Volatile Organic Compounds (VOCs) Removal from Indoor Air by Heterostructures/Composites/Doped Photocatalysts: A Mini-Review
Abstract
:1. Introduction
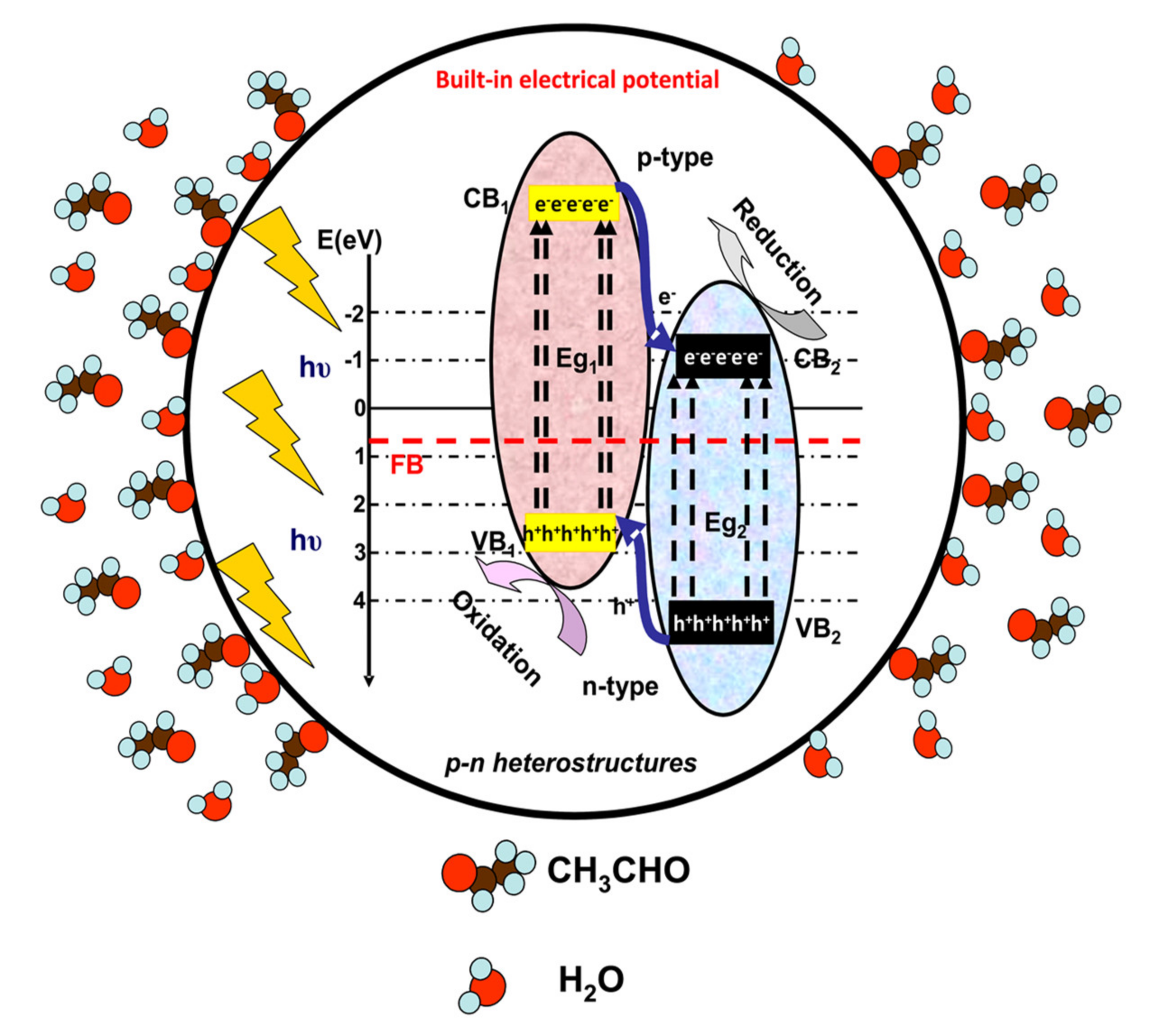
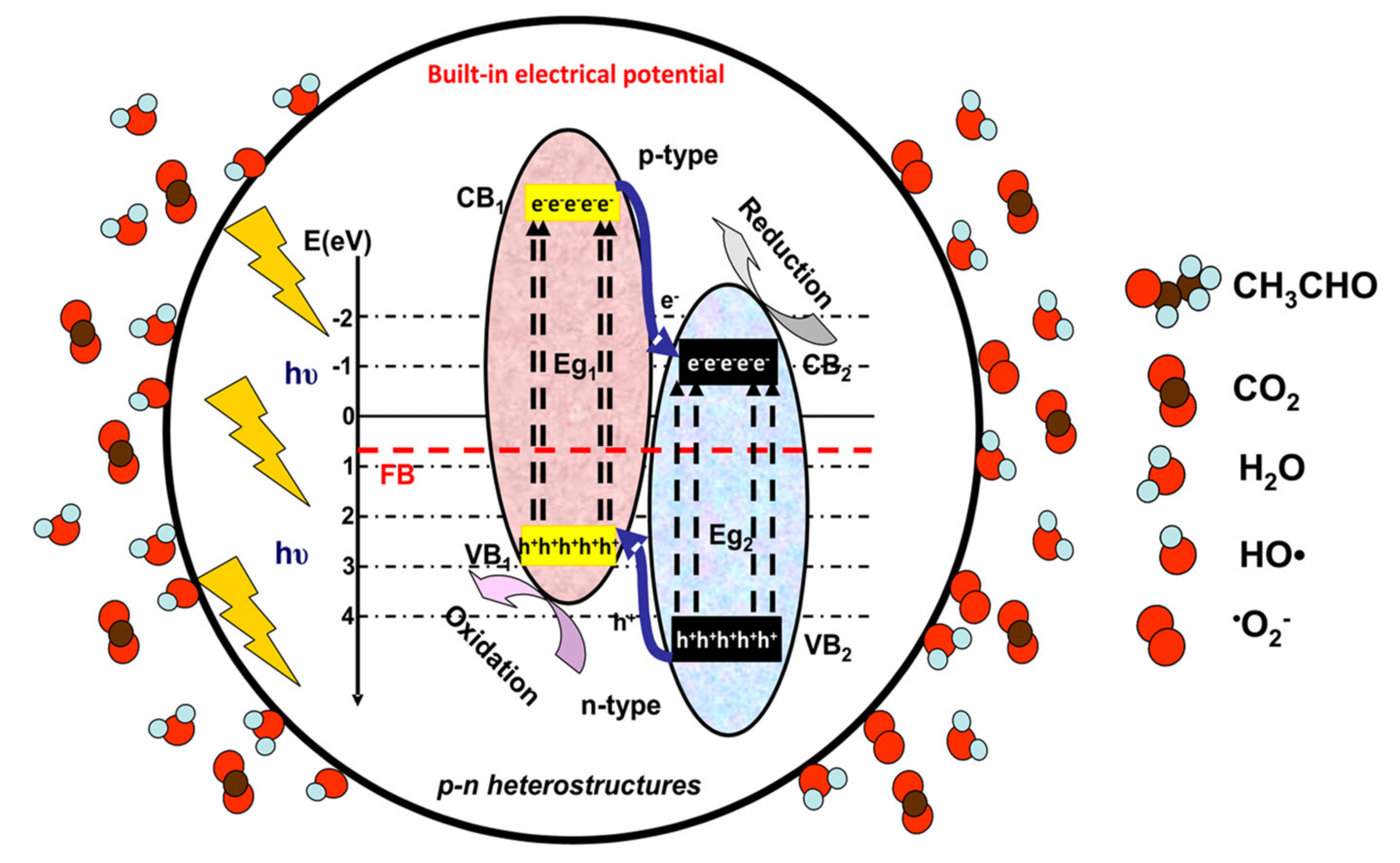
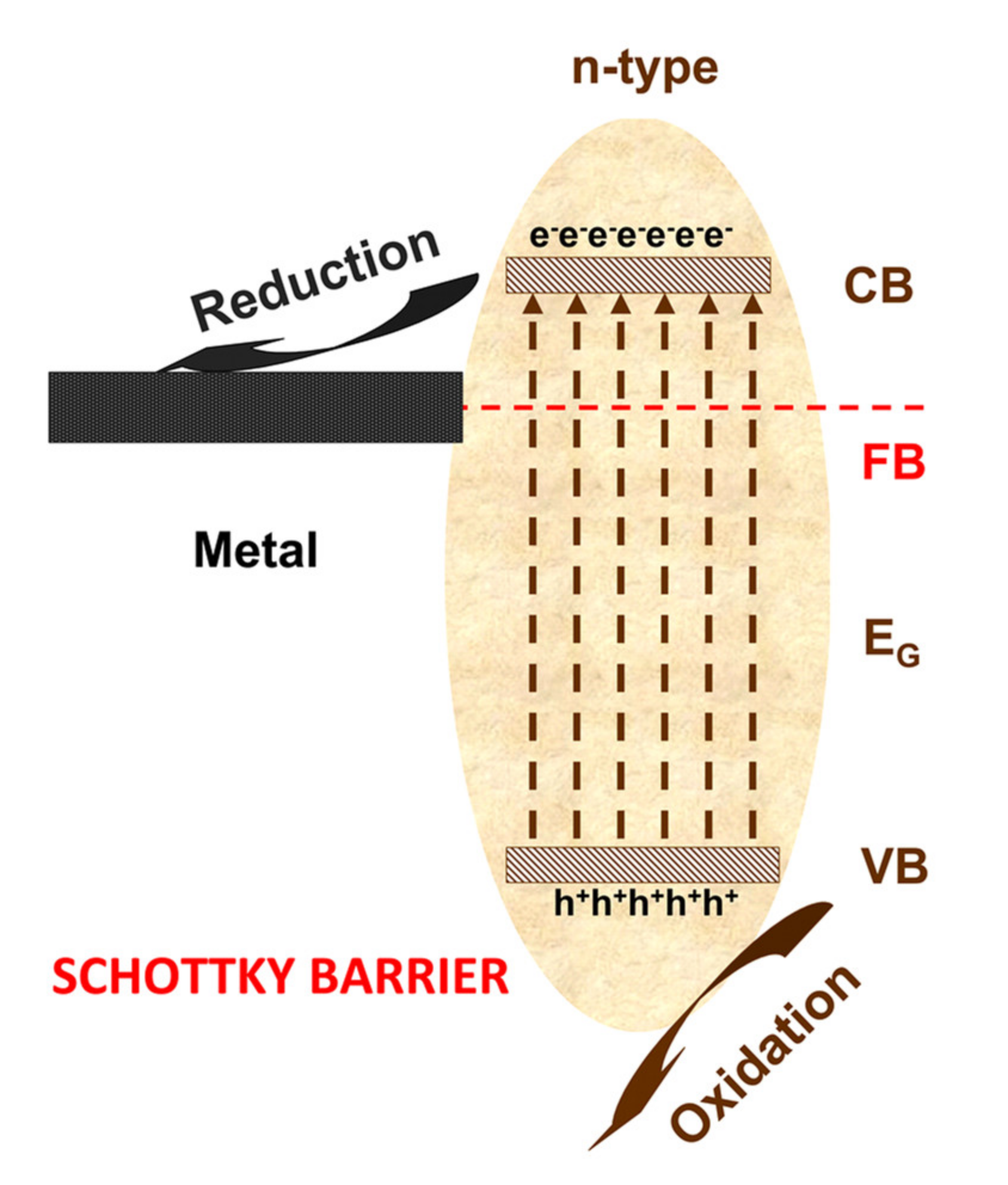
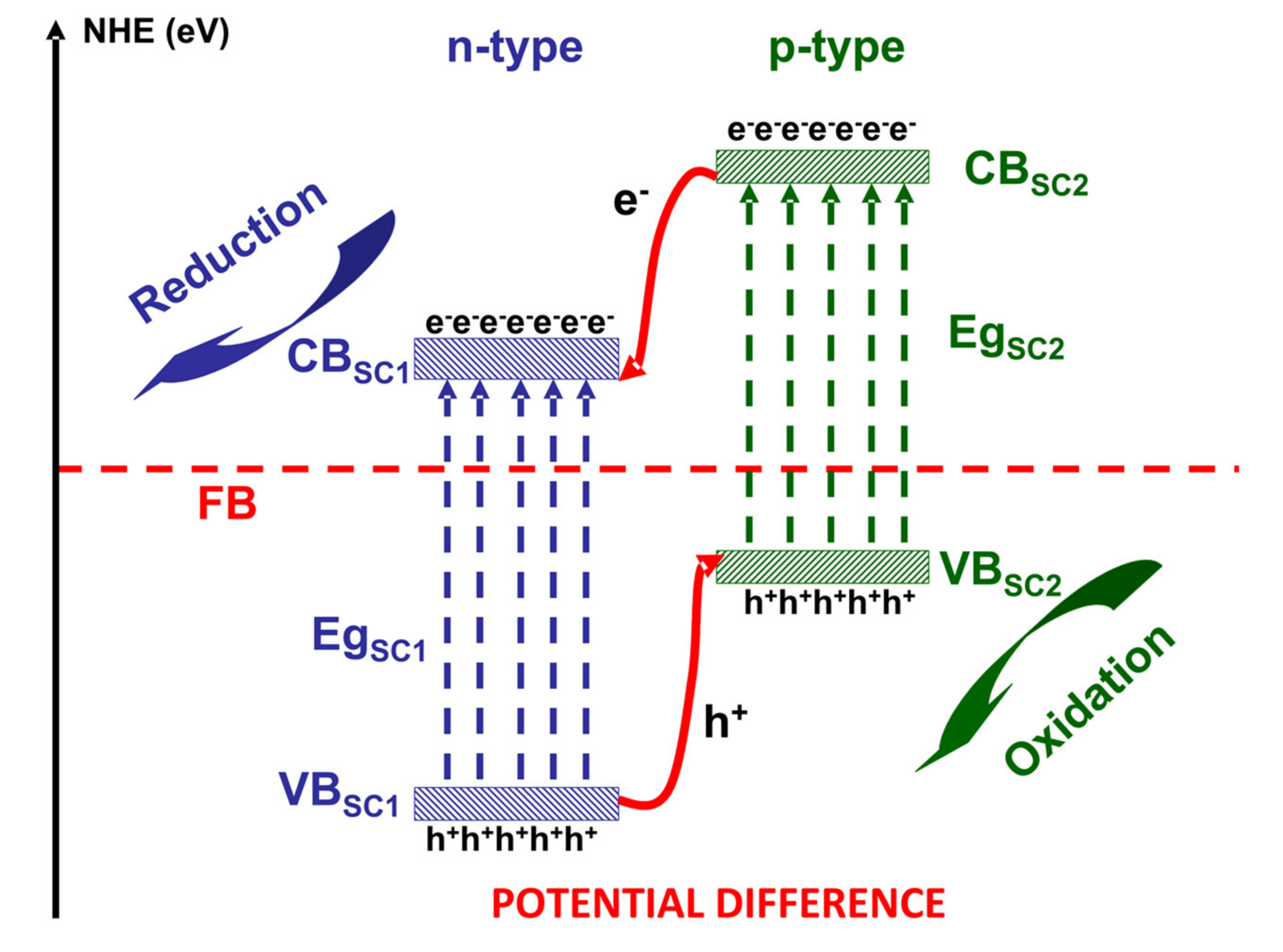
2. Photocatalysis Mechanisms for VOCs Removal
3. VOC Removal Using Heterostructures/Composites/Doped Photocatalysts
3.1. Toluene Photocatalytic Removal
3.2. Photocatalytic Removal of Formaldehyde and O-xylene
3.3. Photocatalytic Removal of Benzene and Ethylbenzene
3.4. Acetaldehyde Photocatalytic Removal
3.5. Photocatalytic Removal of 2-ethyl-1-hexanol, N-decane, N-hexane, Trichloroethylene and Benzaldehyde
| Heterostructure/ Composite/ Doped Photocatalysts | Synthesis Method | Specific Surface (SBET)/Radiation Parameter (Light Spectra, Intensity and Irradiance)/Energy Consumption (EC) | Pollutant/Photocatalytic Parameters | Ref. |
|---|---|---|---|---|
| TiO2-MIL-101 (Cr) | In situ growth on MIL101 (Cr) | SBET = 2128 m2/ g300W Vis, np* Ec = 2400 Wh | Toluene Pollutant concentration: 1000 ppm Time: 480 min Efficiency: ~50% Rate constant: np | [44] |
| CoO/WO3 | Hydrothermal | SBET = np 300W Vis, np Ec = 1200 Wh | Toluene Pollutant concentration:500 ppmTime: 240 min Efficiency: 85.4% Rate constant: 0.0070 min−1 | [45] |
| CaCO3 loading TiO2 | Dip coating | SBET = np 300W UV, 0.29 W/cm2 Ec = 300 Wh | Toluene Pollutant concentration: 50 ppm Time: 60 min Efficiency: 90% Rate constant: np | [46] |
| Ag/TiO2 | Photoreduction method | SBET = np 500 W UV, np Ec = 1500 Wh | Toluene, N,N-dimethylformamide, acetone, dimethyl fumaratePollutant concentration: 2.6 ppm (toluene), 3.3 ppm (N,N-dimethylformamide), 4.2 ppm (acetone), 1.7 ppm (dimethyl fumarate) Time: 180 min Efficiency: 99.6% (toluene), 92.5% (N,N-dimethylformamide), 99.4% (acetone), 99.7% (dimethyl fumarate) Rate constant: np | [47] |
| TiO2@MgAl-layered double hydroxide | In situ hydrolysis | SBET = 94.71 m2/ g500 W Vis, np Real sunlight, np Ec = 1500 Wh | ToluenePollutant concentration: 45.5 ppm Time: 180 min Efficiency: 91.7% (true sunlight), 85.9% (simulated sunlight) Rate constant: 0.0100 min−1 | [48] |
| WO3/TiO2 | Screen printing technique | SBET = np UV, 5 mW/cm2 Ec = np | Toluene Pollutant concentration: 250 ppm Time: 30 min Efficiency: 14% (T/W), 40% (W/T), 70% (W/T with 0.2 V bias) Rate constant: 0.00739 min−1 (T/W), 0.02004 min−1 (W/T) | [49] |
| Graphene oxide (GO)/MnOx/CN | Vacuum filtration | SBET = np 300 W Vis, np Ec = 60 Wh | Formaldehyde Pollutant concentration: 160 ppm Time: 12 min Efficiency:90% Rate constant: 0.202 min−1 | [54] |
| CeO2@ layered double hydroxides | Hydrothermal | SBET = 93 m2/ g500 W Vis, np Ec = 2500 Wh | Formaldehyde Pollutant concentration: 26 ppm Time: 300 min Efficiency: 86.9% Rate constant: 0.00101 min−1 | [55] |
| Reduced graphene oxide (rGO)-TiO2 | Solvothermal | SBET = 227.3 m2/g 200W Vis, np Ec = 533 Wh | Acetaldehyde, o-xylene Pollutant concentration: 25 ppm Time: 160 min Efficiency: 42% (acetaldehyde), 54% (o-xylene). Rate constant: np | [60] |
| TiO2/porous cementitious material | Negative pressure co- stirring method | SBET = 26 m2/g 300W UV, 0.96 mW/cm2 Ec = 900 Wh | Benzene Pollutant concentration: 200 ppm Time: 180 min Efficiency: 63% Rate constant: np | [64] |
| Cu-NiWO4 | Sol-gel | SBET = 12.4 m2/g 100W Vis, 0.025 W/cm2Ec = 200 Wh | Benzene Pollutant concentration: 50 ppm Time: 120 min Efficiency: 96.5% Rate constant: np | [65] |
| BiVO4/TiO2 | Hydrothermal | SBET = 66.49 m2/g 500 W solar simulated light, np 500 W Vis, np 500 W UV, np Ec = 4000 Wh | Benzene Pollutant concentration: 260 ppm Time: 480 min Efficiency: 92% (solar simulated light), 66.8% (Vis), 11% (UV) Rate constant: np | [66] |
