Synthesis and Characterization of CuIn1−xGaxSe2 Semiconductor Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Properties
3.2. Morphological Properties
3.3. Determination of Band Gap Energy
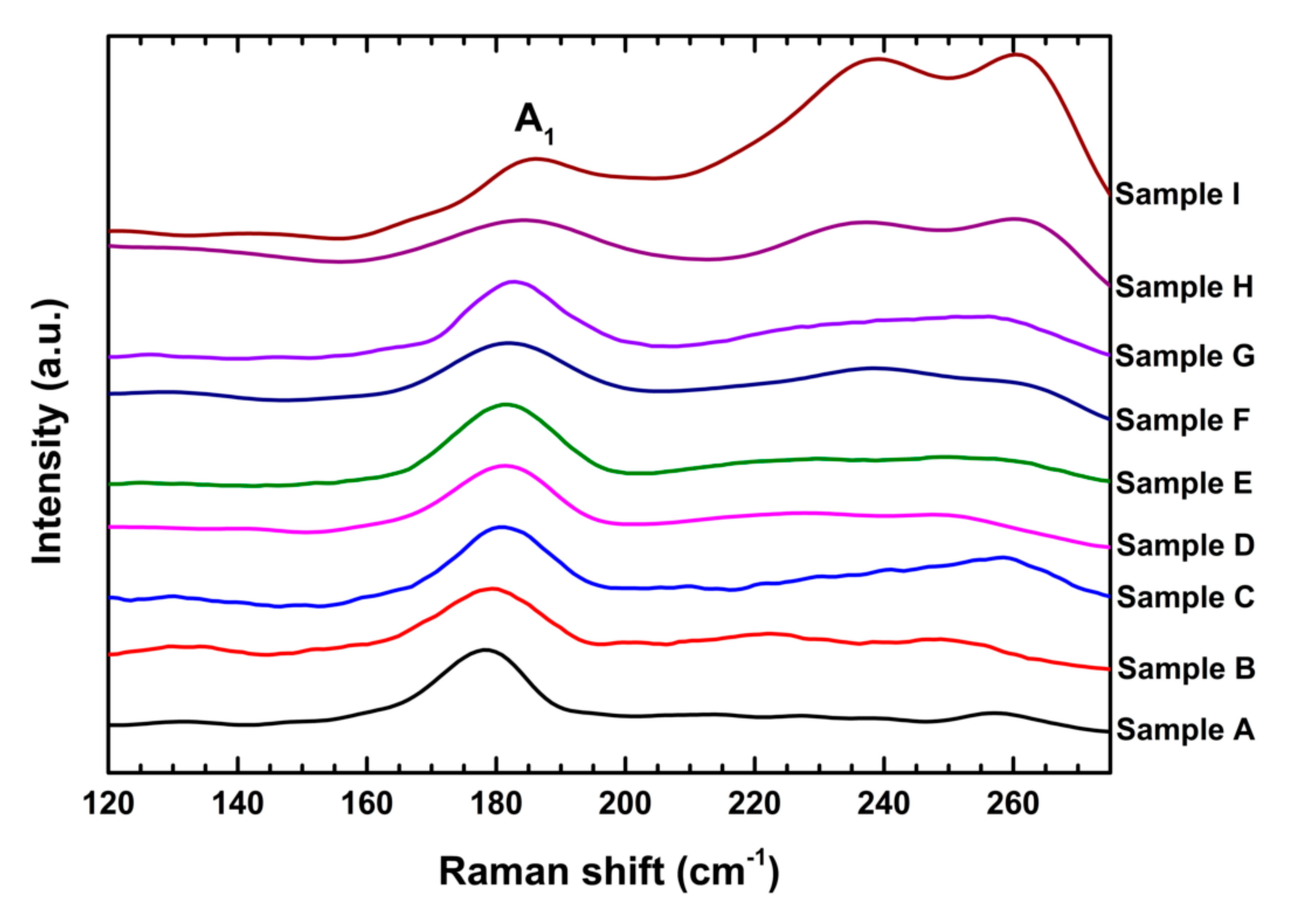
3.4. Raman Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhong, H.; Bai, Z.; Zou, B. Tuning the Luminescence Properties of Colloidal I−III−VI Semiconductor Nanocrystals for Optoelectronics and Biotechnology Applications. J. Phys. Chem. Lett. 2012, 3, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Guillen, C.; Herrero, J. Optical properties of electrochemically deposited CuInSe2 thin films. Sol. Energy Mater. 1991, 23, 31–45. [Google Scholar] [CrossRef]
- Rockett, A.; Liao, D.; Heath, J.T.; Cohen, J.D.; Strzhemechny, Y.M.; Brillson, L.J.; Ramanathan, K.; Shafarman, W.N. Near-surface defect distributions in Cu(In,Ga)Se2. Thin Solid Films 2003, 431–432, 301–306. [Google Scholar] [CrossRef]
- Marai, A.B.; Belgacem, J.B.; Ayadi, Z.B.; Djessas, K.; Alaya, S. Structural and optical properties of CuIn1−xGaxSe2 nanoparticles synthesized by solvothermal route. J. Alloys Compd. 2016, 658, 961–966. [Google Scholar] [CrossRef]
- Latha, M.; Devi, R.A.; Velumani, S. Hot injection synthesis of Cu(In, Ga)Se2 nanocrystals with tunable bandgap. Opt. Mater. 2018, 79, 450–456. [Google Scholar] [CrossRef]
- Miyazaki, H.; Mikami, R.; Yamada, A.; Konagai, M. Cu(InGa)Se2 thin film absorber with high Ga contents and its application to the solar cells. J. Phys. Chem. Solid. 2003, 64, 2055–2058. [Google Scholar] [CrossRef]
