Catalytic Performances of Cu/MCM-22 Zeolites with Different Cu Loadings in NH3-SCR
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Reaction Measurements
3. Results and Discussions
3.1. Textural and Structural Properties
3.2. Acidity of Catalysts
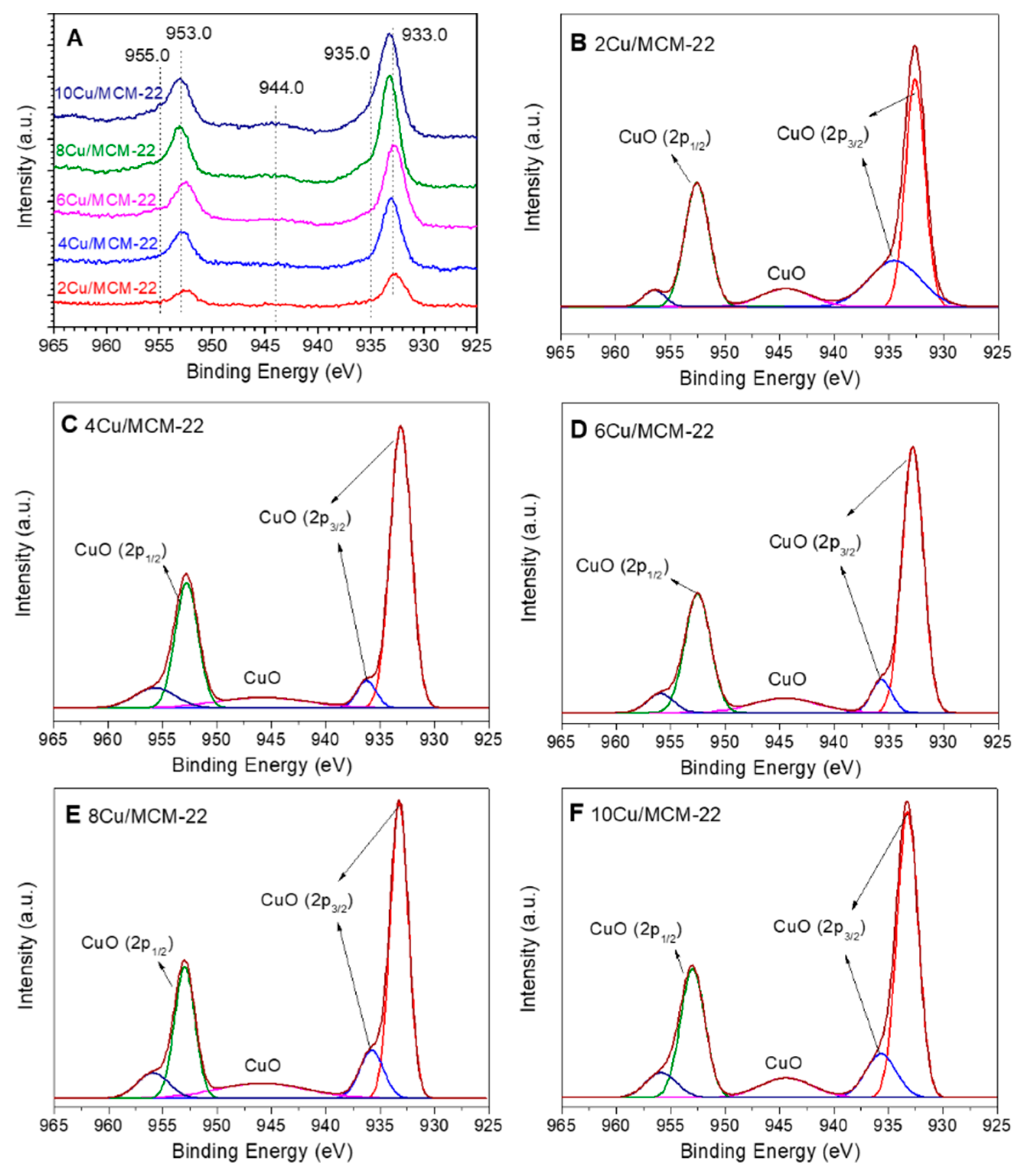
3.3. Characterization of Cu Species
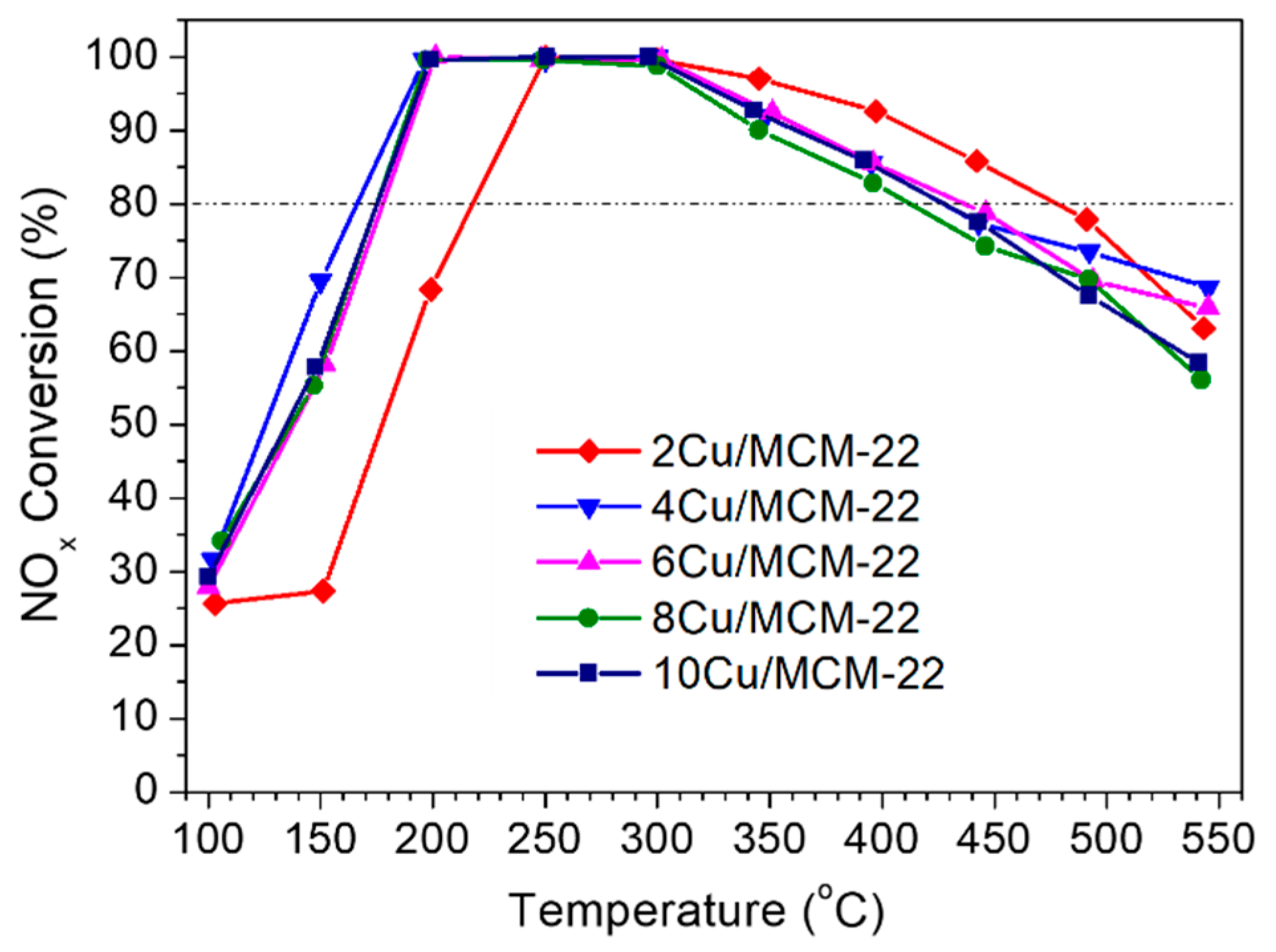
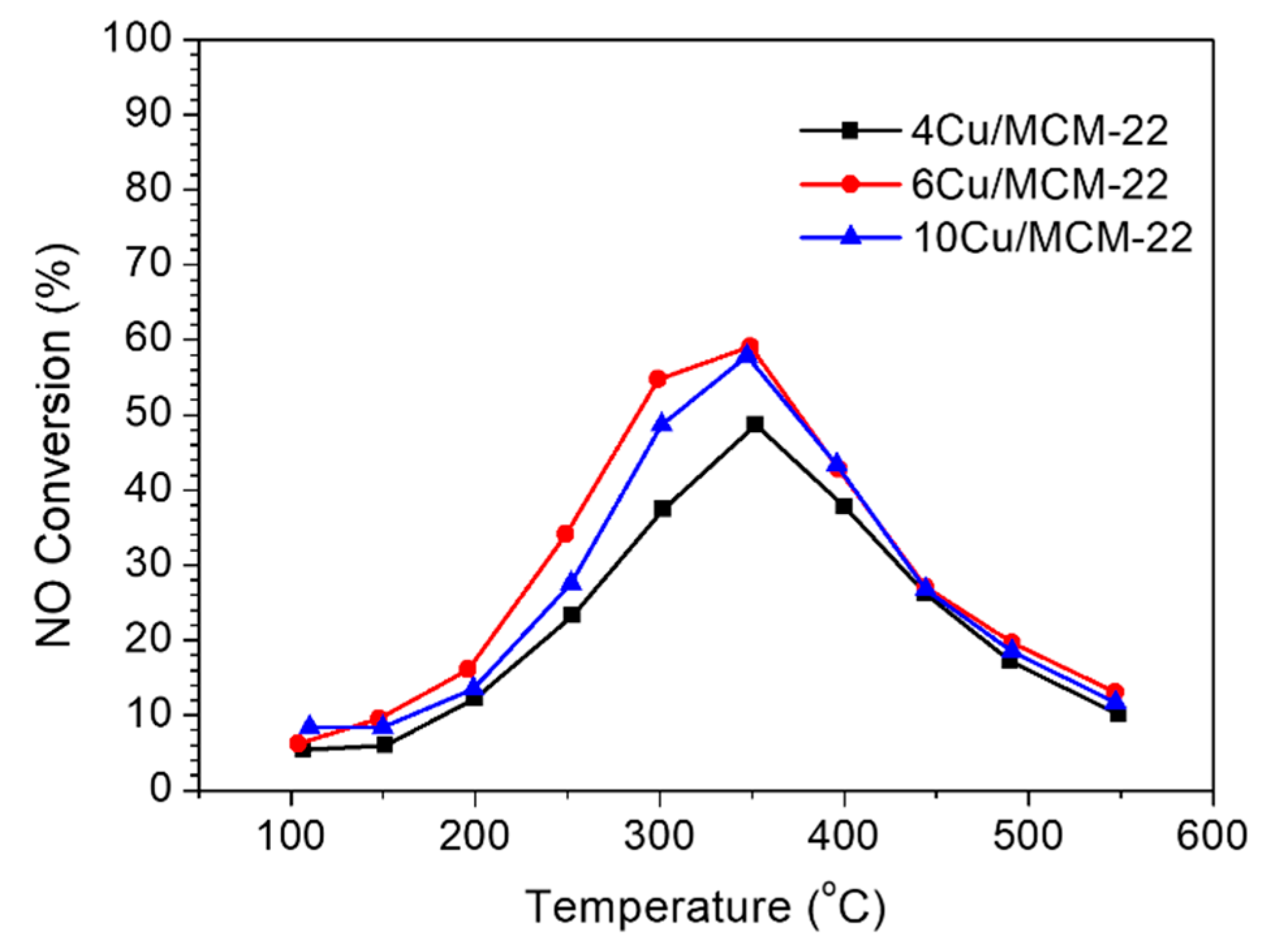
3.4. NH3-SCR Catalytic Performance
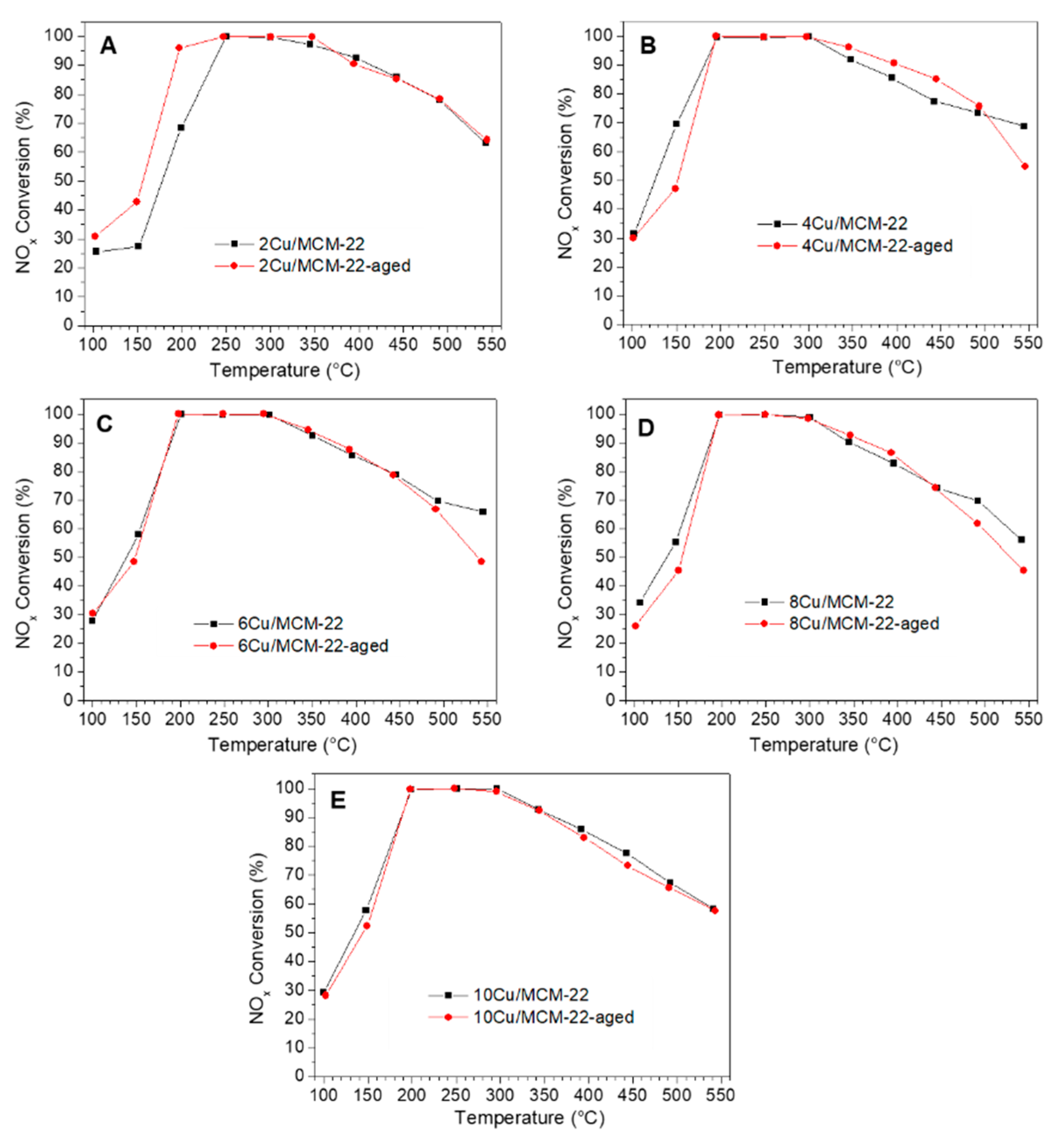
3.5. Hydrothermal Stability of xCu/MCM-22 in NH3-SCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective Transformation of Various Nitrogen-Containing Exhaust Gases toward N2 over Zeolite Catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, R.; Xiong, Y.; Chang, D.; Zhao, H.; Zhang, W.; Zheng, W.; Chen, J.; Wu, X. The Application of Biomass-Based Catalytic Materials in the Synthesis of Cyclic Carbonates from CO2 and Epoxides. Molecules 2020, 25, 3627. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Dou, R.; Wu, Y.; Zhang, R.; Wang, L.; Wang, Y.; Gong, Z.; Chen, J.; Wu, X. From Lignin Waste to Efficient Catalyst: Illuminating the Impact of Lignin Structure on Catalytic Activity of Cycloaddition Reaction. ACS Sustain. Chem. Eng. 2019, 7, 16585–16594. [Google Scholar] [CrossRef]
- Jiang, T.; Lobo, R.F. On the Mechanism of Ammonia SCR over Cu- and Fe-Containing Zeolite Catalysts. In Luminescent and Photoactive Transition Metal Complexes as Biomolecular Probes and Cellular Reagents; Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 155–178. [Google Scholar]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Q.; Zhang, F.; Mueller, U.; Feyen, M.; Dai, D.; Maurer, S.; McGuire, R.; Moini, A.; Parvulescu, A.-N.; et al. Recent advances in the preparation of zeolites for the selective catalytic reduction of NOx in diesel engines. React. Chem. Eng. 2019, 4, 975–985. [Google Scholar] [CrossRef]
- Usui, T.; Liu, Z.; Igarashi, H.; Sasaki, Y.; Shiramata, Y.; Yamada, H.; Ohara, K.; Kusamoto, T.; Wakihara, T. Identifying the Factors Governing the Early-Stage Degradation of Cu-Chabazite Zeolite for NH3-SCR. ACS Omega 2019, 4, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.; Kim, H.; Hong, S.B. Nature of active sites in Cu-LTA NH3-SCR catalysts: A comparative study with Cu-SSZ-13. Appl. Catal. B Environ. 2019, 245, 513–521. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H. Synthesis and evaluation of Cu/SAPO-34 catalysts for NH3-SCR 2: Solid-state ion exchange and one-pot synthesis. Appl. Catal. B Environ. 2015, 162, 501–514. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Moliner, M.; Franch, C.; Kustov, A.; Corma, A. Rational direct synthesis methodology of very active and hydrothermally stable Cu-SAPO-34 molecular sieves for the SCR of NOx. Appl. Catal. B Environ. 2012, 127, 273–280. [Google Scholar] [CrossRef]