| SnOx/Zn2SnO4 | Hydrothermal | SBET = 21.7 m2/g 9 W UV, np Ec = 126 Wh | Benzene Pollutant concentration: 250 ppm Time: 840 min Efficiency: 80.3% Rate constant: 0.0834 min−1 | [67] |
| Fe-TiO2 | Electrospinning technique | SBET = np8W UV, 0.4 mW/cm2 Ec = 0.66 Wh | Benzene, toluene, ethylbenzene and o-xylene Pollutant concentration: 0.1 ppm Time: 5 min Efficiency: 33% (benzene), 68% (toluene), 83% (ethylbenzene) and 91% (o-xylene) Rate constant: np | [68] |
| La-TiO2 | Sol–gel method and hydrothermal technique | SBET = 541.35 m2/g UV, 20.9 mW/cm2 Ec = np | Ethylbenzene Pollutant concentration: 11.5 ppm Catalyst dosage: 1 min Efficiency: 99% Rate constant: 1.1860 min− 1 | [69] |
| Reducend graphene oxide (rGO) with TiO2 | Ultrasonication | SBET = 69.81 m2/g 260 W Vis, np Ec = 260 Wh | Acetaldehyde Pollutant concentration: 500 ppm Time: 60 min Efficiency: 80% Rate constant: np | [74] |
| TiO2/TaS2 | Ultrasonication | SBET = 103 m2/g 260 W Vis, np Ec = 281 Wh | Acetaldehyde Pollutant concentration: 500 ppm Time: 65 min Efficiency: 98% Rate constant: 0.03091 min−1 | [75] |
| Ag@TiO2 | Solvothermal | SBET = 105.93 m2/g 260W UV, 20 mW/cm2 Ec = 20.8 Wh | Acetaldehyde Pollutant concentration: 500 ppm Time: 4.8 min Efficiency: 72% Rate constant: 0.01199 min−1 | [76] |
| Carbon quantum dots/TiO2 | Ultrasonication | SBET = np 260W UV, 20 mW/cm2 400W Vis, np Ec = 520 Wh (UV) Ec = 800 Wh (Vis) | Acetaldehyde Pollutant concentration: 500 ppm Time: 120 min Efficiency: 99% (UV), 30% (Vis) Rate constant: np | [77] |
| SiO2/TiO2 | Fiber impregnation | SBET = 20.5 m2/g 22.6 W UV, 6 mW/cm2 Ec = 52.7 Wh (photocat) Ec = 34 Wh (plasma photocat) | Acetylene Pollutant concentration: 3000 ppm Time: 140min (photocat), 90 min (plasma photocat) Efficiency: 100% Rate constant: np | [78] |
| CuInS2/TiO2/SnO2 | Spray pyrolysis deposition | SBET = 25.3 m2/g 20 W UV + Vis, (2.5 mW/cm2 +0.1 mW/cm2) Ec = 240 Wh | Acetaldehyde Pollutant concentration: 500 ppm Time: 720 min Efficiency: 51.7% Rate constant: 0.0557 min−1. | [79] |
| GO/TiO2 | Ultrasonication | SBET = 100.3 m2/g 8 W Vis, np Ec = 40 Wh | 2-ethyl-1-hexanol Radiation: Vis Pollutant concentration: 0.1 ppm Time: 300 min Efficiency: 99.3% Rate constant: np | [80] |
| Cellulose acetate (CA)/TiO2-P25 | Cold spray | SBET = np 1700 W Vis, 38.4 W/m2 Ec = 8500 Wh | N-decane Pollutant concentration: 320 ppm Time: 300 min Efficiency: 72% Rate constant: np | [81] |
| Bi/BiOBr | Solvothermal | SBET = np 300 W Vis, np Ec = 600 Wh | N-hexane Pollutant concentration: 15 ppm Time: 120 min Efficiency: 97.4% Rate constant: 0.0300 min−1 | [85] |
| TiO2/SiO2 | Dip coating | SBET = 300 m2/g UV (254 nm and 365 nm) Ir254 = 7.3 × 10−3W/cm2 Ir365 = 3.5 × 10−3W/cm2 Ec = np | Trichloroethylene Pollutant concentration: 26 ppm Time: 240 min Efficiency: 100% Rate constant: np | [86] |
| Mesoporous TiO2/conductive carbon felt (OMT/CCF) | Liquid crystal template method with the assistance of ultrasonic deposition | SBET = 148.6 m2/g UV, 40 mW/cm2 Ec = np | Benzaldehyde Pollutant concentration: 100 ppm Time: 325 min Efficiency: ~25% Rate constant: 0.0004 min−1 | [87] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xia, T.; Chen, C. Evolution of pressure drop across electrospun nanofiber filters clogged by solid particles and its influence on indoor particulate air pollution control. J. Hazard. Mater. 2020, 402, 123479. [Google Scholar] [CrossRef] [PubMed]
