelectrocatalytic Oxidation of Phenol on TiO2 Nanotubes under Oxygen Pressure. Russ. J. Electrochem. 2015, 51, 1247–1253. [Google Scholar] [CrossRef]
- Sobola, D.; Ramazanov, S.; Konečný, M.; Orudzhev, F.; Kaspar, P.; Papež, N.; Knápek, A.; Potoček, M. Complementary SEM-AFM of Swelling Bi-Fe-O Film on HOPG Substrate. Materials 2020, 13, 2402. [Google Scholar] [CrossRef] [PubMed]
- Ramazanov, S.; Sobola, D.; Orudzhev, F.; Knápek, A.; Polčák, J.; Potoček, M.; Dallaev, R. Surface Modification and Enhancement of Ferromagnetism in BiFeO3 Nanofilms Deposited on HOPG. Nanomaterials 2020, 10, 1990. [Google Scholar] [CrossRef] [PubMed]
- Leu, C.C.; Lin, T.J.; Chen, S.Y.; Hu, C.T. Effects of bismuth oxide buffer layer on BiFeO3 thin film. J. Am. Ceram. Soc. 2015, 98, 724–731. [Google Scholar] [CrossRef]
- Akbashev, A.R.; Chen, G.; Spanier, J.E. A facile route for producing single-crystalline epitaxial perovskite oxide thin films. Nano Lett. 2014, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.D.; Li, Y.W.; Li, W.M.; Zhang, J.Z.; Hu, Z.G.; Chu, J.H. Growth of Bi2O3 ultrathin films by atomic layer deposition. J. Phys. Chem. C 2012, 116, 3449–3456. [Google Scholar] [CrossRef]
- Aurivillius, B. Mixed Bismuth Oxides with Layer lattices: I. The Structure Type of CaBi2Nb2O9. Ark. Kemi Band I 1949, 54, 463–480. [Google Scholar]
- Lu, C.D.; Chang, L.S.; Lu, Y.F.; Lu, F.H. The growth of interfacial compounds between titanium dioxide and bismuth oxide. Ceram. Int. 2009, 35, 2699–2704. [Google Scholar] [CrossRef]
- Morozov, M.I.; Gusarov, V.V. Synthesis of Am-1Bi2MmO3m+3 compounds in the Bi4Ti3O12-BiFeO3 system. Inorg. Mater. 2002, 38, 723–729. [Google Scholar] [CrossRef]
- Sun, S.; Wang, G.; Huang, Y.; Wang, J.; Peng, R.; Lu, Y. Structural transformation and multiferroic properties in Gd-doped Bi7Fe3Ti3O21 ceramics. RSC Adv. 2014, 4, 30440–30446. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Z.; Wang, G.; Geng, X.; Xiao, Z.; Sun, Z.; Sun, Z.; Peng, R.; Lu, Y. Nanoscale Structural Modulation and Lowerature Magnetic Response in Mixed-layer Aurivillius-type Oxides. Sci. Rep. 2018, 8, 871. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yan, H.; Wang, G.; Wang, J.; Peng, R.; Fu, Z.; Zhai, X.; Mao, X.; Chen, X.; Lu, Y. Room-temperature multiferroic responses arising from 1D phase modulation in correlated Aurivillius-type layer structures. J. Phys. D Appl. Phys. 2016, 49, 125005. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Newnham, R.E. Bismuth titanate solid solutions. Mater. Res. Bull. 1972, 7, 1025–1034. [Google Scholar] [CrossRef]
- Kikuchi, T. Stability of layered bismuth compounds in relation to the structural mismatch. Mater. Res. Bull. 1979, 14, 1561–1569. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Achary, S.N.; Patwe, S.J.; Krishna, P.S.R.; Shinde, A.B.; Tyagi, A.K. Cation disorder and structural studies on Bi4-xNd xTi3O12 (0.0 ≤ × ≤ 2.0). Pramana J. Phys. 2008, 71, 935–940. [Google Scholar] [CrossRef]
- Zhang, S.T.; Lu, M.H.; Wu, D.; Chen, Y.F.; Ming, N.B. Larger polarization and weak ferromagnetism in quenched BiFeO3 ceramics with a distorted rhombohedral crystal structure. Appl. Phys. Lett. 2005, 87, 262907. [Google Scholar] [CrossRef]
- Venkata Ramana, E.; Prasad, N.V.; Figueiras, F.; Lajaunie, L.; Arenal, R.; Otero-Irurueta, G.; Valente, M.A. The growth and improved magnetoelectric response of strain-modified Aurivillius SrBi4.25La0.75Ti4FeO18 thin films. Dalt. Trans. 2019, 48, 13224–13241. [Google Scholar] [CrossRef]
- Giorgi, L.; Dikonimos, T.; Giorgi, R.; Buonocore, F.; Faggio, G.; Messina, G.; Lisi, N. Electrochemical synthesis of self-organized TiO2 crystalline nanotubes without annealing. Nanotechnology 2018, 29, 095604. [Google Scholar] [CrossRef]