- Wang, A.; Arora, P.; Bernin, D.; Kumar, A.; Kamasamudram, K.; Olsson, L. Investigation of the robust hydrothermal stability of Cu/LTA for NH3-SCR reaction. Appl. Catal. B Environ. 2019, 246, 242–253. [Google Scholar] [CrossRef]
- Zhu, N.; Shan, W.; Shan, Y.; Du, J.; Lian, Z.; Zhang, Y.; He, H. Effects of alkali and alkaline earth metals on Cu-SSZ-39 catalyst for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2020, 388, 124250. [Google Scholar] [CrossRef]
- Shan, Y.; Shan, W.; Shi, X.; Du, J.; Yu, Y.; He, H. A comparative study of the activity and hydrothermal stability of Al-rich Cu-SSZ-39 and Cu-SSZ-13. Appl. Catal. B Environ. 2020, 264, 118511. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic Materials for DeNOx Selective Catalytic Reduction. Chem. Cat. Chem. 2017, 10, 29–41. [Google Scholar] [CrossRef]
- Schmieg, S.J.; Oh, S.H.; Kim, C.H.; Brown, D.B.; Lee, J.H.; Peden, C.H.; Kim, D.H. Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal. Today 2012, 184, 252–261. [Google Scholar] [CrossRef]
- Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of the hydrothermal aging temperature and Cu/Al ratio on the hydrothermal stability of CuSSZ-13 catalysts for NH3-SCR. Catal. Sci. Technol. 2017, 7, 703–717. [Google Scholar] [CrossRef]
- Luo, J.; Gao, F.; Kamasamudram, K.; Currier, N.; Peden, C.H.; Yezerets, A. New insights into Cu/SSZ-13 SCR catalyst acidity. Part I: Nature of acidic sites probed by NH3 titration. J. Catal. 2017, 348, 291–299. [Google Scholar] [CrossRef]
- Leistner, K.; Olsson, L. Deactivation of Cu/SAPO-34 during low-temperature NH3-SCR. Appl. Catal. B Environ. 2015, 165, 192–199. [Google Scholar] [CrossRef]
- Woo, J.; Leistner, K.; Bernin, D.; Ahari, H.; Shost, M.; Zammit, M.; Olsson, L. Effect of various structure directing agents (SDAs) on low-temperature deactivation of Cu/SAPO-34 during NH3-SCR reaction. Catal. Sci. Technol. 2018, 8, 3090–3106. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Varga, T.; Wang, Y.; Szanyi, J.; Peden, C.H.F.; Gao, F. Unraveling the mysterious failure of Cu/SAPO-34 selective catalytic reduction catalysts. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Chen, J.; Peng, G.; Zheng, W.; Zhang, W.; Guo, L.; Wu, X. Excellent Performance of One-Pot Synthesized Fe-Containing MCM-22 Zeolites for the Selective Catalytic Reduction of NOx with NH3. Catal. Sci. Technol. 2020, 10, 6583–6598. [Google Scholar] [CrossRef]
