Self-Assembly of Single-Polymer-Tethered Nanoparticle Amphiphiles upon Varying Tail Length
Abstract
1. Introduction
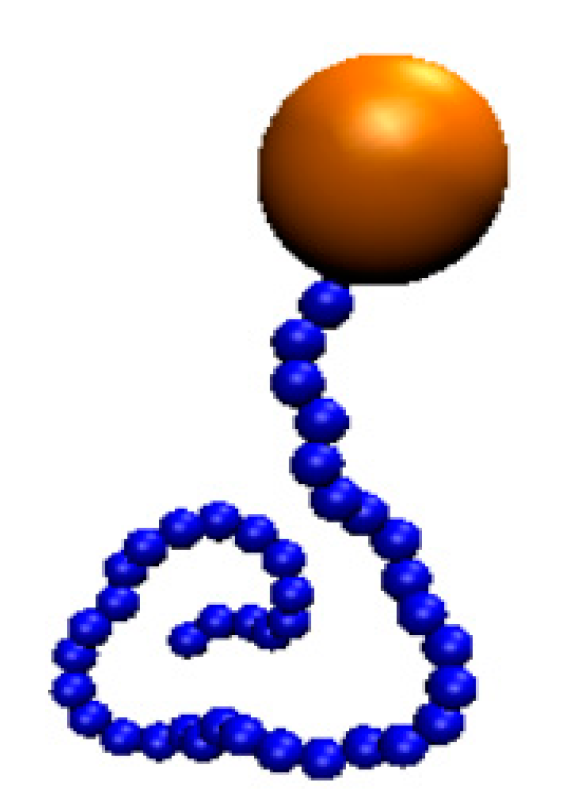
2. Simulation and Method
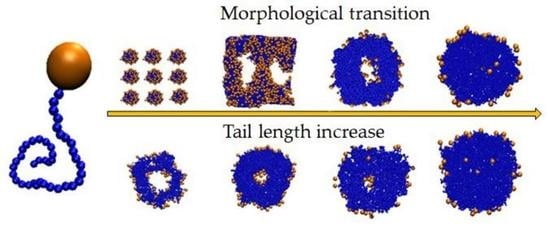
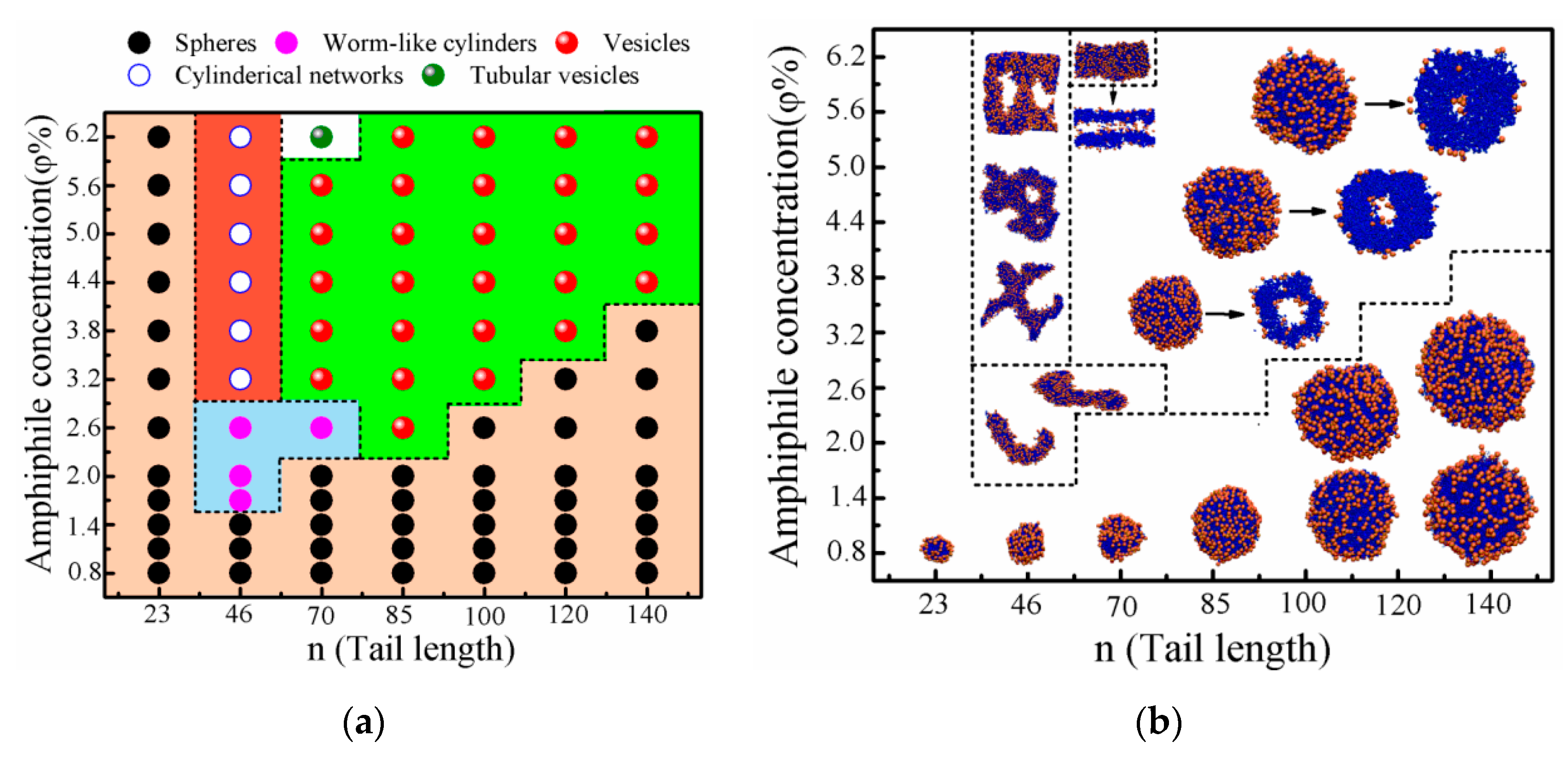
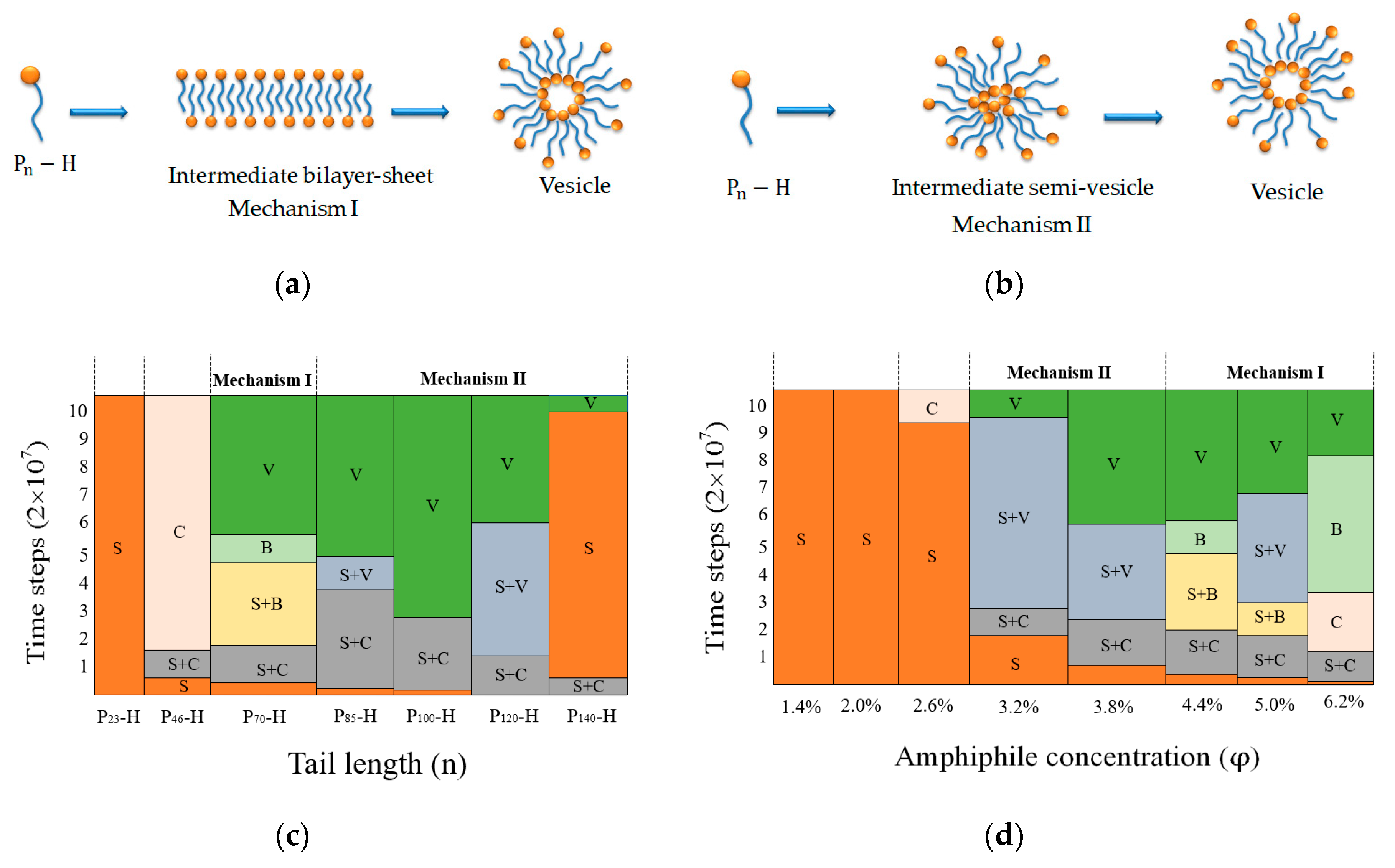
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Zhang, W.B.; Yue, K.; Li, X.; Liu, H.; Xin, Y.; Wang, C.L.; Wesdemiotis, C.; Cheng, S.Z.D. Giant Molecular Shape Amphiphiles Based on Polystyrene-Hydrophilic [60] Fullerene Conjugates: Click Synthesis, Solution Self-Assembly, and Phase Behavior. J. Am. Chem. Soc. 2012, 134, 7780–7787. [Google Scholar] [CrossRef]
- Kawauchi, T.; Kumaki, J.; Yashima, E. Synthesis, Isolation via Self-Assembly, and Single-Molecule Observation of a [60] Fullerene-End-Capped Isotactic Poly(methyl methacrylate). J. Am. Chem. Soc. 2005, 127, 9950–9951. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Dong, X.H.; Yue, K.; Lin, Z.; Feng, X.; Huang, M.; Zhang, W.B.; Cheng, S.Z.D. Giant Surfactants Based on Molecular Nanoparticles: Precise Synthesis and Solution Self-assembly. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1309–1325. [Google Scholar] [CrossRef]
- Zhang, W.B.; Yu, X.; Wang, C.L.; Sun, H.J.; Hsieh, I.F.; Li, Y.; Dong, X.H.; Yue, K.; Van Horn, R.; Cheng, S.Z.D. Molecular Nanoparticles Are Unique Elements for Macromolecular Science: From “Nanoatoms” to Giant Molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Yu, X.; Yue, K.; Hsieh, I.F.; Li, Y.; Dong, X.H.; Liu, C.; Xin, Y.; Wang, H.F.; Shi, A.C.; Newkome, G.R.; et al. Giant Surfactants Provide a Versatile Platform for Sub-10-nm Nanostructure Engineering. Proc. Natl. Acad. Sci. USA 2013, 110, 10078–10083. