Preparation of Fe3+ Doped High-Ordered TiO2 Nanotubes Arrays with Visible Photocatalytic Activities
Abstract
1. Introduction
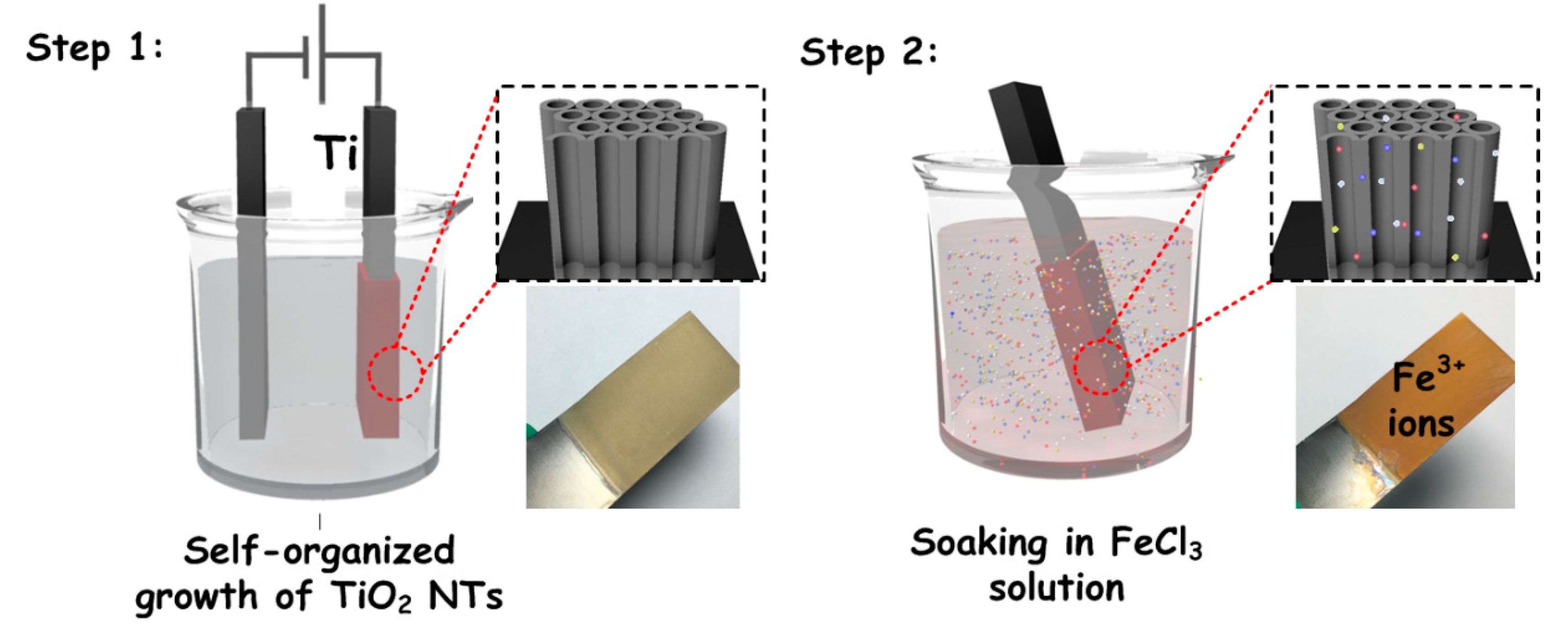
2. Materials and Methods
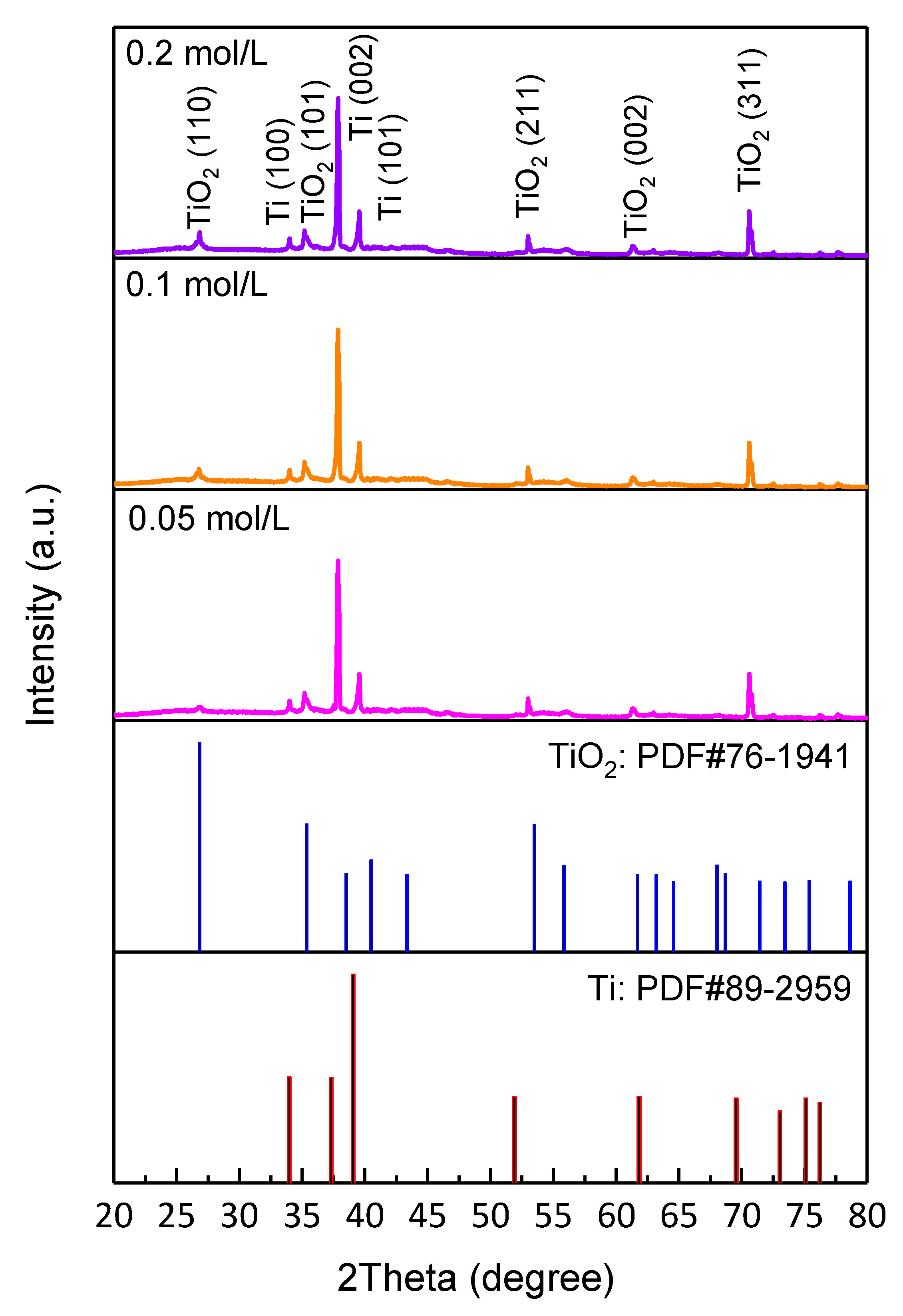
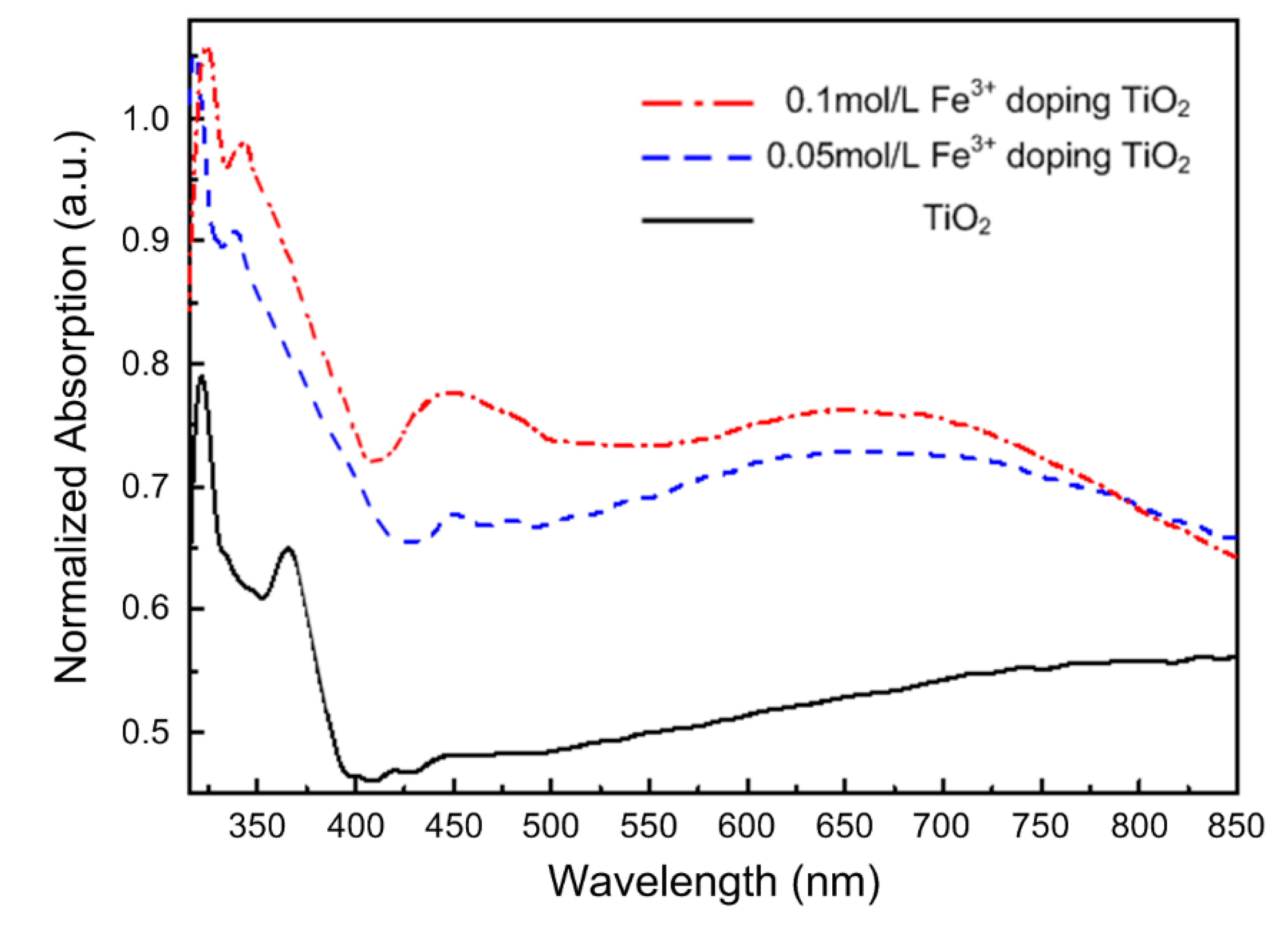
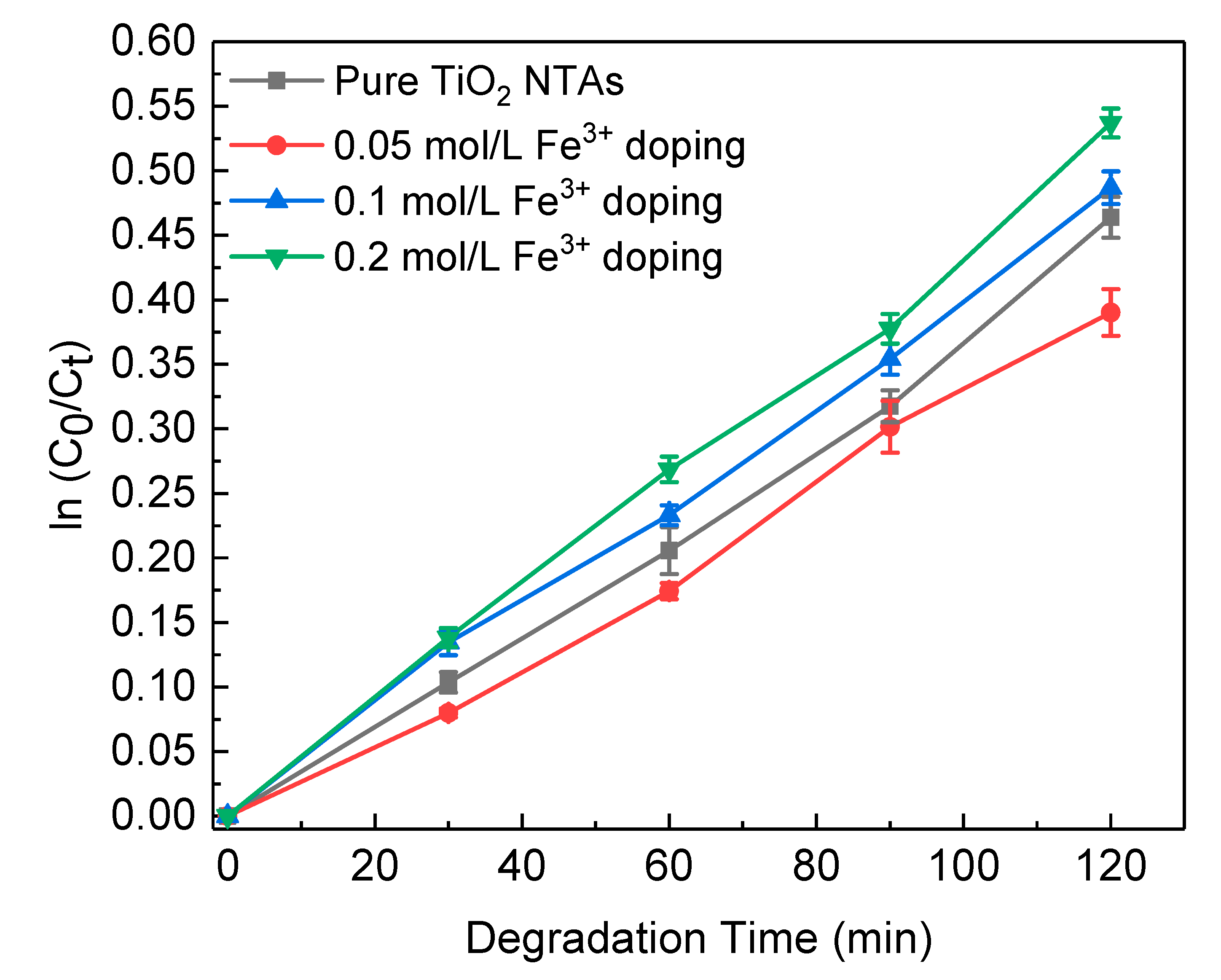
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef]
- Gopalan, A.-I.; Lee, J.-C.; Saianand, G.; Lee, K.-P.; Sonar, P.; Dharmarajan, R.; Hou, Y.-L.; Ann, K.-Y.; Kannan, V.; Kim, W.-J. Recent Progress in the Abatement of Hazardous Pollutants Using Photocatalytic TiO2-Based Building Materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Paulose, M.; Prakasam, H.E.; Varghese, O.K.; Peng, L.; Popat, K.C.; Mor, G.K.; Desai, T.A.; Grimes, C.A. TiO2 Nanotube Arrays of 1000 μm Length by Anodization of Titanium Foil: Phenol Red Diffusion. J. Phys. Chem. C 2007, 111, 14992–14997. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Jaramillo, H.A.; Medina, F. Synthesis of the ZnTiO3/TiO2 Nanocomposite Supported in Ecuadorian Clays for the Adsorption and Photocatalytic Removal of Methylene Blue Dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef]