- Chen, J.; Liang, T.; Li, J.; Wang, S.; Qin, Z.; Wang, P.; Huang, L.; Fan, W.; Wang, J. Regulation of Framework Aluminum Siting and Acid Distribution in H-MCM-22 by Boron Incorporation and Its Effect on the Catalytic Performance in Methanol to Hydrocarbons. ACS Catal. 2016, 6, 2299–2313. [Google Scholar] [CrossRef]
- Paolucci, C.; Di Iorio, J.; Ribeiro, F.; Gounder, R.; Schneider, W.F. Catalysis Science of NOx Selective Catalytic Reduction With Ammonia Over Cu-SSZ-13 and Cu-SAPO-34. Adv. Catal. 2016, 59, 1–107. [Google Scholar]
- Xu, G.; Zhu, X.; Niu, X.; Liu, S.; Xie, S.; Li, X.; Xu, L. One-pot synthesis of high silica MCM-22 zeolites and their performances in catalytic cracking of 1-butene to propene. Micropor. Mesopor. Mater. 2009, 118, 44–51. [Google Scholar] [CrossRef]
- Wang, P.; Huang, L.; Li, J.; Dong, M.; Wang, J.; Tatsumi, T.; Fan, W. Catalytic properties and deactivation behavior of H-MCM-22 in the conversion of methanol to hydrocarbons. RSC Adv. 2015, 5, 28794–28802. [Google Scholar] [CrossRef]
- Wang, Y.; Yokoi, T.; Namba, S.; Kondo, J.N.; Tatsumi, T. Improvement of catalytic performance of MCM-22 in the cracking of n-hexane by controlling the acidic property. J. Catal. 2016, 333, 17–28. [Google Scholar] [CrossRef]
- Corma, A.; Palomares, A.E.; Fornés, V. A comparative study on the activity of metal exchanged MCM22 zeolite in the selective catalytic reduction of NOx. Res. Chem. Intermed. 1998, 24, 613–623. [Google Scholar] [CrossRef]
- Rutkowska, M.; Diaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe modified derivatives of 2D MWW-type zeolites (MCM-22, ITQ-2 and MCM-36) as new catalysts for DeNOx process. Appl. Catal. B Environ. 2015, 531–539. [Google Scholar] [CrossRef]
- Palella, B.I.; Pirone, R.; Russo, G.; Albuquerque, A.; Pastore, H.O.; Cadoni, M.; Frache, A.; Marchese, L. On the activity and hydrothermal stability of CuMCM-22 in the decomposition of nitrogen oxides: A comparison with CuZSM-5. Catal. Commun. 2004, 5, 191–194. [Google Scholar] [CrossRef]
- Gao, F.; Mei, D.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Kollar, M.; Wang, Y.; Szanyi, J.; Peden, C.H. Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 2014, 319, 1–14. [Google Scholar] [CrossRef]
- Liang, T.; Chen, J.; Qin, Z.; Li, J.; Wang, P.; Wang, S.; Wang, G.; Dong, M.; Fan, W.; Wang, J. Conversion of Methanol to Olefins over H-ZSM-5 Zeolite: Reaction Pathway Is Related to the Framework Aluminum Siting. ACS Catal. 2016, 6, 7311–7325. [Google Scholar] [CrossRef]
- Liang, T.; Fadaeerayeni, S.; Shan, J.; Li, T.; Wang, H.; Cheng, J.; Toghiani, H.; Xiang, Y. Ethane Aromatization over Zn-HZSM-5: Early-Stage Acidity/Performance Relationships and Deactivation Kinetics. Ind. Eng. Chem. Res. 2019, 58, 17699–17708. [Google Scholar] [CrossRef]