- Du, Y.L.; Chen, G.; Zhang, M.S. Grain size effects in Bi4Ti3O12 nanocrystals investigated by Raman spectroscopy. Solid State Commun. 2004, 132, 175–179. [Google Scholar] [CrossRef]
- Rodríguez Aranda, M.D.C.; Rodríguez-Vázquez, Á.G.; Salazar-Kuri, U.; Mendoza, M.E.; Navarro-Contreras, H.R. Raman effect in multiferroic Bi5Fe1+xTi3-xO15 solid solutions: A temperature study. J. Appl. Phys. 2018, 123, 084101. [Google Scholar] [CrossRef]
- Rodrigues, H.O.; Pires Junior, G.F.M.; Sales, A.J.M.; Silva, P.M.O.; Costa, B.F.O.; Alcantara, P.; Moreira, S.G.C.; Sombra, A.S.B. BiFeO3 ceramic matrix with Bi2O3 or PbO added: Mössbauer, Raman and dielectric spectroscopy studies. Phys. B Condens. Matter 2011, 406, 2532–2539. [Google Scholar] [CrossRef]
- Quintana-Cilleruelo, J.Á.; Veerapandiyan, V.K.; Deluca, M.; Algueró, M.; Castro, A. Mechanosynthesis of the whole Y1-xBixMn1-xFexO3 perovskite system: Structural characterization and study of phase transitions. Materials 2019, 12, 1515. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Hu, J.; Li, X.; Li, M. Cysteine modified anatase TiO2 hollow microspheres with enhanced visible-light-driven photocatalytic activity. J. Mol. Catal. A Chem. 2012, 356, 78–84. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T.; Liu, S. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electron-transfer channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Coy, E.; Viter, R.; Nowaczyk, G.; Jancelewicz, M.; Baleviciute, I.; Załeski, K.; Jurga, S. Study on Structural, Mechanical, and Optical Properties of Al2O3-TiO2 Nanolaminates Prepared by Atomic Layer Deposition. J. Phys. Chem. C 2015, 119, 20591–20599. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-light-driven N-F-codoped TiO2 photocatalysts. 2. Optical characterization, photocatalysis, and potential application to air purification. Chem. Mater. 2005, 17, 2596–2602. [Google Scholar] [CrossRef]
- Zhu, A.; Zhao, Q.; Li, X.; Shi, Y. BiFeO3/TiO2 Nanotube Arrays Composite Electrode: Construction, Characterization, and Enhanced Photoelectrochemical Properties. ACS Appl. Mater. Interfaces 2014, 6, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cai, L.; Zhang, Y.; Wei, Y. Bi5+, Bi(3−x)+, and Oxygen Vacancy Induced BiOClxI1−x Solid Solution toward Promoting Visible-Light Driven Photocatalytic Activity. Chem. Eur. J. 2018, 24, 7434–7444. [Google Scholar] [CrossRef]
- Yuan, B.; Yang, J.; Song, D.P.; Zuo, X.Z.; Tang, X.W.; Zhu, X.B.; Dai, J.M.; Song, W.H.; Sun, Y.P. Structural, magnetic, and dielectric studies of the Aurivillius compounds SrBi5Ti4MnO18 and SrBi5Ti4Mn0.5Co0.5O18. J. Appl. Phys. 2015, 117, 023907. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, S.; Bai, J.; He, E.; Hui, Z.; Zhang, P.; Song, D.; Song, W.; Yang, J.; Zhu, X.; et al. Enhanced multiferroicity and narrow band gap in B-site Co-doped Aurivillius Bi5FeTi3O15. Ceram. Int. 2019, 45, 137–143. [Google Scholar] [CrossRef]
- Wanger, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979; 190p. [Google Scholar]
- Song, D.; Zuo, X.; Yuan, B.; Tang, X.; Song, W.; Yang, J.; Zhu, X.; Sun, Y. Enhanced remnant polarization in ferroelectric Bi6Fe2Ti3O18 thin films. CrystEngComm 2015, 17, 1609–1614. [Google Scholar] [CrossRef]
- Liu, X.; Xu, L.; Huang, Y.; Qin, C.; Qin, L.; Seo, H.J. Improved photochemical properties of Aurivillius Bi5Ti3FeO15 with partial substitution of Ti4+ with Fe3+. Ceram. Int. 2017, 43, 12372–12380. [Google Scholar] [CrossRef]
