Recent Advances in Nanostructured Transition Metal Carbide- and Nitride-Based Cathode Electrocatalysts for Li–O2 Batteries (LOBs): A Brief Review
Abstract
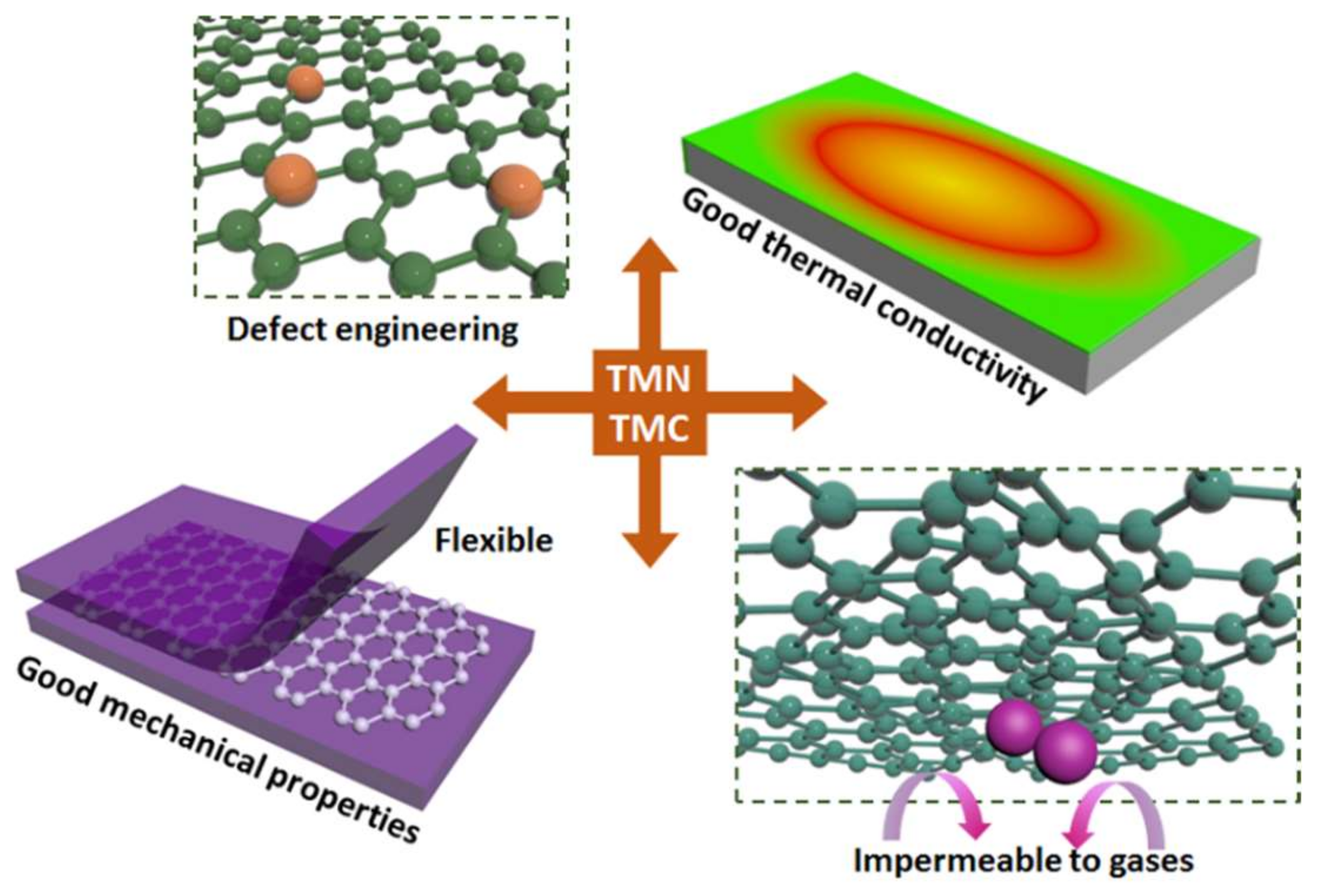
1. Introduction
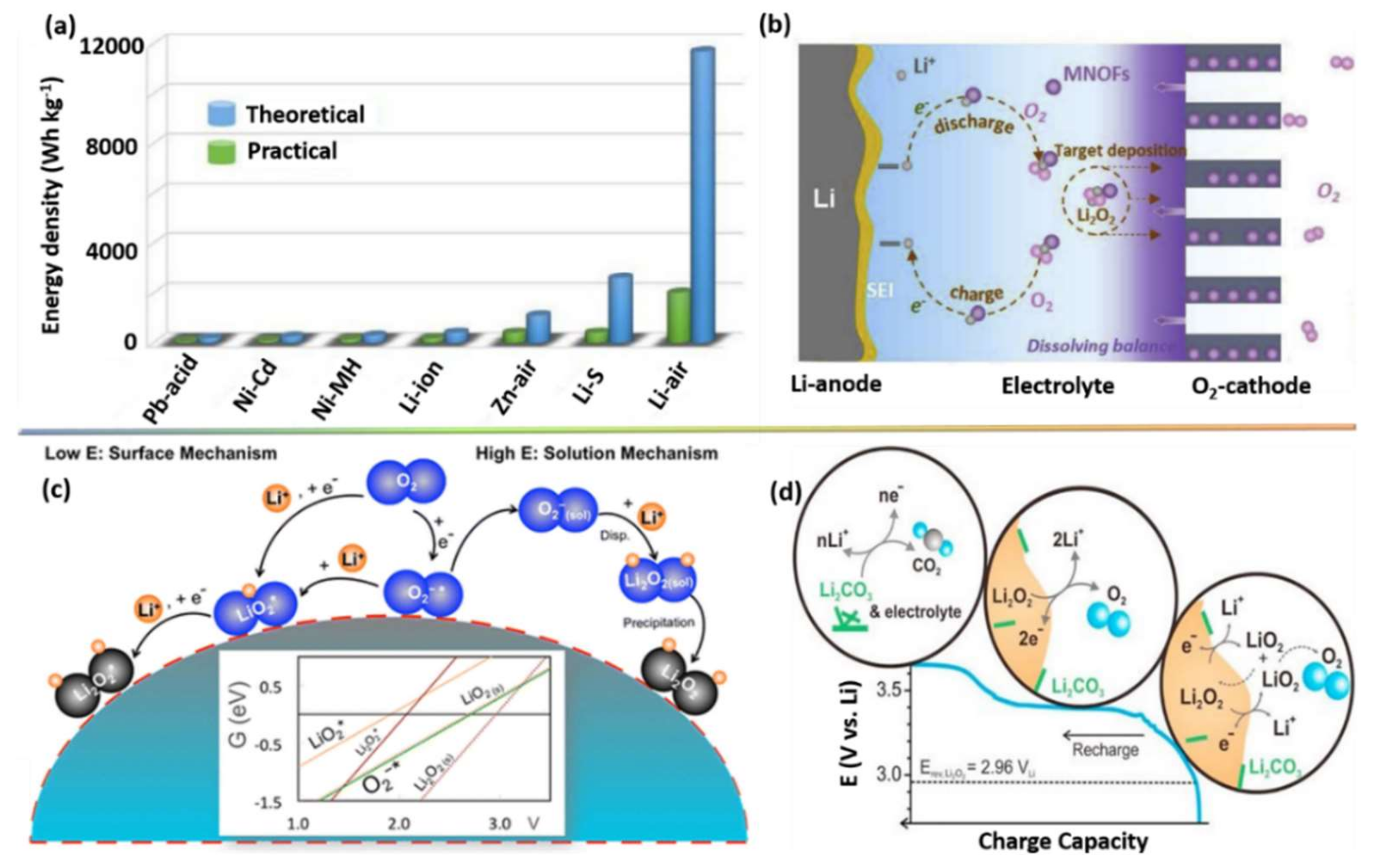
2. Basic Principles of Li–O2 Batteries
2.1. Basic Requirements for an Air Cathode
- High electrochemical and chemical stability are necessary to reduce the overpotential during charging, which results in a reversible electrochemical reaction at satisfactory charge–discharge potentials, with less chance of irreversible sponging reactions.
- A high specific surface area with a mesoporous structure enhances the discharge capacity even at high current densities, which is an important criterion for the high storage of Li2O2.
- To increase the rechargeability, a large packed electrode with a lower void volume is essential because it can prevent electrolyte penetration and improve the electrochemical reaction at catalytic sites due to the enhanced transportation of active O2 and Li+. Therefore, an electrode with a porosity that is appropriate for the size of Li2O2 exhibits greater rechargeability.
- Electrical conductivity is a deciding factor for renewable energy storage performance; hence, a cathode with greater electrical conductivity is required to allow the consistent transportation of electrons from the insulator and Li2O2 to the surface of the cathode.