- Liang, T.; Toghiani, H.; Xiang, Y. Transient Kinetic Study of Ethane and Ethylene Aromatization over Zinc-Exchanged HZSM-5 Catalyst. Ind. Eng. Chem. Res. 2018, 57, 15301–15309. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward Rational Design of Cu/SSZ-13 Selective Catalytic Reduction Catalysts: Implications from Atomic-Level Understanding of Hydrothermal Stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Gao, F.; Szanyi, J. On the hydrothermal stability of Cu/SSZ-13 SCR catalysts. Appl. Catal. A Gen. 2018, 560, 185–194. [Google Scholar] [CrossRef]
- Luo, J.; Wang, D.; Kumar, A.; Li, J.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. Identification of two types of Cu sites in Cu/SSZ-13 and their unique responses to hydrothermal aging and sulfur poisoning. Catal. Today 2016, 267, 3–9. [Google Scholar] [CrossRef]
- Xing, E.; Shi, Y.; Zheng, A.; Zhang, J.; Gao, X.; Liu, D.; Xin, M.; Xie, W.; Zhang, F.; Mu, X.; et al. Transformation from NaA to MCM-49 Zeolite and Its Catalytic Alkylation Performance. Ind. Eng. Chem. Res. 2015, 54, 3123–3135. [Google Scholar] [CrossRef]
- Lee, K.; Kosaka, H.; Sato, S.; Yokoi, T.; Choi, B. Effect of Cu content and zeolite framework of n-C4H10-SCR catalysts on de-NOx performances. Chem. Eng. Sci. 2019, 203, 28–42. [Google Scholar] [CrossRef]
- Lee, K.; Kosaka, H.; Sato, S.; Yokoi, T.; Choi, B.; Kim, D. Effects of Cu loading and zeolite topology on the selective catalytic reduction with C3H6 over Cu/zeolite catalysts. J. Ind. Eng. Chem. 2019, 72, 73–86. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Schmieg, S.; Weng, D. Role of Brønsted acidity in NH3 selective catalytic reduction reaction on Cu/SAPO-34 catalysts. J. Catal. 2015, 324, 98–106. [Google Scholar] [CrossRef]
- Sazama, P.; Pilar, R.; Mokrzycki, L.; Vondrova, A.; Kaucky, D.; Plsek, J.; Sklenak, S.; Šťastný, P.; Klein, P. Remarkably enhanced density and specific activity of active sites in Al-rich Cu-, Fe- and Co-beta zeolites for selective catalytic reduction of NOx. Appl. Catal. B Environ. 2016, 189, 65–74. [Google Scholar] [CrossRef]
- Boroń, P.; Chmielarz, L.; Gurgul, J.; Łątka, K.; Gil, B.; Marszałek, B.; Dzwigaj, S. Influence of iron state and acidity of zeolites on the catalytic activity of FeHBEA, FeHZSM-5 and FeHMOR in SCR of NO with NH3 and N2O decomposition. Microporous Mesoporous Mater. 2015, 203, 73–85. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, Z.; Wang, H.F.; Hu, P. Understanding Catalytic Reactions over Zeolites: A Density Functional Theory Study of Selective Catalytic Reduction of NOx by NH3 over Cu-SAPO-34. ACS Catal. 