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhong, S.; Li, X.; Tu, Y.; Yang, S.; Van Horn, R.M.; Ni, C.; Pochan, D.J.; Quirk, R.P.; Wesdemiotis, C.; et al. A Giant Surfactant of Polystyrene-(Carboxylic Acid-Functionalized Polyhedral Oligomeric Silsesquioxane) Amphiphile with Highly Stretched Polystyrene Tails in Micellar Assemblies. J. Am. Chem. Soc. 2010, 132, 16741–16744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, B.; Walther, A.; Müller, A.H.E. Synthesis via RAFT Polymerization of Tadpole-Shaped Organic/Inorganic Hybrid Poly(acrylic acid) Containing Polyhedral Oligomeric Silsesquioxane (POSS) and Their Self-assembly in Water. Macromolecules 2009, 42, 2563–2569. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Dong, X.H.; Yu, X.; Guo, K.; Su, H.; Yue, K.; Wesdemiotis, C.; Cheng, S.Z.D.; Zhang, W.B. Giant Gemini Surfactants Based on Polystyrene-hydrophilic Polyhedral Oligomeric Silsesquioxane Shape Amphiphiles: Sequential “Click” Chemistry and Solution Self-assembly. Chem. Sci. 2013, 4, 1345–1352. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H. Surfactant Behavior of Amphiphilic Polymer-Tethered Nanoparticles. Langmuir 2016, 32, 3567–3579. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, J.; Zhang, Y.; Yang, Y.; Zhao, H. Controlled Self-assembly of Amphiphilic Monotailed Single-chain Nanoparticles. Polym. Chem. 2014, 5, 4032–4038. [Google Scholar] [CrossRef]
- Ma, S.; Hu, Y.; Wang, R. Self-Assembly of Polymer Tethered Molecular Nanoparticle Shape Amphiphiles in Selective Solvents. Macromolecules 2015, 48, 3112–3120. [Google Scholar] [CrossRef]
- Wang, C.; Ma, S.; Hu, Y.; Wang, R. Hierarchical Colloidal Polymeric Structure from Surfactant-Like Amphiphiles in Selective Solvents. Langmuir 2017, 33, 3427–3433. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z.; Yin, Y.; Jiang, R.; Li, B. Self-assembly of Giant Amphiphiles Based on Polymer-tethered Nanoparticle in Selective Solvents. Macromolecules 2018, 51, 3050–3058. [Google Scholar] [CrossRef]
- Iacovella, C.R.; Glotzer, S.C. Phase Behavior of Ditethered Nanospheres. Soft Matter 2009, 5, 4492–4498. [Google Scholar] [CrossRef]