- Sharbati, S.; Gharibshahian, I.; Orouji, A.A. Proposed suitable electron reflector layer materials for thin-film CuIn1−xGaxSe2 solar cells. Opt. Mater. 2018, 75, 216–223. [Google Scholar] [CrossRef]
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of heavy alkali elements in Cu(In,Ga)Se2 solar cells with efficiencies up to 22.6%. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Yarema, O.; Yarema, M.; Wood, V. Tuning the composition of multicomponent semiconductor nanocrystals: The case of I–III–VI materials. Chem. Mater. 2018, 30, 1446–1461. [Google Scholar] [CrossRef] [Green Version]
- Dilena, E.; Xie, Y.; Brescia, R.; Prato, M.; Maserati, L.; Krahne, R.; Paolella, A.; Bertoni, G.; Povia, M.; Moreels, I.; et al. CuInxGa1−xS2 nanocrystals with tunable composition and band gap synthesized via a phosphine-free and scalable procedure. Chem. Mater. 2013, 25, 3180–3187. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid Nanorod-Polymer Solar Cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gur, I.; Fromer, N.A.; Geier, M.L.; Alivisatos, A.P. Air-stable all-inorganic nanocrystal solar cells processed from solution. Science 2005, 310, 462–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, A.; Gratzel, M. Low cost photovoltaic modules based on dye sensitized nanocrystalline titanium dioxide and carbon powder. Sol. Energy Mater. Sol. Cells 1996, 44, 99–117. [Google Scholar] [CrossRef]