- O’Lenick, C.R.; Wilhelmi, O.V.; Michael, R.; Hayden, M.H.; Baniassadi, A.; Wiedinmyer, C.; Monaghan, A.J.; Crank, P.J.; Sailor, D.J. Urban heat and air pollution: A framework for integrating population vulnerability and indoor exposure in health risk analyses. Sci. Total Environ. 2019, 660, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Shrubsole, C.; Symonds, P.; Mackenzie, I.; Davies, M. Application of an indoor air pollution metamodel to a spatially-distributed housing stock. Sci. Total Environ. 2019, 667, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.W.; Li, W. A Review on Catalytic Nanomaterials for Volatile Organic Compounds VOC Removal and Their Applications for Healthy Buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, C.; Chen, W.; Guo, M.; Weng, M.; Shen, X. Law and features of TVOC and Formaldehyde pollution in urban indoor air. Atmos. Environ. 2016, 132, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Barron, M.; Torero, M. Household electrification and indoor air pollution. J. Environ. Econ. Manag. 2017, 86, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Jeleńska, M.; Górka-Kostrubiec, B.; Werner, T.; Kądziałko-Hofmokl, M.; Szwarczewski, P. Evaluation of indoor/outdoor urban air pollution by magnetic, chemical and microscopic studies. Atmos. Pollut. Res. 2017, 8, 754–766. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Luo, Y.; Xu, Y.; Liu, J.; Lin, Y. (002) Oriented Bi2O2CO3 Nanosheets with Enhanced Photocatalytic Performance for Toluene Removal in Air. Catalysts 2020, 10, 389. [Google Scholar] [CrossRef] [Green Version]
- Ruan, T.; Rim, D. Indoor air pollution in office buildings in mega-cities: Effects of filtration efficiency and outdoor air ventilation rates. Sustain. Cities Soc. 2019, 49, 101609. [Google Scholar] [CrossRef]
- Adamová, T.; Hradecký, J.; Prajer, M. VOC Emissions from Spruce Strands and Hemp Shive: In Search for a Low Emission Raw Material for Bio-Based Construction Materials. Materials 2019, 12, 2026. [Google Scholar] [CrossRef] [Green Version]
- Ke, S.; Liu, Q.; Deng, M.; Zhang, X.; Sui, G. Cytotoxicity analysis of indoor air pollution from biomass combustion in anmal keratinocytes on a multilayered dynamic cell culture platform. Chemosphere 2018, 208, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yu, Z.; Ma, X.; Zhang, G.; Feng, G. Study on the influence of pollution source location on indoor pollutant distribution under different air supply. Proc. Eng. 2017, 205, 2623–2630. [Google Scholar] [CrossRef]
- Zadi, T.; Azizi, M.; Nasrallah, N.; Bouzaza, A.; Assadi, A.A. Indoor air treatment of refrigerated food chambers with synergetic association between cold plasma and photocatalysis: Process performance and photocatalytic poisoning. Chem. Eng. J. 2020, 382, 122951. [Google Scholar] [CrossRef]
- Saoud, W.A.; Assadi, A.A.; Kane, A.; Jung, A.V.; Wolbert, D. Integrated process for the removal of indoor VOCs from food industry manufacturing: Elimination of Butane-2,3-dione and Heptan-2-one by cold plasma-photocatalysis combination. J. Photochem. Photobiol. A 2020, 386, 112071. [Google Scholar] [CrossRef]
- Kulathunga, K.M.; Yan, C.F.; Bandara, J. Photocatalytic removal of airborne indoor pollutants by IR illuminated silver coated TiO2 catalyst: Advantage of one-dimensional TiO2 nanostructures in IR active photocatalysis. Colloid. Surf. A 2020, 590, 124509. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, P.; Li, K.; Liu, X.; Zhang, P. Nano Copper Oxide-Modified Carbon Cloth as Cathode for a Two-Chamber Microbial Fuel Cell. Nanomaterials 2016, 6, 238. [Google Scholar] [CrossRef] [Green Version]
- Jo, W.K.; Park, K.H. Heterogeneous photocatalysis of aromatic and chlorinated volatile organic compounds (VOCs) for non-occupational indoor air application. Chemosphere 2004, 57, 555–565. [Google Scholar] [CrossRef]