- Iacovella, C.R.; Glotzer, S.C. Complex Crystal Structures Formed by the Self-Assembly of Ditethered Nanospheres. Nano Lett. 2009, 9, 1206–1211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iacovella, C.R.; Horsch, M.A.; Zhang, Z.; Glotzer, S.C. Phase Diagrams of Self-Assembled Mono-Tethered Nanospheres from Molecular Simulation and Comparison to Surfactants. Langmuir 2005, 21, 9488–9494. [Google Scholar] [CrossRef]
- Zhang, Z.; Horsch, M.A.; Lamm, M.H.; Glotzer, S.C. Tethered Nano Building Blocks: Toward a Conceptual Framework for Nanoparticle Self-Assembly. Nano Lett. 2003, 3, 1341–1346. [Google Scholar] [CrossRef]
- Horsch, M.A.; Zhang, Z.; Glotzer, S.C. Self-Assembly of Laterally-Tethered Nanorods. Nano Lett. 2006, 6, 2406–2413. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, E.R.; Glotzer, S.C. Self-assembled Morphologies of Monotethered Polyhedral Oligomeric Silsesquioxane Nanocubes from Computer Simulation. J. Chem. Phys. 2005, 123, 184718. [Google Scholar] [CrossRef]
- Horsch, M.A.; Zhang, Z.; Glotzer, S.C. Self-Assembly of Polymer-Tethered Nanorods. Phys. Rev. Lett. 2005, 95, 056105. [Google Scholar] [CrossRef]
- Horsch, M.A.; Zhang, Z.; Glotzer, S.C. Simulation Studies of Self-assembly of End-tethered Nanorods in Solution and Role of Rod Aspect Ratio and Tether Length. J. Chem. Phys. 2006, 125, 184903. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Glotzer, S.C. Effect of Nanoparticle Polydispersity on the Self-assembly of Polymer Tethered Nanospheres. J. Chem. Phys. 2012, 137, 104901. [Google Scholar] [CrossRef]
- Phillips, C.L.; Iacovella, C.R.; Glotzer, S.C. Stability of the Double Gyroid Phase to Nanoparticle Polydispersity in Polymer-tethered Nanosphere Systems. Soft Matter 2010, 6, 1693–1703. [Google Scholar] [CrossRef]
- Floudas, G.; Hadjichristidis, N.; Stamm, M.; Likhtman, A.E.; Semenov, A.N. Microphase separation in block copolymer/homopolymer blends: Theory and experiment. J. Chem. Phys. 1997, 106, 3318–3328. [Google Scholar] [CrossRef]
- Rosales, A.M.; Mcculloch, B.L.; Zuckermann, R.N.; Segalman, R.A. Tunable Phase Behavior of Polystyrene-Polypeptoid Block Copolymers. Macromolecules 2012, 45, 6027–6035. [Google Scholar] [CrossRef]