- Guo, Q.; Ford, G.M.; Yang, W.-C.; Walker, B.C.; Stach, E.A.; Hillhouse, H.W.; Agrawal, R. Fabrication of 7.2% efficient CZTSSe solar cells using CZTS nanocrystals. J. Am. Chem. Soc. 2010, 132, 17384–17386. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ford, G.M.; Hillhouse, H.W.; Agrawal, R. Sulfide nanocrystal inks for dense Cu(In1-xGax)(S1-ySey)2 absorber films and their photovoltaic performance. Nano Lett. 2009, 9, 3060–3065. [Google Scholar] [CrossRef] [PubMed]
- Graetzel, M.; Janssen, R.A.J.; Mitzi, D.B.; Sargent, E.H. Materials interface engineering for solution-processed photovoltaics. Nature 2012, 488, 304–312. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibaánñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound copper chalcogenide nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef]
- Ahmadi, M.; Pramana, S.S.; Xi, L.; Boothroyd, C.; Lam, Y.M.; Mhaisalkar, S. Evolution pathway of CIGSe nanocrystals for solar cell applications. J. Phys. Chem. C 2012, 116, 8202–8209. [Google Scholar] [CrossRef]
- Panthani, M.G.; Akhavan, V.; Goodfellow, M.B.; Schmidtke, J.P.; Dunn, L.; Dodabalapur, A.; Barbara, P.F.; Korgel, B.A. Synthesis of CuInS2, CuInSe2, and Cu(InxGa1-x)Se2 (CIGS) Nanocrystal “Inks” for Printable Photovoltaics. J. Am. Chem. Soc. 2008, 130, 16770–16777. [Google Scholar] [CrossRef]
- Lauth, J.; Marbach, J.; Meyer, A.; Dogan, S.; Klinke, C.; Kornowski, A.; Weller, H. Virtually bare nanocrystal surfaces: Significantly enhanced electrical transport in CuInSe2 and CuIn1−xGaxSe2 thin films upon ligand exchange with thermally degradable 1-ethyl-5-thiotetrazole. Adv. Funct. Mater. 2014, 24, 1081–1088. [Google Scholar] [CrossRef] [Green Version]
