Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Radionuclides
2.3. Cell Lines
2.4. Instrumentation
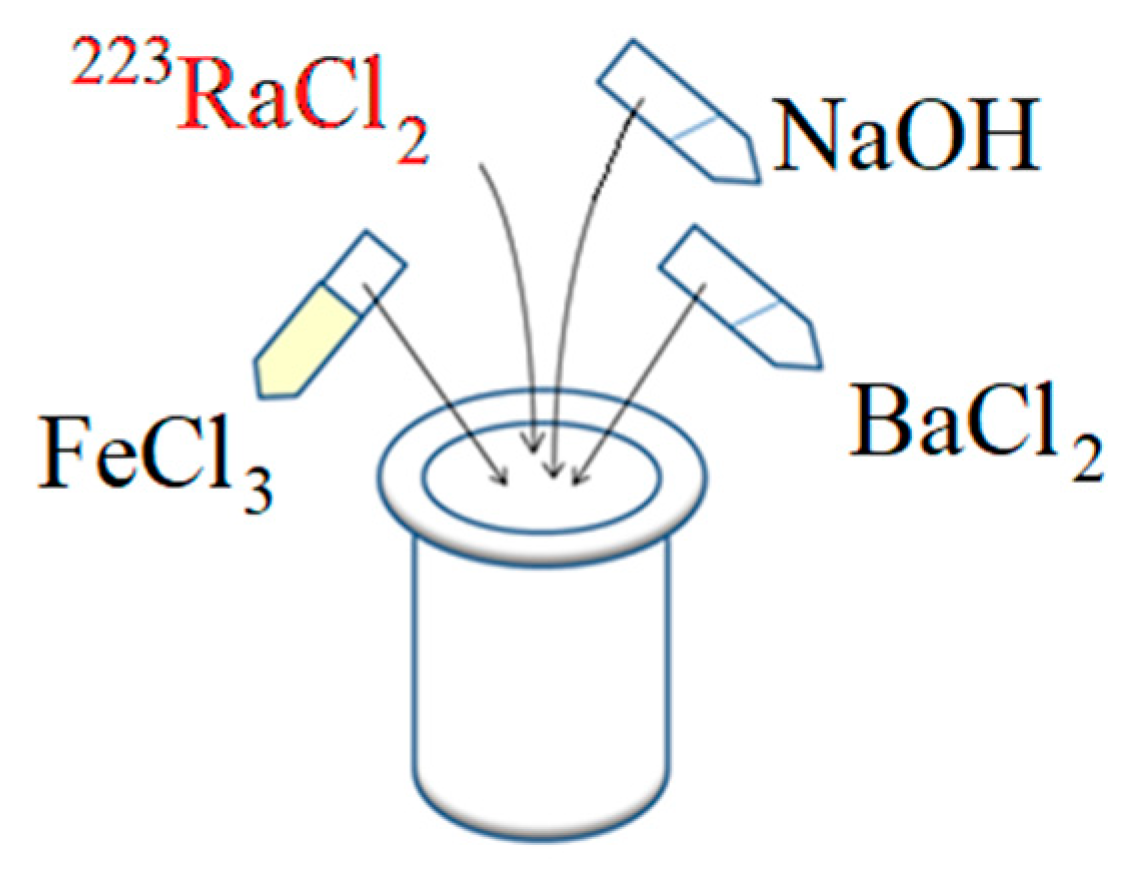
2.5. Synthesis of Barium Ferrite Nanoparticles Labeled with 223Ra
2.6. Synthesis of [223Ra]BaFe–CEPA–Trastuzumab
2.7. Stability Studies of [223Ra]BaFeNPs and [223Ra]BaFe–CEPA–Trastuzumab
2.8. Quantification of the Number of Trastuzumab Molecules per One Barium Ferrite Nanoparticle
2.9. Binding Specificity Assay
2.10. Internalization Studies
2.11. Confocal Imaging
2.12. In Vitro Cytotoxicity Assay
2.13. Spheroids
3. Results and Discussion
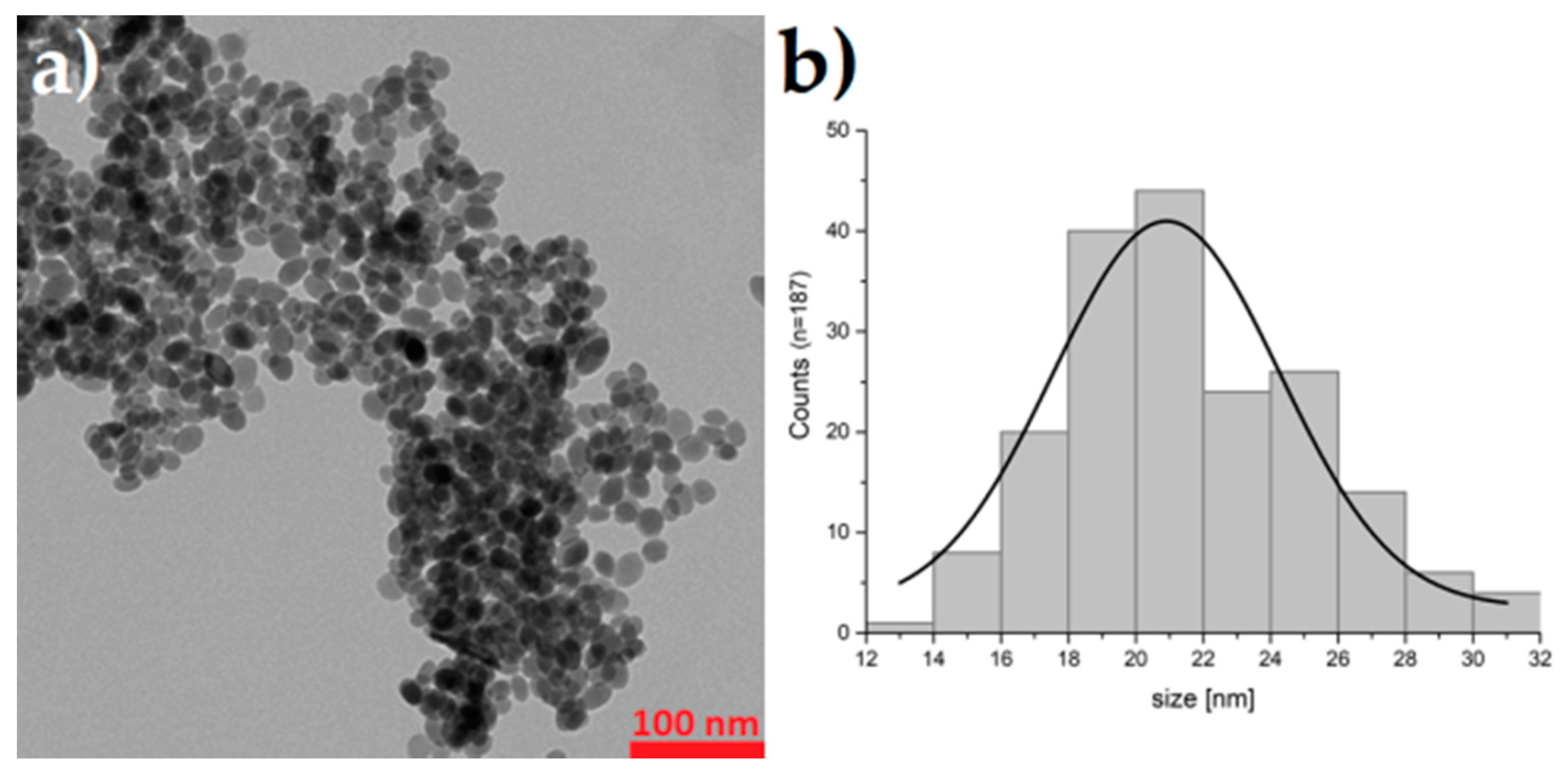
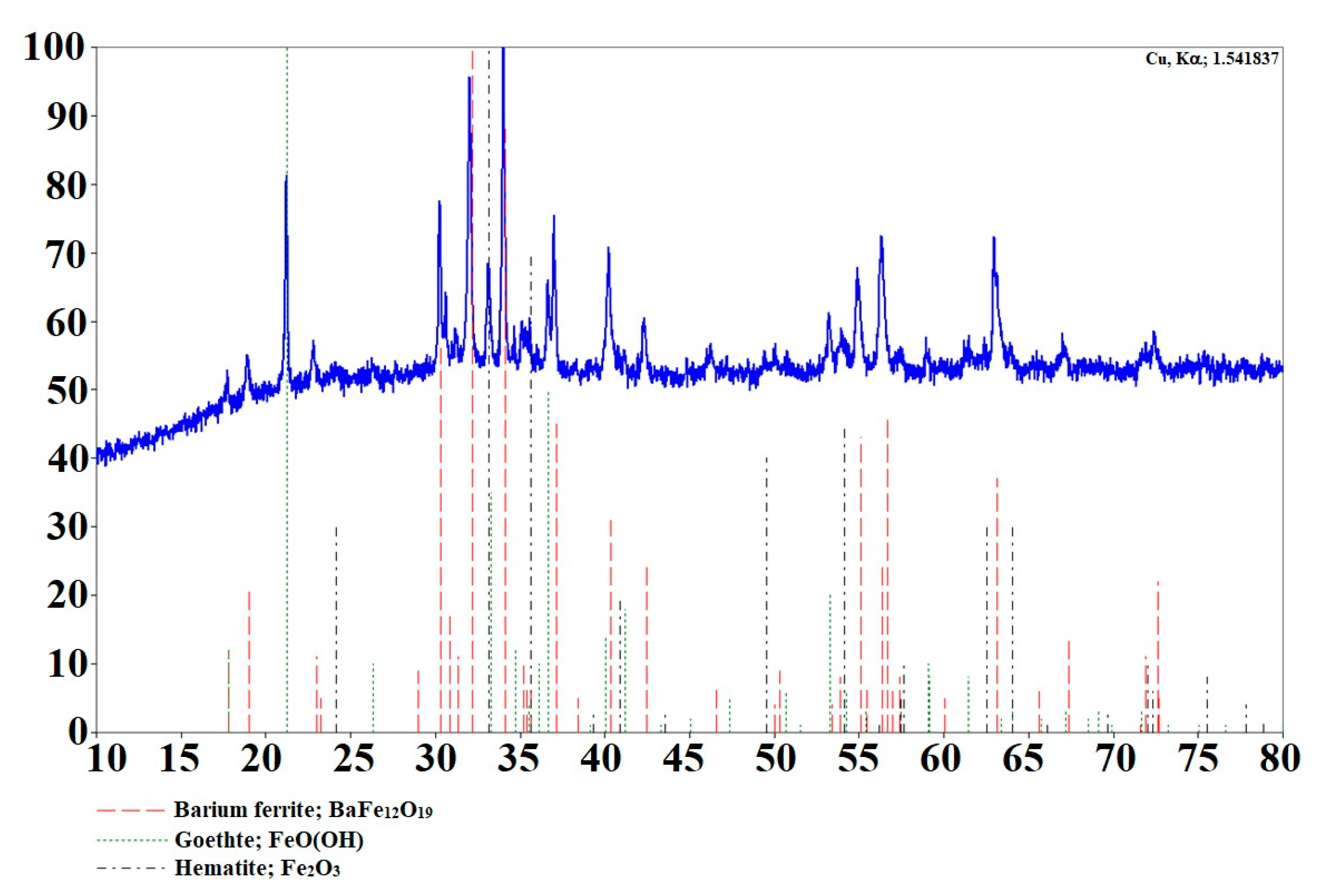
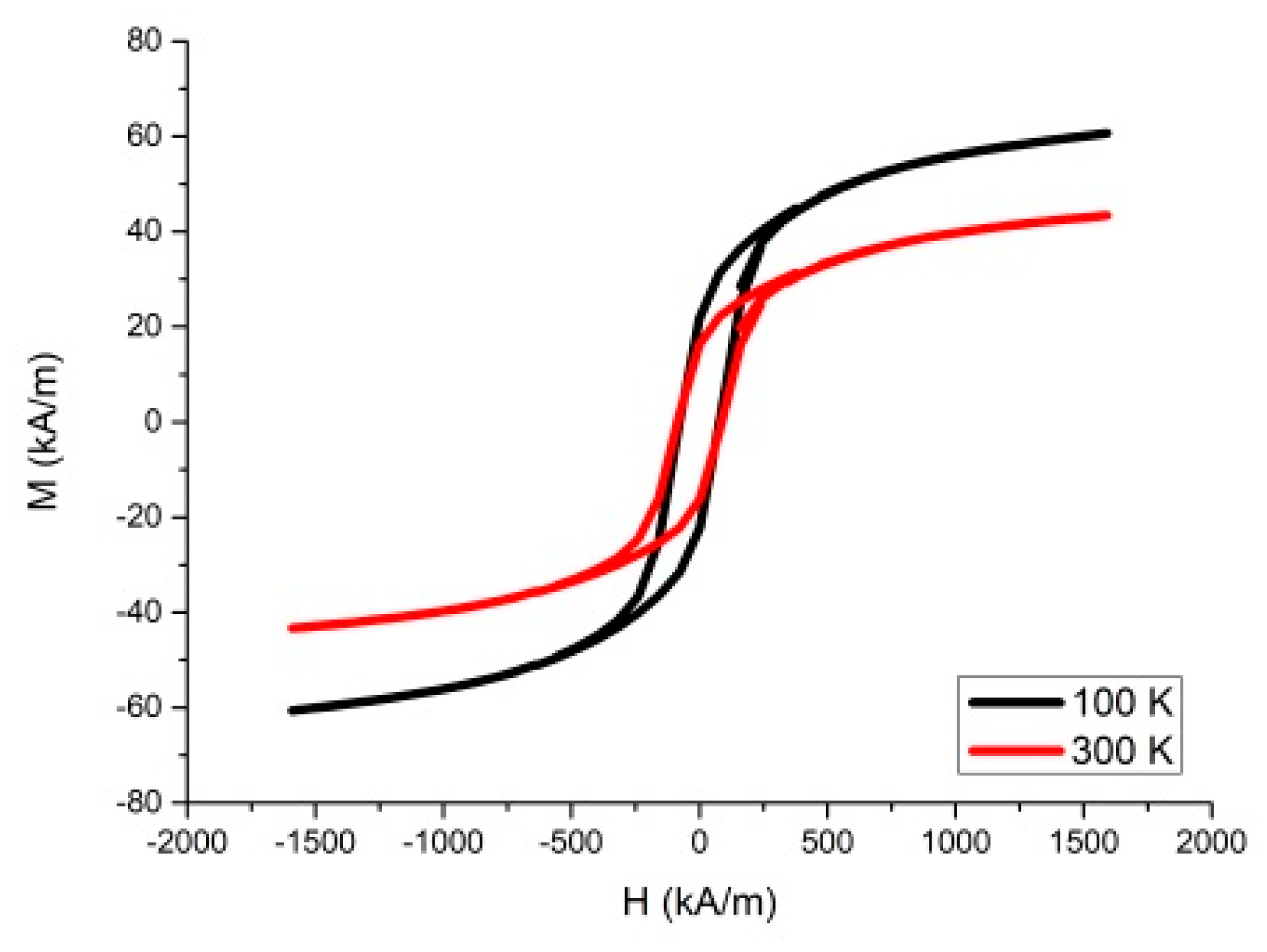
3.1. Synthesis and Characterization of Barium Ferrite Nanoparticles
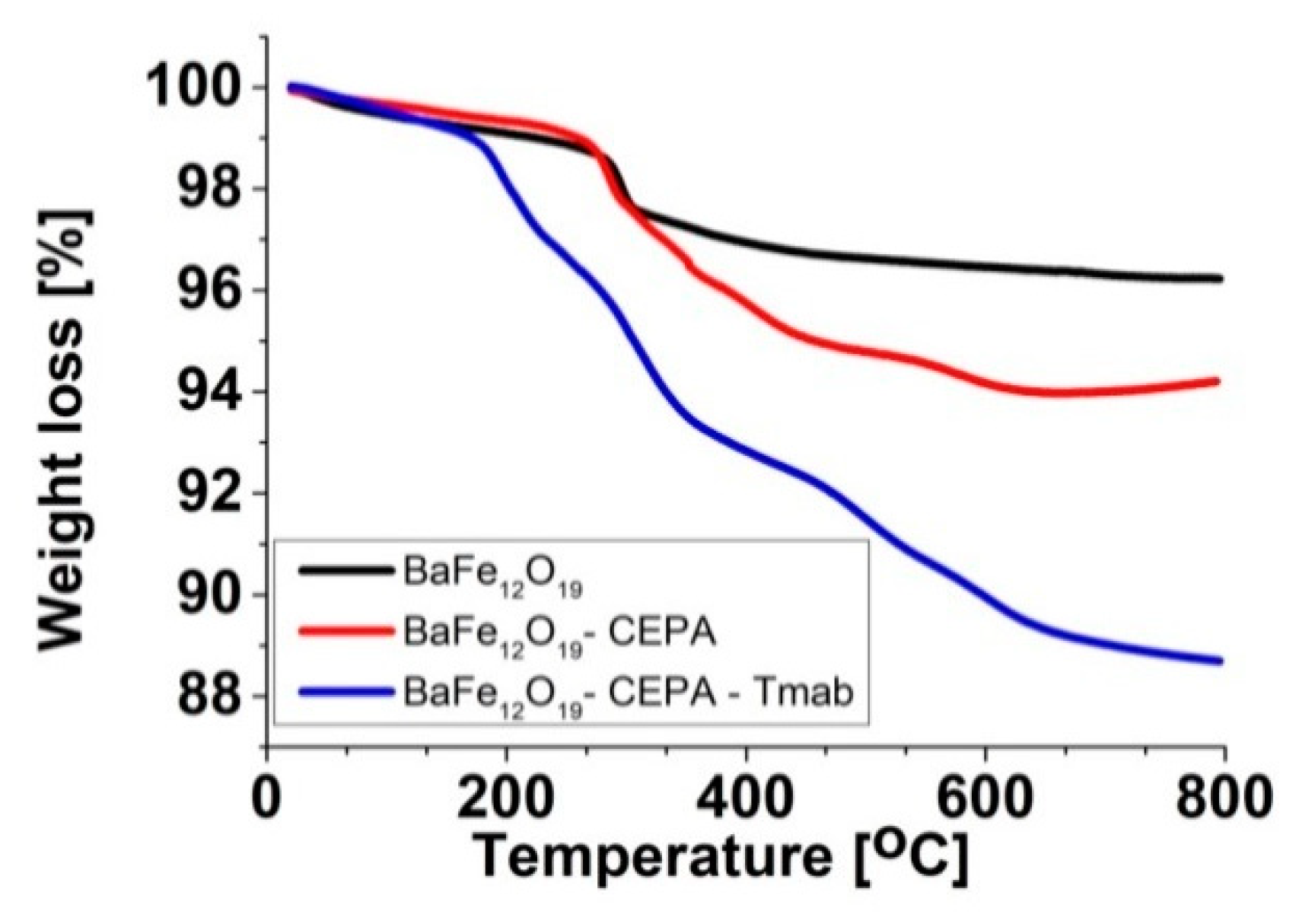
3.2. Functionalization of BaFeNPs
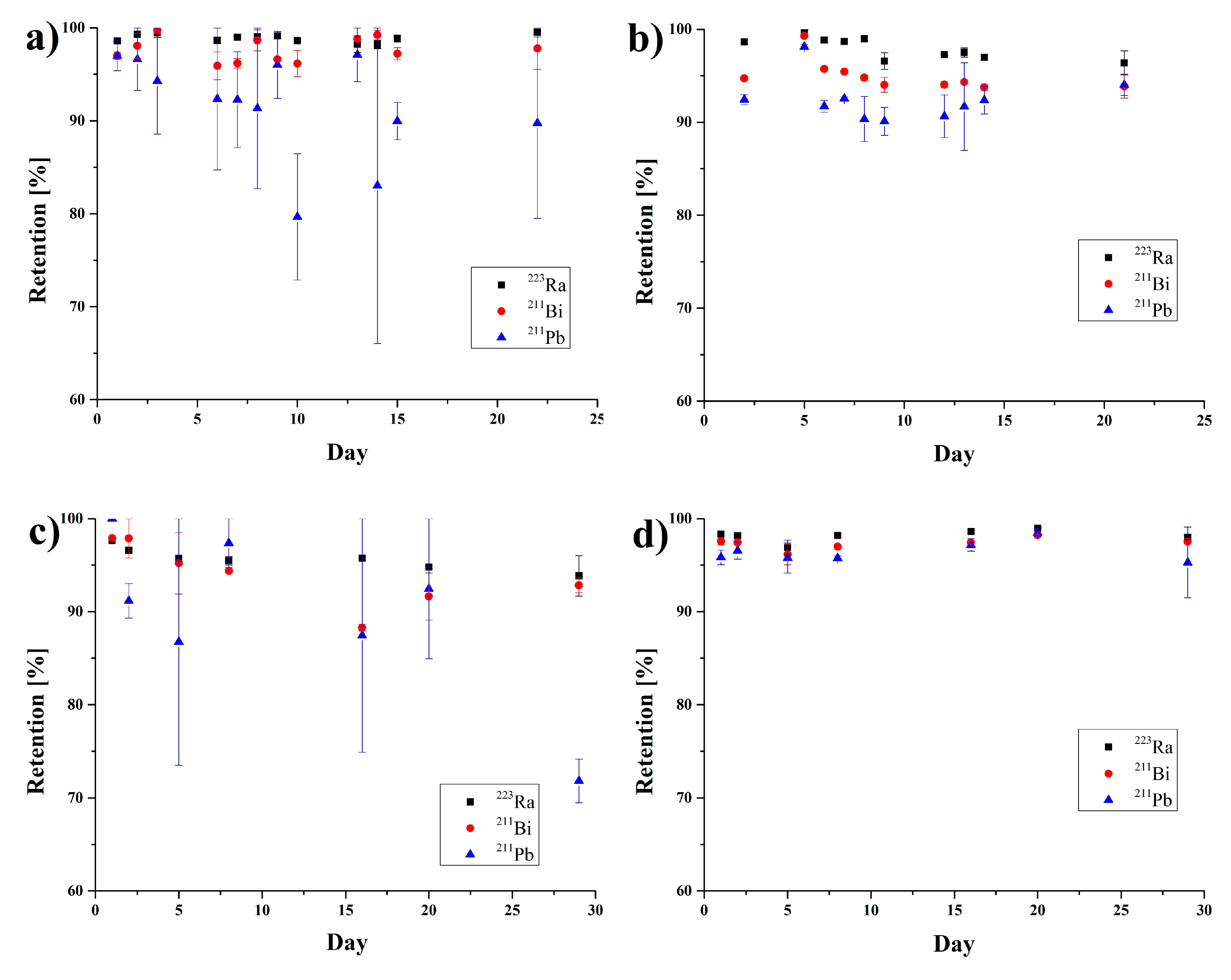
3.3. Stability Studies
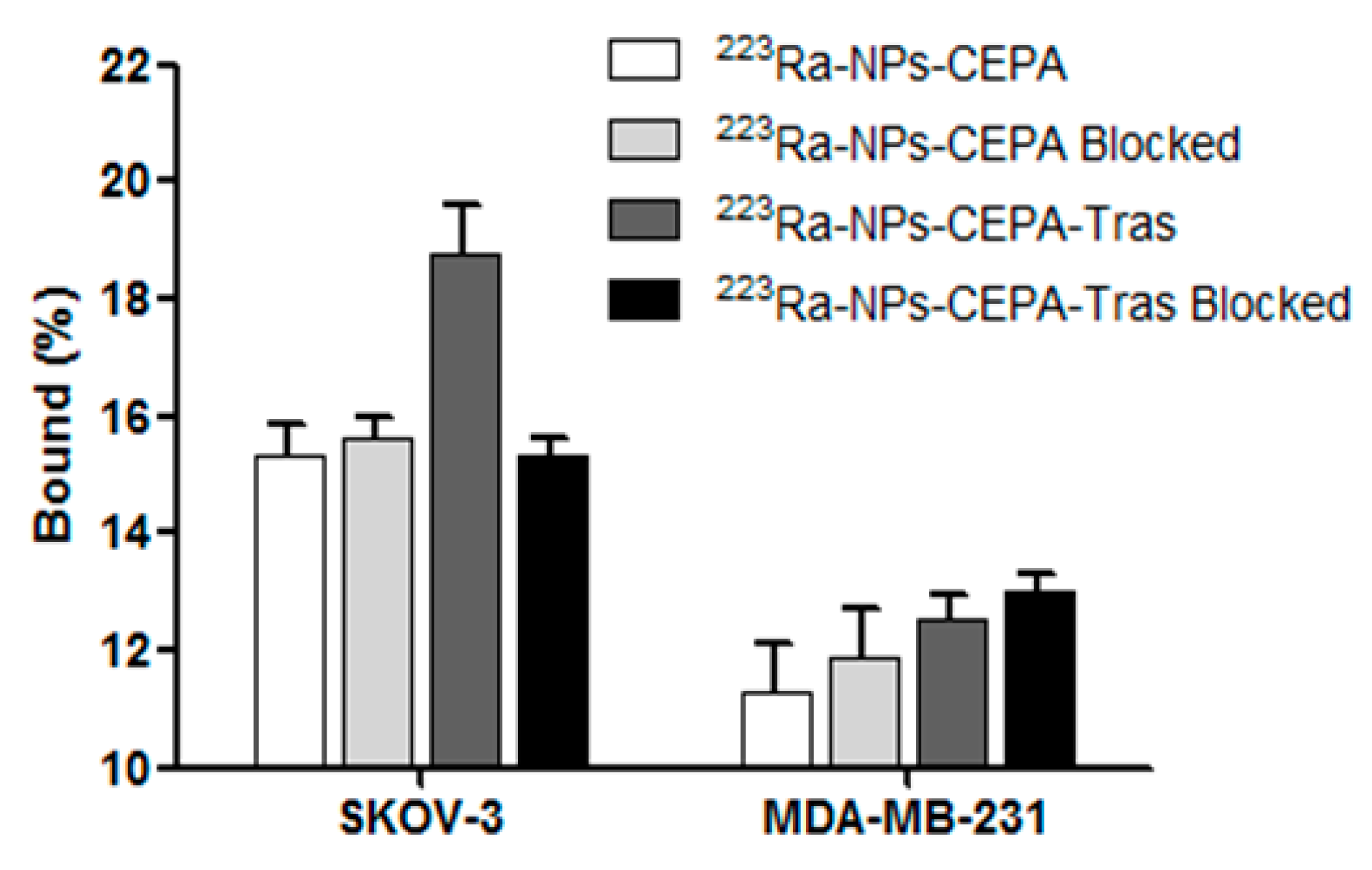
3.4. Specificity of Binding
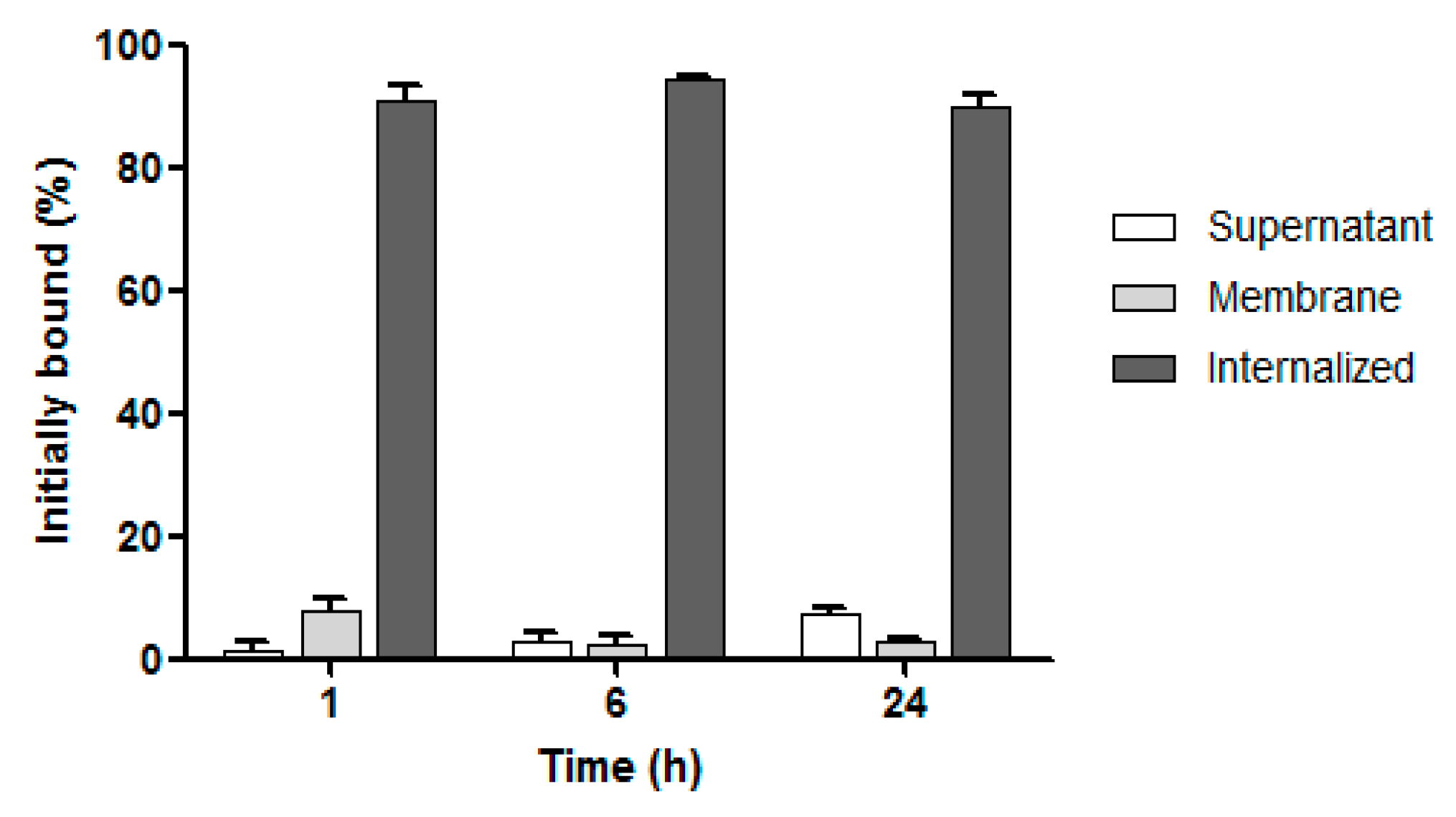
3.5. Internalization Studies
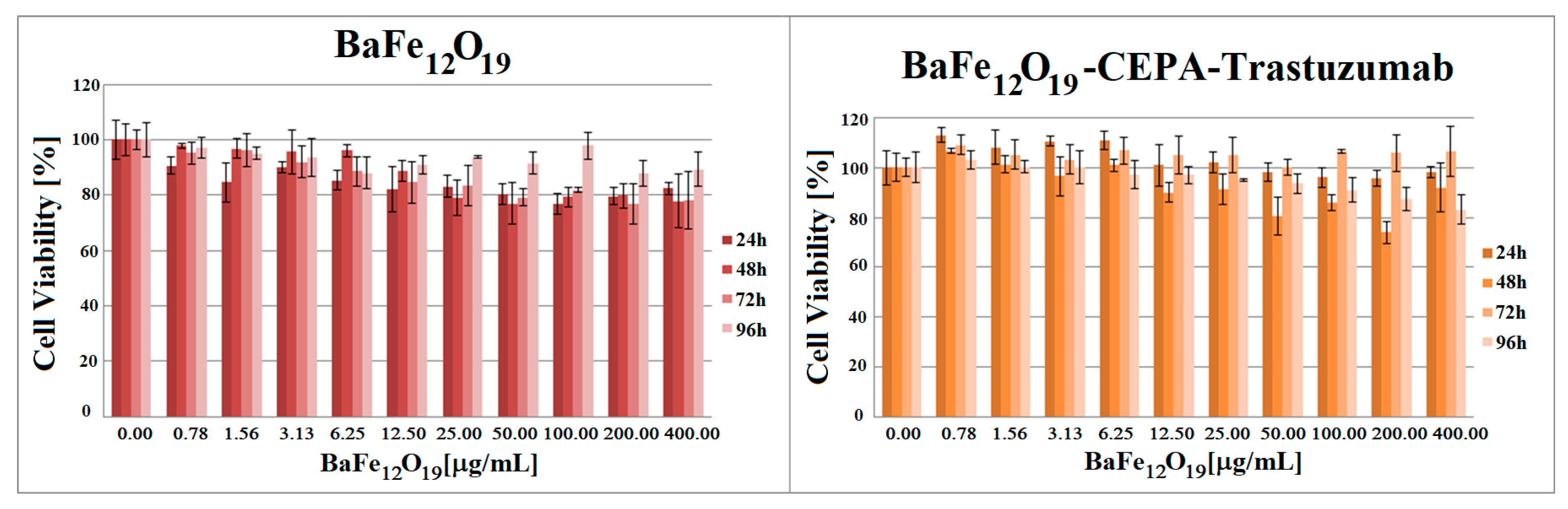
3.6. In Vitro Cytotoxicity Assay
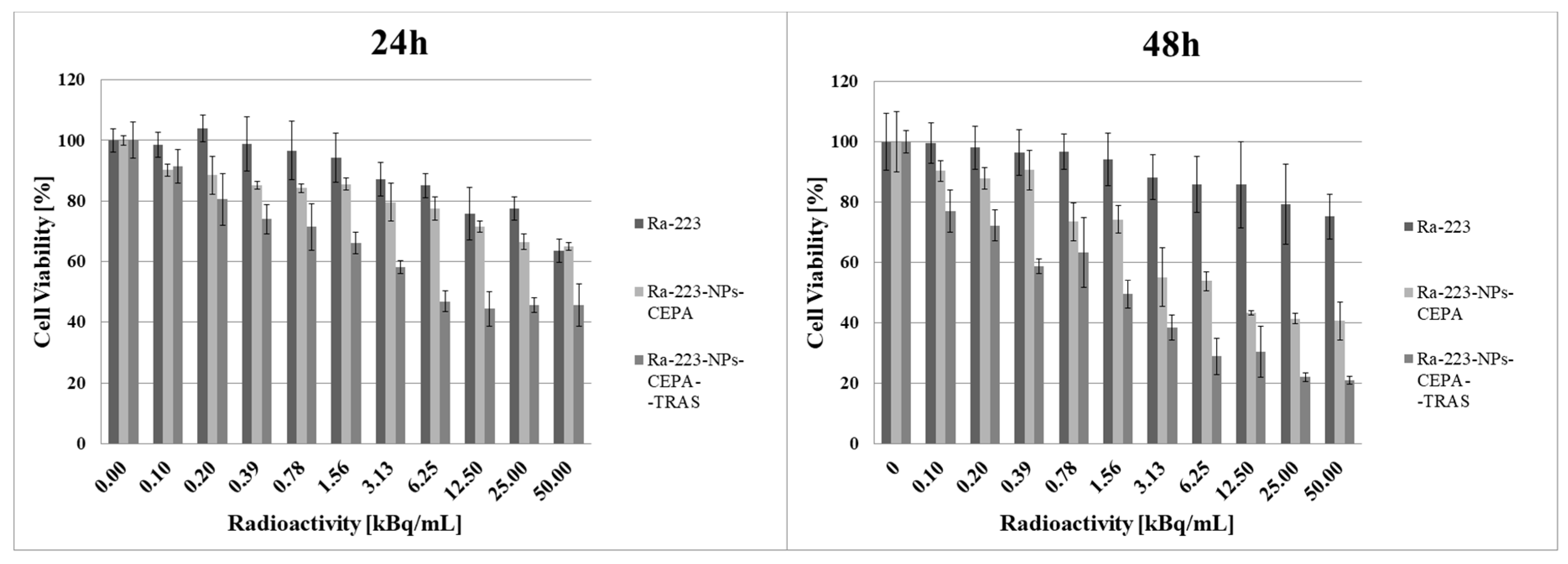
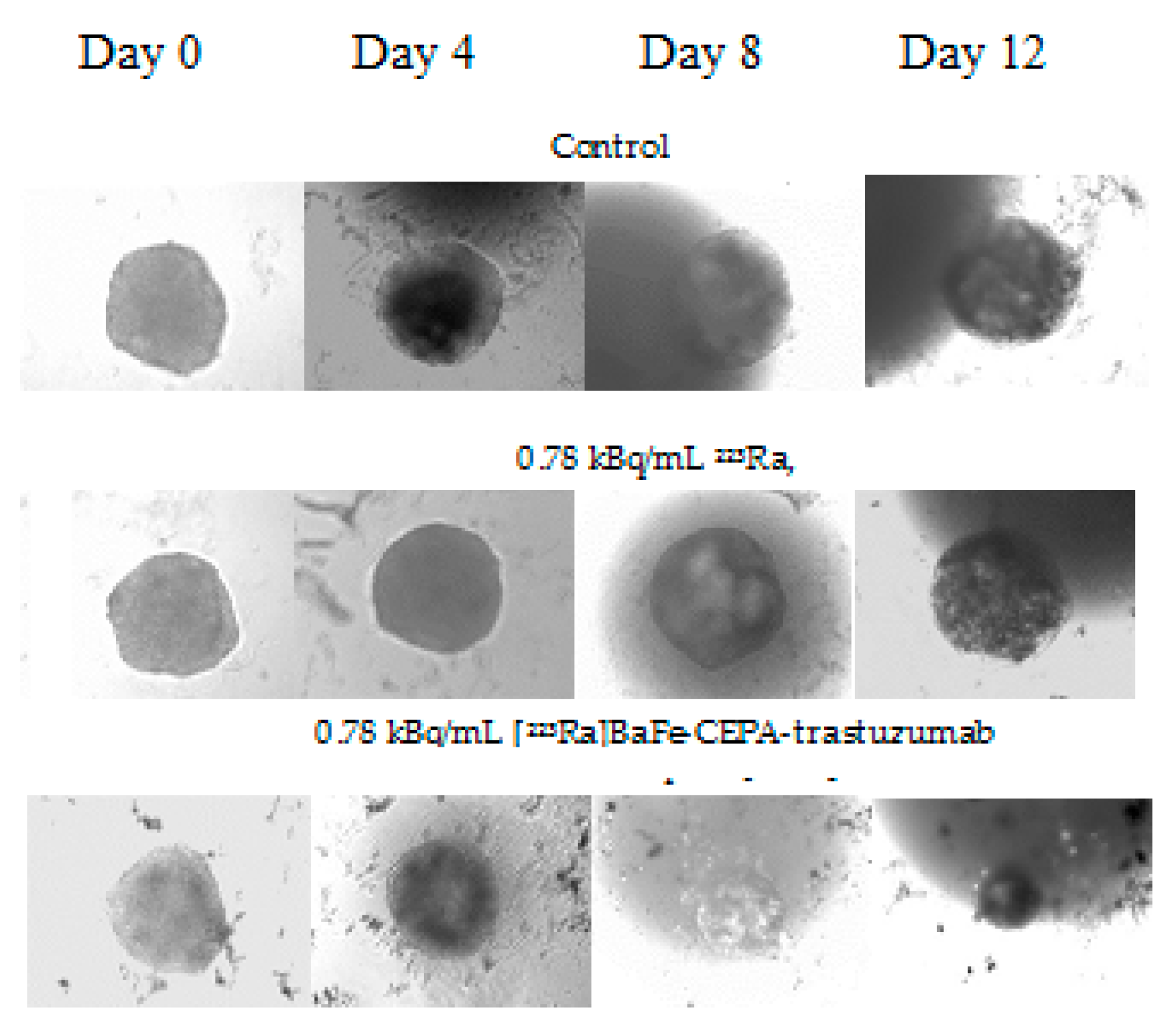
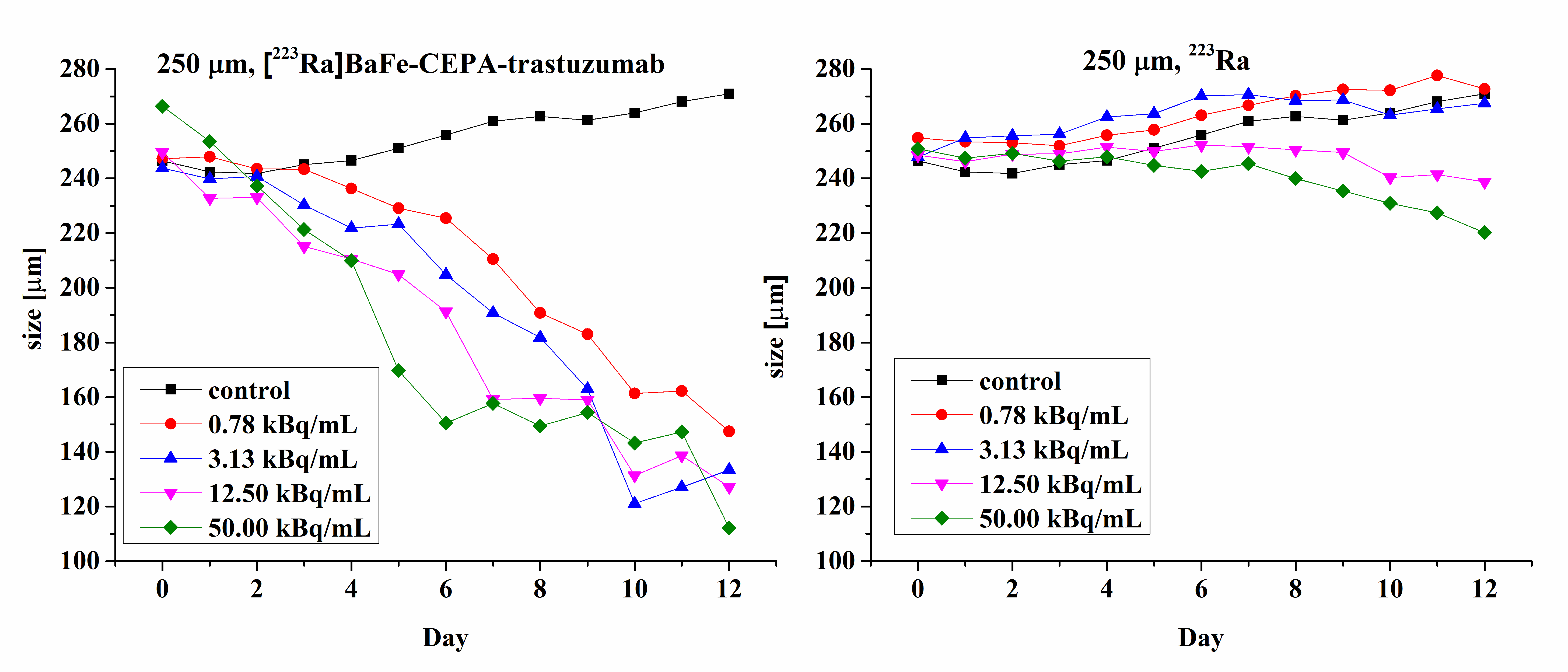
3.7. Radiotoxicity Studies on Cell Spheroids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baidoo, K.E.; Yong, K.; Brechbiel, M.W. Molecular pathways: Targeted alpha-particle radiation therapy. Clin. Cancer Res. 2013, 19, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R. Radionuclide therapy. In Radiochemistry and Radiopharmaceutical Chemistry in Life Sciences; Rösch, F., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 315–348. [Google Scholar]
- Bandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Anti–prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular α-particle therapy of cancer. J. Nucl. Med. 2014, 55, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, M.G.; Radchenko, V.; Wilbur, D.S. Radiochemical aspects of alpha emitting radionuclides for medical application. Radiochim. Acta 2019, 107, 1065–1085. [Google Scholar] [CrossRef]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-emitters and targeted alpha therapy in oncology: From basic science to clinical investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An overview of targeted alpha therapy with 225actinium and 213bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Swimmer-plot analysis suggests efficacy regarding duration of tumor control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. Ac-225-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Colletti, P.M. New treatment option: 223Ra chloride, the first approved unsealed α-emitting radiopharmaceutical. Clin. Nucl. Med. 2013, 38, 724–725. [Google Scholar] [CrossRef]
- Chang, C.A. Selectivity of macrocyclic aminocarboxylates for alkaline-earth metal ions and stability of their complexes. J. Chem. Soc. Dalton Trans. 1996, 2347–2350. [Google Scholar] [CrossRef]
- Henriksen, G.; Hoff, P.; Larsen, R.H. Evaluation of potential chelating agents for radium. Appl. Radiat. Isot. 2002, 56, 667–671. [Google Scholar] [CrossRef]
- Gott, M.; Yang, P.; Kortz, U.; Stephan, H.; Pietzsch, H.-J.; Mamat, C. A 224Ra-labeled polyoxopalladate as a putative radiopharmaceutical. Chem. Commun. 2019, 55, 7631–7634. [Google Scholar] [CrossRef]
- Aboul, D.; Thiele, N.; Villmer, A.; Gustche, N.; Escorcia, F.; Wilson, J.; Thorek, D. MACROPA highly stable chelator of Radium-223 and functionalization attempts for targeted treatment of cancer. J. Nucl. Med. 2020, 61 (Suppl. 1), 587. [Google Scholar]
- Henriksen, G.; Schoultz, B.W.; Michaelsen, T.E.; Bruland, Ø.S.; Larsen, R.H. Sterically stabilized liposomes as a carrier for α-emitting radium and actinium radionuclides. Nucl. Med. Biol. 2004, 31, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jonasdottir, T.J.; Fisher, D.R.; Borrebæk, J.; Bruland, Ø.; Larsen, R.H. First in vivo evaluation of liposome-encapsulated 223Ra as a potential alpha-particle-emitting cancer therapeutic agent. Anticancer Res. 2006, 26, 2841–2848. [Google Scholar] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 752. [Google Scholar] [CrossRef]