2016, 6, 7882–7891. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.; He, C.; Miao, J.; Chen, J. In situ growth synthesis of CuO@Cu-MOFs core-shell materials as novel low-temperature NH3-SCR catalysts. ChemCatChem 2018, 11, 979–984. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, R.T. N2O Formation Pathways over Zeolite-Supported Cu and Fe Catalysts in NH3-SCR. Energy Fuels 2018, 32, 2170–2182. [Google Scholar] [CrossRef]
- Ming, S.; Chen, Z.; Fan, C.; Pang, L.; Guo, W.; Albert, K.B.; Liu, P.; Li, T. The effect of copper loading and silicon content on catalytic activity and hydrothermal stability of Cu-SAPO-18 catalyst for NH3-SCR. Appl. Catal. A Gen. 2018, 559, 47–56. [Google Scholar] [CrossRef]
- Leistner, K.; Xie, K.; Kamasamudram, K.; Olsson, L.; Kumar, A. Ammonia Desorption Peaks Can Be Assigned to Different Copper Sites in Cu/SSZ-13. Catal. Lett. 2017, 147, 1882–1890. [Google Scholar] [CrossRef]
- Praliaud, H. Surface and bulk properties of Cu–ZSM-5 and Cu/Al2O3 solids during redox treatments. Correlation with the selective reduction of nitric oxide by hydrocarbons. Appl. Catal. B Environ. 1998, 16, 359–374. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, R.; Zhao, R.; Shi, C.; Gies, H.; Xiao, F.-S.; De Vos, D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: Effects of Na+ ions on the activity and hydrothermal stability. Appl. Catal. B Environ. 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Lezcano-Gonzalez, I.; Deka, U.; Van Der Bij, H.; Paalanen, P.; Arstad, B.; Weckhuysen, B.; Beale, A. Chemical deactivation of Cu-SSZ-13 ammonia selective catalytic reduction (NH3-SCR) systems. Appl. Catal. B Environ. 2014, 339–349. [Google Scholar] [CrossRef]
- Dědecěk, J.; Wichterlová, B. Role of Hydrated Cu Ion Complexes and Aluminum Distribution in the Framework on the Cu Ion Siting in ZSM-5. J. Phys. Chem. B 1997, 101, 10233–10240. [Google Scholar] [CrossRef]
- Korhonen, S.T.; Fickel, D.W.; Lobo, R.F.; Weckhuysen, B.M.; Beale, A.M. Isolated Cu2+ ions: Active sites for selective catalytic reduction of NO. Chem. Commun. 2011, 47, 800–802. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, Q.-H.; Shen, S.-C.; Kawi, S.; Hidajat, K. Catalytic oxidation of pyridine on the supported copper catalysts in the presence of excess oxygen. J. Catal. 2004, 225, 128–137. [Google Scholar] [CrossRef]
- Torreabreu, C.; Ribeiro, M.F.; Henriques, C.; Delahay, G. NO TPD and H2-TPR studies for characterisation of CuMOR catalysts: The role of Si/Al ratio, copper content and cocation. Appl. Catal. B Environ. 1997, 14, 261–272. [Google Scholar] [CrossRef]