- Latha, M.; Devi, R.A.; Velumani, S.; Oza, G.; Reyes-Figueroa, P.; Rohini, M.; Becerril-Juarez, I.G.; Yi, J. Synthesis of CuIn1−xGaxSe2 nanoparticles by thermal decomposition method with tunable Ga content. J. Nanosci. Nanotechnol. 2015, 15, 8388–8394. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.D.; Wang, L.T.; Gau, C. Synthesis of CuInGaSe2 nanoparticles by modified polyol route. Sol. Energy Mater. Sol. Cells 2012, 98, 404–408. [Google Scholar]
- Rohini, M.; Reyes, P.; Velumani, S.; Latha, M.; Oza, G.; Becerril-Juarez, I.; Asomoza, R. Parametric optimization of mechanochemical process for synthesis of Cu(In,Ga)0.5Se2 nanoparticles. Mater. Sci. Semicond. Process. 2015, 37, 151–158. [Google Scholar] [CrossRef]
- Cui, X.; Yun, D.; Zhong, C.; Chen, W.; Cheng, Q.; Feng, J.; Zhang, F. A facile route for synthesis of CuInxGa1−xSe2 nanocrystals with tunable composition for photovoltaic application. J. Sol-Gel Technol. 2015, 76, 469–475. [Google Scholar] [CrossRef]
- Houck, D.W.; Nandu, S.V.; Siegler, T.D.; Korgel, B.A. CuGaSe2 and CuInxGa1−xSe2 nanocrystals with sphalerite or wurtzite phase for optoelectronic applications. ACS Appl. Nano Mater. 2019, 2, 4673–4680. [Google Scholar] [CrossRef]
- Wang, Y.-H.A.; Zhang, X.; Bao, N.; Lin, B.; Gupta, A. Synthesis of shape-controlled monodisperse wurtzite CuInxGa1−xS2 semiconductor nanocrystals with tunable band gap. J. Am. Chem. Soc. 2011, 133, 11072–11075. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, G.S. Structure and Bonding in Crystalline Materials; Cambridge University Press: Cambridge, UK, 2001; p. 478. [Google Scholar]