- Reissig, F.; Hübner, R.; Steinbach, J.; Pietzsch, H.-J.; Mamat, C. Facile preparation of radium-doped, functionalized nanoparticles as carriers for targeted alpha therapy. Inorg. Chem. Front. 2019, 6, 1341–1349. [Google Scholar] [CrossRef]
- Reissig, F.; Zarschler, K.; Hübner, R.; Pietzsch, H.-J.; Kopka, K.; Mamat, C. Sub-10 nm Radiolabeled barium sulfate nanoparticles as carriers for theranostic applications and targeted alpha therapy. ChemistryOpen 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Severin, A.V.; Vasiliev, A.N.; Gopin, A.V.; Vlasova, I.E.; Chernykh, E.V. Dynamics of sorption–desorption of 223Ra therapeutic α-emitter on granulated hydroxyapatite. Radiochemistry 2019, 61, 339–346. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Severin, A.; Lapshina, E.; Chernykh, E.; Ermolaev, S.; Kalmykov, S. Hydroxyapatite particles as carriers for 223Ra. J. Radioanal. Nucl. Chem. 2017, 311, 1503–1509. [Google Scholar] [CrossRef]
- Suchankova, P.; Kukleva, E.; Stamberg, K.; Nykl, P.; Vlk, M.; Kozempel, J. Study of 223Ra uptake mechanism on hydroxyapatite and titanium dioxide nanoparticles as a function of pH. RSC Adv. 2020, 10, 3659–3666. [Google Scholar] [CrossRef]
- Kozempel, J.; Vlk, M.; Malkova, E.; Bajzıkova, A.; Barta, J.; Santos-Oliveira, R.; Malta Rossi, A. Prospective carriers of 223Ra for targeted alpha therapy. J. Radioanal. Nucl. Chem. 2015, 304, 443–447. [Google Scholar] [CrossRef]