- Torre-Abreu, C.; Ribeiro, M.F.; Henriques, C.; Delahay, G. Characterisation of CuMFI catalysts by temperature programmed desorption of NO and temperature programmed reduction. Effect of the zeolite Si/Al ratio and copper loading. Appl. Catal. B Environ. 1997, 12, 249–262. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Wasowicz, T.; Prakash, A.M.; Kevan, L. Electron Spin Resonance and Electron Spin Echo Modulation Studies of Cu(II) Cation Locations and Adsorbate Interactions in Ion-exchanged Cu-MCM-22 Zeolite. Micropor. Mater. 1997, 12, 107–116. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Zhao, S.; Wang, B.; Ma, X.; Gong, J. A copper-phyllosilicate core-sheath nanoreactor for carbon–oxygen hydrogenolysis reactions. Nat. Commun. 2013, 4, 2339. [Google Scholar] [CrossRef]
- Yangab, H.; Cuia, X.; Liab, S.; Cenc, Y.; Denga, T.; Wangab, J.; Olsbyed, U.; Fana, W. Developing a general method for encapsulation of metal oxide nanoparticles in mesoporous silica shell by unraveling its formation mechanism. Micropor. Mesopor. Mater. 2020, 305, 110381. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Cui, X.; Wang, G.; Cen, Y.; Deng, T.; Yan, W.; Gao, J.; Zhu, S.; Olsbye, U.; et al. A Highly Stable Copper-Based Catalyst for Clarifying the Catalytic Roles of Cu0 and Cu+ Species in Methanol Dehydrogenation. Angew. Chem. Int. Ed. 2018, 57, 1836–1840. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-F.; Guo, P.-J.; Qiao, M.-H.; Yan, S.-R.; Li, H.-X.; Shen, W.; Xu, H.-L.; Fan, K.-N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of Ethanol via Syngas on Cu/SiO2 Catalysts with Balanced Cu0-Cu+ Sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Lian, Z.; Zhang, Y.; Shan, W.; He, H. The promotional effect of H2 reduction treatment on the low-temperature NH3-SCR activity of Cu/SAPO-18. Appl. Surf. Sci. 2019, 483, 536–544. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; De La Torre, U.; Illán-Gómez, M.J.; Bueno-López, A.; González-Velasco, J.R. Role of the different copper species on the activity of Cu/zeolite catalysts for SCR of NOx with NH3. Appl. Catal. B Environ. 2014, 147, 420–428. [Google Scholar] [CrossRef]
- Marberger, A.; Petrov, A.W.; Steiger, P.; Elsener, M.; Kröcher, O.; Nachtegaal, M.; Ferri, D. Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13. Nat. Catal. 2018, 1, 221–227. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Kollár, M.; Washton, N.M.; Szanyi, J.; Peden, C.H. A comparative kinetics study between Cu/SSZ-13 and Fe/SSZ-13 SCR catalysts. Catal. Today 2015, 258, 347–358. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, Y.; Kukkadapu, R.K.; Wang, Y.; Walter, E.D.; Schwenzer, B.; Szanyi, J.; Peden, C.H. Iron Loading Effects in Fe/SSZ-13 NH3-SCR Catalysts: Nature of the Fe Ions and Structure-Function Relationships. ACS Catal. 2016, 6, 2939–2954. [Google Scholar] [CrossRef]