- Balboul, M.R.; Schock, H.W.; Fayak, S.A.; El-Aal, A.A.; Werner, J.H. Correlation of structure parameters of absorber layer with efficiency of Cu(In, Ga)Se2 solar cell. Appl. Phys. A 2008, 92, 557–563. [Google Scholar] [CrossRef]
- Benabdeslem, M.; Sehli, H.; Rahal, S.; Benslim, N.; Bechiri, L.; Djekoun, A.; Touam, T.; Boujnah, M.; El Kenz, A.; Benyoussef, A.; et al. Ab initio calculations and experimental properties of CuAlx Ga1−x Te2 for photovoltaic solar cells. J. Electron. Mater. 2016, 45, 1035–1040. [Google Scholar] [CrossRef]
- El-Fadl, A.A.; Hassan, A.M.; Kassem, M.A. Structural and spectroscopic studies of nanocrystalline Ni1−xMgxFe2O4 ferrites synthesized by a microwave-assisted combustion route. Phys. Scr. 2020, 95, 055813. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, Q.; Lei, S.; Lai, L.; Xiong, J.; Bian, Q.; Xiao, Y.; Cheng, B. Preparation of quinary CuNixZn2−xInS4 nanocrystals with wurtzite structure and tunable band gap. J. Alloys Compd. 2020, 820, 153436. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2001; p. 264. [Google Scholar]
- Ghali, M.; Elnimr, M.; Ali, G.F.; Yousif, B. Colloidal CuInSe2 nanocrystals and thin films for low-cost photovoltaics. Opt. Mater. 2016, 55, 145–152. [Google Scholar] [CrossRef]
- Bhattacharya, P. Semiconductor Optoelectronic Devices, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1997; p. 124. [Google Scholar]