- Quan, X.; Yang, S.; Ruan, X.; Zhao, H. Preparation of Titania Nanotubes and Their Environmental Applications as Electrode. Environ. Sci. Technol. 2005, 39, 3770–3775. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kuk, Y.-S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Huang, B. Fabrication and Characterization of Visible-Light-Driven Plasmonic Photocatalyst Ag/AgCl/TiO2 Nanotube Arrays. J. Phys. Chem. C 2009, 113, 16394–16401. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiya, H.; Schmuki, P. High-Aspect-Ratio TiO2 Nanotubes by Anodization of Titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Mark, K.; Schmuki, P. Nanosize and Vitality: TiO2 Nanotube Diameter Directs Cell Fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Basham, J.I.; Allam, N.K.; Varghese, O.K.; Mor, G.K.; Feng, X.; Paulose, M.; Seabold, J.A.; Choi, K.S.; Grimes, C.A. Recent Advances in the Use of TiO2 Nanotube and Nanowire Arrays for Oxidative Photoelectrochemistry. J. Phys. Chem. C 2009, 113, 6327–6359. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Bard, A.J. Novel Carbon-Doped TiO2 Nanotube Arrays with High Aspect Ratios for Efficient Solar Water Splitting. Nano Lett. 2006, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhou, B.; Bai, J.; Li, L.; Jin, Z.; Zhang, J.; Li, J.; Liu, Y.; Cai, W.; Zhu, X. Self-Organized TiO2 Nanotube Array Sensor for the Determination of Chemical Oxygen Demand. Adv. Mater. 2008, 20, 1044–1049. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Jeon, T.H.; Choi, W. Hydrogenated heterojunction of boron nitride and titania enables the photocatalytic generation of H2 in the absence of noble metal catalysts. Appl. Catal. B-Environ. 2018, 237, 772–782. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Lin, L.; Jeon, T.H.; Lin, S.; Wang, X.; Choi, W. Formation of heterostructures via direct growth CN on h-BN porous nanosheets for metal-free photocatalysis. Nano Energy 2017, 42, 58–68. [Google Scholar] [CrossRef]
- Klosek, S.; Raftery, D. Visible Light Driven V-Doped TiO2 Photocatalyst and Its Photooxidation of Ethanol. J. Phys. Chem. B 2001, 105, 2815–2819. [Google Scholar] [CrossRef]
- Liu, Z.; Pesic, B.; Raja, K.S.; Rangaraju, R.R.; Misra, M. Hydrogen generation under sunlight by self ordered TiO2 nanotube arrays. Int. J. Hydrogen Energy 2009, 34, 3250–3257. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, X.; Wang, C. Electrospun mesoporous W6+-doped TiO2 thin films for efficient visible-light photocatalysis. Mater. Lett. 2009, 63, 331–333. [Google Scholar] [CrossRef]
- Asiltürk, M.; Sayılkan, F.; Arpaç, E. Effect of Fe3+ ion doping to TiO2 on the photocatalytic degradation of Malachite Green dye under UV and vis-irradiation. J. Photochem. Photobiol. A 2009, 203, 64–71. [Google Scholar] [CrossRef]