- Dundar, I.; Krichevskaya, M.; Katerski, A.; Krunks, M.; Oja Acik, I. Photocatalytic Degradation of Different VOCs in the Gas-Phase over TiO2 Thin Films Prepared by Ultrasonic Spray Pyrolysis. Catalysts 2019, 9, 915. [Google Scholar] [CrossRef] [Green Version]
- Karafas, E.S.; Romanias, M.N.; Stefanopoulos, V.; Binas, V.; Papagiannakopoulos, P. Effect of metal doped and co-doped TiO2 photocatalysts oriented to degrade indoor/outdoor pollutants for air quality improvement. A kinetic and product study using acetaldehyde as probe molecule. J. Photochem. Photobiol. A 2019, 371, 255–263. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L.; Duta, A. The influence of surfactants on the crystalline structure, electrical and photocatalytic properties of hybrid multi-structured (SnO2, TiO2 and WO3) thin films. Appl. Surf. Sci. 2012, 258, 4339–4346. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.-H. Sonochemical Synthesis of Ce-doped TiO2 Nanostructure: A Visible-Light-Driven Photocatalyst for Degradation of Toluene and O-Xylene. Materials 2019, 12, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energ. Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photochem. Photobiol. C 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Vikrant, K.; Park, C.M.; Kim, K.H.; Kumar, S.; Jeon, E.C. Recent advancements in photocatalyst-based platforms for the destruction of gaseous benzene: Performance evaluation of different modes of photocatalytic operations and against adsorption techniques. J. Photochem. Photobiol. C 2019, 41, 100316. [Google Scholar] [CrossRef]
- Zeng, Y.; Xie, R.; Cao, J.; Chen, Z.; Huang, H. Simultaneous removal of multiple indoor-air pollutants using a combined process of electrostatic precipitation and catalytic decomposition. Chem. Eng. J. 2020, 388, 124219. [Google Scholar] [CrossRef]
- Wang, J.; Deng, H.; Li, X.; Yang, C.; Xia, Y. Visible-light photocatalysis enhanced room-temperature formaldehyde gas sensing by MoS2/rGO hybrids. Sens. Actuat. B 2020, 304, 127317. [Google Scholar] [CrossRef]
- Magudieshwaran, R.; Ishii, J.; Raja, K.C.N.; Terashima, C.; Pitchaimuthu, S. Green and chemical synthesized CeO2 nanoparticles for photocatalytic indoor air pollutant degradation. Mater. Lett. 2019, 239, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Zhang, W.; Sun, Y.; Li, J.; Dong, F. Visible-light-induced charge transfer pathway and photocatalysis mechanism on Bi semimetal@defective BiOBr hierarchical microspheres. J. Catal. 2018, 357, 41–50. [Google Scholar] [CrossRef]
- Tomer, V.K.; Malik, R.; Chaudhary, V.; Mishra, Y.K.; Lin, L. Superior visible light photocatalysis and low-operating temperature VOCs sensor using cubic Ag(0)-MoS2 loaded g-CN 3D porous hybrid. Appl. Mater. Today 2019, 16, 193–203. [Google Scholar] [CrossRef]
- Kamaei, M.; Rashedi, H.; Dastgheib, S.M.M.; Tasharrofi, S. Comparing Photocatalytic Degradation of Gaseous Ethylbenzene Using N-doped and Pure TiO2 Nano-Catalysts Coated on Glass Beads under Both UV and Visible Light Irradiation. Catalysts 2018, 8, 466. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Xie, Y.; Xia, Y.; Zou, N.; Wang, J. Facile photo-driven strategy for the regeneration of a hierarchical C@MnO2 sponge for the removal of indoor toluene. Appl. Surf. Sci. 2019, 481, 404–413. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L.; Duta, A. Optimization of Opto-Electrical and Photocatalytic Properties of SnO2 Thin Films Using Zn2+ and W6+ Dopant Ions. Catal. Lett. 2012, 142, 224–230. [Google Scholar] [CrossRef]
