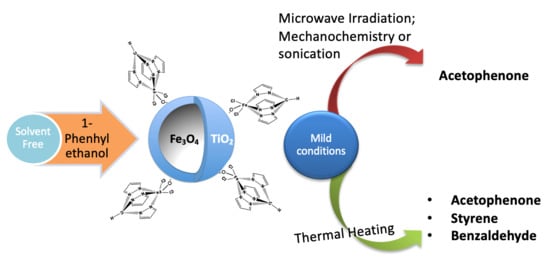
Catalytic Performance of a Magnetic Core-Shell Iron(II) C-Scorpionate under Unconventional Oxidation Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis of Magnetic Fe3O4
2.3. Synthesis of Magnetic Core-Shell Fe3O4/TiO2
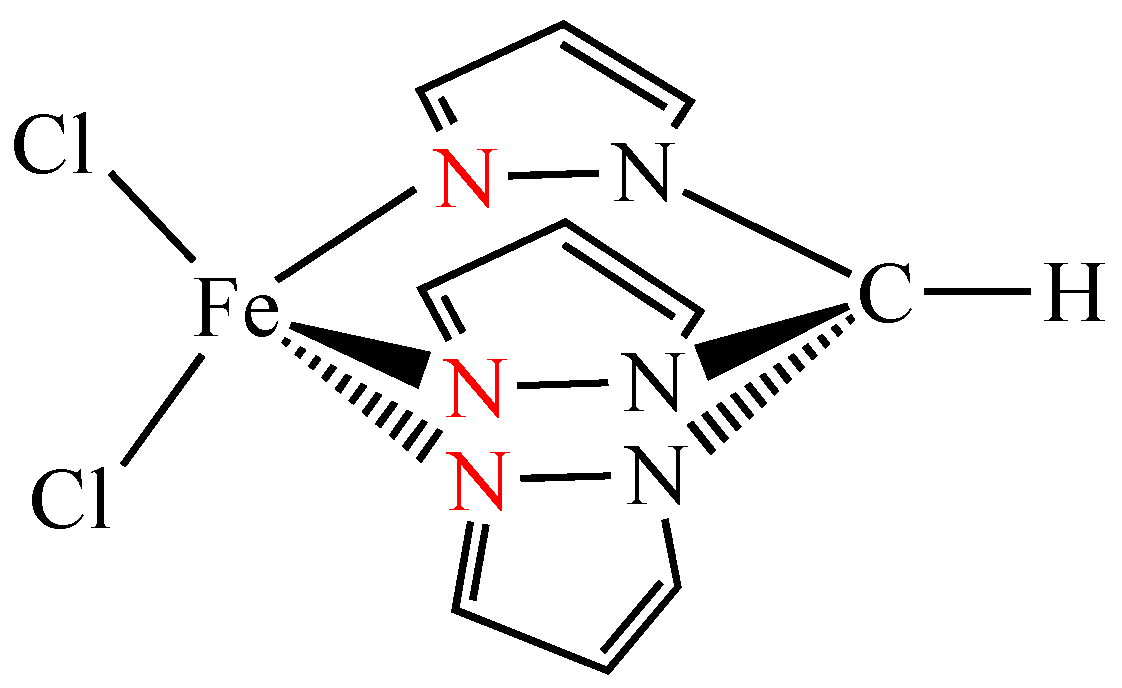
2.4. Synthesis of Magnetic Fe3O4/TiO2/[FeCl2{κ3-HC(pz)3}]
2.5. Oxidation of 1-Phenylethanol
2.5.1. Oxidation of 1-Phenylethanol Using Conventional Thermal Heating
2.5.2. Microwave-Assisted Oxidation of 1-Phenylethanol
2.5.3. Ultrasounds-Assisted Oxidation of 1-Phenylethanol
2.5.4. Mechanochemical Oxidation of 1-Phenylethanol
3. Results
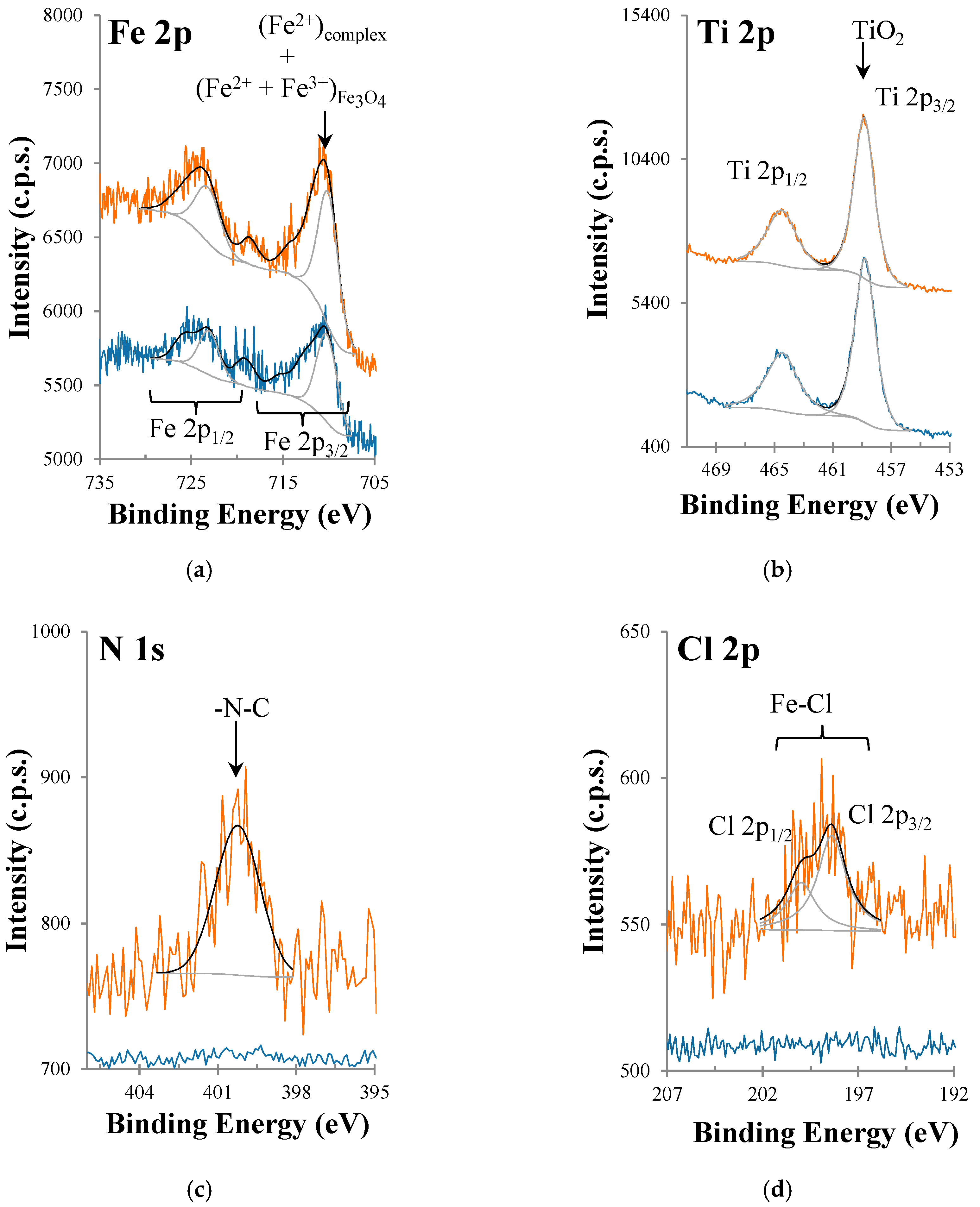
3.1. X-ray Photoelectron Spectroscopy (XPS)
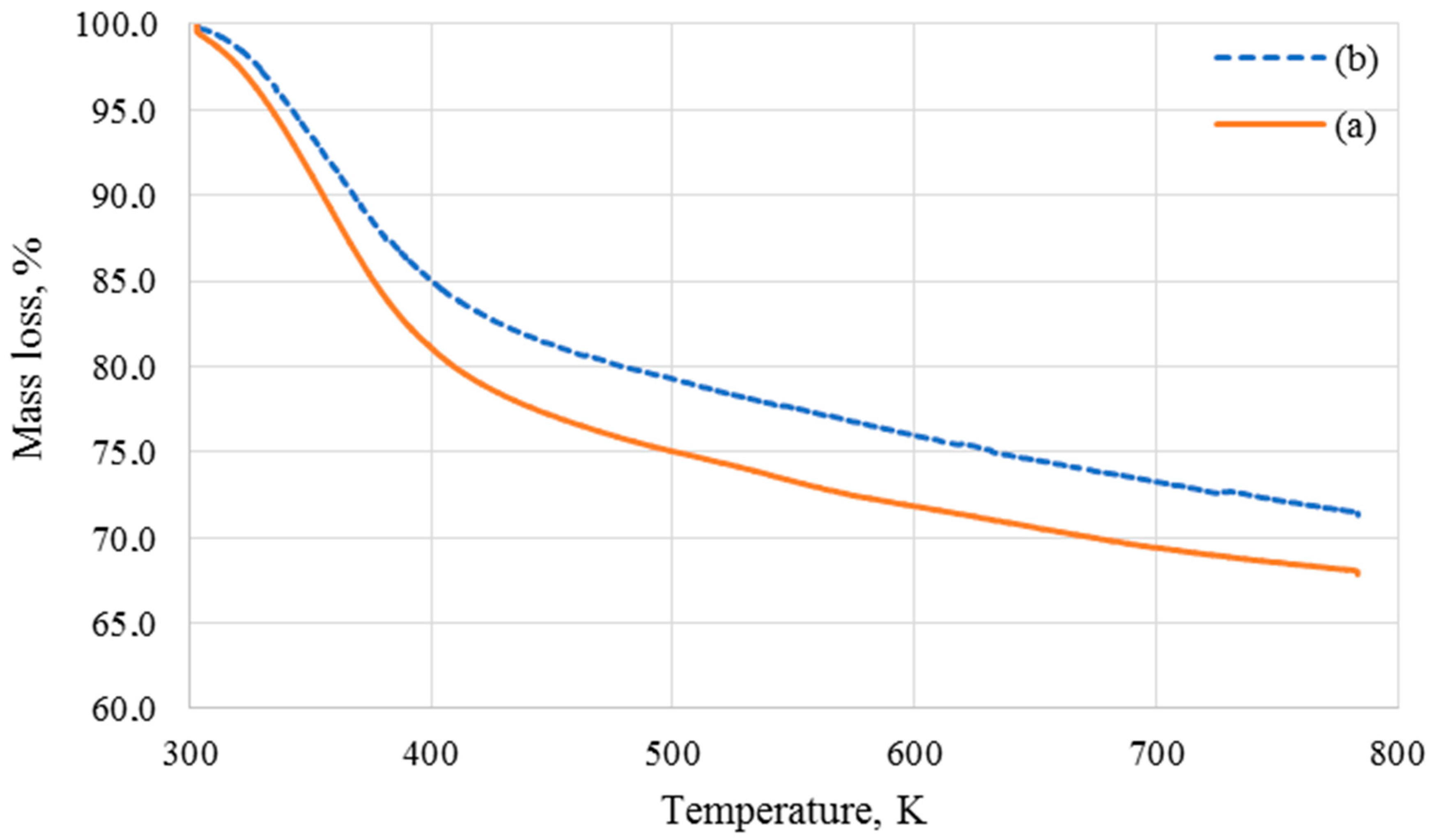
3.2. Thermogravimetric Analysis (TGA)
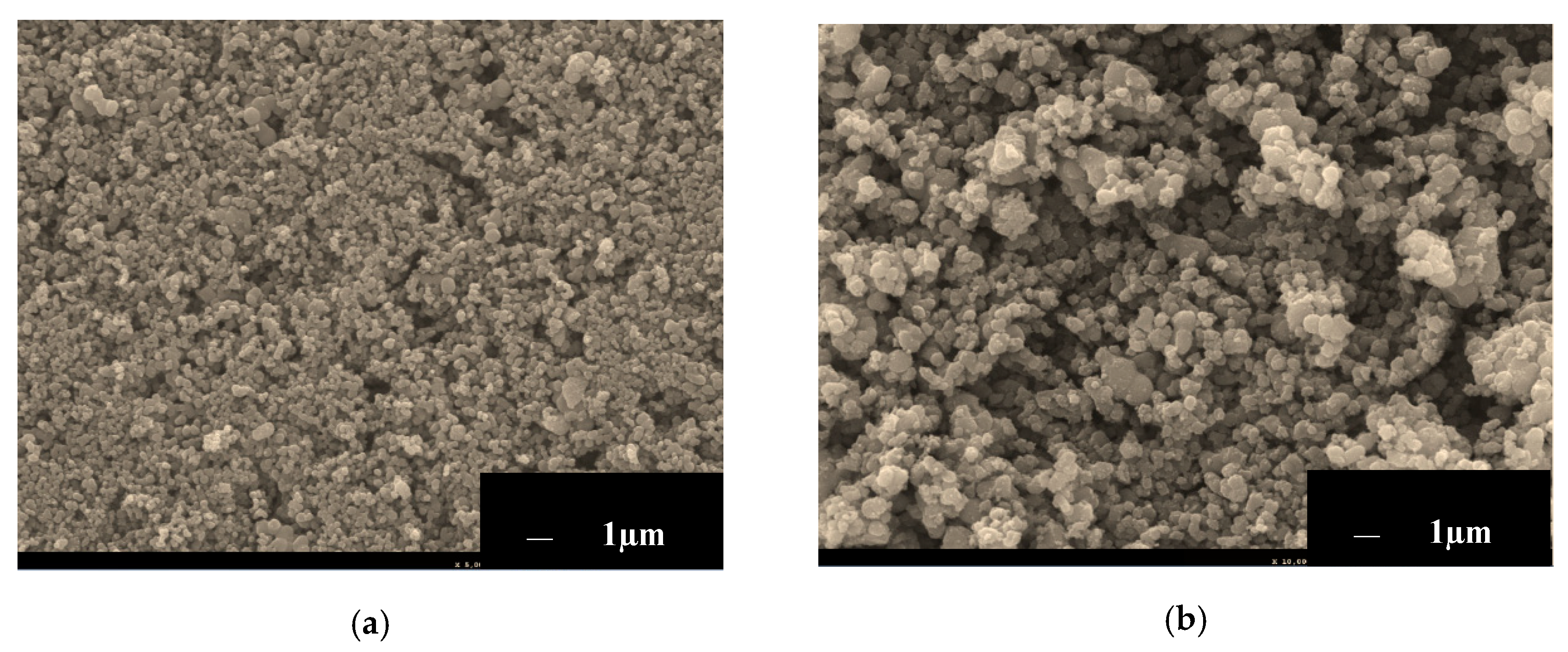
3.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX)
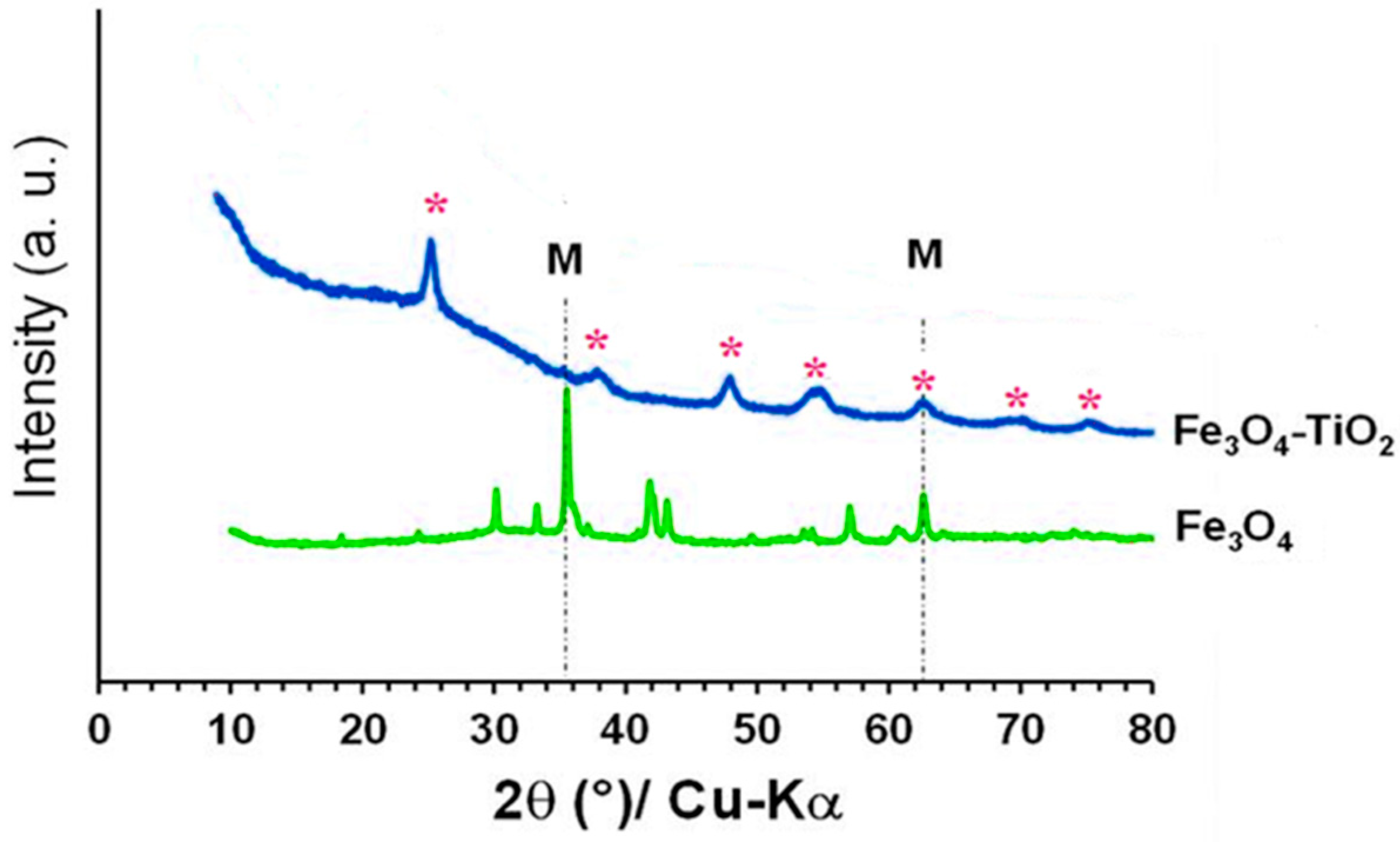
3.4. X-ray Diffraction (XRD)
3.5. Fourier Transform Infrared Spectroscopy (FTIR)
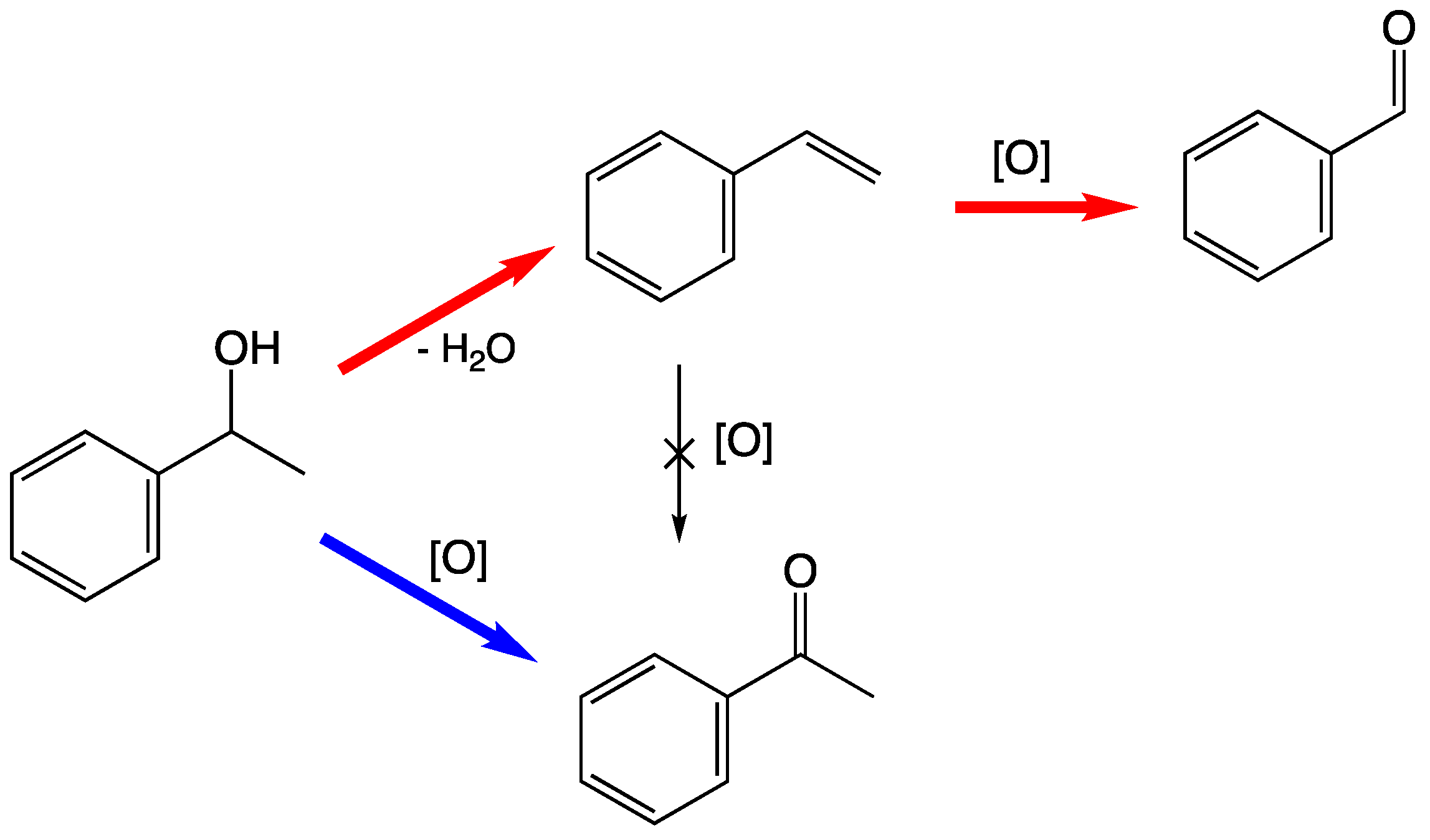
3.6. Catalytic Oxidation of 1-Phenylethanol
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lee, I.; Joo, J.B.; Zaera, F.; Yin, Y. Core–Shell Nanostructured Catalysts. Acc. Chem. Res. 2012, 46, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Guo, X.; Luo, Y.; Li, Q.; Zhu, R.; Pang, H. Core-shell materials for advanced batteries. Chem. Eng. J. 2019, 355, 208–237. [Google Scholar] [CrossRef]