- Tager, A.A.; Adamova, L.V.; Kolmakova, L.K.; Nokhrina, N.N.; Valetskii, P.M.; Rogovina, L.Z.; Starozhuk, I.P. Thermodynamics of the compatibility of blocks in block copolymers. Polym. Sci. USSR 1982, 24, 2331–2339. [Google Scholar] [CrossRef]
- Liu, F.Q.; Sun, T.J.; Tang, P.; Zhang, H.D.; Qiu, F. Understanding chain folding morphology of semicrystalline polymers based on a rod-coil multiblock model. Soft Matter 2017, 13, 8250–8263. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Goswami, M.; Kumar, R.; Sumpter, B.G.; Mays, J. Morphologies of block copolymers composed of charged and neutral blocks. Soft Matter 2012, 8, 3036–3052. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Drechsler, M.; Garamus, V.M.; Mutafchieva, R.; Lesieur, S. Identification of large channels in cationic PEGylated cubosome nanoparticles by synchrotron radiation SAXS and Cryo-TEM imaging. Soft Matter 2015, 11, 3686–3692. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Eisenberg, A. Thermodynamic vs Kinetic Aspects in the Formation and Morphological Transitions of Crew-Cut Aggregates Produced by Self-Assembly of Polystyrene-b-poly(acrylic acid) Block Copolymers in Dilute Solution. Macromolecules 1999, 32, 2239–2249. [Google Scholar] [CrossRef]
- Shen, H.; Eisenberg, A. Morphological Phase Diagram for a Ternary System of Block Copolymer PS310-b-PAA52/Dioxane/H2O. J. Phys. Chem. B 1999, 103, 9473–9487. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Eisenberg, A. Morphogenic Effect of Solvent on Crew-Cut Aggregates of Apmphiphilic Diblock Copolymers. Macromolecules 1998, 31, 1144–1154. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. J. Chem. Soc. Faraday Trans. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Du, B.; Mei, A.; Yin, K.; Zhang, Q.; Xu, J.; Fan, Z. Vesicle Formation of PLAx-PEG44 Diblock Copolymers. Macromolecules 2009, 42, 8477–8484. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. Multiple Morphologies of “Crew-cut” Aggregates of Polystyrene-b-poly(acrylic acid) Block Copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef]