- Peyghambarian, N.; Koch, S.W.; Mysyrowicz, A. Introduction to Semiconductor Optics; Prentice-Hall: Englewood Cliffs, NJ, USA, 1993; p. 127. [Google Scholar]
- Tauc, J.; Grigorvici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Brus, L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403. [Google Scholar] [CrossRef] [Green Version]
- Kayanuma, Y. Quantum-size effects of interacting electrons and holes in semiconductor microcrystals with spherical shape. Phys. Rev. B 1988, 38, 9797–9805. [Google Scholar] [CrossRef]
- Omata, T.; Nose, K.; Otsuka-Yao-Matsuo, S. Size dependent optical band gap of ternary I-III-VI2 semiconductor nanocrystals. J. Appl. Phys. 2009, 105, 073106. [Google Scholar] [CrossRef]
- Rincon, C.; Gonzalez, J. Acoustic deformation potentials in AIBIIIC2VI chalcopyrite semiconductors. Phys. Rev. B 1989, 40, 8552–8554. [Google Scholar] [CrossRef]
- Persson, C. Anisotropic hole-mass tensor of CuIn1−xGax(S,Se)2: Presence of free carriers narrows the energy gap. Appl. Phys. Lett. 2008, 93, 072106. [Google Scholar] [CrossRef]
- Wei, S.-H.; Zunger, A. Band offsets and optical bowings of chalcopyrites and Zn-based II-VI alloys. J. Appl. Phys. 1995, 78, 3846–3856. [Google Scholar] [CrossRef] [Green Version]
- Jeong, A.R.; Jo, W.; Song, M.; Yoon, S. Crystalline ordered states of CuIn1−xGaxSe2 (x = 0, 0.3, and 1.0) thin-films on different substrates investigated by Raman scattering spectroscopy. Mater. Chem. Phys. 2012, 134, 1030–1035. [Google Scholar] [CrossRef]