- Mélinon, P.; Begin-Colin, S.; Duvail, J.L.; Gauffre, F.; Boime, N.H.; Ledoux, G.; Plain, J.; Reiss, P.; Silly, F.; Warot-Fonrose, B. Engineered inorganic core/shell nanoparticles. Phys. Rep. 2014, 543, 163–197. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Tung, S.O.; Tang, Z.; Li, L.; Ding, L.; Xi, X.; Liu, Y.; Zhang, L.; Zhang, J. A review of core-shell nanostructured electrocatalysts for oxygen reduction reaction. Energy Storage Mater. 2018, 12, 260–276. [Google Scholar] [CrossRef]
- Pandey, G.; Singh, S.; Hitkari, G. Synthesis and characterization of polyvinyl pyrrolidone (PVP)-coated Fe3O4 nanoparticles by chemical co-precipitation method and removal of Congo red dye by adsorption process. Int. Nano Lett. 2018, 8, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Khashan, S.; Dagher, S.; Tit, N.; Alazzam, A.; Obaidat, I. Novel method for synthesis of Fe3O4@TiO2 core/shell nanoparticles. Surf. Coat. Technol. 2017, 322, 92–98. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Organic synthesis via magnetic attraction: Benign and sustainable protocols using magnetic nanoferrites. Green Chem. 2013, 15, 398–417. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Efficient cyclohexane oxidation with hydrogen peroxide catalysed by a C-scorpionate iron(II) complex immobilized on desilicated MOR zeolite. Appl. Catal. A Gen. 2013, 464–465, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.M.D.R.S.; De Almeida, M.P.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-Scorpionate Fe(II) Complex on Carbon Materials for Cyclohexane Oxidation with Hydrogen Peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Tuning Cyclohexane Oxidation: Combination of Microwave Irradiation and Ionic Liquid with the C-Scorpionate [FeCl2(Tpm)] Catalyst. Organometallics 2016, 36, 192–198. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. N2O-Free single-pot conversion of cyclohexane to adipic acid catalysed by an iron(II) scorpionate complex. Green Chem. 2017, 19, 1499–1501. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Carbon dioxide-to-methanol single-pot conversion using a C-scorpionate iron(II) catalyst. Green Chem. 2017, 19, 4811–4815. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenized C-Scorpionate Iron(II) Complex on Nanostructured Carbon Materials as Recyclable Catalysts for Microwave-Assisted Oxidation Reactions. ChemCatChem 2018, 10, 1821–1828. [Google Scholar] [CrossRef]
- Van-Dúnem, V.; Carvalho, A.P.; Martins, L.M.D.R.S.; Martins, A. Improved Cyclohexane Oxidation Catalyzed by a Heterogenized Iron(II) Complex on Hierarchical Y Zeolite through Surfactant Mediated Technology. ChemCatChem 2018, 10, 4058–4066. [Google Scholar] [CrossRef]
- Andrade, M.A.; Mestre, A.S.; Carvalho, A.P.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. The role of nanoporous carbon materials in catalytic cyclohexane oxidation. Catal. Today 2019, 357, 46–55. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Ribeiro, A.P.C.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Highly efficient and reusable CNT supported iron(II) catalyst for microwave assisted alcohol oxidation. Dalton Trans. 2016, 45, 6816–6819. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.P.C.; Matias, I.A.; Alegria, E.C.B.A.; Ferraria, A.M.; Rego, A.M.B.D.