- Denkova, A.G.; Bomans, P.H.H.; Coppens, M.O.; Sommerdijk, N.A.J.M.; Mendes, E. Complex Morphologies of Self-assembled Block Copolymer Micelles in Binary Solvent Mixtures: The Role of Solvent-solvent Correlations. Soft Matter 2011, 7, 6622–6628. [Google Scholar] [CrossRef]
- Zhong, S.; Cui, H.; Chen, Z.; Wooleyb, K.L.; Pochan, D.J. Helix Self-assembly Through the Coiling of Cylindrical Micelles. Soft Matter 2008, 4, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chung, B.; Jung, J.; Park, H.W.; Chang, T. Toroidal Micelles of Uniform Size from Diblock Copolymers. Angew. Chem. Int. Ed. 2009, 48, 4594–4597. [Google Scholar] [CrossRef]
- Marrink, S.J.; Mark, A.E. Molecular Dynamics Simulation of the Formation, Structure, and Dynamics of Small Phospholipid Vesicles. J. Am. Chem. Soc. 2003, 125, 15233–15242. [Google Scholar] [CrossRef]
- Noguchi, H.; Takasu, M. Self-assembly of Amphiphiles into Vesicles: A Brownian Dynamics Simulation. Phys. Rev. E 2001, 64, 041913. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Maruyama, Y.; Hyodo, S. Dissipative Particle Dynamics Study of Spontaneous Vesicle Formation of Amphiphilic Molecules. J. Chem. Phys. 2002, 116, 5842–5849. [Google Scholar] [CrossRef]
- Uneyama, T. Density Functional Simulation of Spontaneous Formation of Vesicle in Block Copolymer Solutions. J. Chem. Phys. 2007, 126, 114902. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xia, G.; Wang, R.; Xie, D. Controlling the Self-assembly Pathways of Amphiphilic Block Copolymers into Vesicles. Soft Matter 2012, 8, 7865–7874. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Qian, C. Simulation Study on the Formation of Vesicle and Influence of Solvent. J. Chem. Phys. 2009, 131, 234902. [Google Scholar] [CrossRef]
- Han, Y.; Yu, H.; Du, H.; Jiang, W. Effect of Selective Solvent Addition Rate on the Pathways for Spontaneous Vesicle Formation of ABA Amphiphilic Triblock Copolymers. J. Am. Chem. Soc. 2010, 132, 1144–1150. [Google Scholar] [CrossRef]