- Rincon, C.; Ramirez, F.J. Lattice vibrations of CuInSe2 and CuGaSe2 by Raman microspectrometry. J. Appl. Phys. 1992, 72, 4321–4324. [Google Scholar] [CrossRef]
- Matsushita, H.; Endo, S.; Irie, T. Raman-scattering properties of I-III-VI2 group chalcopyrite semiconductors. Jpn. J. Appl. Phys. 1992, 31, 18–22. [Google Scholar] [CrossRef]
- Witt, W.; Kniese, R.; Powalla, M. Raman investigations of Cu(In,Ga)Se2 thin films with various copper contents. Thin Solid Films 2008, 517, 867–869. [Google Scholar] [CrossRef]
- Roy, S.; Guha, P.; Kundu, S.N.; Hanzawa, H.; Chaudhuri, S.; Pal, A.K. Characterization of Cu(In,Ga)Se2 films by Raman scattering. Mater. Chem. Phys. 2002, 73, 24–30. [Google Scholar] [CrossRef]
- Olejnicek, J.; Kamler, C.A.; Mirasano, A.; Martinez-Skinner, A.L.; Ingersoll, M.A.; Exstrom, C.L.; Darveau, S.A.; Huguenin-Love, J.L.; Diaz, M.; Ianno, N.J.; et al. A non-vacuum process for preparing nanocrystalline CuIn1−xGaxSe2 materials involving an open-air solvothermal reaction. Sol. Energy Mater. Sol. Cells 2010, 94, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1996; p. 79. [Google Scholar]
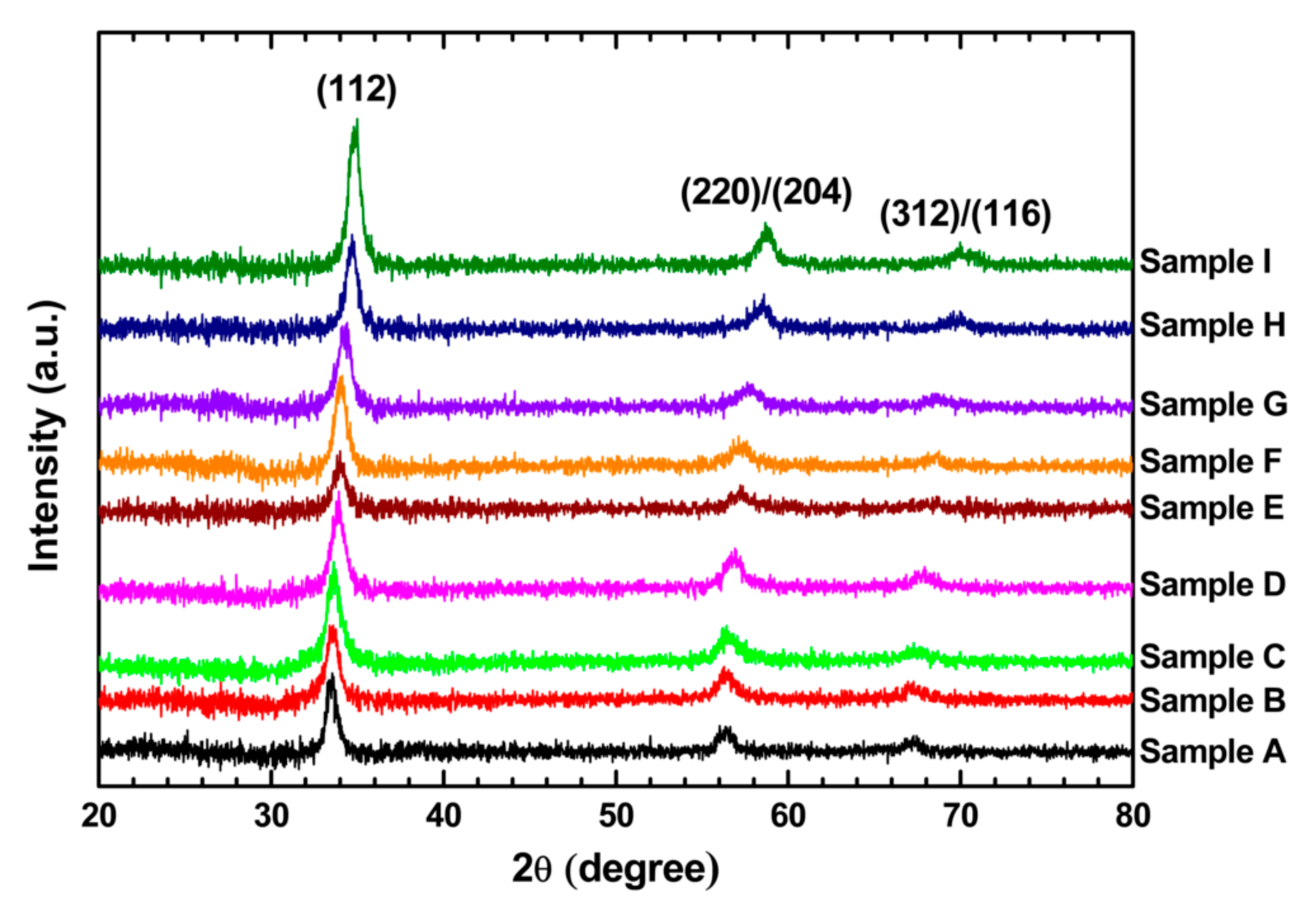
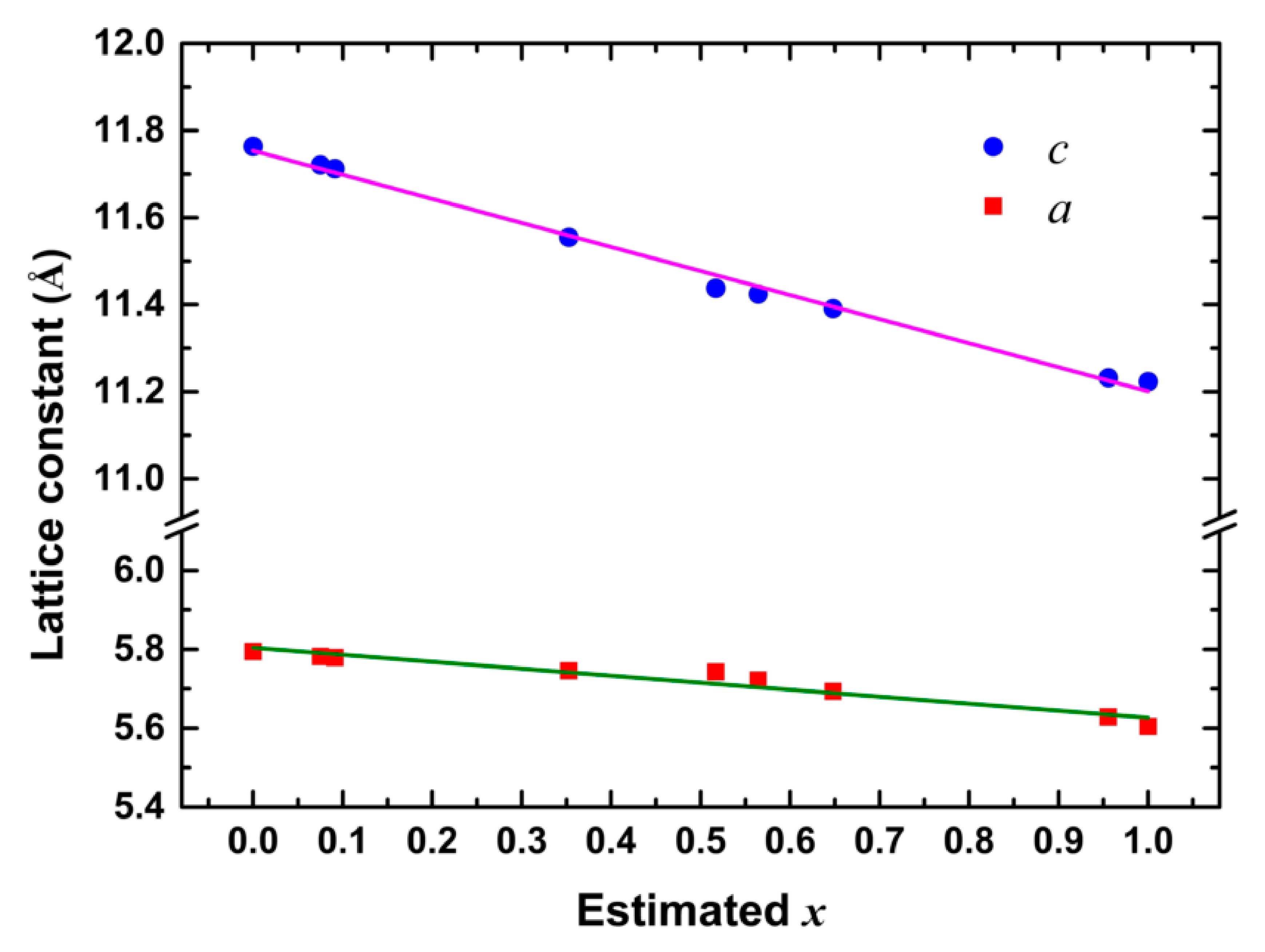
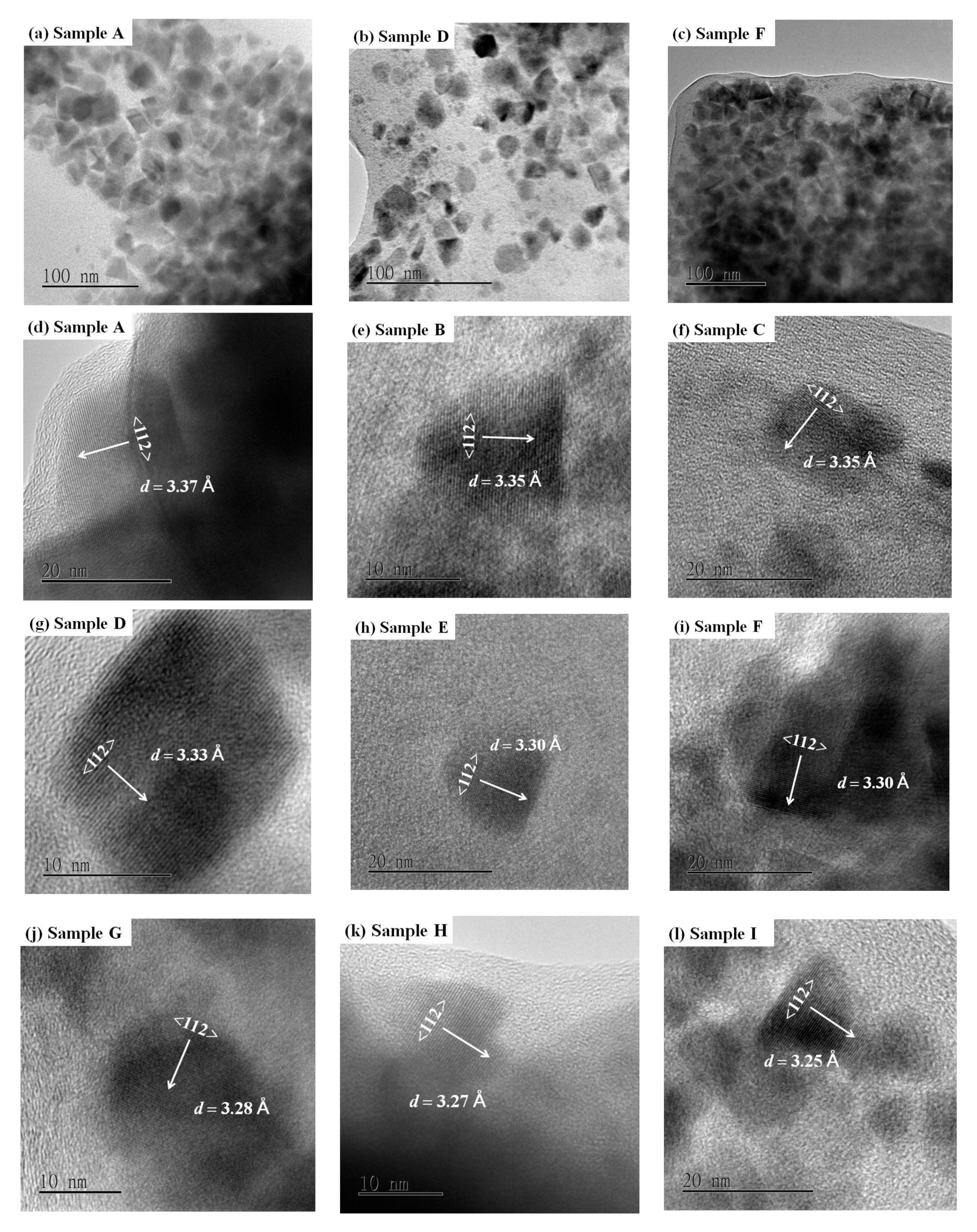

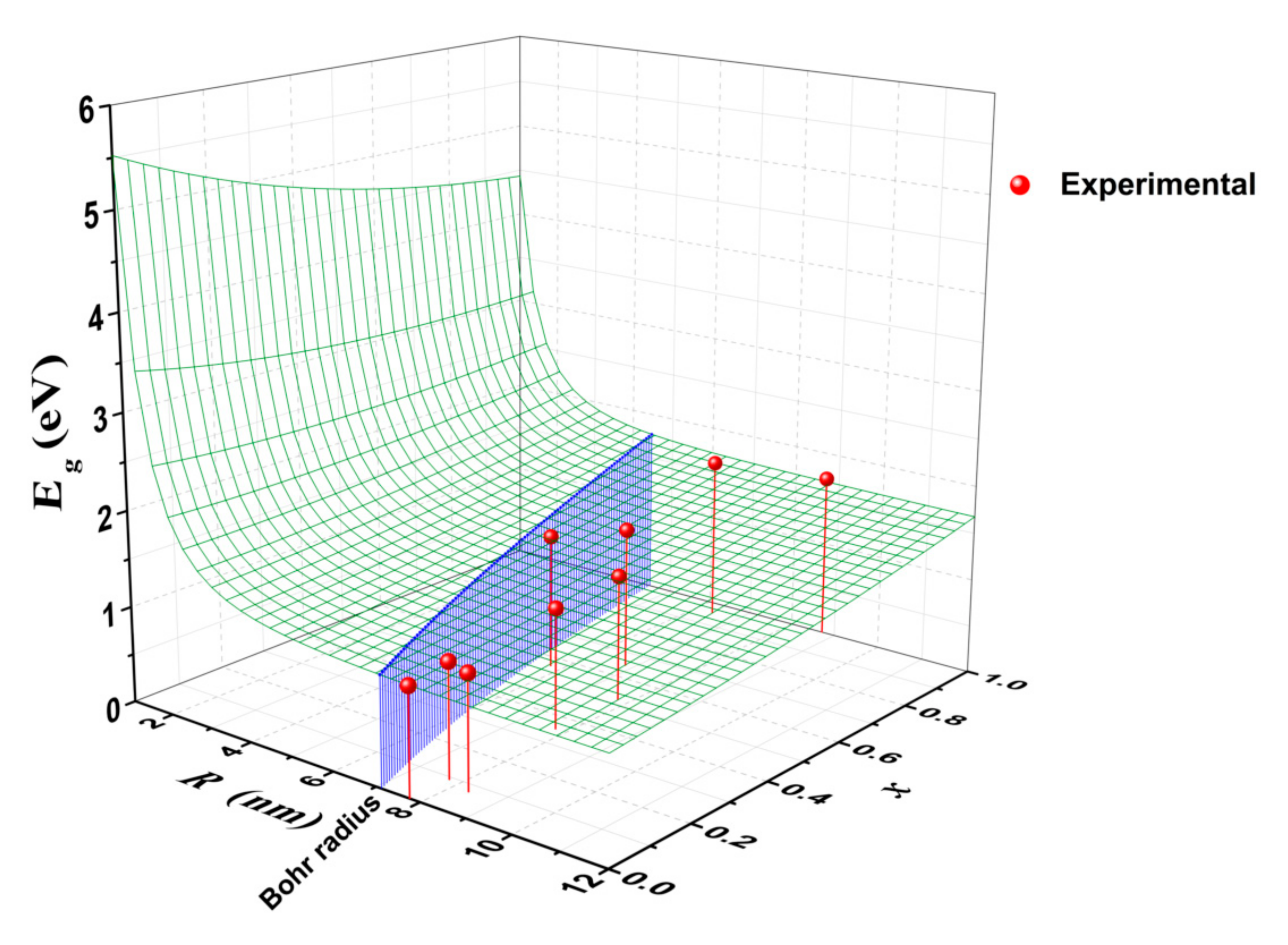

| Sample | Nominal x | d112 (Å) | a (Å) | c (Å) | Estimated x | Crystallite Size (nm) | Gap Energy (eV) | Raman Shift (cm−1) |
|---|---|---|---|---|---|---|---|---|
| A | 0 | 3.362 | 5.794 | 11.764 | 0 | 15.528 | 1.108 ± 0.01 | 177.97 ± 1.52 |