- Verma, A.A.; Bates, S.A.; Anggara, T.; Paolucci, C.; Parekh, A.A.; Kamasamudram, K.; Yezerets, A.; Miller, J.T.; Delgass, W.N.; Schneider, W.F.; et al. NO oxidation: A probe reaction on Cu-SSZ-13. J. Catal. 2014, 312, 179–190. [Google Scholar] [CrossRef]
- Selleri, T.; Gramigni, F.; Nova, I.; Tronconi, E. NO oxidation on Fe- and Cu-zeolites mixed with BaO/Al2O3: Free oxidation regime and relevance for the NH3-SCR chemistry at low temperature. Appl. Catal. B Environ. 2018, 225, 324–331. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wei, Z.; Kollar, M.; Gao, F.; Wang, Y.; Szanyi, J.; Peden, C.H. NO oxidation on zeolite supported Cu catalysts: Formation and reactivity of surface nitrates. Catal. Today 2016, 267, 17–27. [Google Scholar] [CrossRef]
- Wang, D.; Jangjou, Y.; Liu, Y.; Sharma, M.K.; Luo, J.; Li, J.; Kamasamudram, K.; Epling, W.S. A Comparison of Hydrothermal Aging Effects on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 Catalysts. Appl. Catal. B Environ. 2015, 165, 438–445. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Zhao, H.; Liu, M.; Ma, Y.; Yong, X.; Chen, H.; Li, Y. The Cu migration of Cu-SAPO-34 catalyst for ammonia selective catalytic reduction of NOx during high temperature hydrothermal aging treatment. Catal. Today 2019, 327, 126–133. [Google Scholar] [CrossRef]
- Niu, C.; Shi, X.; Liu, F.; Liu, K.; Xie, L.; You, Y.; He, H. High hydrothermal stability of Cu-SAPO-34 catalysts for the NH3-SCR of NOx. Chem. Eng. J. 2016, 294, 254–263. [Google Scholar] [CrossRef]
- Wang, L.; Gaudet, J.R.; Li, W.; Weng, D. Migration of Cu species in Cu/SAPO-34 during hydrothermal aging. J. Catal. 2013, 306, 68–77. [Google Scholar] [CrossRef]
- Peden, C.H.; Kwak, J.H.; Burton, S.D.; Tonkyn, R.G.; Kim, D.H.; Lee, J.-H.; Jen, H.-W.; Cavataio, G.; Cheng, Y.; Lambert, C.K. Possible origin of improved high temperature performance of hydrothermally aged Cu/beta zeolite catalysts. Catal. Today 2012, 184, 245–251. [Google Scholar] [CrossRef]
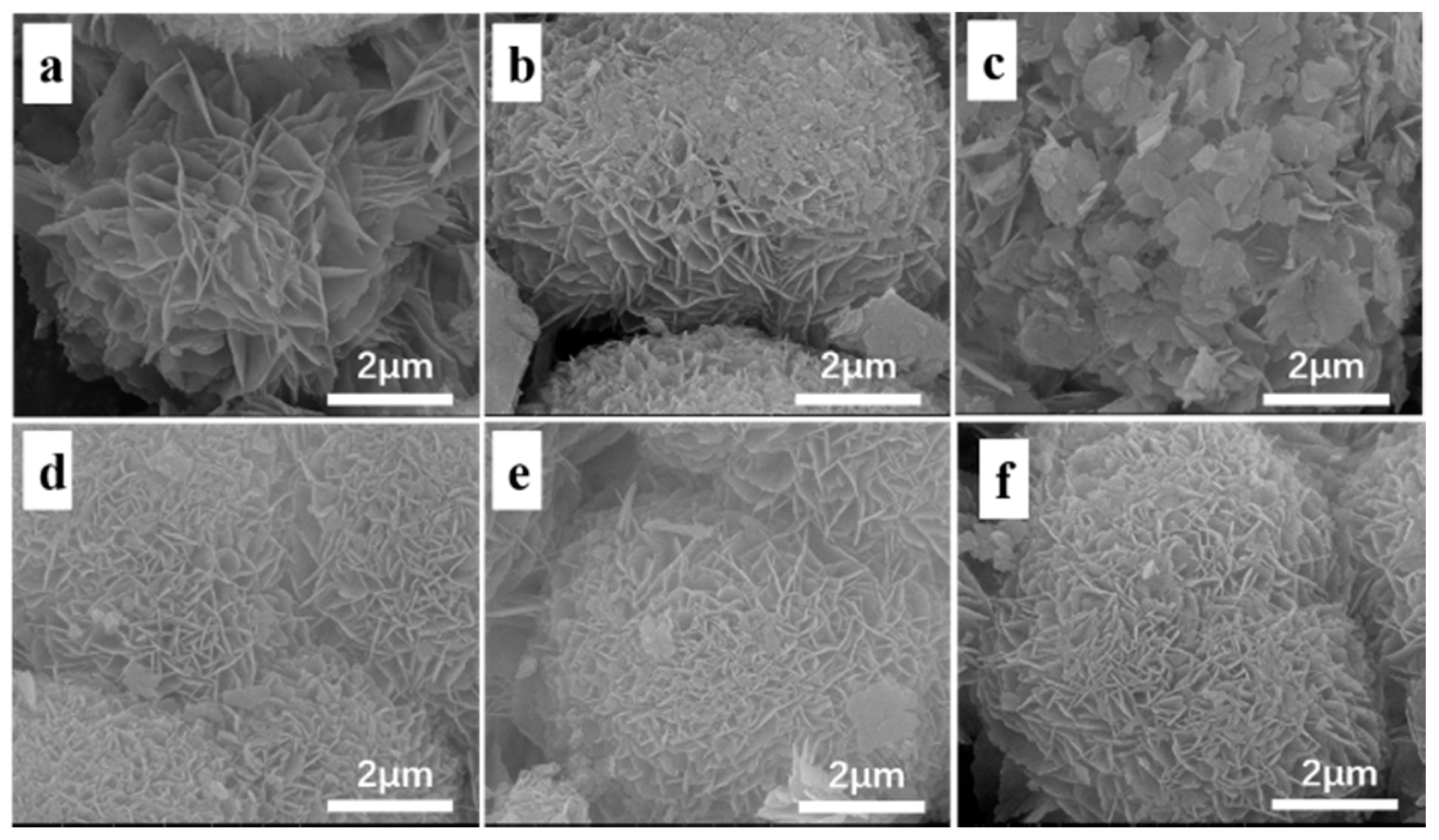
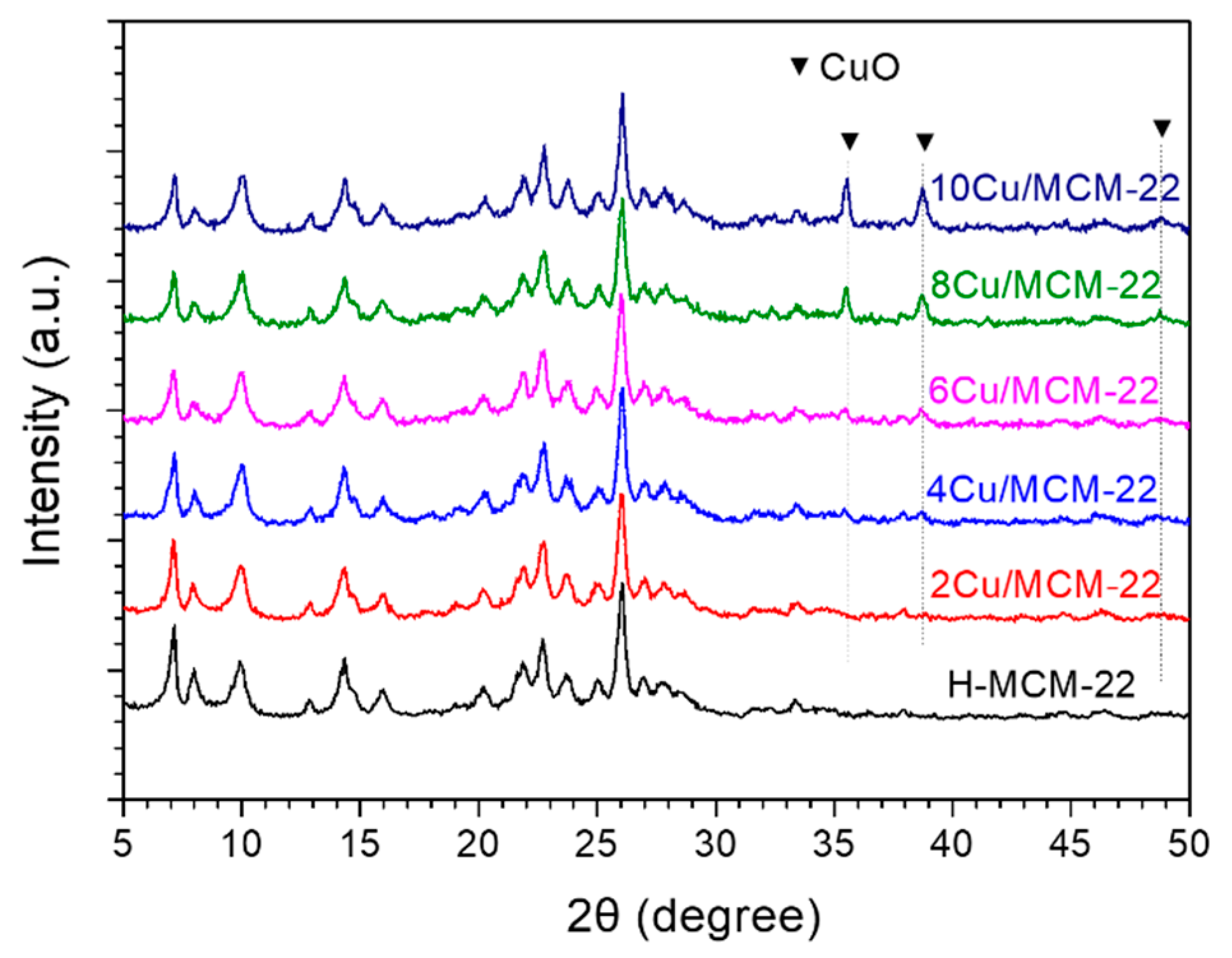
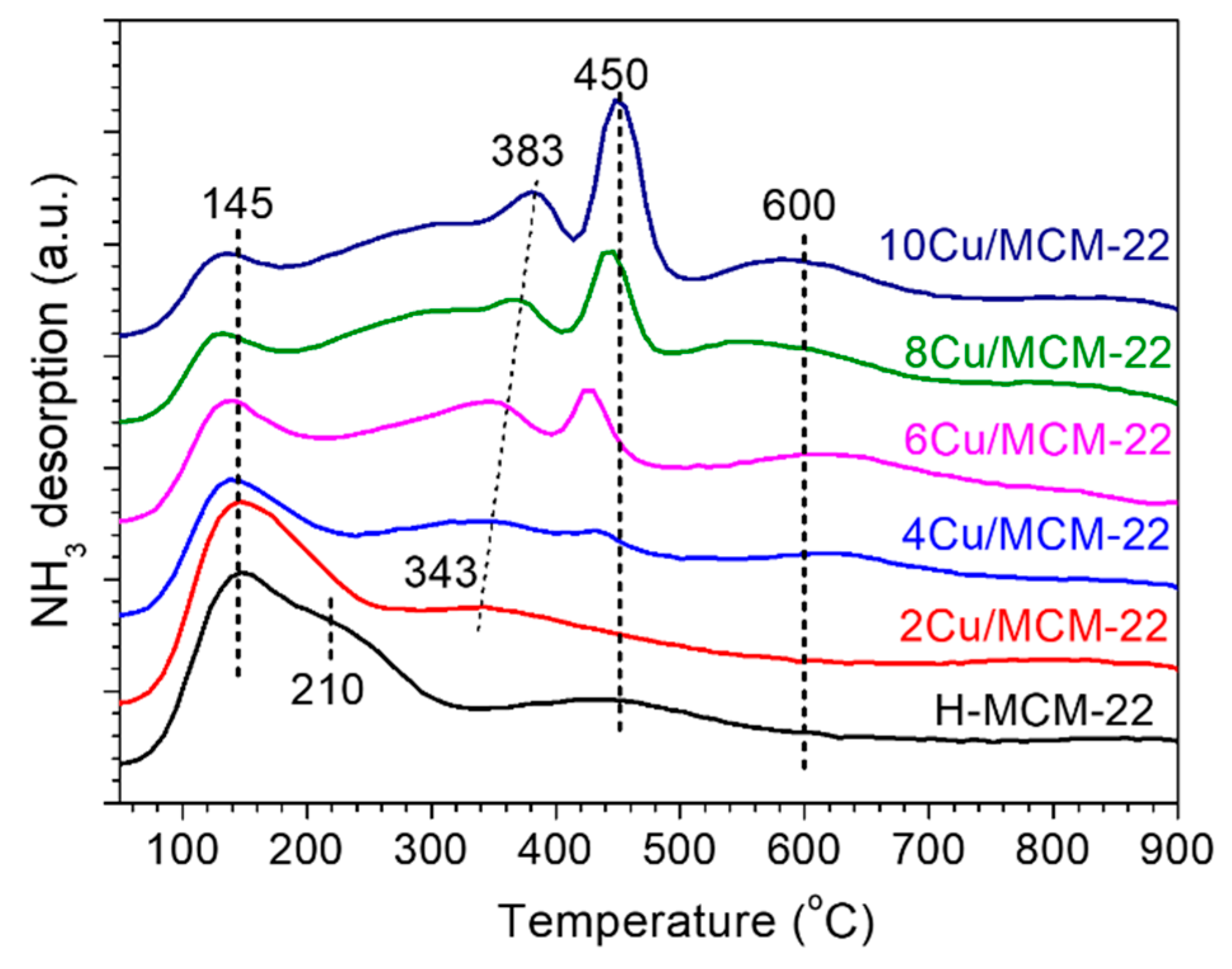

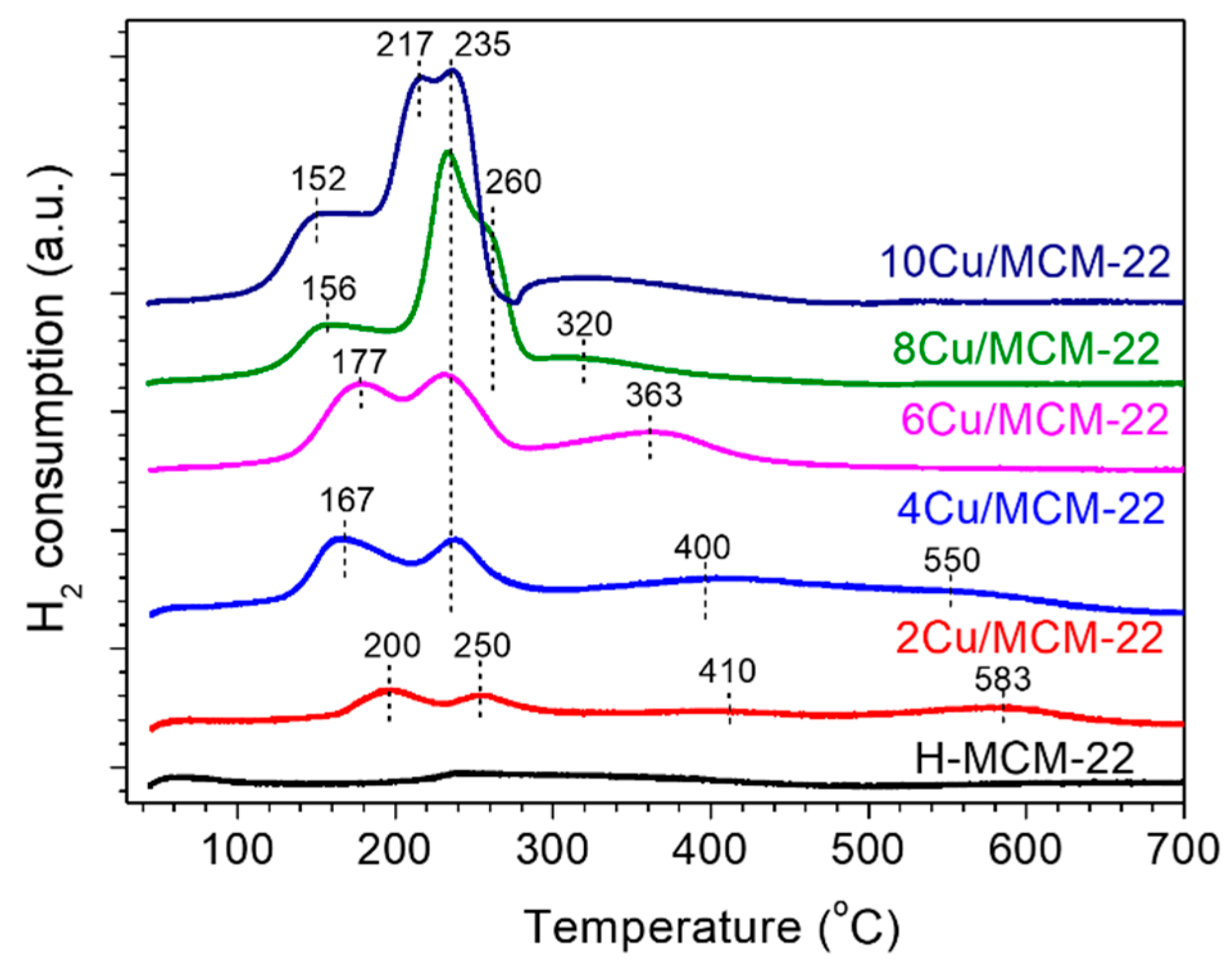
| Zeolites | Cu a (wt%) | RC b (%) | Surface Area c (m2 g−1) | Pore Volume c (cm3 g−1) | ||||
|---|---|---|---|---|---|---|---|---|
| SBET | Smicro | Sext | Vtotal | Vmicro | Vmeso | |||
| H-MCM-22 | 0 | 100 | 500 | 348 | 152 | 0.50 | 0.163 | 0.337 |
| 2Cu/MCM-22 | 2.1 | 93 | 404 | 281 | 123 | 0.40 | 0.133 | 0.267 |