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized NaA nanozeolites labeled with 224,225Ra for targeted alpha therapy. J. Nanopart. Res. 2013, 15, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Meczynska-Wielgosz, S.A.; Majkowska-Pilip, A.; Kozminski, P.; Wójciuk, G.; Cędrowska, E.; Bruchertseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with Ra-223 for targeted alpha therapy. Nucl. Med. Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.; Fracasso, G.; Pruszyński, M.; Bilewicz, A.; Kruszewski, M.; Majkowska-Pilip, A.; Lankoff, A. Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy–Part, I. Materials 2020, 13, 3875. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.F.; Robertson, D.; Pevsner, P.H.; Wall, J.S.; Mirzadeh, S.; Kennel, S.J. LnPO4 nanoparticles doped with Ac-225 and sequestered daughters for targeted alpha therapy. Cancer Biother. Radiopharm. 2014, 29, 34–41. [Google Scholar] [CrossRef]
- Woodward, J.; Kennel, S.J.; Stuckey, A.; Osborne, D.; Wall, J.; Rondinone, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO4 nanoparticles doped with actinium-225 that partially sequester daughter radionuclides. Bioconjug. Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef]
- McLaughlin, M.F.; Woodward, J.; Boll, R.A.; Rondinone, A.J.; Mirzadeh, S.; Robertson, J.D. Gold-coated lanthanide phosphate nanoparticles for an 225Ac in vivo alpha generator. Radiochim. Acta 2013, 101, 595–600. [Google Scholar] [CrossRef]
- McLaughlin, M.F.; Woodward, J.; Boll, R.A.; Wall, J.S.; Rondinone, A.J.; Kennel, S.J.; Mirzadeh, S.; Robertson, D. Gold coated lanthanide phosphate nanoparticles for targeted alpha generator radiotherapy. PLoS ONE 2013, 8, e54531. [Google Scholar] [CrossRef]
- Toro-Gonzalez, M.; Dame, A.N.; Mirzadeh, S.; Rojas, J.V. Gadolinium vanadate nanocrystals as carriers of α-emitters (Ac-225, Th-227) and contrast agents. J. Appl. Phys. 2019, 125, 214901. [Google Scholar] [CrossRef]
- Rojas, J.V.; Woodward, J.D.; Chen, N.; Rondinone, A.J.; Castano, C.H.; Mirzadeh, S. Synthesis and characterization of lanthanum phosphate nanoparticles as carriers for 223Ra and 225Ra for targeted alpha therapy. Nucl. Med. Biol. 2015, 42, 614–620. [Google Scholar] [CrossRef]