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. New Trendy Magnetic C-Scorpionate Iron Catalyst and Its Performance towards Cyclohexane Oxidation. Catalysts 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Assal, M.E.; Al-Warthan, A.A.; Khan, M.; Shaik, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Labis, J.P.; Adil, S.F. Comparative Catalytic Evaluation of Nano-ZrOx Promoted Manganese Catalysts: Kinetic Study and the Effect of Dopant on the Aerobic Oxidation of Secondary Alcohols. Adv. Mater. Sci. Eng. 2017, 2017, 3958319. [Google Scholar] [CrossRef] [Green Version]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.; Martins, L.M.D.R.S.; Pombeiro, A.J. Catalytic Oxidation of Alcohols. Adv. Organomet. Chem. 2015, 63, 91–174. [Google Scholar]
- Karabach, Y.Y.; Kopylovich, M.N.; Mahmudov, K.T.; Pombeiro, A.J.L. Microwave-assisted catalytic oxidation of alcohols to carbonyl compounds. In Advances in Organometallic Chemistry and Catalysis: The Silver/Gold Jubilee International Conference on Organometallic Chemistry Celebratory Book; Pombeiro, A.J.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 18, pp. 233–246. [Google Scholar]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A. Mechanochemical activation and catalysis. In Noncovalent Interactions in Catalysis; Mahmudov, K.T., Kopylovich, M.N., Guedes da Silva, M.F.C., Pombeiro, A.J.L., Eds.; RCS Publishing: Washington, DC, USA, 2019; Volume 25, pp. 548–563. [Google Scholar]