2.2. Characterization Techniques for TMCs and TMNs Cathode Catalysts
2.2.1. Physicochemical Characterization Techniques
2.2.2. Electrochemical Characterizations
3. Metal Carbides for LOBs
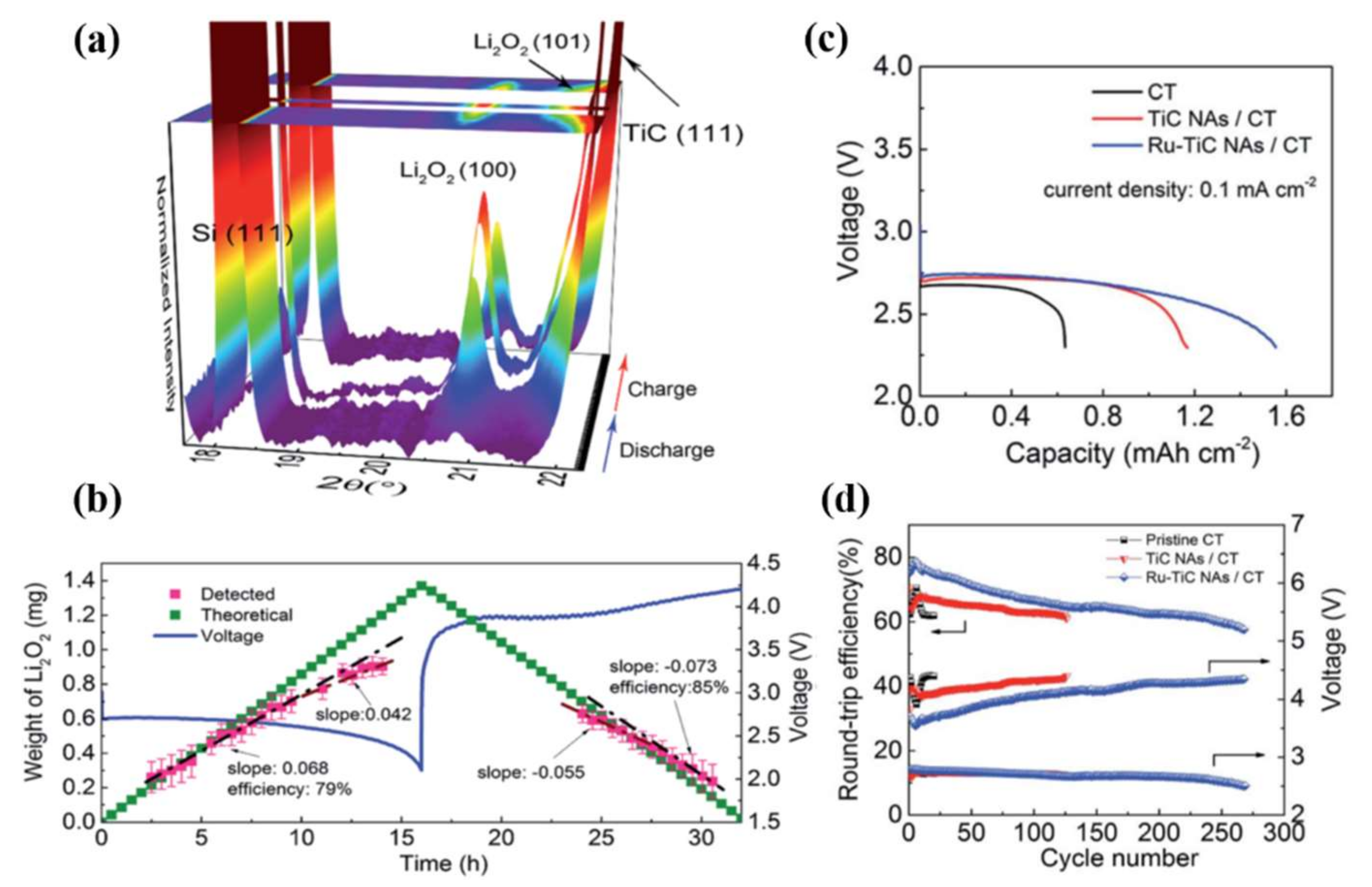
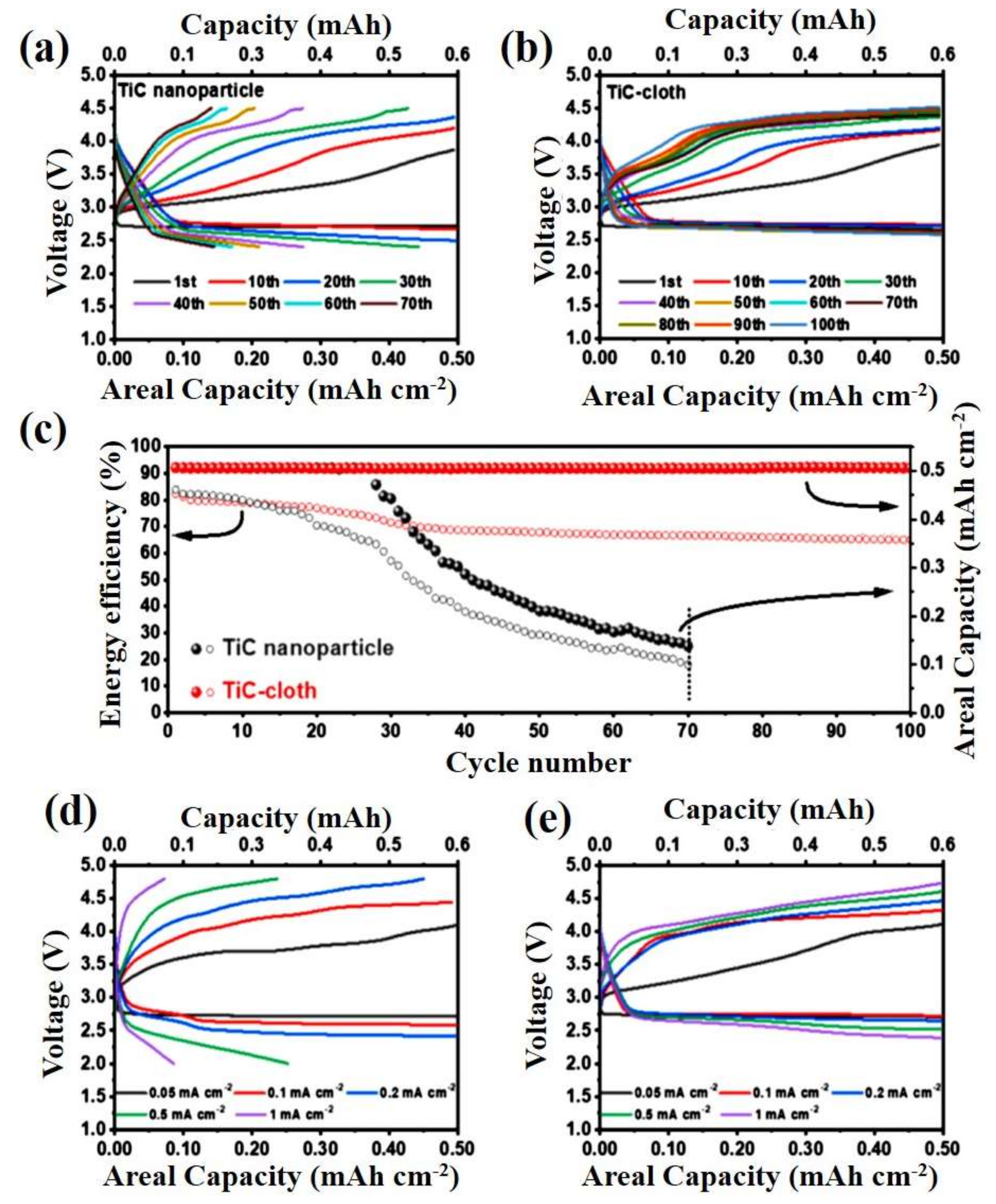
3.1. Titanium Carbide
3.2. Iron Carbide
3.3. Metal Nitrides
3.3.1. Titanium Nitride (TiN)
3.3.2. Vanadium Nitride
3.4. 2D Layered Transition Metal Carbides and Nitrides (MXenes)
3.4.1. 2D Layered Titanium Carbide (Ti3C2Tx)
3.4.2. 2D Layered Molybdenum Carbides (Mo2CTx)
3.4.3. 2D Layered Transition Metal Nitrides
4. Summary, Outlook, and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Vikraman, D.; Hussain, S.; Prasanna, K.; Karuppasamy, K.; Jung, J.; Kim, H.-S. Facile method to synthesis hybrid phase 1T@2H MoSe2 nanostructures for rechargeable lithium ion batteries. J. Electroanal. Chem. 2019, 833, 333–339. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Vikraman, D.; Jeon, J.-H.; Ramesh, S.; Yadav, H.M.; Jothi, V.R.; Bose, R.; Kim, H.S.; Alfantazi, A.; Kim, H.-S. Highly porous, hierarchical microglobules of Co3O4 embedded N-doped carbon matrix for high performance asymmetric supercapacitors. Appl. Surf. Sci. 2020, 529, 147147. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tosi, M.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Chang, Z.; Xu, J.; Zhang, X. Recent Progress in Electrocatalyst for Li-O2Batteries. Adv. Energy Mater. 2017, 7, 1700875. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, G.; Jin, J.; Zhang, L.; Wenab, Z. Coupling solid and soluble catalysts toward stable Li anode for high-performance Li–O2 batteries. Energy Storage Mater. 2020, 28, 342–349. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Prasanna, K.; Ilango, P.R.; Vikraman, D.; Bose, R.; Alfantazi, A.; Kim, H.-S. Biopolymer phytagel-derived porous nanocarbon as efficient electrode material for high-performance symmetric solid-state supercapacitors. J. Ind. Eng. Chem. 2019, 80, 258–264. [Google Scholar] [CrossRef]
- Hussain, S.; Rabani, I.; Vikraman, D.; Feroze, A.; Karuppasamy, K.; Haq, Z.U.; Seo, Y.-S.; Chun, S.-H.; Kim, H.-S.; Jung, J. Hybrid Design Using Carbon Nanotubes Decorated with Mo2C and W2C Nanoparticles for Supercapacitors and Hydrogen Evolution Reactions. ACS Sustain. Chem. Eng. 2020, 8, 12248–12259. [Google Scholar] [CrossRef]
- Zakeri, B.; Syri, S. Electrical energy storage systems: A comparative life cycle cost analysis. Renew. Sustain. Energy Rev. 2015, 42, 569–596. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nat. Cell Biol. 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liang, J.; Chen, Y. An Overview of the Applications of Graphene-Based Materials in Supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and Graphene-Based Materials for Energy Storage Applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Jothi, V.R.; Nichelson, A.; Vikraman, D.; Tanveer, W.H.; Kim, H.-S.; Yi, S.-C. Chapter 14—Nanostructured transition metal sulfide/selenide anodes for high-performance sodium-ion batteries. In Nanostructured, Functional, and Flexible Materials for Energy Conversion and Storage Systems; Pandikumar, A., Rameshkumar, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 437–464. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Feroze, A.; Kathalingam, A.; Sanmugam, A.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering the novel MoSe2-Mo2C hybrid nanoarray electrodes for energy storage and water splitting applications. Appl. Catal. B Environ. 2020, 264, 118531. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes Promising Anode Materials for Li Ion Batteries? Computational Studies on Electronic Properties and Li Storage Capability of Ti3C2 and Ti3C2X2 (X = F, OH) Monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef]
- Ghidiu, M.; Kota, S.; Halim, J.; Sherwood, A.W.; Nedfors, N.; Rosen, J.; Mochalin, V.N.; Barsoum, M.W. Alkylammonium Cation Intercalation into Ti3C2 (MXene): Effects on Properties and Ion-Exchange Capacity Estimation. Chem. Mater. 2017, 29, 1099–1106. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Durai, G.; Karuppasamy, K.; Arunachalam, P.; Elakkiya, V.; Kuppusami, P.; Maiyalagan, T.; Kim, H.-S. Recent advances in 2-D nanostructured metal nitrides, carbides, and phosphides electrodes for electrochemical supercapacitors—A brief review. J. Ind. Eng. Chem. 2018, 67, 12–27. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.U.H.S.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.-S. Recent Advances in Metal Chalcogenides (MX.; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials 2018, 8, 256. [Google Scholar] [CrossRef]