- Fu, L.; Liu, X.; Zhang, Y.; Dravid, V.P.; Mirkin, C.A. Nanopatterning of “hard” magnetic nanostructures via dip-pen nanolithography and a sol-based ink. Nano Lett. 2003, 3, 757–760. [Google Scholar] [CrossRef]
- Kappiyoor, R.; Liangruksa, M.; Ganguly, R.; Puri, I.K. The effects of magnetic nanoparticle properties on magnetic fluid hyperthermia. J. Appl. Phys. 2010, 108, 094702. [Google Scholar] [CrossRef]
- Belous, A.G. Synthesis and properties of ferromagnetic nanostructures and their possible use in medicine and microwave engineering. In Proceedings of the International Conference on Oxide Materials for Electronic Engineering—Fabrication, Properties and Applications (OMEE-2014), Lviv, Ukraine, 26–30 May 2014; pp. 69–70. [Google Scholar] [CrossRef]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Yu, Z.; Vujaskovic, D.; Sanders, L.L.; Dewhirst, M.W. Randomized trial of hyperthermia and radiation for superficial tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Bu, J.; Chen, J.; Zheng, G.; DaCosta, R.S. Dual in vivo photoacoustic and fluorescence imaging of HER2 expression in breast tumors for diagnosis, margin assessment, and surgical guidance. Mol. Imaging 2014, 13, 1–9. [Google Scholar] [CrossRef]
- Drofenik, M.; Ban, I.; Makovec, D.; Znidarsic, A.; Jaglicic, Z.; Hanzel, D.; Lisjak, D. The hydrothermal synthesis of super-paramagnetic barium hexaferrite particles. Mater. Chem. Phys. 2011, 127, 415–441. [Google Scholar] [CrossRef]
- Cędrowska, E.; Pruszyński, M.; Gawęda, W.; Żuk, M.; Krysiński, P.; Bruchertseifer, F.; Morgenstern, A.; Karageorgou, M.-A.; Bouziotis, P.; Bilewicz, A. Trastuzumab conjugated superparamagnetic iron oxide nanoparticles labeled with 225Ac as a perspective tool for combined radioimmunotherapy and magnetic hyperthermia of HER2-positive breast cancer. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-modified gold nanoparticles labeled with 211At as a prospective tool for local treatment of HER2-positive breast cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef]