- Devi, L.G.; Kottam, N.; Murthy, B.N.; Kumar, S.G. Enhanced photocatalytic activity of transition metal ions Mn2+, Ni2+ and Zn2+ doped polycrystalline titania for the degradation of Aniline Blue under UV/solar light. J. Mol. Catal. A Chem. 2010, 328, 44–52. [Google Scholar] [CrossRef]
- Kuljanin-Jakovljević, J.; Radoičić, M.; Radetić, T.; Konstantinović, Z.; Šaponjić, Z.V.; Nedeljković, J. Presence of Room Temperature Ferromagnetism in Co2+ Doped TiO2 Nanoparticles Synthesized through Shape Transformation. J. Phys. Chem. C 2009, 113, 21029–21033. [Google Scholar] [CrossRef]
- Lin, H.J.; Yang, T.S.; Hsi, C.S.; Wang, M.C.; Lee, K.C. Optical and photocatalytic properties of Fe3+-doped TiO2 thin films prepared by a sol–gel spin coating. Ceram. Int. 2014, 40, 10633–10640. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Xu, Y.; Niu, X.; Zheng, L.; Ding, X. The preparation of Zn2+-doped TiO2 nanoparticles by sol–gel and solid phase reaction methods respectively and their photocatalytic activities. Chemosphere 2005, 59, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Miyauchi, M. Ce-doped ZnO (CexZn1−xO) becomes an efficient visible-light-sensitive photocatalyst by co-catalyst (Cu2+) grafting. Phys. Chem. Chem. Phys. 2011, 13, 14937–14945. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Wang, C.L.; Li, S.F.; Chen, H.B.; Lin, C.J. An electrochemical strategy of doping Fe3+ into TiO2 nanotube array films for enhancement in photocatalytic activity. Sol. Energy Mater. Sol. Cells 2009, 93, 1875–1880. [Google Scholar] [CrossRef]
- Tong, T.; Zhang, J.; Tian, B.; Chen, F.; He, D. Preparation of Fe3+-doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J. Hazard. Mater. 2008, 155, 572–579. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, J.; Deng, P.; Wang, X.; Li, Y.; Wen, T.; Li, Y.; Xiang, Q.; Liao, Y. Hydrogen evolution promotion of Au-nanoparticles-decorated TiO2 nanotube arrays prepared by dip-loading approach. J. Am. Ceram. Soc. 2019, 102, 5873–5880. [Google Scholar] [CrossRef]
- Buschow, K.H.J. Intermetallic compounds of rare-earth and 3d transition metals. Rep. Prog. Phys. 1977, 40, 1179. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Modification of the surface electronic and chemical properties of Pt (111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic Effect of Nanoparticle 3d-Transition Metals on Hydrogen Storage Properties in Magnesium Hydride MgH2 Prepared by Mechanical Milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Mittal, M.; Sharma, M.; Pandey, O.P. UV–Visible light induced photocatalytic studies of Cu doped ZnO nanoparticles prepared by co-precipitation method. Sol. Energy 2014, 110, 386–397. [Google Scholar] [CrossRef]