- Silva, T.F.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Half-Sandwich Scorpionate Vanadium, Iron and Copper Complexes: Synthesis and Application in the Catalytic Peroxidative Oxidation of Cyclohexane under Mild Conditions. Adv. Synth. Catal. 2008, 350, 706–716. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, G.; Wu, J.; Li, D.; Liu, Z. Study on enhanced photocatalytic activity of magnetically recoverable Fe3O4@C@TiO2 nanocomposites with core–shell nanostructure. Opt. Mater. 2015, 46, 52–58. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, Y.; Zhang, Y.; Li, Y.; Wang, C.; Zhou, Y.-L. Direct liquid phase deposition fabrication of waxberry-like magnetic Fe3O4@TiO2 core-shell microspheres. Mater. Chem. Phys. 2016, 181, 391–396. [Google Scholar] [CrossRef]
- Morozov, Y.; Sathasivam, S.; Belousova, O.V.; Shishkovsky, I.; Kuznetcov, M.V. Room temperature ferromagnetism in mixed-phase titania nanoparticles produced by the levitation–jet generator. J. Mater. Sci. Mater. Electron. 2018, 29, 3304–3316. [Google Scholar] [CrossRef]
- Timokhin, I.; Pettinari, R.; Marchetti, F.; Pettinari, R.; Condello, F.; Galli, S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Novel Coordination Polymers with (Pyrazolato)-Based Tectons: Catalytic Activity in the Peroxidative Oxidation of Alcohols and Cyclohexane. Cryst. Growth Des. 2015, 15, 2303–2317. [Google Scholar] [CrossRef]
- Sabbatini, A.; Martins, L.M.D.R.S.; Mahmudov, K.T.; Kopylovich, M.N.; Drew, M.G.B.; Pettinari, R.; Pombeiro, A.J.L. Microwave-assisted and solvent-free peroxidative oxidation of 1-phenylethanol to acetophenone with a Cu(II)–TEMPO catalytic system. Catal. Commun. 2014, 48, 69–72. [Google Scholar] [CrossRef]