- He, X.; Liang, H.; Huang, L.; Pan, C. Complex Microstructures of Amphiphilic Diblock Copolymer in Dilute Solution. J. Phys. Chem. B 2004, 108, 1731–1735. [Google Scholar] [CrossRef]
- He, X.; Schmid, F. Dynamics of Spontaneous Vesicle Formation in Dilute Solutions of Amphiphilic Diblock Copolymers. Macromolecules 2006, 39, 2654–2662. [Google Scholar] [CrossRef]
- He, X.; Schmid, F. Spontaneous Formation of Complex Micelles from a Homogeneous Solution. Phys. Rev. Lett. 2008, 100, 137802. [Google Scholar] [CrossRef]
- Anderson, J.A.; Travesset, A. Coarse-Grained Simulations of Gels of Nonionic Multiblock Copolymers with Hydrophobic Groups. Macromolecules 2006, 39, 5143–5151. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.; Qian, H.J.; Lu, Z.Y. Coarse-grained Simulation Study on the Self-assembly of Miktoarm Star-like Block Copolymers in Various Solvent Conditions. Soft Matter 2014, 10, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Grest, G.S.; Kremer, K. Molecular Dynamics Simulation for Polymers in the Presence of a Heat Bath. Phys. Rev. A 1986, 33, 3628–3631. [Google Scholar] [CrossRef]
- Li, B.; Zhu, Y.L.; Liu, H.; Lu, Z.Y. Brownian Dynamics Simulation Study on the Self-assembly of Incompatible Star-like Block Copolymers in Dilute Solution. Phys. Chem. Chem. Phys. 2012, 14, 4964–4970. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Lorenz, C.D.; Travesset, A. General Purpose Molecular Dynamics Simulations Fully Implemented on Graphics Processing Units. J. Comput. Phys. 2008, 227, 5342–5359. [Google Scholar] [CrossRef]
- Glaser, J.; Nguyen, T.D.; Anderson, J.A.; Lui, P.; Spiga, F.; Millan, J.A.; Morse, D.C.; Glotzer, S.C. Strong Scaling of General-purpose Molecular Dynamics Simulations on GPUs. Comput. Phys. Commun. 2015, 192, 97–107. [Google Scholar] [CrossRef]
- HOOMD-Blue Web Page. Available online: http://glotzerlab.engin.umich.edu/hoomd-blue (accessed on 28 October 2018).