| B | 0.125 | 3.353 | 5.782 | 11.721 | 0.075 | 16.711 | 1.175 ± 0.01 | 179.32 ± 0.53 |
| C | 0.25 | 3.351 | 5.779 | 11.712 | 0.091 | 15.533 | 1.177 ± 0.01 | 180.34 ± 1.29 |
| D | 0.375 | 3.323 | 5.745 | 11.555 | 0.353 | 15.530 | 1.250 ± 0.01 | 181.44 ± 1.10 |
| E | 0.5 | 3.311 | 5.743 | 11.438 | 0.517 | 15.537 | 1.307 ± 0.01 | 181.94 ± 0.48 |
| F | 0.625 | 3.302 | 5.722 | 11.424 | 0.564 | 11.501 | 1.409 ± 0.01 | 182.41 ± 0.53 |
| G | 0.75 | 3.288 | 5.693 | 11.391 | 0.648 | 13.659 | 1.476 ± 0.01 | 182.90 ± 0.48 |
| H | 0.875 | 3.247 | 5.628 | 11.232 | 0.956 | 12.818 | 1.709 ± 0.01 | 183.76 ± 0.68 |
| I | 1 | 3.237 | 5.604 | 11.223 | 1 | 17.,442 | 1.719 ± 0.01 | 186.03 ± 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, Y.-T.; Tsai, Y.-C.; Lin, D.-Y. Synthesis and Characterization of CuIn1−xGaxSe2 Semiconductor Nanocrystals. Nanomaterials 2020, 10, 2066. https://doi.org/10.3390/nano10102066
Shih Y-T, Tsai Y-C, Lin D-Y. Synthesis and Characterization of CuIn1−xGaxSe2 Semiconductor Nanocrystals. Nanomaterials. 2020; 10(10):2066. https://doi.org/10.3390/nano10102066
Chicago/Turabian StyleShih, Yu-Tai, Yu-Ching Tsai, and Der-Yu Lin. 2020. "Synthesis and Characterization of CuIn1−xGaxSe2 Semiconductor Nanocrystals" Nanomaterials 10, no. 10: 2066. https://doi.org/10.3390/nano10102066
APA StyleShih, Y.-T., Tsai, Y.-C., & Lin, D.-Y. (2020). Synthesis and Characterization of CuIn1−xGaxSe2 Semiconductor Nanocrystals. Nanomaterials, 10(10), 2066. https://doi.org/10.3390/nano10102066