- Lei, C.-J.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Baek, D.S.; Joo, S.H.; Kim, T. MXene: An emerging two-dimensional material for future energy conversion and storage applications. J. Mater. Chem. A 2017, 5, 24564–24579. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Sun, S.; Liao, C.; Hafez, A.M.; Zhu, H.; Wu, S. Two-dimensional MXenes for energy storage. Chem. Eng. J. 2018, 338, 27–45. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, S.; Heo, J.; Park, C.M.; Her, N.; Jang, M.; Huang, Y.; Han, J.; Yoon, Y. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano Res. 2018, 12, 471–487. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; Zhou, Z. Lithium-air batteries: Challenges coexist with opportunities. APL Mater. 2019, 7, 040701. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, C.; Xiao, Y.; Xu, R.; Xu, J.; Huang, J.; Zhang, Q.; Yu, X.; Li, H. Safe Lithium-Metal Anodes for Li–O2 Batteries: From Fundamental Chemistry to Advanced Characterization and Effective Protection. Batter. Supercaps 2019, 2, 638–658. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Wu, M.; Zhao, H.; Li, Q.; Wu, Z.-S. Recent advances and future perspectives of two-dimensional materials for rechargeable Li-O2 batteries. Energy Storage Mater. 2020, 31, 470–491. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Balaish, M.; Kraytsberg, A.; Ein-Eli, Y. A critical review on lithium–air battery electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-N.; Kim, J.; Yamauchi, Y.; Park, M.; Lee, J.-W.; Kim, J.H. Rechargeable lithium–air batteries: A perspective on the development of oxygen electrodes. J. Mater. Chem. A 2016, 4, 14050–14068. [Google Scholar] [CrossRef]
- Littauer, E.L.; Tsai, K.C. Anodic Behavior of Lithium in Aqueous Electrolytes: II. Mechanical Passivation. J. Electrochem. Soc. 1976, 123, 964–969. [Google Scholar] [CrossRef]
- Abraham, K.M.; Jiang, Z. A Polymer Electrolyte-Based Rechargeable Lithium/Oxygen Battery. J. Electrochem. Soc. 2019, 143, 1–5. [Google Scholar] [CrossRef]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 2016, 1, 16128. [Google Scholar] [CrossRef]
- GirishKumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium–Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Wang, J.; McKee, W.C.; Xu, Y.; Peng, Z. Potential-Dependent Generation of O2– and LiO2 and Their Critical Roles in O2 Reduction to Li2O2 in Aprotic Li–O2 Batteries. J. Phys. Chem. C 2016, 120, 3690–3698. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Shao-Horn, Y. Probing the Reaction Kinetics of the Charge Reactions of Nonaqueous Li–O2 Batteries. J. Phys. Chem. Lett. 2012, 4, 93–99. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, X.; Li, L.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Zhang, J. A review of cathode materials and structures for rechargeable lithium–air batteries. Energy Environ. Sci. 2015, 8, 2144–2198. [Google Scholar] [CrossRef]
- Kim, M.-C.; So, J.-Y.; Moon, S.-H.; Han, S.-B.; Choi, S.; Kim, E.-S.; Shin, Y.-K.; Lee, J.-E.; Kwak, D.-H.; Lee, C.; et al. Nature inspired cathodes using high-density carbon papers with an eddy current effect for high-rate performance lithium–air batteries. J. Mater. Chem. A 2018, 6, 9550–9560. [Google Scholar] [CrossRef]
- Yang, N.; Hu, D.-R.; Cao, B.-K.; Chen, Y.; Li, D.; Chen, D.-M. Preparation of three-dimensional hierarchical porous carbon microspheres for use as a cathode material in lithium-air batteries. Carbon 2018, 130, 847–848. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Xu, J.-J.; Chang, Z.-W.; Bao, D.; Yin, Y.-B.; Liu, T.; Yan, J.-M.; Liu, D.-P.; Zhang, Y.; Zhang, X.-B. Blood-Capillary-Inspired, Free-Standing, Flexible, and Low-Cost Super-Hydrophobic N-CNTs@SS Cathodes for High-Capacity, High-Rate, and Stable Li-Air Batteries. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Cheng, Z.; Li, C.; Zhang, X.; Guo, S.; He, P.; Zhou, H. Intensive investigation on all-solid-state Li-air batteries with cathode catalysts of single-walled carbon nanotube/RuO2. J. Power Sources 2018, 395, 439–443. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, Y.; Wan, W.; Yan, Y.; Wang, Y.; He, X.; Lü, Z. CNF-grafted carbon fibers as a binder-free cathode for Lithium Oxygen batteries with a superior performance. Int. J. Hydrogen Energy 2018, 43, 739–747. [Google Scholar] [CrossRef]
- Hu, S.-J.; Fan, X.-P.; Chen, J.; Peng, J.-M.; Hu, S.; Huang, Y.-G.; Li, Q.-Y. Carbon Nanotubes/Carbon Fiber Paper Supported MnO2 Cathode Catalyst for Li–Air Batteries. ChemElectroChem 2017, 4, 2997–3003. [Google Scholar] [CrossRef]
- Xiao, J.; Mei, D.; Li, X.; Xu, W.; Wang, D.; Graff, G.L.; Bennett, W.D.; Nie, Z.; Saraf, L.V.; Aksay, I.A.; et al. Hierarchically Porous Graphene as a Lithium–Air Battery Electrode. Nano Lett. 2011, 11, 5071–5078. [Google Scholar] [CrossRef]
- Jiang, Y.; Cheng, J.; Zou, L.; Li, X.; Huang, Y.; Jia, L.; Chi, B.; Pu, J.; Li, J. Graphene Foam Decorated with Ceria Microspheres as a Flexible Cathode for Foldable Lithium-Air Batteries. ChemCatChem 2017, 9, 4231–4237. [Google Scholar] [CrossRef]
- Sun, B.; Wang, B.; Su, D.; Xiao, L.; Ahn, H.; Wang, G. Graphene nanosheets as cathode catalysts for lithium-air batteries with an enhanced electrochemical performance. Carbon 2012, 50, 727–733. [Google Scholar] [CrossRef]