- Zhu, Y.L.; Liu, H.; Li, Z.W.; Qian, H.J.; Milano, G.; Lu, Z.Y. GALAMOST: GPU-accelerated Large-scale Molecular Simulation Toolkit. J. Comput. Chem. 2013, 34, 2197–2211. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xiao, M.; Wang, R. Formation and Structural Characteristics of Thermosensitive Multiblock Copolymer Vesicles. Langmuir 2013, 29, 16010–16017. [Google Scholar] [CrossRef]

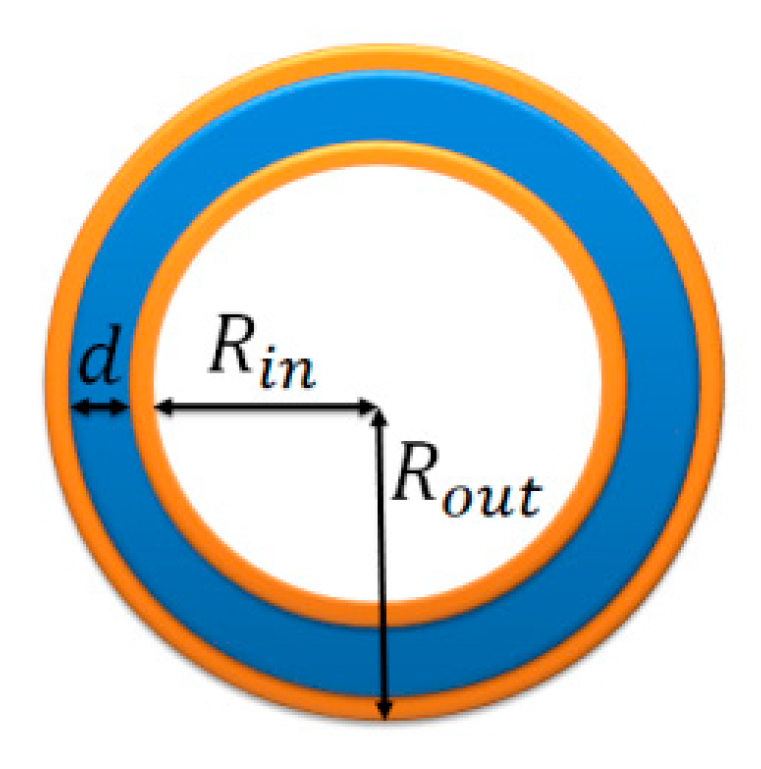
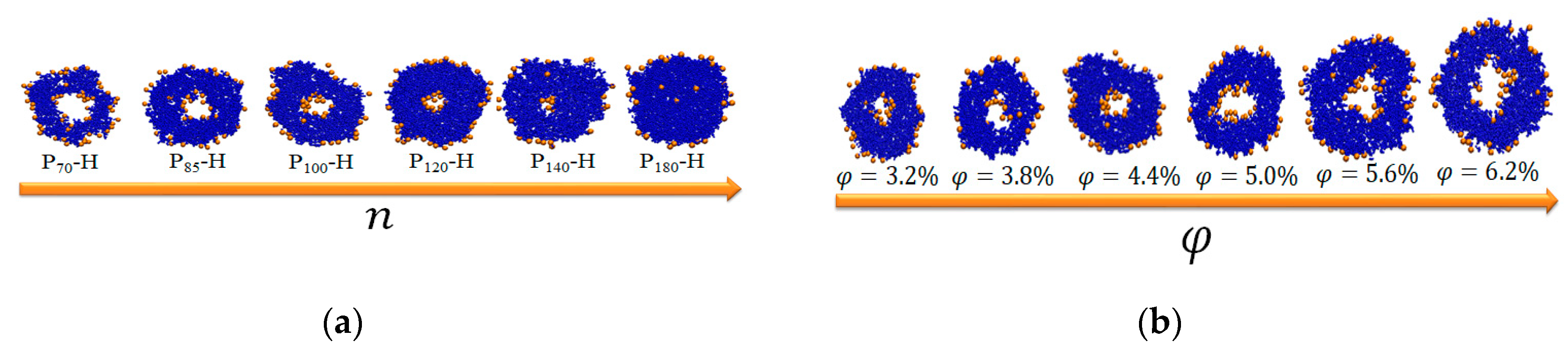
| n | 1 | |||
|---|---|---|---|---|
| 70 | 867 | 15.02 | 35.42 | 20.40 |
| 85 | 751 | 14.20 | 35.74 | 21.54 |
| 100 | 662 | 12.17 | 36.38 | 24.21 |
| 120 | 572 | 8.71 | 36.71 | 28.00 |
| 140 | 504 | 3.32 | 36.73 | 33.41 |
| 180 | 406 | 0.00 | 37.17 | 37.17 |
| 1 | ||||
|---|---|---|---|---|
| 3.2% | 482 | 7.05 | 31.49 | 24.44 |
| 3.8% | 572 | 10.04 | 34.33 | 24.29 |
| 4.4% | 662 | 12.17 | 36.38 | 24.21 |
| 5.0% | 753 | 13.04 | 37.19 | 24.15 |
| 5.6% | 843 | 14.92 | 39.02 | 24.10 |