- Alexandru, M.; Cazacu, M.; Arvinte, A.; Shova, S.; Turta, C.; Simionescu, B.C.; Dobrov, A.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; et al. μ-Chlorido-Bridged Dimanganese(II) Complexes of the Schiff Base Derived from [2+2] Condensation of 2,6-Diformyl-4-methylphenol and 1,3-Bis(3-aminopropyl)tetramethyldisiloxane: Structure, Magnetism, Electrochemical Behaviour, and Catalytic Oxidation of Sec. Eur. J. Inorg. Chem. 2013, 2014, 120–131. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Silva, M.D.F.C.G.D.; Pombeiro, A.J.L. Oxidovanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohols. Appl. Catal. A Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. C-scorpionate complexes: Ever young catalytic tools. Co-Ord. Chem. Rev. 2019, 396, 89–102. [Google Scholar] [CrossRef]
- Martins, N.M.; Pombeiro, A.J.; Martins, L.M.D.R.S. A green methodology for the selective catalytic oxidation of styrene by magnetic metal-transition ferrite nanoparticles. Catal. Commun. 2018, 116, 10–15. [Google Scholar] [CrossRef]
- Duarte, T.A.G.; Carvalho, A.P.; Martins, L.M.D.R.S. Ultra-fast and selective oxidation of styrene to benzaldehyde catalyzed by a C-scorpionate Cu(II) complex. Catal. Sci. Technol. 2018, 8, 2285–2288. [Google Scholar] [CrossRef]
- Duarte, T.A.G.; Carvalho, A.P.; Martins, L.M.D.R.S. Styrene oxidation catalyzed by copper(II) C-scorpionates in homogenous medium and immobilized on sucrose derived hydrochars. Catal. Today 2019, 357, 56–63. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Fang, W.-Y.; Li, Y.-G.; Leng, J.; Chen, X.; Qin, H.-L. SO2F2-Mediated Oxidative Dehydrogenation and Dehydration of Alcohols to Alkynes. J. Am. Chem. Soc. 2018, 140, 17666–17673. [Google Scholar]