- Jung, H.-G.; Jeong, Y.S.; Park, J.-B.; Sun, Y.-K.; Scrosati, B.; Lee, Y.J. Ruthenium-Based Electrocatalysts Supported on Reduced Graphene Oxide for Lithium-Air Batteries. ACS Nano 2013, 7, 3532–3539. [Google Scholar] [CrossRef]
- Sun, C.; Li, F.; Ma, C.; Wang, Y.; Ren, Y.; Yang, W.; Ma, Z.; Li, J.; Chen, Y.; Kim, Y.; et al. Graphene–Co3O4 nanocomposite as an efficient bifunctional catalyst for lithium–air batteries. J. Mater. Chem. A 2014, 2, 7188–7196. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Lu, Y.; Xu, M.; Zhang, D.; Ruoff, R.S.; Stevenson, K.J.; Goodenough, J.B. CoMn2O4 Spinel Nanoparticles Grown on Graphene as Bifunctional Catalyst for Lithium-Air Batteries. J. Electrochem. Soc. 2011, 158, A1379–A1382. [Google Scholar] [CrossRef]
- Wu, D.; Guo, Z.; Yin, X.; Pang, Q.; Tu, B.; Zhang, L.; Wang, Y.; Li, Q. Metal-Organic Frameworks as Cathode Materials for Li-O2 Batteries. Adv. Mater. 2014, 26, 3258–3262. [Google Scholar] [CrossRef] [PubMed]
- García-Lastra, J.M.; Myrdal, J.S.G.; Christensen, R.; Thygesen, K.S.; Vegge, T. DFT+U Study of Polaronic Conduction in Li2O2 and Li2CO3: Implications for Li–Air Batteries. J. Phys. Chem. C 2013, 117, 5568–5577. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Speidel, A.; Scheffler, R.; Miller, D.C.; Viswanathan, V.; Hummelshøj, J.S.; Nørskov, J.K.; Luntz, A.C. Twin Problems of Interfacial Carbonate Formation in Nonaqueous Li–O2 Batteries. J. Phys. Chem. Lett. 2012, 3, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Xu, Z.; Gasteiger, H.A.; Chen, S.; Hamad-Schifferli, K.; Shao-Horn, Y. Platinum–Gold Nanoparticles: A Highly Active Bifunctional Electrocatalyst for Rechargeable Lithium–Air Batteries. J. Am. Chem. Soc. 2010, 132, 12170–12171. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kim, B.K.; Yoon, W.Y. Catalytic behavior of V2O5 in rechargeable Li–O2 batteries. J. Appl. Electrochem. 2012, 42, 1045–1048. [Google Scholar] [CrossRef]
- Yang, W.; Salim, J.; Ma, C.; Ma, Z.; Sun, C.; Li, J.; Chen, L.; Kim, Y. Flowerlike Co3O4 microspheres loaded with copper nanoparticle as an efficient bifunctional catalyst for lithium–air batteries. Electrochem. Commun. 2013, 28, 13–16. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, H.; Byun, J.; Kim, B.K.; Yoon, W.Y. Electrochemical and catalytic properties of V2O5/Al2O3 in rechargeable Li–O2 batteries. Electrochimica Acta 2013, 107, 681–685. [Google Scholar] [CrossRef]
- Truong, T.T.; Liu, Y.; Ren, Y.; Trahey, L.; Sun, Y. Morphological and Crystalline Evolution of Nanostructured MnO2 and Its Application in Lithium–Air Batteries. ACS Nano 2012, 6, 8067–8077. [Google Scholar] [CrossRef]
- Lee, A.; Krishnamurthy, D.; Viswanathan, V. Exploring MXenes as Cathodes for Non-Aqueous Lithium–Oxygen Batteries: Design Rules for Selectively Nucleating Li2O2. ChemSusChem 2018, 11, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Guo, X.; Wu, W.; Wang, G. 2D Metal Carbides and Nitrides (MXenes) as High-Performance Electrode Materials for Lithium-Based Batteries. Adv. Energy Mater. 2018, 8, 1801897. [Google Scholar] [CrossRef]
- Zheng, H.; Xiao, D.; Li, X.; Liu, Y.; Wu, Y.; Wang, J.; Jiang, K.; Chen, C.; Gu, L.; Wei, X.; et al. New Insight in Understanding Oxygen Reduction and Evolution in Solid-State Lithium–Oxygen Batteries Using an in Situ Environmental Scanning Electron Microscope. Nano Lett. 2014, 14, 4245–4249. [Google Scholar] [CrossRef]
- Liang, Z.; Zou, Q.; Wang, Y.; Lu, Y.-C. Recent Progress in Applying In Situ/Operando Characterization Techniques to Probe the Solid/Liquid/Gas Interfaces of Li-O2 Batteries. Small Methods 2017, 1, 1700150. [Google Scholar] [CrossRef]
- Shui, J.-L.; Karan, N.K.; Balasubramanian, M.; Li, S.-Y.; Liu, D.-J. Fe/N/C Composite in Li–O2 Battery: Studies of Catalytic Structure and Activity toward Oxygen Evolution Reaction. J. Am. Chem. Soc. 2012, 134, 16654–16661. [Google Scholar] [CrossRef]
- Thotiyl, M.M.O.; Freunberger, S.A.; Peng, Z.; Chen, Y.; Liu, Z.; Bruce, P.G. A stable cathode for the aprotic Li–O2 battery. Nat. Mater. 2013, 12, 1050–1056. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, X.; Qin, Y.; Wang, X.; Yao, M.; Qin, Z.; Huang, H. Oxygen Evolution Reaction on Pristine and Oxidized TiC (100) Surface in Li–O2 Battery. J. Phys. Chem. C 2018, 122, 12665–12672. [Google Scholar] [CrossRef]
- Raz, K.; Tereshchuk, P.; Golodnitsky, D.; Natan, A. Adsorption of Li2O2, Na2O2, and NaO2 on TiC(111) Surface for Metal–Air Rechargeable Batteries: A Theoretical Study. J. Phys. Chem. C 2018, 122, 16473–16480. [Google Scholar] [CrossRef]
- Kozmenkova, A.Y.; Kataev, E.Y.; Belova, A.I.; Amati, M.; Gregoratti, L.; Velasco-Vélez, J.; Knop-Gericke, A.; Senkovskiy, B.V.; Vyalikh, D.V.; Itkis, D.M.; et al. Tuning Surface Chemistry of TiC Electrodes for Lithium–Air Batteries. Chem. Mater. 2016, 28, 8248–8255. [Google Scholar] [CrossRef]