- Parrino, F.; Camera-Roda, G.; Loddo, V.; Palmisano, G.; Augugliaro, V. Combination of ozonation and photocatalysis for purification of aqueous effluents containing formic acid as probe pollutant and bromide ion. Water Res. 2014, 50, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellingiri, K.; Vikrant, K.; Kumar, V.; Kim, K.H. Advances in thermocatalytic and photocatalytic techniques for the room/low temperature oxidative removal of formaldehyde in air. Chem. Eng. J. 2020, 399, 125759. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Destaillats, H.; Sleiman, M.; Sullivan, D.P.; Jacquiod, C.; Molins, L. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Appl. Catal. B 2012, 128, 159–170. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Guillard, C.; Bui, T.H.; Felix, C.; Moules, V.; Lejeune, P. Microbiological disinfection of water and air by photocatalysis. C.R. Chimie 2008, 11, 107–113. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Yu, J.Z.; Xu, J.H. Photodegradation of formaldehyde by photocatalyst TiO2: Effects on the presences of NO, SO2 and VOCs. Appl. Catal. B 2004, 54, 41–50. [Google Scholar] [CrossRef]
- Kopelovich, J.; Perez, A.L.; Jacobs, N.; Mendelsohn, E.; Keenan, J.J. Screening-level human health risk assessment of toluene and dibutyl phthalate in nail lacquers. Food Chem. Toxicol. 2015, 81, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gericke, C.; Hanke, B.; Beckmann, G.; Baltes, M.M. Multicenter field trial on possible health effects of toluene: III. Evaluation of effects after long-term exposure. Toxicology 2001, 168, 185–209. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Wi, S.; Yun, B.Y.; Kim, S. Field study on the improvement of indoor air quality with toluene adsorption finishing materials in an urban residential apartment. Environ. Pollut. 2020, 261, 114137. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Shi, X.; Bi, F.; Yang, Y.; Wang, Y. Synergistic effects of octahedral TiO2-MIL-101(Cr) with two heterojunctions for enhancing visible-light photocatalytic degradation of liquid tetracycline and gaseous toluene. J. Colloid Interface Sci. 2020, 579, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Dong, Y.; Ke, J.; Ge, H.; Chen, D.; Sun, H.; Cui, Y. Cobalt monoxide/tungsten trioxide p-n heterojunction boosting charge separation for efficient visible-light-driven gaseous toluene degradation. Chem. Eng. J. 2020, 400, 125919. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Chen, L.; Dong, X.; Wang, H.; Sheng, J.; Sun, Y.; Zhou, Y.; Dong, F. Nature-inspired CaCO3 loading TiO2 composites for efficient and durable photocatalytic mineralization of gaseous toluene. Sci. Bull. 2020, 65, 1626–1634. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, L.; Chang, W.; Huang, Z.; Feng, X.; Qi, X.; Li, Z. Efficient photocatalytic degradation of gaseous N,N-dimethylformamide in tannery waste gas using doubly open-ended Ag/TiO2 nanotube array membranes. Appl. Surf. Sci. 2018, 444, 610–620. [Google Scholar] [CrossRef]
- Wang, L.; Gao, X.; Cheng, Y.; Zhang, X.; Wang, G.; Zhang, Q.; Su, J. TiO2@MgAl-layered double hydroxide with enhanced photocatalytic activity towards degradation of gaseous toluene. J. Photochem. Photobiol. A 2019, 369, 44–53. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, C.; Li, H.; Chen, H.; Zou, T.; Zeng, D. Improvement of gaseous pollutant photocatalysis with WO3/TiO2 heterojunctional-electrical layered system. J. Hazard. Mater. 2011, 196, 52–58. [Google Scholar] [CrossRef]