- Jacobo, S.E.; Civale, L.; Blesa, M.A. Evolution of the magnetic properties during the thermal treatment of barium hexaferrite precursors obtained by coprecipitation from barium ferrate (VI) solutions. J. Magn. Magn. Mater. 2003, 260, 37–41. [Google Scholar] [CrossRef]
- Zahari, M.H.B.; Guan, B.H.; Chuan, L.K. Structural and magnetic properties of hexagonal barium ferrite synthesized through the sol-gel combustion route. AIP Conf. Proc. 2016, 1787, 070002. [Google Scholar] [CrossRef]
- Yang, P.F.; Wang, M.G.; Meng, F.J.; Yhang, J.H.; Sun, A.J.; Lu, C.X. Preparation of rodlike barium ferrite particles by inverse microemulsion method. Chin. J. Inorg. Chem. 2005, 21, 607–611. [Google Scholar]
- Sankaranarayanan, V.K.; Khan, D.C. Mechanism of the formation of nanoscale M-type barium hexaferrite in the citrate precursor method. J. Magn. Magn. Mater. 1996, 153, 337–346. [Google Scholar] [CrossRef]
- Mishra, D.; Anand, S.; Panda, R.K.; Das, R.P. Studies on characterization, microstructures and magnetic properties of nano-size barium hexa-ferrite prepared through a hydrothermal precipitation-calcination route. Mater. Chem. Phys. 2004, 86, 132–136. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Gan, L.-M.; Ng, S.-C. Improving the magnetic properties of hydrothermally synthesized barium ferrite. J. Magn. Magn. Mater. 1999, 195, 452–459. [Google Scholar] [CrossRef]
- Che, S.; Wang, J.; Chen, Q. Soft magnetic nanoparticles of BaFe12O19 fabricated under mild conditions. J. Phys. Condens. Matter. 2003, 15, L335–L339. [Google Scholar] [CrossRef]
- Cheon, J.; Lee, J.-H. Magnetic Nanoparticles in Biomedical Application in Inorganic Nanoprobes for Biological Sensing and Imaging; Mattoussi, H., Cheon, J., Eds.; Artech House: London, UK, 2009. [Google Scholar]
- Mokhodoeva, O.; Vlk, M.; Malkova, E.; Kukleva, E.; Micolova, P.; Stamberg, K.; Slouf, M.; Dzhenloda, R.; Kozempel, J. Study of 223Ra uptake mechanism by Fe3O4 nanoparticles: Towards new prospective theranostic SPIONs. J. Nanopart. Res. 2016, 18, 301–313. [Google Scholar] [CrossRef]