- Adams, B.D.; Black, R.; Radtke, C.; Williams, Z.; Mehdi, B.L.; Browning, N.D.; Nazar, L.F. The Importance of Nanometric Passivating Films on Cathodes for Li–Air Batteries. ACS Nano 2014, 8, 12483–12493. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhu, K.; Frank, A.J.; Grimes, C.A.; Mallouk, T.E. Rapid Charge Transport in Dye-Sensitized Solar Cells Made from Vertically Aligned Single-Crystal Rutile TiO2 Nanowires. Angew. Chem. Int. Ed. 2012, 51, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.K.; Schmuki, P. Chapter 3. Electrochemistry at TiO2 nanotubes and other semiconductor nanostructures. Electrochemistry 2013, 12, 87–131. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, Z.; Brant, W.R.; Younesi, R.; Ma, Y.; Edström, K.; Gustafsson, T.; Zhu, J.; Brant, W.R. A free standing Ru–TiC nanowire array/carbon textile cathode with enhanced stability for Li–O2 batteries. J. Mater. Chem. A 2018, 6, 23659–23668. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Kwak, W.-J.; Shin, H.-J.; Sun, Y.-K.; Jung, H.-G. Perpendicularly Aligned TiC-Coated Carbon Cloth Cathode for High-Performance Li-O2 Batteries. Chem. Eng. J. 2020, 399, 125699. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Lee, J.-S.; Park, G.S.; Kim, S.T.; Liu, M.; Cho, J. A Highly Efficient Electrocatalyst for the Oxygen Reduction Reaction: N-Doped Ketjenblack Incorporated into Fe/Fe3C-Functionalized Melamine Foam. Angew. Chem. Int. Ed. 2012, 52, 1026–1030. [Google Scholar] [CrossRef]
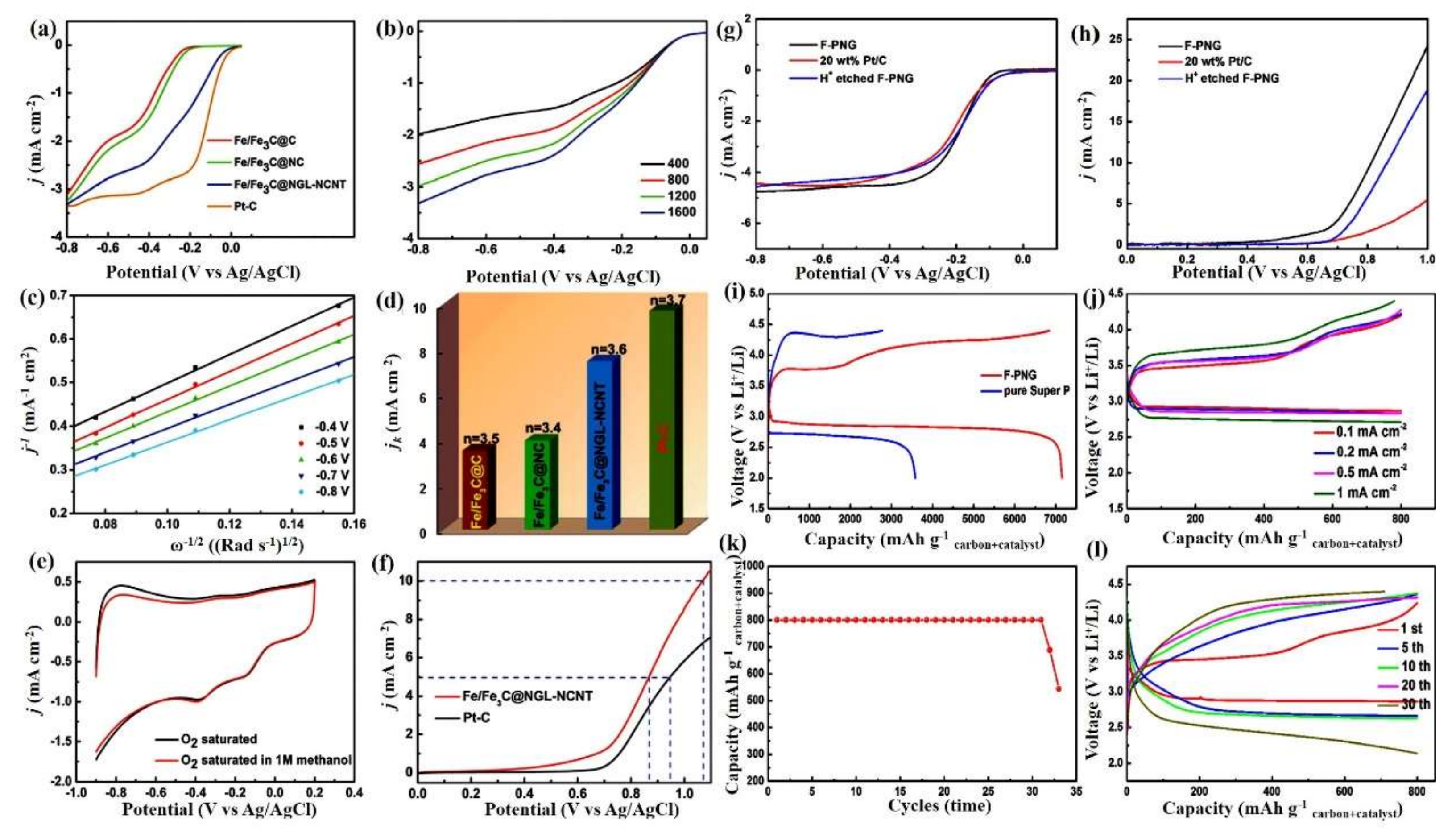
- Wen, Z.; Ci, S.; Zhang, F.; Feng, X.; Cui, S.; Mao, S.; Luo, S.; He, Z.; Chen, J. Nitrogen-Enriched Core-Shell Structured Fe/Fe3C-C Nanorods as Advanced Electrocatalysts for Oxygen Reduction Reaction. Adv. Mater. 2012, 24, 1399–1404. [Google Scholar] [CrossRef]
- Hu, M.Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow Spheres of Iron Carbide Nanoparticles Encased in Graphitic Layers as Oxygen Reduction Catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef]
- Li, J.-S.; Li, S.-L.; Tang, Y.-J.; Han, M.; Dai, Z.; Bao, J.; Lan, Y.-Q. Nitrogen-doped Fe/Fe3C@graphitic layer/carbon nanotube hybrids derived from MOFs: Efficient bifunctional electrocatalysts for ORR and OER. Chem. Commun. 2015, 51, 2710–2713. [Google Scholar] [CrossRef]
- Yang, J.; Hu, J.; Weng, M.; Tan, R.; Tian, L.; Yang, J.; Amine, J.; Zheng, J.; Chen, H.; Pan, F. Fe-Cluster Pushing Electrons to N-Doped Graphitic Layers with Fe3C(Fe) Hybrid Nanostructure to Enhance O2 Reduction Catalysis of Zn-Air Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4587–4596. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.Y.; Yu, L.; Lou, X.W.D. A dual-metal–organic-framework derived electrocatalyst for oxygen reduction. Energy Environ. Sci. 2016, 9, 3092–3096. [Google Scholar] [CrossRef]
- Lai, Y.; Chen, W.; Zhang, Z.; Qu, Y.; Gan, Y.; Li, J. Fe/Fe3C decorated 3-D porous nitrogen-doped graphene as a cathode material for rechargeable Li–O2 batteries. Electrochim. Acta 2016, 191, 733–742. [Google Scholar] [CrossRef]
- Lai, Y.; Jiao, Y.; Song, J.; Zhang, K.; Li, J.; Zhang, Z. Fe/Fe3C@graphitic carbon shell embedded in carbon nanotubes derived from Prussian blue as cathodes for Li–O2 batteries. Mater. Chem. Front. 2018, 2, 376–384. [Google Scholar] [CrossRef]
- Li, J.; Zou, M.; Chen, L.; Huang, Z.; Guan, L. An efficient bifunctional catalyst of Fe/Fe3C carbon nanofibers for rechargeable Li–O2 batteries. J. Mater. Chem. A 2014, 2, 10634–10638. [Google Scholar] [CrossRef]
- Wei, L.; Sun, H.; Yang, T.; Deng, S.; Wu, M.; Li, Z. Iron carbide encapsulated by porous carbon nitride as bifunctional electrocatalysts for oxygen reduction and evolution reactions. Appl. Surf. Sci. 2018, 439, 439–446. [Google Scholar] [CrossRef]
- Ren, W.; Xu, L.; Zhu, L.; Wang, X.; Ma, X.; Wang, D. Cobalt-Doped Vanadium Nitride Yolk–Shell Nanospheres @ Carbon with Physical and Chemical Synergistic Effects for Advanced Li–S Batteries. ACS Appl. Mater. Interfaces 2018, 10, 11642–11651. [Google Scholar] [CrossRef]
- Jothi, V.R.; Karuppasamy, K.; Maiyalagan, T.; Rajan, H.; Jung, C.; Yi, S.C. Corrosion and Alloy Engineering in Rational Design of High Current Density Electrodes for Efficient Water Splitting. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Bose, R.; Jothi, V.R.; Karuppasamy, K.; Alfantazi, A.; Yi, S.C. High performance multicomponent bifunctional catalysts for overall water splitting. J. Mater. Chem. A 2020, 8, 13795–13805. [Google Scholar] [CrossRef]
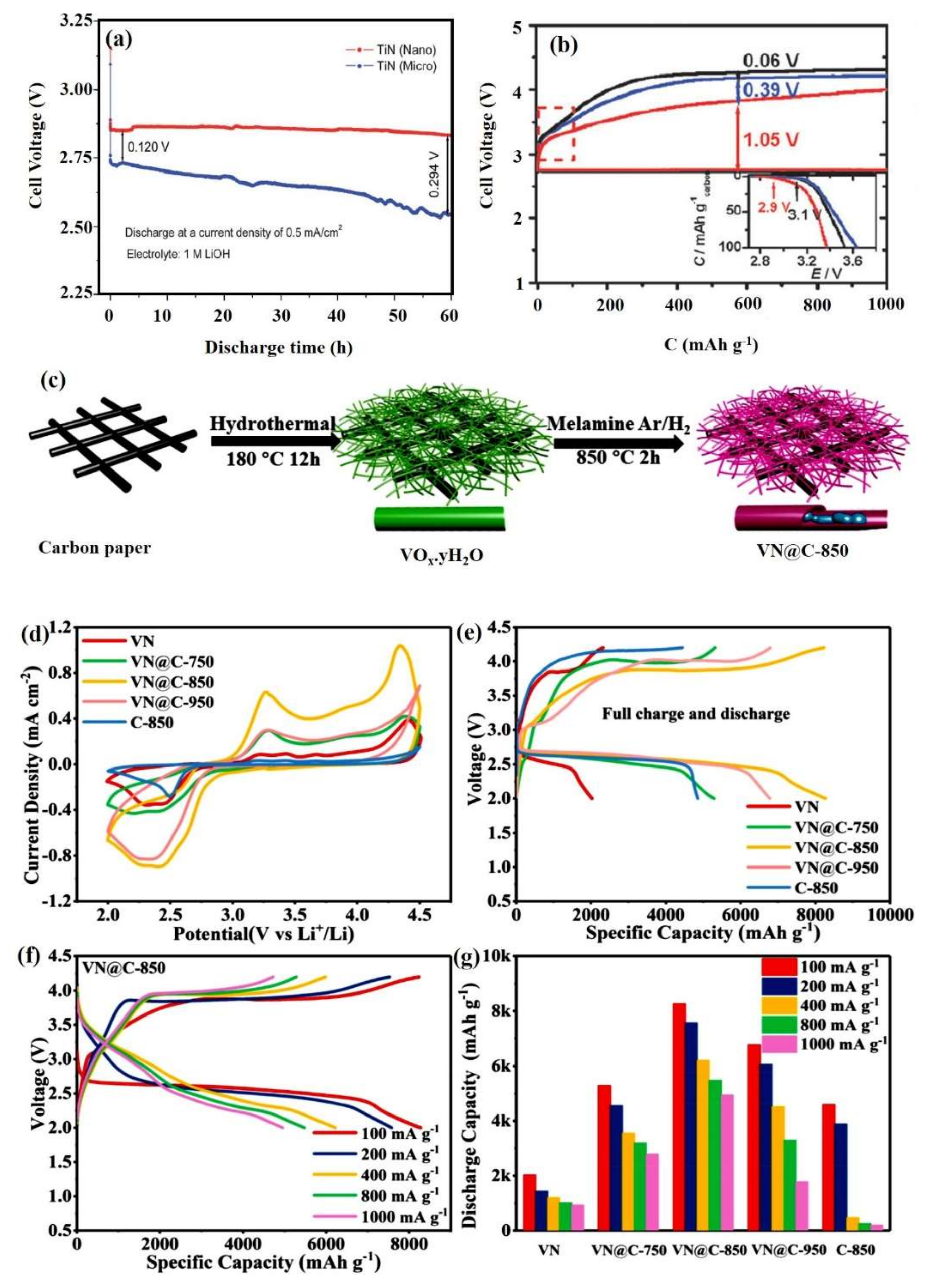
- Chen, J.; Takanabe, K.; Ohnishi, R.; Lu, D.; Okada, S.; Hatasawa, H.; Morioka, H.; Antonietti, M.; Kubota, J.; Domen, K. Nano-sized TiN on carbon black as an efficient electrocatalyst for the oxygen reduction reaction prepared using an mpg-C3N4 template. Chem. Commun. 2010, 46, 7492–7494. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wang, Y.; Zhou, H. Titanium nitride catalyst cathode in a Li–air fuel cell with an acidic aqueous solution. Chem. Commun. 2011, 47, 10701. [Google Scholar] [CrossRef]
- Wang, Y.; Ohnishi, R.; Yoo, E.; He, P.; Kubota, J.; Domen, K.; Zhou, H. Nano- and micro-sized TiN as the electrocatalysts for ORR in Li–air fuel cell with alkaline aqueous electrolyte. J. Mater. Chem. 2012, 22, 15549. [Google Scholar] [CrossRef]