- Lei, C.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar]
- Yin, X.; Guo, Y.; Xie, H.; Que, W.; Kong, L.B. Nickel Oxide as Efficient Hole Transport Materials for Perovskite Solar Cells. Sol. RRL 2019, 3, 1900001. [Google Scholar] [CrossRef]
- Alexandrescu, R.; Morjan, I.; Scarisoreanu, M.; Birjega, R.; Popovici, E.; Soare, I.; Gavrila-Florescu, L.; Voicu, I.; Sandu, I.; Dumitrache, F.; et al. Structural investigations on TiO2 and Fe-doped TiO2 nanoparticles synthesized by laser pyrolysis. Thin Solid Films 2007, 515, 8438–8445. [Google Scholar] [CrossRef]
- Vignesh, K.; Rajarajan, M.; Suganthi, A. Visible light assisted photocatalytic performance of Ni and Th co-doped ZnO nanoparticles for the degradation of methylene blue dye. J. Ind. Eng. Chem. 2014, 20, 3826–3833. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Z.; Gong, C.; Xiao, W.; Sun, L.; Lin, C. Fe3+-Doped TiO2 Nanotube Arrays on Ti-Fe Alloys for Enhanced Photoelectrocatalytic Activity. Nanomaterials 2016, 6, 107. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z. Fe3+ doped TiO2 nanotubes for combined adsorption–sonocatalytic degradation of real textile wastewater. Appl. Catal. B-Environ. 2013, 129, 473–481. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmidt, B.; Kunze, J.; Schmuki, P. Photoresponse in the visible range from Cr doped TiO2 nanotubes. Chem. Phys. Lett. 2007, 433, 323–326. [Google Scholar] [CrossRef]