- Reingruber, H.; Pontel, L.B. Formaldehyde metabolism and its impact on human health. Curr. Opin. Toxicol. 2018, 9, 28–34. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Rezende, L.C.; Barbosa, A.S.; Carvalho, P.; Carvalho, A.A. Economic, human and environmental health benefits of replacing formaldehyde in the preservation of corpses. Ecotoxicol. Environ. Safety 2017, 145, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, W.; Cai, J.; Wang, X.; Sun, C. Household formaldehyde exposure and its associations with dwelling characteristics, lifestyle behaviours, and childhood health outcomes in Shanghai, China. Build. Environ. 2017, 125, 143–152. [Google Scholar] [CrossRef]
- Noisel, N.; Bouchard, M.; Carrier, G. Evaluation of the health impact of lowering the formaldehyde occupational exposure limit for Quebec workers. Regul. Toxicol. Pharm. 2007, 48, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, H.; Xiao, Y.; Zhang, L.; Guo, L.; Zhang, L.; Dong, X. Free-standing composite films of multiple 2D nanosheets: Synergetic photothermocatalysis/photocatalysis for efficient removal of formaldehyde under ambient condition. Chem. Eng. J. 2020, 394, 125014. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, G.; Meng, Y.; Yang, C.; Ni, Z.; Hu, J. Kinetic and mechanistic analysis for the photodegradation of gaseous formaldehyde by core-shell CeO2@LDHs. Appl. Catal. B 2020, 278, 119266. [Google Scholar] [CrossRef]
- Armenta-Reséndiz, M.; Ríos-Leal, E.; Rivera-García, M.T.; López-Rubalcava, C.; Cruz, S.L. Structure-activity study of acute neurobehavioral effects of cyclohexane, benzene, m-xylene, and toluene in rats. Toxicol. Appl. Pharm. 2019, 376, 38–45. [Google Scholar] [CrossRef]
- Singh, M.P.; Mishra, M.; Sharma, A.; Shukla, A.K.; Kar Chowdhuri, D. Genotoxicity and apoptosis in Drosophila melanogaster exposed to benzene, toluene and xylene: Attenuation by quercetin and curcumin. Toxicol. Appl. Pharm. 2011, 253, 14–30. [Google Scholar] [CrossRef]
- Lee, E.; Ahn, S.; Jin, S.H.; Lee, M.; Noh, M. CXCL14 downregulation in human keratinocytes is a potential biomarker for a novel in vitro skin sensitization test. Toxicol. Appl. Pharm. 2020, 386, 114828. [Google Scholar] [CrossRef]
- Jenkins, L.J.; Jones, R.A.; Siegel, J. Long-term inhalation screening studies of benzene, toluene, o-xylene, and cumene on experimental plants. Toxicol. Appl. Pharm. 1970, 16, 818–823. [Google Scholar] [CrossRef]
- Lin, W.; Xie, X.; Wang, X.; Wang, Y.; Segets, D.; Sun, J. Efficient adsorption and sustainable degradation of gaseous acetaldehyde and o-xylene using rGO-TiO2 photocatalyst. Chem. Eng. J. 2018, 349, 708–718. [Google Scholar] [CrossRef]
- Edokpolo, B.; Yu, Q.J.; Connell, D. Use of toxicant sensitivity distributions (TSD) for development of exposure guidelines for risk to human health from benzene. Environ. Pollut. 2019, 250, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Mostafalou, S.; Abdollahi, M. Current understandings and perspectives on non-cancer health effects of benzene: A global concern. Toxicol. Appl. Pharm. 2014, 276, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edokpolo, B.; Yu, Q.J.; Connell, D. Health risk characterization for exposure to benzene in service stations and petroleum refineries environments using human adverse response data. Toxicol. Rep. 2015, 2, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, F.; Shu, C.; Liu, P.; Zhang, W.; Hu, S. TiO2/porous cementitious composites: Influences of porosities and TiO2 loading levels on photocatalytic degradation of gaseous benzene. Constr. Build. Mater. 2017, 150, 774–780. [Google Scholar] [CrossRef]