- Li, F.; Ohnishi, R.; Yamada, Y.; Kubota, J.; Domen, K.; Yamada, A.; Zhou, H. Carbon supported TiN nanoparticles: An efficient bifunctional catalyst for non-aqueous Li–O2 batteries. Chem. Commun. 2013, 49, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, T.; Zhou, H. Challenges of non-aqueous Li–O2 batteries: Electrolytes, catalysts, and anodes. Energy Environ. Sci. 2013, 6, 1125–1141. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Zeng, X.; Tian, X.; Song, H.; Cui, Z.; Shu, T.; Wang, H.-S.; Ren, J.; Liao, S. Enhanced cyclability of Li–O2 batteries with cathodes of Ir and MnO2 supported on well-defined TiN arrays. Nanoscale 2018, 10, 2983–2989. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.R.; Shin, K.; Park, J.; Cho, S.-H.; Kim, C.; Jung, J.-W.; Cheong, J.Y.; Byon, H.R.; Lee, H.M.; Kim, I.-D. Brush-Like Cobalt Nitride Anchored Carbon Nanofiber Membrane: Current Collector-Catalyst Integrated Cathode for Long Cycle Li–O2 Batteries. ACS Nano 2017, 12, 128–139. [Google Scholar] [CrossRef]
- Pan, C.; Xu, J.; Wang, Y.; Li, D.; Zhu, Y. Dramatic Activity of C3N4/BiPO4 Photocatalyst with Core/Shell Structure Formed by Self-Assembly. Adv. Funct. Mater. 2012, 22, 1518–1524. [Google Scholar] [CrossRef]
- Fu, G.; Cui, Z.; Chen, Y.; Xu, L.; Tang, Y.; Goodenough, J.B. Hierarchically mesoporous nickel-iron nitride as a cost-efficient and highly durable electrocatalyst for Zn-air battery. Nano Energy 2017, 39, 77–85. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Yin, L.; Hu, G.; Fang, R.; Cheng, H.-M.; Li, F. Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium-sulfur batteries. Nat. Commun. 2017, 8, 14627. [Google Scholar] [CrossRef]
- Li, X.; Ding, K.; Gao, B.; Li, Q.; Li, Y.; Fu, J.; Zhang, X.; Chu, P.K.; Huo, K. Freestanding carbon encapsulated mesoporous vanadium nitride nanowires enable highly stable sulfur cathodes for lithium-sulfur batteries. Nano Energy 2017, 40, 655–662. [Google Scholar] [CrossRef]
- Mosavati, N.; Salley, S.O.; Ng, K.S. Characterization and electrochemical activities of nanostructured transition metal nitrides as cathode materials for lithium sulfur batteries. J. Power Sources 2017, 340, 210–216. [Google Scholar] [CrossRef]
- Sun, K.; Liu, M.; Yu, S.; Song, H.; Zeng, J.; Li, X.; Liao, S. In-situ grown vanadium nitride coated with thin layer of nitrogen-doped carbon as a highly durable binder-free cathode for Li–O2 batteries. J. Power Sources 2020, 460, 228109. [Google Scholar] [CrossRef]
- Okubo, M.; Sugahara, A.; Kajiyama, S.; Yamada, A. MXene as a Charge Storage Host. Accounts Chem. Res. 2018, 51, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, L.; Abdolhosseinzadeh, S.; Heier, J. Two-dimensional MXenes for lithium-sulfur batteries. InfoMat 2020, 2, 613–638. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Li, L.; Guo, M.; Qi, Z.; Zhang, Q.; Zhou, Y. Intercalated MXene-based layered composites: Preparation and application. Chin. Chem. Lett. 2020, 31, 961–968. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horizons 2020, 5, 235–258. [Google Scholar] [CrossRef]
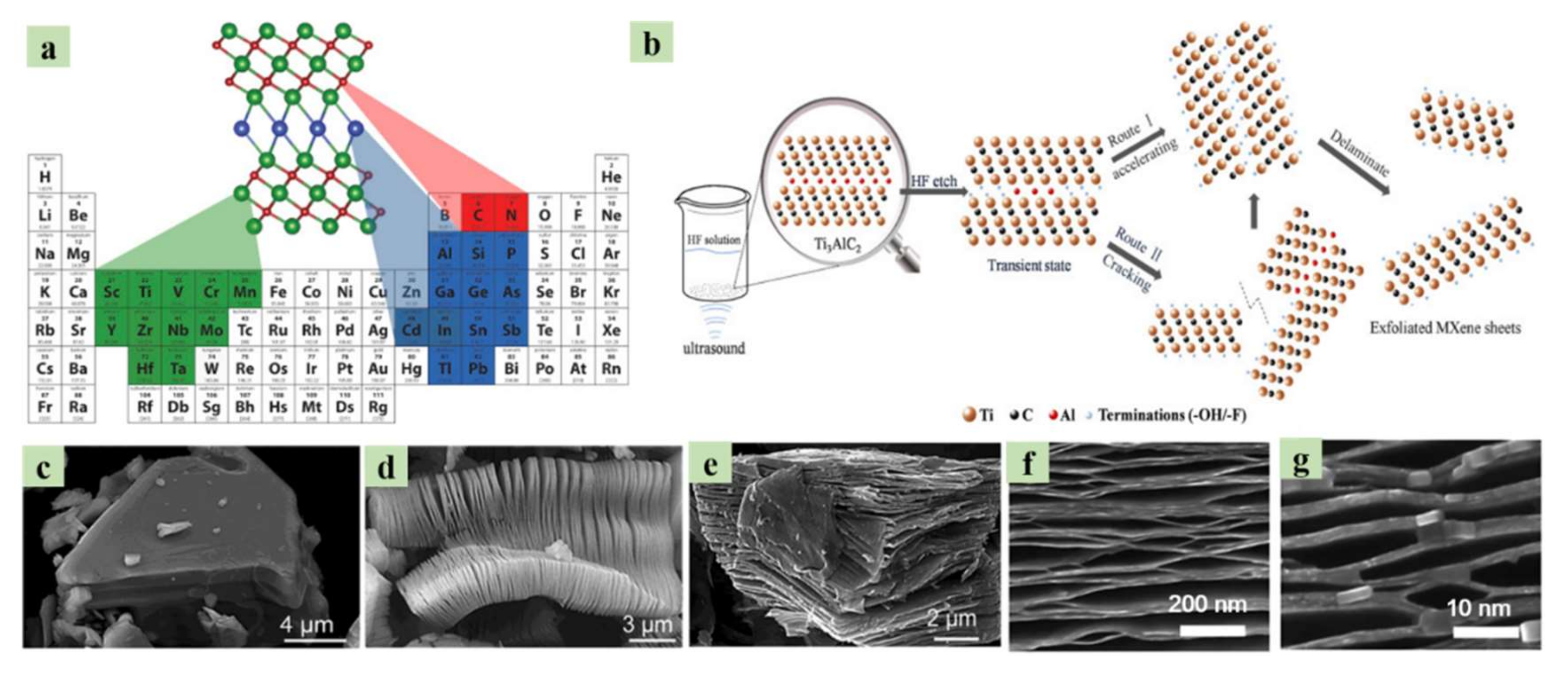
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2013, 26, 992–1005. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Gao, G.; Ding, G.; Li, J.; Yao, K.; Wu, M.; Qian, M. Monolayer MXenes: Promising half-metals and spin gapless semiconductors. Nanoscale 2016, 8, 8986–8994. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Xu, S.; Wei, G.; Li, J.; Han, W.; Gogotsi, Y. Flexible MXene–graphene electrodes with high volumetric capacitance for integrated co-cathode energy conversion/storage devices. J. Mater. Chem. A 2017, 5, 17442–17451. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Luo, H.; Wang, Y.; Zeng, S.; Tan, Y.; Zhang, H.; Peng, S. Ultrasonic assisted etching and delaminating of Ti3C2 Mxene. Ceram. Int. 2018, 44, 7084–7087. [Google Scholar] [CrossRef]
- Xue, Q.; Pei, Z.; Huang, Y.; Zhu, M.; Tang, Z.; Li, H.; Li, N.; Zhang, H.; Zhi, C. Mn3O4 nanoparticles on layer-structured Ti3C2MXene towards the oxygen reduction reaction and zinc–air batteries. J. Mater. Chem. A 2017, 5, 20818–20823. [Google Scholar] [CrossRef]
- Zou, H.; He, B.; Kuang, P.; Yu, J.; Fan, K. Metal–Organic Framework-Derived Nickel–Cobalt Sulfide on Ultrathin Mxene Nanosheets for Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 22311–22319. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Dong, S.; Ye, Z.; Guo, Y. One-step hydrothermal synthesis of a TiO2-Ti3C2Tx nanocomposite with small sized TiO2 nanoparticles. Ceram. Int. 2017, 43, 11065–11070. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Abeyweera, S.C.; Thenuwara, A.C.; Anasori, B.; Gogotsi, Y.; Sun, Y.; Strongin, D.R. Vertically aligned MoS2 on Ti3C2 (MXene) as an improved HER catalyst. J. Mater. Chem. A 2018, 6, 16882–16889. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, B.; Hu, L.; Xu, M.; Li, Y.; Liu, D.; Xu, M. Synthesis of SnS nanoparticle-modified MXene (Ti3C2Tx) composites for enhanced sodium storage. J. Alloys Compd. 2018, 732, 448–453. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene hybrids with ultrahigh volumetric capacity as an anode material for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Ma, T.Y.; Cao, J.L.; Jaroniec, M.; Qiao, S.Z. Interacting Carbon Nitride and Titanium Carbide Nanosheets for High-Performance Oxygen Evolution. Angew. Chem. Int. Ed. 2015, 55, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cao, M.; Qin, Y.; Zhu, J.; Wang, L.; Tang, Y. ZnO nanoparticle-decorated two-dimensional titanium carbide with enhanced supercapacitive performance. RSC Adv. 2016, 6, 88934–88942. [Google Scholar] [CrossRef]