| 4Cu/MCM-22 | 3.7 | 100 | 404 | 282 | 122 | 0.40 | 0.132 | 0.268 |
| 6Cu/MCM-22 | 6.7 | 100 | 391 | 273 | 118 | 0.39 | 0.128 | 0.262 |
| 8Cu/MCM-22 | 8.5 | 93 | 365 | 253 | 112 | 0.38 | 0.118 | 0.262 |
| 10Cu/MCM-22 | 10.4 | 102 | 290 | 188 | 102 | 0.34 | 0.085 | 0.255 |
| Zeolites | Acid Sites Density (μmol/g) | |||
|---|---|---|---|---|
| Total a | Weak b | Medium b | Strong b | |
| H-MCM-22 | 1062 | 255 | 446 | 361 |
| 2Cu/MCM-22 | 1216 | 207 | 328 | 681 |
| 4Cu/MCM-22 | 1124 | 202 | 247 | 674 |
| 6Cu/MCM-22 | 1152 | 173 | 265 | 714 |
| 8Cu/MCM-22 | 1197 | 96 | 323 | 778 |
| 10Cu/MCM-22 | 1276 | 102 | 140 | 1034 |
| Catalyst | Reduction Process 1a (Cu2+ to Cu+) | Reduction Process 2a (CuO to Cu0) | Reduction Process 3a (Cu+ to Cu0) | H2 Consumpt-ionb (µmol/g) | H2/Cu c | |||
|---|---|---|---|---|---|---|---|---|
| Peak Temperature (°C) | Relative Peak Area (%) | Peak Temper-ature (°C) | Relative Peak Area (%) | Peak Temper-ature (°C) | Relative Peak Area (%) | |||
| 2Cu/MCM-22 | 196 | 21 | 256 | 14 | 386 580 | 65 | 241 | 0.73 |
| 4Cu/MCM-22 | 170 | 23 | 235 | 19 | 417 580 | 58 | 428 | 0.74 |
| 6Cu/MCM-22 | 177 | 34 | 235 | 31 | 352 | 35 | 622 | 0.59 |
| 8Cu/MCM-22 | 170 | 27 | 235 260 | 48 | 300 | 25 | 792 | 0.59 |
| 10Cu/MCM-22 | 161 | 29 | 217 242 | 58 | 338 | 13 | 976 | 0.60 |
| Zeolites | Si/Al a mol/mol | Si/Cu a mol/mol | Si/Al b mol/mol | Si/Cu b mol/mol |
|---|---|---|---|---|
| 2Cu/MCM-22 | 10.3 | 121.8 | 11.0 | 33.5 |
| 4Cu/MCM-22 | 5.9 | 53.2 | 19.6 | |
| 6Cu/MCM-22 | 6.8 | 50.6 | 7.4 | |
| 8Cu/MCM-22 | 7.7 | 46.3 | 11.3 | |
| 10Cu/MCM-22 | 6.3 | 41.4 | 7.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Peng, G.; Liang, T.; Zhang, W.; Zheng, W.; Zhao, H.; Guo, L.; Wu, X. Catalytic Performances of Cu/MCM-22 Zeolites with Different Cu Loadings in NH3-SCR. Nanomaterials 2020, 10, 2170. https://doi.org/10.3390/nano10112170
Chen J, Peng G, Liang T, Zhang W, Zheng W, Zhao H, Guo L, Wu X. Catalytic Performances of Cu/MCM-22 Zeolites with Different Cu Loadings in NH3-SCR. Nanomaterials. 2020; 10(11):2170. https://doi.org/10.3390/nano10112170
Chicago/Turabian StyleChen, Jialing, Gang Peng, Tingyu Liang, Wenbo Zhang, Wei Zheng, Haoran Zhao, Li Guo, and Xiaoqin Wu. 2020. "Catalytic Performances of Cu/MCM-22 Zeolites with Different Cu Loadings in NH3-SCR" Nanomaterials 10, no. 11: 2170. https://doi.org/10.3390/nano10112170
APA StyleChen, J., Peng, G., Liang, T., Zhang, W., Zheng, W., Zhao, H., Guo, L., & Wu, X. (2020). Catalytic Performances of Cu/MCM-22 Zeolites with Different Cu Loadings in NH3-SCR. Nanomaterials, 10(11), 2170. https://doi.org/10.3390/nano10112170