- de Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A critical review of alpha radionuclide therapy—How to deal with recoiling daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ataie, A.; Sheibani, S. Chromium (VI) ions adsorption onto barium hexaferrite magnetic nano-adsorbent. Adv. Mater. Lett. 2016, 77, 579–586. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Arsenic removal by magnetic nanocrystalline barium hexaferrite. J. Nanopart. Res. 2012, 14, 881–887. [Google Scholar] [CrossRef]
- Holzwarth, U.; Jimenez, I.O.; Calzolai, L. A random walk approach to estimate the confinement of α-particle emitters in nanoparticles for targeted radionuclide therapy. EJNMMI Radiopharm. Chem. 2018, 3, 9. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Gawęda, W.; Żelechowska-Matysiak, K.; Wawrowicz, K.; Bilewicz, A. Nanoparticles in targeted alpha therapy. Nanomaterials 2020, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an anti-HER2 nanobody labeled with 225 Ac for targeted α-particle therapy of cancer. Mol. Pharm. 2018, 15, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N. Targeting breast carcinoma with radioiodinated anti-HER2 Nanobody. Nucl. Med. Biol. 2013, 40, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yook, S.; Lu, Y.; Bergstrom, D.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Local radiation treatment of HER2-positive breast cancer using trastuzumab-modified gold nanoparticles labeled with 177Lu. Pharm. Res. 2017, 34, 579–590. [Google Scholar] [CrossRef]
- Cai, Z.; Chattopadhyay, N.; Yang, K.; Kwon, Y.L.; Yook, S.; Pignol, J.-P.; Reilly, R.M. In-labeled trastuzumab-modified gold nanoparticles are cytotoxic in vitro to HER2-positive breast cancer cells and arrest tumor growth in vivo in athymic mice after intratumoral injection. Nucl. Med. Biol. 2016, 43, 818–826. [Google Scholar] [CrossRef]