- Chen, S.; Xiang, Y.; Xu, W.; Peng, C. A novel MnO2/MXene composite prepared by electrostatic self-assembly and its use as an electrode for enhanced supercapacitive performance. Inorg. Chem. Front. 2019, 6, 199–208. [Google Scholar] [CrossRef]
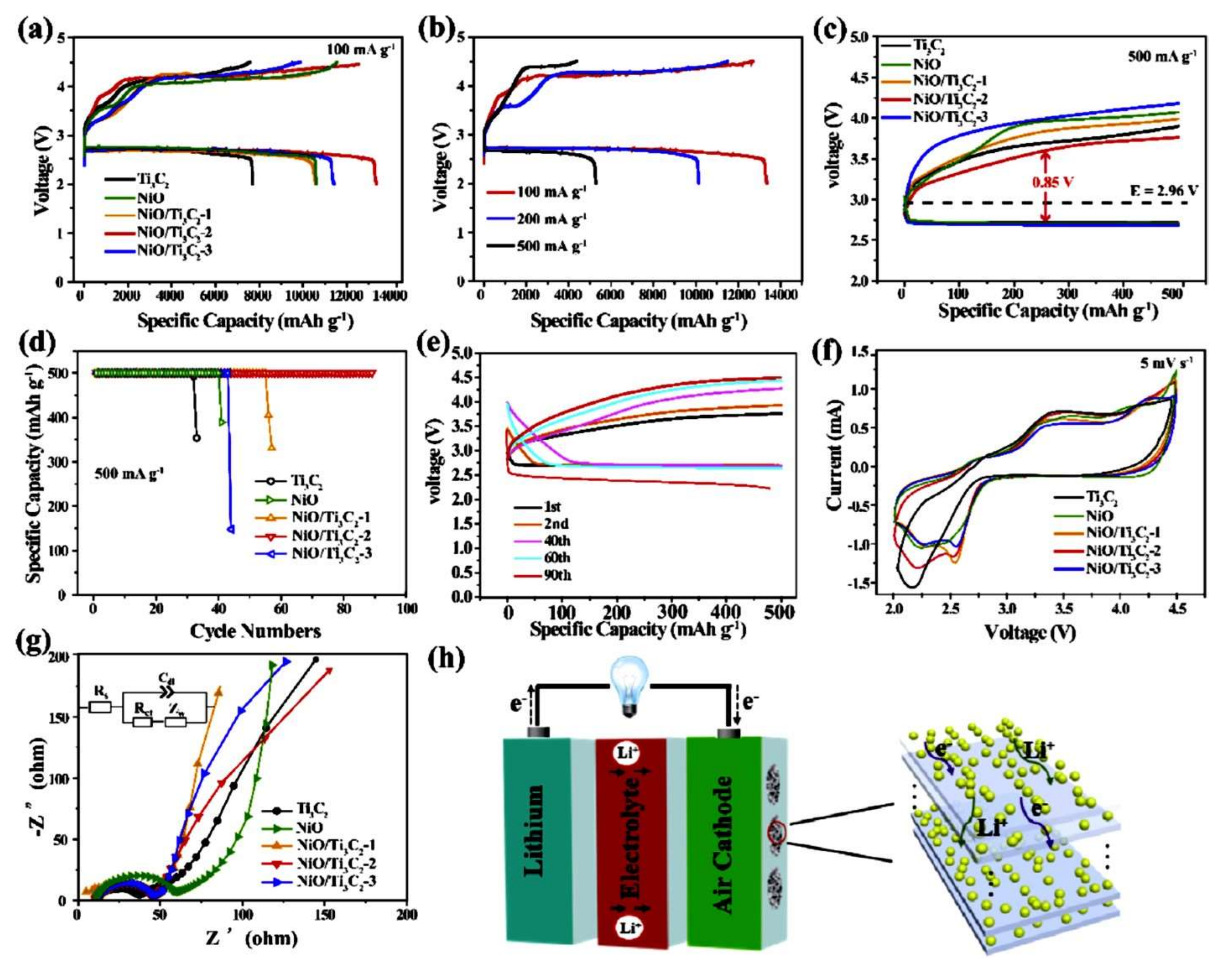
- Zheng, R.; Shu, C.; Hou, Z.; Hu, A.; Hei, P.; Yang, T.; Li, J.; Liang, R.; Long, J. In Situ Fabricating Oxygen Vacancy-Rich TiO2 Nanoparticles via Utilizing Thermodynamically Metastable Ti Atoms on Ti3C2Tx MXene Nanosheet Surface To Boost Electrocatalytic Activity for High-Performance Li–O2 Batteries. ACS Appl. Mater. Interfaces 2019, 11, 46696–46704. [Google Scholar] [CrossRef]
- Li, X.; Wen, C.; Yuan, M.; Sun, Z.; Wei, Y.; Ma, L.; Li, H.; Sun, G. Nickel oxide nanoparticles decorated highly conductive Ti3C2 MXene as cathode catalyst for rechargeable Li–O2 battery. J. Alloys Compd. 2020, 824, 153803. [Google Scholar] [CrossRef]
- Li, X.; Wen, C.; Li, H.; Sun, G. In situ decoration of nanosized metal oxide on highly conductive MXene nanosheets as efficient catalyst for Li-O2 battery. J. Energy Chem. 2020, 47, 272–280. [Google Scholar] [CrossRef]
- Yu, H.; Dinh, K.N.; Sun, Y.; Fan, H.; Wang, Y.; Jing, Y.; Li, S.; Srinivasan, M.; Tan, H.T. Performance-improved Li-O2 batteries by tailoring the phases of MoxC porous nanorods as an efficient cathode. Nanoscale 2018, 10, 14877–14884. [Google Scholar] [CrossRef]
- Wu, M.; Kim, D.Y.; Park, H.; Cho, K.M.; Kim, J.Y.; Kim, S.J.; Choi, S.; Kang, Y.; Kim, J.; Jung, H.-T. Formation of toroidal Li2O2 in non-aqueous Li–O2 batteries with Mo2CTx MXene/CNT composite. RSC Adv. 2019, 9, 41120–41125. [Google Scholar] [CrossRef]
- Yang, Z.-D.; Chang, Z.-W.; Zhang, Q.; Huang, K.; Zhang, X.-B. Decorating carbon nanofibers with Mo2C nanoparticles towards hierarchically porous and highly catalytic cathode for high-performance Li-O2 batteries. Sci. Bull. 2018, 63, 433–440. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, Q.; Wu, T.; Lu, W.; Bao, M.; Sun, L.-Q.; Xie, H.; Liu, J. 3D Foam-Like Composites of Mo2C Nanorods Coated by N-Doped Carbon: A Novel Self-Standing and Binder-Free O2 Electrode for Li–O2 Batteries. ACS Appl. Mater. Interfaces 2018, 10, 6327–6335. [Google Scholar] [CrossRef]
- Jiao, W.; Su, Q.; Ge, J.; Dong, S.; Wang, D.; Zhang, M.; Ding, S.; Du, G.; Xu, B. Mo2C quantum dots decorated ultrathin carbon nanosheets self-assembled into nanoflowers toward highly catalytic cathodes for Li-O2 batteries. Mater. Res. Bull. 2021, 133, 111020. [Google Scholar] [CrossRef]
- Zakharchenko, T.K.; Kozmenkova, A.Y.; Isaev, V.V.; Itkis, D.M.; Yashina, L.V. Positive Electrode Passivation by Side Discharge Products in Li–O2 Batteries. Langmuir 2020, 36, 8716–8722. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Kim, J.H.; Lee, J.Y.; Kang, Y.C.; Kang, Y.C. Design of house centipede-like MoC–Mo2C nanorods grafted with N-doped carbon nanotubes as bifunctional catalysts for high-performance Li–O2 batteries. Chem. Eng. J. 2020, 384, 123344. [Google Scholar] [CrossRef]
- Zhu, Q.-C.; Xu, S.-M.; Harris, M.M.; Ma, C.; Liu, Y.-S.; Wei, X.; Xu, H.-S.; Zhou, Y.-X.; Cao, Y.-C.; Wang, K.-X.; et al. A Composite of Carbon-Wrapped Mo2C Nanoparticle and Carbon Nanotube Formed Directly on Ni Foam as a High-Performance Binder-Free Cathode for Li-O2 Batteries. Adv. Funct. Mater. 2016, 26, 8514–8520. [Google Scholar] [CrossRef]
- Lu, Y.; Ang, H.; Yan, Q.; Fong, E. Bioinspired Synthesis of Hierarchically Porous MoO2/Mo2C Nanocrystal Decorated N-Doped Carbon Foam for Lithium–Oxygen Batteries. Chem. Mater. 2016, 28, 5743–5752. [Google Scholar] [CrossRef]
- Wua, C.; Houb, Y.; Jianga, J.; Guoa, H.; Liua, H.-K.; Chenb, J.; Wanga, J. Heterostructured Mo2C–MoO2 as highly efficient catalyst for rechargeable Li–O2 battery. J. Power Sources 2020, 470, 228317. [Google Scholar] [CrossRef]
- Shein, I.; Ivanovskii, A. Graphene-like titanium carbides and nitrides Tin+1Cn, Tin+1Nn (n=1, 2, and 3) from de-intercalated MAX phases: First-principles probing of their structural, electronic properties and relative stability. Comput. Mater. Sci. 2012, 65, 104–114. [Google Scholar] [CrossRef]
- Venkateshalu, S.; Grace, A.N. MXenes—A new class of 2D layered materials: Synthesis, properties, applications as supercapacitor electrode and beyond. Appl. Mater. Today 2020, 18, 100509. [Google Scholar] [CrossRef]