- Fry, W.J.; Fry, R.B. Determination of Absolute Sound Levels and Acoustic Absorption Coefficients by Thermocouple Probes—Theory. J. Acoust. Soc. Am. 1954, 26, 294–310. [Google Scholar] [CrossRef] [Green Version]


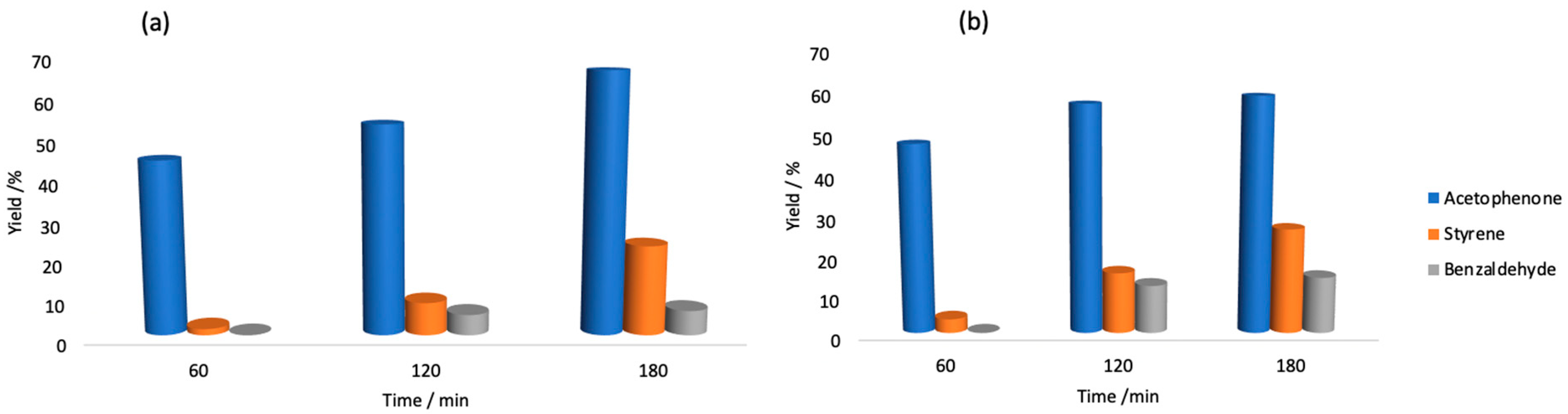

| Entry | t/min | T/°C | Conversion/% | Yield/% b | Total TON c | Total TOF/h−1 d | ||
|---|---|---|---|---|---|---|---|---|
 |  | 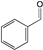 | ||||||
| 1 | 60 | 80 | 48.3 | 44.8 | 1.6 | - | 744 | 744 |
| 2 | 120 | 69.1 | 53.8 | 8.4 | 5.3 | 1082 | 541 | |
| 3 | 180 | 97.1 | 67.2 | 23.1 | 6.4 | 1548 | 516 | |
| 4 e | 180 | 4.0 | 2.5 | - | - | 40 | 13 | |
| 5 f | 180 | 46.9 | 41.7 | 5.2 | - | 2255 | 752 | |
| 6 g | 180 | 96.4 | 83.2 | 10.3 | 2.9 | 772 | 257 | |
| 7 h | 180 | 83.9 | 67.3 | 9.4 | 5.3 | 657 | 219 | |
| 8 | 60 | 120 | 53.1 | 47.6 | 3.5 | - | 820 | 820 |
| 9 | 120 | 95.3 | 57.4 | 15.3 | 12.1 | 1359 | 680 | |
| 10 | 180 | 100.0 | 59.3 | 26.4 | 14.2 | 1601 | 534 | |
| 11 i | 120 | 100.0 | - | - | 3 | 37 | 12 | |
| 12 i | 180 | 9.9 | - | - | 7 | 86 | 29 | |
| Cycle | Yield/% b | Total TON c | Total TOF / h−1 d | Conversion/% | ||
|---|---|---|---|---|---|---|
 |  | 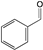 | ||||
| 1st | 59.3 | 26.4 | 14.2 | 3202 | 1067 | 100.0 |
| 2nd | 22.4 | 1.6 | - | 769 | 256 | 25.9 |
| 3rd | 8.1 | - | - | 260 | 87 | 8.3 |
| Entry | Time/min | Temperature/°C | Yield/% b | TON c | TOF/h−1 d | Conversion/% |
|---|---|---|---|---|---|---|
| 1 | 60 | 80 | 1.6 | 51 | 51 | 1.8 |
| 2 | 120 | 2.6 | 83 | 42 | 2.7 | |