- Ballangrud, M.; Yang, W.-H.; Palm, S.; Enmon, R.; Borchardt, P.E.; Pellegrini, V.A.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Alpha-particle emitting atomic generator (Actinium-225)-labeled trastuzumab (herceptin) targeting of breast cancer spheroids: Efficacy versus HER2/neu expression. Clin. Cancer Res. 2004, 13, 4489–4497. [Google Scholar] [CrossRef] [PubMed]

| BaFeNPs | BaFe–CEPA | BaFe–CEPA–Trastuzumab | |
|---|---|---|---|
| Hydrodynamic diameter (nm) | 144.7 ± 6.6 | 59.4 ± 2.4 | 99.9 ± 3.0 |
| Zeta potential (mV) | +26.4 ± 1.6 | −23.1 ± 1.3 | +27.2 ± 0.7 |
| BaFeNPs | BaFe–CEPA | BaFe–CEPA–Trastuzumab | |
|---|---|---|---|
| Hydrodynamic diameter (nm) | 116.6 ± 6.0 | 60.9 ± 6.6 | 218.3 ± 3.7 |
| Zeta potential (mV) | −40.0 ± 1.1 | −49.6 ± 0.9 | −8.8 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawęda, W.; Pruszyński, M.; Cędrowska, E.; Rodak, M.; Majkowska-Pilip, A.; Gaweł, D.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy. Nanomaterials 2020, 10, 2067. https://doi.org/10.3390/nano10102067
Gawęda W, Pruszyński M, Cędrowska E, Rodak M, Majkowska-Pilip A, Gaweł D, Bruchertseifer F, Morgenstern A, Bilewicz A. Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy. Nanomaterials. 2020; 10(10):2067. https://doi.org/10.3390/nano10102067
Chicago/Turabian StyleGawęda, Weronika, Marek Pruszyński, Edyta Cędrowska, Magdalena Rodak, Agnieszka Majkowska-Pilip, Damian Gaweł, Frank Bruchertseifer, Alfred Morgenstern, and Aleksander Bilewicz. 2020. "Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy" Nanomaterials 10, no. 10: 2067. https://doi.org/10.3390/nano10102067
APA StyleGawęda, W., Pruszyński, M., Cędrowska, E., Rodak, M., Majkowska-Pilip, A., Gaweł, D., Bruchertseifer, F., Morgenstern, A., & Bilewicz, A. (2020). Trastuzumab Modified Barium Ferrite Magnetic Nanoparticles Labeled with Radium-223: A New Potential Radiobioconjugate for Alpha Radioimmunotherapy. Nanomaterials, 10(10), 2067. https://doi.org/10.3390/nano10102067