- Shen, X.Z.; Guo, J.; Liu, Z.C.; Xie, S.M. Visible-light-driven titania photocatalyst co-doped with nitrogen and ferrum. Appl. Surf. Sci. 2008, 254, 4726–4731. [Google Scholar] [CrossRef]
- Su, Y.L.; Xiao, Y.T.; Li, Y.; Du, Y.X.; Zhang, Y.L. Preparation, photocatalytic performance and electronic structures of visible-light-driven Fe–N-codoped TiO2 nanoparticles. Mater. Chem. Phys. 2011, 126, 761–768. [Google Scholar] [CrossRef]
- Xin, B.; Ren, Z.; Wang, P.; Liu, J.; Jing, L.; Fu, H. Study on the mechanisms of photoinduced carriers separation and recombination for Fe3+–TiO2 photocatalysts. Appl. Surf. Sci. 2007, 253, 4390–4395. [Google Scholar] [CrossRef]

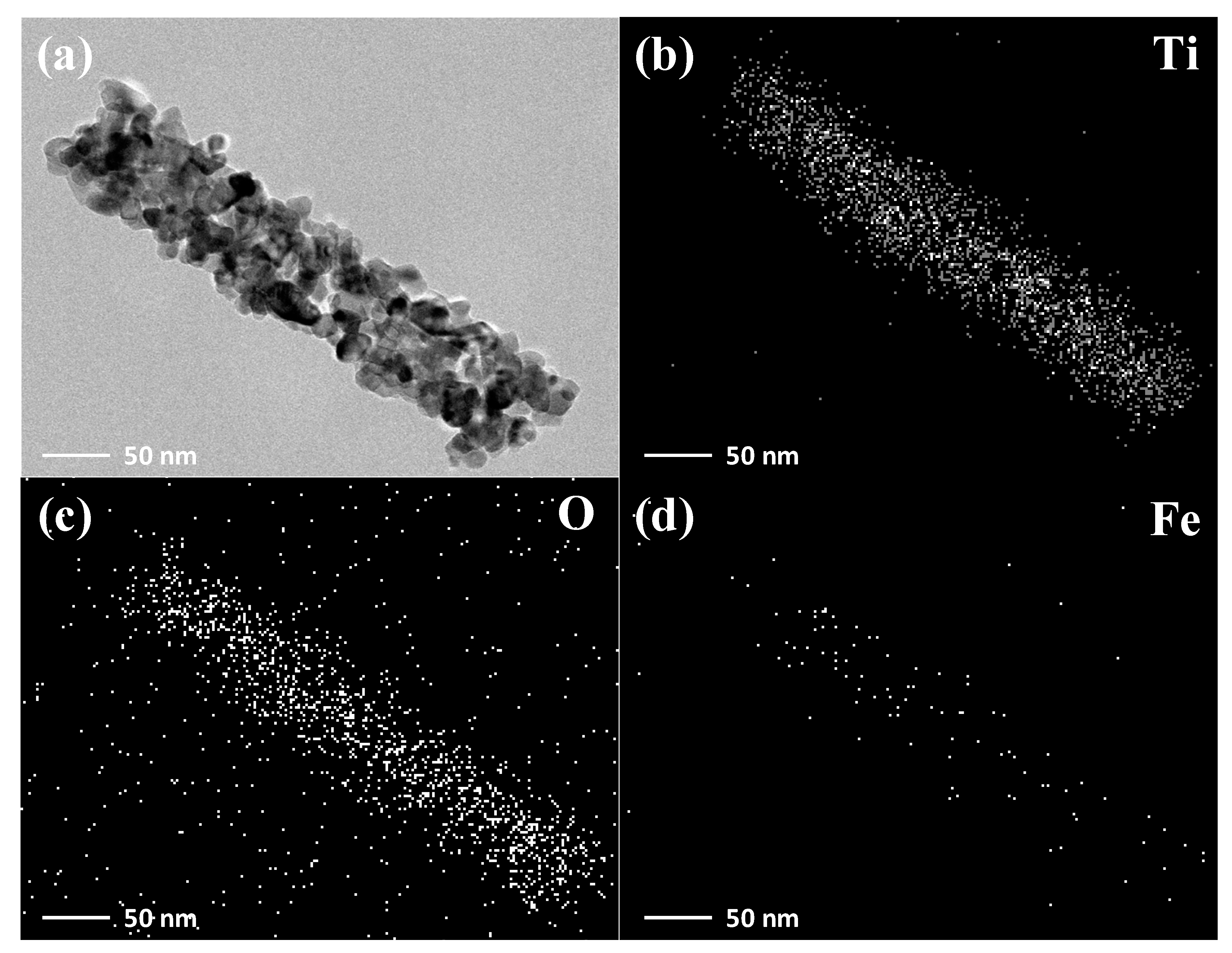


| Samples | Ti | O | Fe |
|---|---|---|---|
| 0.05 mol/L Fe3+ | 47.64 | 50.02 | 2.34 |
| 0.1 mol/L Fe3+ | 46.18 | 49.36 | 4.46 |
| 0.2 mol/L Fe3+ | 40.98 | 50.49 | 8.53 |
| Samples | a = b | c | d Value (Å) | ||||
|---|---|---|---|---|---|---|---|
| (110) | (101) | (211) | (002) | (311) | |||
| Pure | 4.645 | 2.992 | 3.285 | 2.515 | 1.706 | 1.496 | 1.319 |
| 0.05 mol/L Fe3+ | 4.642 | 2.991 | 3.282 | 2.514 | 1.705 | 1.496 | 1.318 |