- Tri, N.L.; Duc, D.S.; Thuan, D.V.; Tahtamouni, T.A.; Pham, T.D.; Tran, D.T.; Phuong Le Chi, N.T.; Nguyen, V.N. Superior photocatalytic activity of Cu doped NiWO4 for efficient degradation of benzene in air even under visible radiation. Chem. Phys. 2019, 525, 110411. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Zheng, Y.; Chen, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 nanocrystalline heterostructure: A wide spectrum responsive photocatalyst towards the highly efficient decomposition of gaseous benzene. Appl. Catal. B 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Meng, S.; Zhang, L.; Fu, X.; Chen, S. One-pot hydrothermal synthesis of highly efficient SnOx/Zn2SnO4 composite photocatalyst for the degradation of methyl orange and gaseous benzene. Appl. Catal. B 2017, 200, 19–30. [Google Scholar] [CrossRef]
- Chun, H.H.; Lee, j.Y.; Jo, W.K. Photocatalysis of low-concentration gaseous organic pollutants over electrospun iron-doped titanium dioxide nanofibers. Solid State Sci. 2013, 25, 103–109. [Google Scholar] [CrossRef]
- Cheng, Z.W.; Feng, L.; Chen, J.M.; Yu, J.M.; Jiang, Y.F. Photocatalytic conversion of gaseous ethylbenzene on lanthanum-doped titanium dioxide nanotubes. J. Hazard. Mater. 2013, 254, 354–363. [Google Scholar] [CrossRef]
- Delikhoon, M.; Fazlzadeh, M.; Sorooshian, A.; Baghani, A.N.; Barkhordari, A. Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran. Environ. Pollut. 2018, 242, 938–951. [Google Scholar] [CrossRef]
- Tian, X.; Shen, Z.; Zhou, Y.; Wang, K. Inhibition on biological acidification and microbial community by high-strength acetaldehyde. Process. Saf. Environ. 2020, 143, 231–238. [Google Scholar] [CrossRef]
- Fan, G.; Xie, J.; Yoshino, H.; Zhang, H.; Liu, J. Concentration characteristics of gaseous carbonyl compounds in urban houses in two different climatic zones of China and health risk assessment for schoolchildren. Sustain. Cities Soc. 2020, 60, 102270. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Salaspuro, M. ALDH2-deficiency as genetic epidemiologic and biochemical model for the carcinogenicity of acetaldehyde. Regul. Toxicol. Pharm. 2017, 86, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Xie, X.; Wang, X.; Lu, G.; Li, H.; Lee, S.C.; Sun, J. New insights into the synergistic effect of active radicals and adsorptive ability on the photodegradation of gaseous acetaldehyde over reduced graphene Oxide/P25 composite. J. Hazard. Mater. 2019, 380, 120814. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, X.; Xie, X.; Lu, G.; Wang, Y.; Lee, S.C.; Sun, J. TiO2/TaS2 with superior charge separation and adsorptive capacity to the photodegradation of gaseous acetaldehyde. Chem. Eng. J. 2020, 379, 122395. [Google Scholar] [CrossRef]
- Zeng, Q.; Xie, X.; Wang, X.; Wang, Y.; Lu, G.; Pui, D.Y.H.; Sun, J. Enhanced photocatalytic performance of Ag@TiO2 for the gaseous acetaldehyde photodegradation under fluorescent lamp. Chem. Eng. J. 2018, 341, 83–92. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, X.; Wang, X.; Wang, Y.; Zeng, Y.; Pui, D.Y.H.; Sun, J. Visible-Light Upconversion Carbon Quantum Dots Decorated TiO2 for the Photodegradation of Flowing Gaseous Acetaldehyde. Appl. Surf. Sci. 2018, 440, 266–274. [Google Scholar] [CrossRef]
- Thevenet, F.; Guaitell, O.; Puzenat, E.; Herrmann, J.M.; Rousseau, A.; Guillard, C. Oxidation of acetylene by photocatalysis coupled with dielectric barrier discharge. Catal. Today 2007, 122, 186–194. [Google Scholar] [CrossRef]
- Enesca, A.; Yamaguchi, Y.; Terashima, C.; Fujishima, A.; Nakata, K.; Duta, A. Enhanced UV–Vis photocatalytic performance of the CuInS2/TiO2/SnO2 hetero-structure for air decontamination. J. Catal. 2017, 350, 174–181. [Google Scholar] [CrossRef]
- Chun, H.H.; Jo, W.K. Adsorption and photocatalysis of 2-ethyl-1-hexanol over graphene oxide–TiO2 hybrids post-treated under various thermal conditions. Appl. Catal. B 2016, 180, 740–750. [Google Scholar] [CrossRef]