- Wei, Y.; Soomro, R.A.; Xie, X.; Xu, B. Design of efficient electrocatalysts for hydrogen evolution reaction based on 2D MXenes. J. Energy Chem. 2020, 55, 244–255. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef]
- Soundiraraju, B.; George, B.K. Two-Dimensional Titanium Nitride (Ti2N) MXene: Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef] [PubMed]
- Urbankowski, P.; Anasori, B.; Hantanasirisakul, K.; Yang, L.; Zhang, L.; Haines, B.; May, S.J.; Billinge, S.J.L.; Gogotsi, Y. 2D molybdenum and vanadium nitrides synthesized by ammoniation of 2D transition metal carbides (MXenes). Nanoscale 2017, 9, 17722–17730. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-Templated Synthesis of 2D Metallic MoN and Other Nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef]
- Chao, F.; Wang, B.; Ren, J.; Lu, Y.; Zhang, W.; Wang, X.; Cheng, L.; Lou, Y.; Chen, J. Micro-meso-macroporous FeCo-N-C derived from hierarchical bimetallic FeCo-ZIFs as cathode catalysts for enhanced Li-O2 batteries performance. J. Energy Chem. 2019, 35, 212–219. [Google Scholar] [CrossRef]
- Shukla, V.; Jena, N.K.; Naqvi, S.R.; Luo, W.; Ahuja, R. Modelling high-performing batteries with Mxenes: The case of S-functionalized two-dimensional nitride Mxene electrode. Nano Energy 2019, 58, 877–885. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Yin, R.; Li, B.; Huang, X.; Zhao, L.; Qian, L. Single-atom Pt supported on holey ultrathin g-C3N4 nanosheets as efficient catalyst for Li-O2 batteries. J. Colloid Interface Sci. 2020, 564, 28–36. [Google Scholar] [CrossRef] [PubMed]

| Materials | Morphology | CD-Potential Gap (V) | Capacity (mAh g−1)/Current Density (mA g−1) | Capacity Retention | Ref |
|---|---|---|---|---|---|
| TiC | Nanosheets | 1 V | 500 at 1 mA cm−2 | 98% after 100 cycles | [67] |
| Ru-TiC | Nanowire | 0.91 V | 1.6 mAh cm−2 at 0.1 mA cm−2 | ~61% after 270 cycles | [74] |
| TiC-cloth | Nanowire | 0.6 V | 0.5 mAh cm−2 at 0.1 mA cm−2 | ~92% after 100 cycles | [75] |
| graphene@Fe/Fe3C | Three-dimensional porous structure | 0.61 V | 7150 at 0.1 mA cm−2 | n.a | [83] |
| F@NG-NCNT | Bamboo tubular | 1.1 V | 6966 at 0.1 mA cm−2 | 100% after 30 cycles | [84] |
| Fe/Fe3C–CNFs | Nanofibers | 1.05 V | 6920/80 | n.a | [85] |
| (V-TiO2/Ti3C2Tx) | Nanosheet | 0.21 V | 11,487/100 | 79% after 200 cycles | [127] |
| NiO/Ti3C2 | Accordion nanosheets | 1.07 V | 13,350/100 | Maintains stable capacity after 90 cycles | [128] |
| CoO/Ti3C2Tx | Layered nanosheet | 1.02 V | 16,220/100 | n.a | [129] |
| Carbon-wrapped Mo2C/Ni-foam | Nanoparticles | 0.9 V | 10,400/100 | Maintains stable capacity after 200 cycles | [137] |
| Mo2C/C | Nanoflowers | 1.2 V | 7500/100 | Maintains stable capacity after 104 cycles | [134] |
| Mo2C/CNF | Nanoparticles | 1.0 V | 10,509/100 | Maintains stable capacity after 124 cycles | [132] |
| Mo2CTx MXene/CNT | Nanoporous | 2.0 V | 5950/100 | Maintains stable capacity after 40 cycles | [131] |
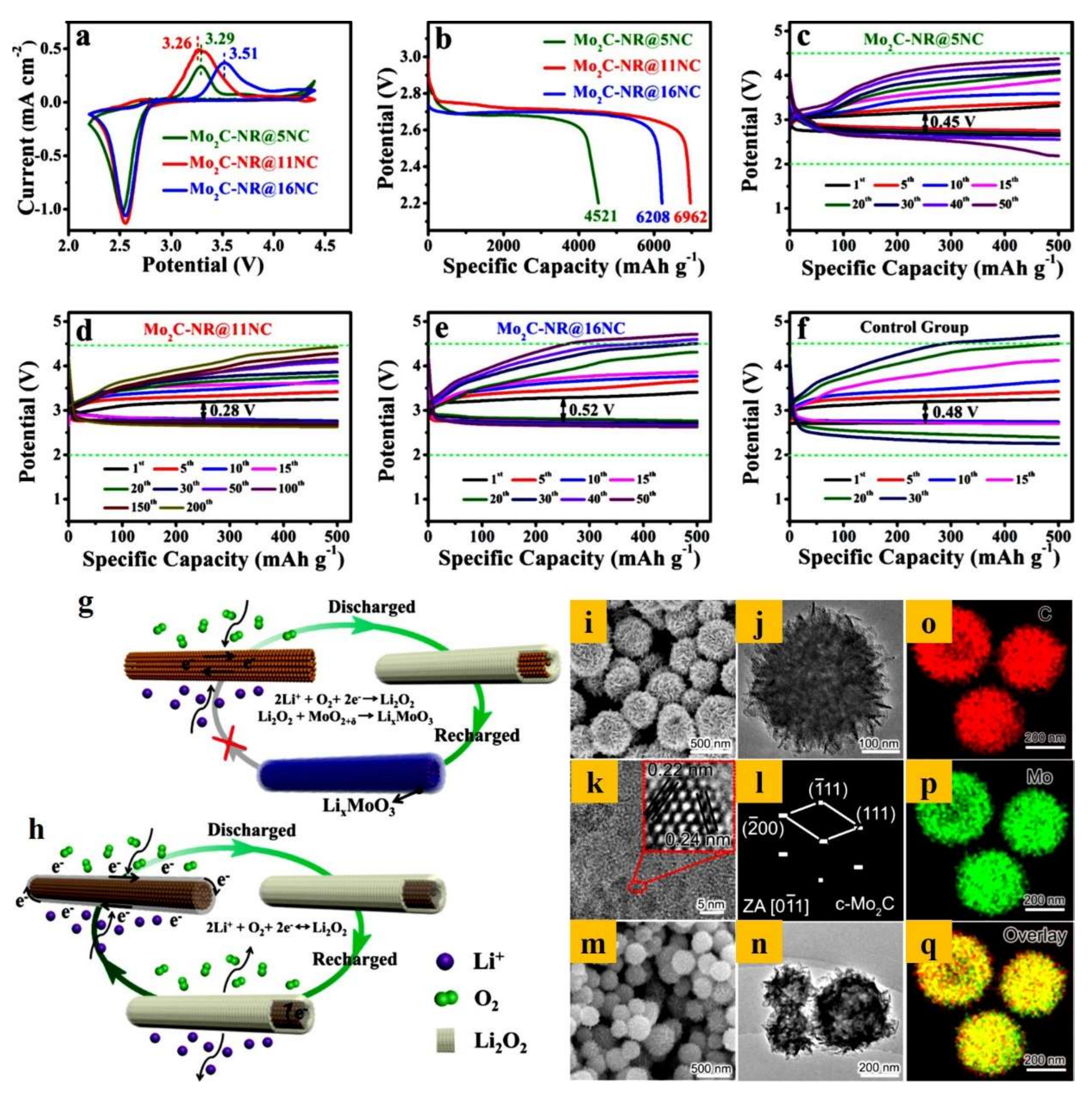
| Mo2C-NR@NC | Nanorods | 0.28 V | 6962/100 | n.a | [133] |
| Mo2C-MoC | House penticide | n.a | 34,862/200 | Maintains stable capacity after 162 cycles | [136] |
| MoO2/Mo2C | Porous nanocrystals | 0.21 V | 5000/1000 | Maintains stable capacity after 40 cycles | [138] |
| Mo2C-MoO2 | Porous nanosheet | 0.56 V | 2365/200 | Maintains stable capacity after 100 cycles | [139] |
| n-TiN/VC | Nanoparticles | 0.39 V | 6407/50 | n.a | [90] |
| VN @C | nanoribbon | 0.88 V | 1000/100 | Maintains stable capacity after 183 cycles | [102] |
| Fe, Co–co-doped C-N | polyhedra | 1.0 V | 800/500 | Maintains stable capacity after 56 cycles | [147] |
| Pt supported g-C3N4 | nanosheet | 1.5 V | 17,059.5/100 | Maintains stable capacity after 100 cycles | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppasamy, K.; Prasanna, K.; Jothi, V.R.; Vikraman, D.; Hussain, S.; Hwang, J.-H.; Kim, H.-S. Recent Advances in Nanostructured Transition Metal Carbide- and Nitride-Based Cathode Electrocatalysts for Li–O2 Batteries (LOBs): A Brief Review. Nanomaterials 2020, 10, 2106. https://doi.org/10.3390/nano10112106
Karuppasamy K, Prasanna K, Jothi VR, Vikraman D, Hussain S, Hwang J-H, Kim H-S. Recent Advances in Nanostructured Transition Metal Carbide- and Nitride-Based Cathode Electrocatalysts for Li–O2 Batteries (LOBs): A Brief Review. Nanomaterials. 2020; 10(11):2106. https://doi.org/10.3390/nano10112106
Chicago/Turabian StyleKaruppasamy, K., K. Prasanna, Vasanth Rajendiran Jothi, Dhanasekaran Vikraman, Sajjad Hussain, Jung-Hoon Hwang, and Hyun-Seok Kim. 2020. "Recent Advances in Nanostructured Transition Metal Carbide- and Nitride-Based Cathode Electrocatalysts for Li–O2 Batteries (LOBs): A Brief Review" Nanomaterials 10, no. 11: 2106. https://doi.org/10.3390/nano10112106
APA StyleKaruppasamy, K., Prasanna, K., Jothi, V. R., Vikraman, D., Hussain, S., Hwang, J.-H., & Kim, H.-S. (2020). Recent Advances in Nanostructured Transition Metal Carbide- and Nitride-Based Cathode Electrocatalysts for Li–O2 Batteries (LOBs): A Brief Review. Nanomaterials, 10(11), 2106. https://doi.org/10.3390/nano10112106