| 0.1 mol/L Fe3+ | 4.640 | 2.988 | 3.281 | 2.512 | 1.704 | 1.494 | 1.317 |
| 0.2 mol/L Fe3+ | 4.638 | 2.987 | 3.280 | 2.511 | 1.704 | 1.494 | 1.317 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, C.; Li, S.; Xi, Y.; Cai, C.; Liu, W.; Golosov, D.; Zavadski, S.; Melnikov, S. Preparation of Fe3+ Doped High-Ordered TiO2 Nanotubes Arrays with Visible Photocatalytic Activities. Nanomaterials 2020, 10, 2107. https://doi.org/10.3390/nano10112107
Zhang J, Yang C, Li S, Xi Y, Cai C, Liu W, Golosov D, Zavadski S, Melnikov S. Preparation of Fe3+ Doped High-Ordered TiO2 Nanotubes Arrays with Visible Photocatalytic Activities. Nanomaterials. 2020; 10(11):2107. https://doi.org/10.3390/nano10112107
Chicago/Turabian StyleZhang, Jin, Chen Yang, Shijie Li, Yingxue Xi, Changlong Cai, Weiguo Liu, Dmitriy Golosov, Sergry Zavadski, and Siarhei Melnikov. 2020. "Preparation of Fe3+ Doped High-Ordered TiO2 Nanotubes Arrays with Visible Photocatalytic Activities" Nanomaterials 10, no. 11: 2107. https://doi.org/10.3390/nano10112107
APA StyleZhang, J., Yang, C., Li, S., Xi, Y., Cai, C., Liu, W., Golosov, D., Zavadski, S., & Melnikov, S. (2020). Preparation of Fe3+ Doped High-Ordered TiO2 Nanotubes Arrays with Visible Photocatalytic Activities. Nanomaterials, 10(11), 2107. https://doi.org/10.3390/nano10112107