- Costa Filho, B.M.; Araujo, A.L.P.; Silva, G.V.; Boaventura, R.A.R.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J.P. Intensification of heterogeneous TiO2 photocatalysis using an innovative micro-meso-structured-photoreactor for n-decane oxidation at gas phase. Chem. Eng. J. 2017, 310, 331–341. [Google Scholar] [CrossRef]
- McKee, R.H.; Nessel, C.S.; Carrillo, J.C. An investigation of the acute central nervous system effects of n-decane. Regul. Toxicol. Pharmacol. 2019, 107, 104421. [Google Scholar] [CrossRef] [PubMed]
- Kjærgaard, S.; Mølhave, L.; Pedersen, O.F. Human reactions to indoor air pollutants: N-decane. Environ. Int. 1989, 15, 273–282. [Google Scholar] [CrossRef]
- Akinola, J.O.; Olawusi-Peters, O.O.; Apkambang, V.O. Human health risk assessment of TPHs in brackish water prawn (Nematopalaemon hastatus, AURIVILLUS, 1898). Heliyon 2020, 6, e03234. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, J.; Li, Y.; Wen, M.; Liu, H.; Li, G.; An, T. In-situ decoration of metallic Bi on BiOBr with exposed (110) facets and surface oxygen vacancy for enhanced solar light photocatalytic degradation of gaseous n-hexane. Chinese J. Catal. 2020, 41, 1603–1612. [Google Scholar] [CrossRef]
- Mohseni, M. Gas phase trichloroethylene (TCE) photooxidation and byproduct formation: Photolysis vs. titania/silica based photocatalysis. Chemosphere 2005, 59, 335–342. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Chen, F.; Lin, X.; Feng, Q. Electrically enhanced photocatalysis for gas-phase benzaldehyde degradation by ordered mesoporous titania/conductive carbon felts. Electrochim. Acta 2016, 216, 517–527. [Google Scholar] [CrossRef]
- Sayed, M.; Arooj, A.; Shah, N.S.; Khan, J.A.; Shah, L.A.; Rehman, F.; Arandiyan, H.; Khan, A.M.; Khan, A.R. Narrowing the band gap of TiO2 by co-doping with Mn2+ and Co2+ for efficient photocatalytic degradation of enoxacin and its additional peroxidase like activity: A mechanistic approach. J. Molec. Liq. 2018, 272, 403–412. [Google Scholar] [CrossRef]
- Gul, I.; Sayed, M.; Shah, N.S.; Khan, J.A.; Polychronopoulou, K.; Iqbal, J.; Rehman, F. Solar light responsive bismuth doped titania with Ti3+ for efficient photocatalytic degradation of flumequine: Synergistic role of peroxymonosulfate. Chem. Eng. J. 2020, 384, 123255. [Google Scholar] [CrossRef]
- Khan, J.A.; Sayed, M.; Shah, N.S.; Khan, S.; Zhang, Y.; Boczkaj, G.; Khan, H.M.; Dionysiou, D.D. Synthesis of eosin modified TiO2 film with co-exposed {001} and {101} facets for photocatalytic degradation of para-aminobenzoic acid and solar H2 production. App. Catal. B Environ. 2019, 265, 118557. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Cazan, C. Volatile Organic Compounds (VOCs) Removal from Indoor Air by Heterostructures/Composites/Doped Photocatalysts: A Mini-Review. Nanomaterials 2020, 10, 1965. https://doi.org/10.3390/nano10101965
Enesca A, Cazan C. Volatile Organic Compounds (VOCs) Removal from Indoor Air by Heterostructures/Composites/Doped Photocatalysts: A Mini-Review. Nanomaterials. 2020; 10(10):1965. https://doi.org/10.3390/nano10101965
Chicago/Turabian StyleEnesca, Alexandru, and Cristina Cazan. 2020. "Volatile Organic Compounds (VOCs) Removal from Indoor Air by Heterostructures/Composites/Doped Photocatalysts: A Mini-Review" Nanomaterials 10, no. 10: 1965. https://doi.org/10.3390/nano10101965
APA StyleEnesca, A., & Cazan, C. (2020). Volatile Organic Compounds (VOCs) Removal from Indoor Air by Heterostructures/Composites/Doped Photocatalysts: A Mini-Review. Nanomaterials, 10(10), 1965. https://doi.org/10.3390/nano10101965