- Sanjinés, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Lévy, F. Electronic structure of anatase TiO2 oxide. J. Appl. Phys. 1994, 75, 2945. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; Sullivan, J.L.; Saied, S.O. Surface characterisation of plasma-nitrided titanium: An XPS study. Appl. Surf. Sci. 1995, 84, 357–371. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Di Mo, S.; Ching, W.Y. Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Phys. Rev. B 1995, 51, 13023–13032. [Google Scholar]
- Li, X.; Ju, Z.; Li, F.; Huang, Y.; Xie, Y.; Fu, Z.; Knize, R.J.; Lu, Y. Visible light responsive Bi7Fe3Ti3O21 nanoshelf photocatalysts with ferroelectricity and ferromagnetism. J. Mater. Chem. A 2014, 2, 13366–13372. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Ilican, S.; Caglar, Y.; Caglar, M.; Kundakci, M.; Ates, A. Photovoltaic solar cell properties of CdxZn1-xO films prepared by sol-gel method. Int. J. Hydrog. Energy 2009, 34, 5201–5207. [Google Scholar] [CrossRef]
- Bai, W.; Xu, W.F.; Wu, J.; Zhu, J.Y.; Chen, G.; Yang, J.; Lin, T.; Meng, X.J.; Tang, X.D.; Chu, J.H. Investigations on electrical, magnetic and optical behaviors of five-layered Aurivillius Bi6Ti3Fe2O18 polycrystalline films. Thin Solid Films 2012, 525, 195–199. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen defect dependent variation of band gap, Urbach energy and luminescence property of anatase, anatase-rutile mixed phase and of rutile phases of TiO2 nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 364–371. [Google Scholar] [CrossRef]
- Kim, S.; Ko, K.C.; Lee, J.Y.; Illas, F. Single oxygen vacancies of (TiO2)35 as a prototype reduced nanoparticle: Implication for photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 23755–23762. [Google Scholar] [CrossRef]
- Ouyang, B.; Zhang, K.; Yang, Y. Photocurrent Polarity Controlled by Light Wavelength in Self-Powered ZnO Nanowires/SnS Photodetector System. IScience 2018, 1, 16–23. [Google Scholar] [CrossRef]
- Morgan, B.J.; Watson, G.W. Intrinsic n-type defect formation in TiO2: A comparison of rutile and anatase from GGA+U calculations. J. Phys. Chem. C 2010, 114, 2321–2328. [Google Scholar] [CrossRef]
- Takahashi, M.; Noguchi, Y.; Miyayama, M. Electrical conduction mechanism in Bi4Ti3O12 single crystal. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2002, 41, 7053–7056. [Google Scholar] [CrossRef]
- Long, M.; Cai, W.; Cai, J.; Zhou, B.; Chai, X.; Wu, Y. Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation. J. Phys. Chem. B 2006, 110, 20211–20216. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orudzhev, F.; Ramazanov, S.; Sobola, D.; Isaev, A.; Wang, C.; Magomedova, A.; Kadiev, M.; Kaviyarasu, K. Atomic Layer Deposition of Mixed-Layered Aurivillius Phase on TiO2 Nanotubes: Synthesis, Characterization and Photoelectrocatalytic Properties. Nanomaterials 2020, 10, 2183. https://doi.org/10.3390/nano10112183
Orudzhev F, Ramazanov S, Sobola D, Isaev A, Wang C, Magomedova A, Kadiev M, Kaviyarasu K. Atomic Layer Deposition of Mixed-Layered Aurivillius Phase on TiO2 Nanotubes: Synthesis, Characterization and Photoelectrocatalytic Properties. Nanomaterials. 2020; 10(11):2183. https://doi.org/10.3390/nano10112183
Chicago/Turabian StyleOrudzhev, Farid, Shikhgasan Ramazanov, Dinara Sobola, Abdulgalim Isaev, Chuanyi Wang, Asiyat Magomedova, Makhmud Kadiev, and Kasinathan Kaviyarasu. 2020. "Atomic Layer Deposition of Mixed-Layered Aurivillius Phase on TiO2 Nanotubes: Synthesis, Characterization and Photoelectrocatalytic Properties" Nanomaterials 10, no. 11: 2183. https://doi.org/10.3390/nano10112183
APA StyleOrudzhev, F., Ramazanov, S., Sobola, D., Isaev, A., Wang, C., Magomedova, A., Kadiev, M., & Kaviyarasu, K. (2020). Atomic Layer Deposition of Mixed-Layered Aurivillius Phase on TiO2 Nanotubes: Synthesis, Characterization and Photoelectrocatalytic Properties. Nanomaterials, 10(11), 2183. https://doi.org/10.3390/nano10112183