| 3 | 180 | 4.6 | 147 | 49 | 4.8 | |
| 4 | 60 | 120 | 9.5 | 305 | 305 | 10.0 |
| 5 | 120 | 12.2 | 391 | 196 | 12.2 | |
| 6 | 180 | 15.5 | 497 | 166 | 15.6 |
| Entry | Energy Input | Time/min | Yield/% b | TON c | TOF/h−1 d | Selectivity/% |
|---|---|---|---|---|---|---|
| 1 | Mechanical | 60 | 2.5 | 80 | 80 | 96 |
| 2 | 180 | 6.0 | 192 | 64 | 94 | |
| 3 | Ultrasounds | 10 | 1.7 | 54 | 327 | 98 |
| 4 | 15 | 3.9 | 125 | 500 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matias, I.A.S.; Ribeiro, A.P.C.; Ferraria, A.M.; Rego, A.M.B.d.; Martins, L.M.D.R.S. Catalytic Performance of a Magnetic Core-Shell Iron(II) C-Scorpionate under Unconventional Oxidation Conditions. Nanomaterials 2020, 10, 2111. https://doi.org/10.3390/nano10112111
Matias IAS, Ribeiro APC, Ferraria AM, Rego AMBd, Martins LMDRS. Catalytic Performance of a Magnetic Core-Shell Iron(II) C-Scorpionate under Unconventional Oxidation Conditions. Nanomaterials. 2020; 10(11):2111. https://doi.org/10.3390/nano10112111
Chicago/Turabian StyleMatias, Inês A. S., Ana P. C. Ribeiro, Ana M. Ferraria, Ana M. Botelho do Rego, and Luísa M. D. R. S. Martins. 2020. "Catalytic Performance of a Magnetic Core-Shell Iron(II) C-Scorpionate under Unconventional Oxidation Conditions" Nanomaterials 10, no. 11: 2111. https://doi.org/10.3390/nano10112111
APA StyleMatias, I. A. S., Ribeiro, A. P. C., Ferraria, A. M., Rego, A. M. B. d., & Martins, L. M. D. R. S. (2020). Catalytic Performance of a Magnetic Core-Shell Iron(II) C-Scorpionate under Unconventional Oxidation Conditions. Nanomaterials, 10(11), 2111. https://doi.org/10.3390/nano10112111