| 6.2% | 933 | 15.49 | 39.51 | 24.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhu, Y.-L.; Zhang, X.; Xu, K.; Wang, J.; Li, Z.; Bao, Y. Self-Assembly of Single-Polymer-Tethered Nanoparticle Amphiphiles upon Varying Tail Length. Nanomaterials 2020, 10, 2108. https://doi.org/10.3390/nano10112108
Li Q, Zhu Y-L, Zhang X, Xu K, Wang J, Li Z, Bao Y. Self-Assembly of Single-Polymer-Tethered Nanoparticle Amphiphiles upon Varying Tail Length. Nanomaterials. 2020; 10(11):2108. https://doi.org/10.3390/nano10112108
Chicago/Turabian StyleLi, Qingxiao, You-Liang Zhu, Xinhui Zhang, Kaidong Xu, Jina Wang, Zhixin Li, and Yun Bao. 2020. "Self-Assembly of Single-Polymer-Tethered Nanoparticle Amphiphiles upon Varying Tail Length" Nanomaterials 10, no. 11: 2108. https://doi.org/10.3390/nano10112108
APA StyleLi, Q., Zhu, Y.-L., Zhang, X., Xu, K., Wang, J., Li, Z., & Bao, Y. (2020). Self-Assembly of Single-Polymer-Tethered Nanoparticle Amphiphiles upon Varying Tail Length. Nanomaterials, 10(11), 2108. https://doi.org/10.3390/nano10112108