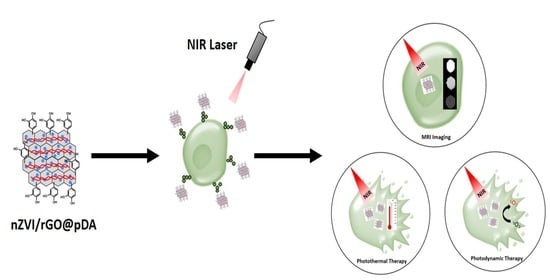
Preparation of Multifunctional Dopamine-Coated Zerovalent Iron/Reduced Graphene Oxide for Targeted Phototheragnosis in Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of nZVI/rGO@pDA
2.3. Cell Cultures
2.4. Cytotoxic Potential of nZVI/rGO@pDA
2.5. Photothermal and Photodynamic Ability of nZVI/rGO@pDA
2.6. In Vitro Efficacy of PTT/PDT
2.7. Targeting Ability of nZVI/rGO@pDA
2.8. Magnetic Resonance Imaging Ability of nZVI/rGO@pDA
2.9. Statistical Analysis
3. Results and Discussion
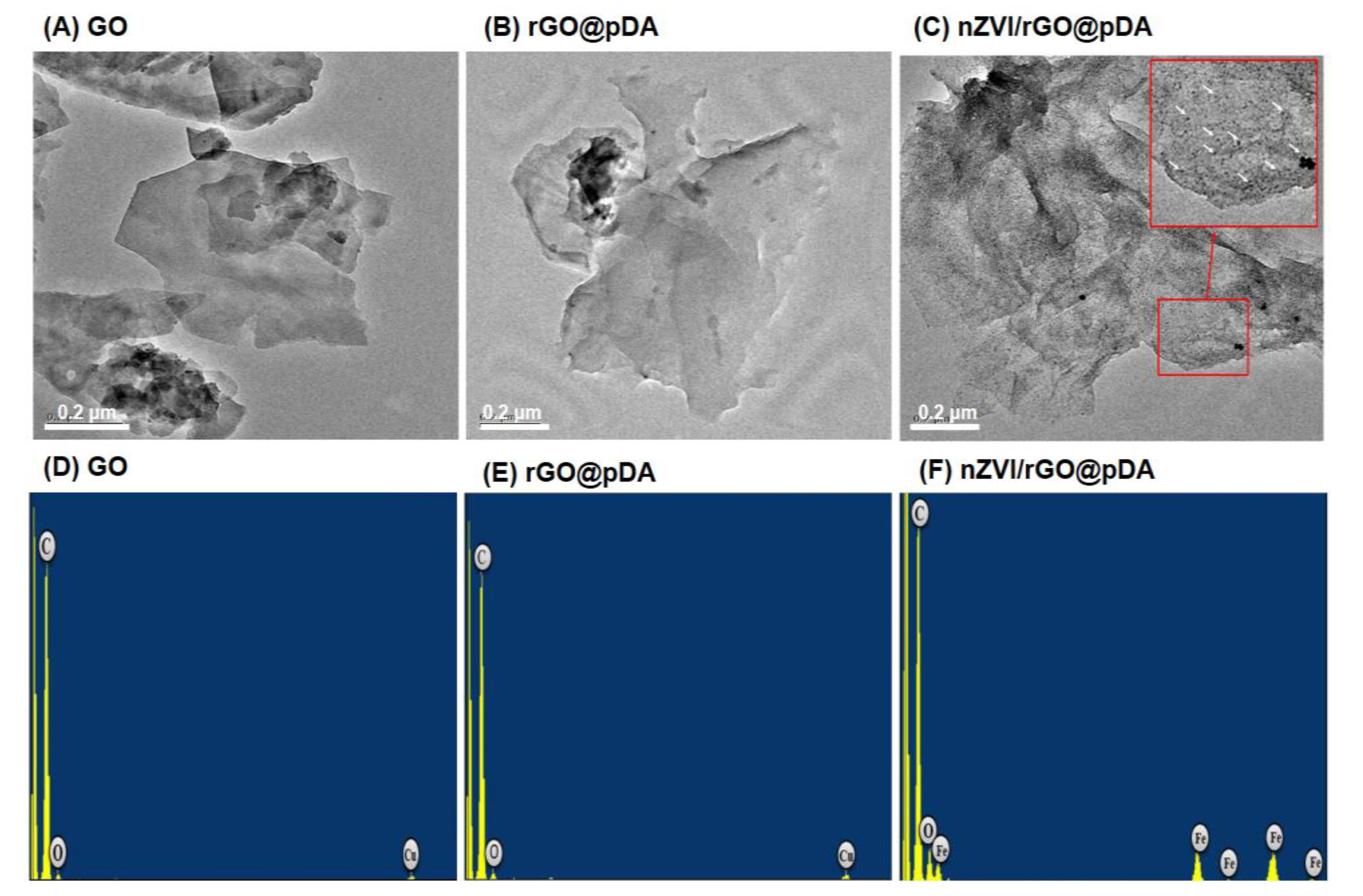
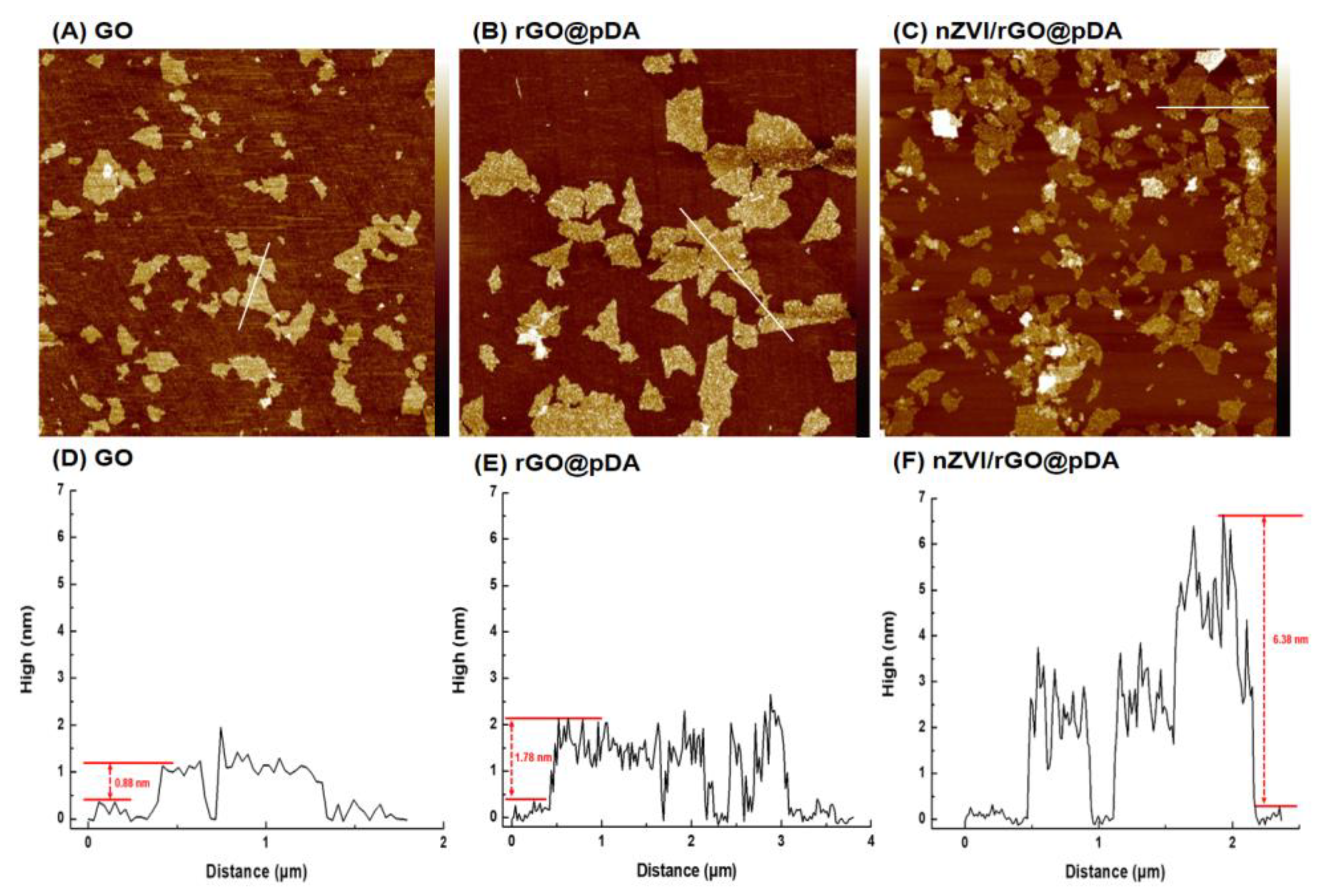
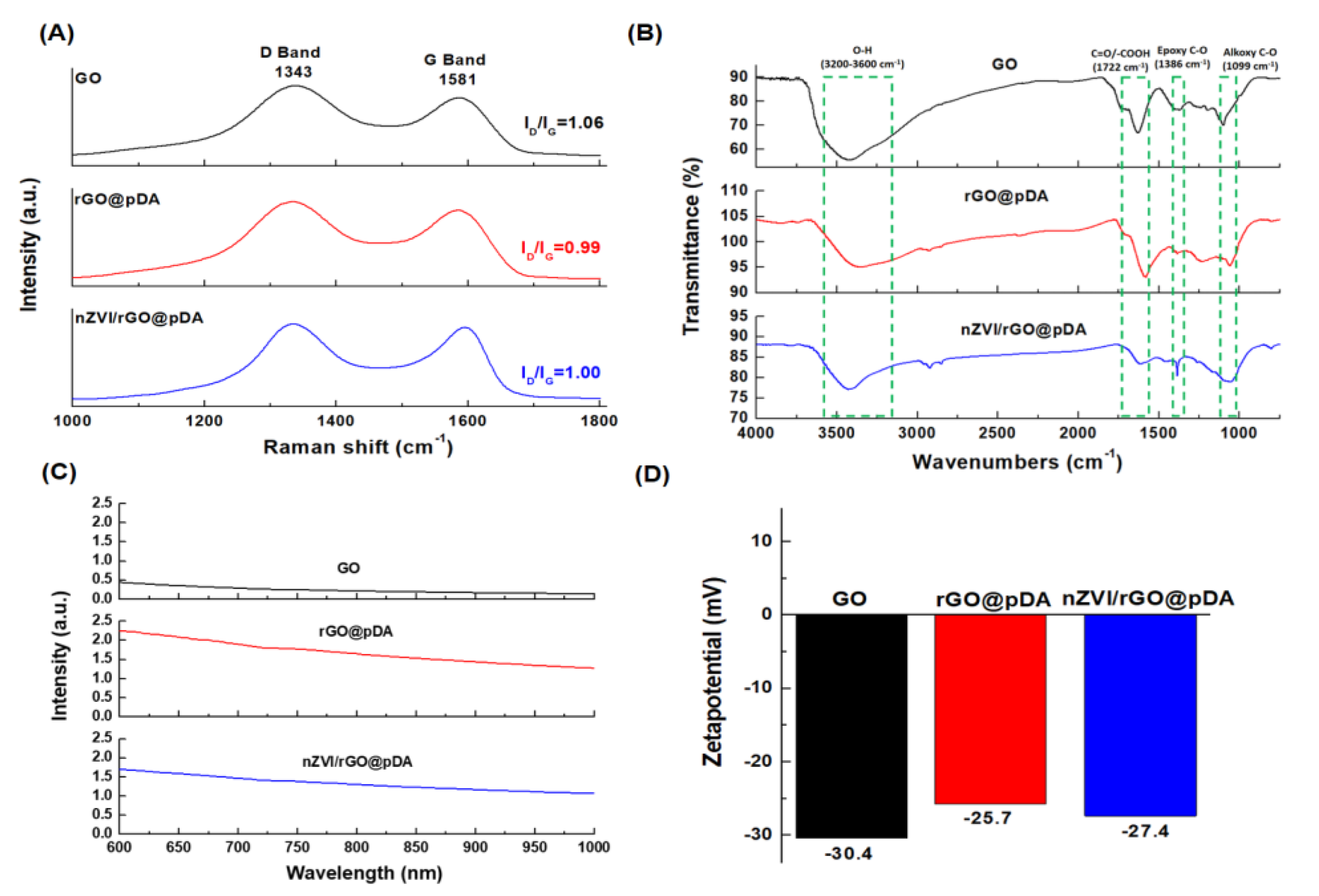
3.1. Preparation of nZVI/rGO@pDA
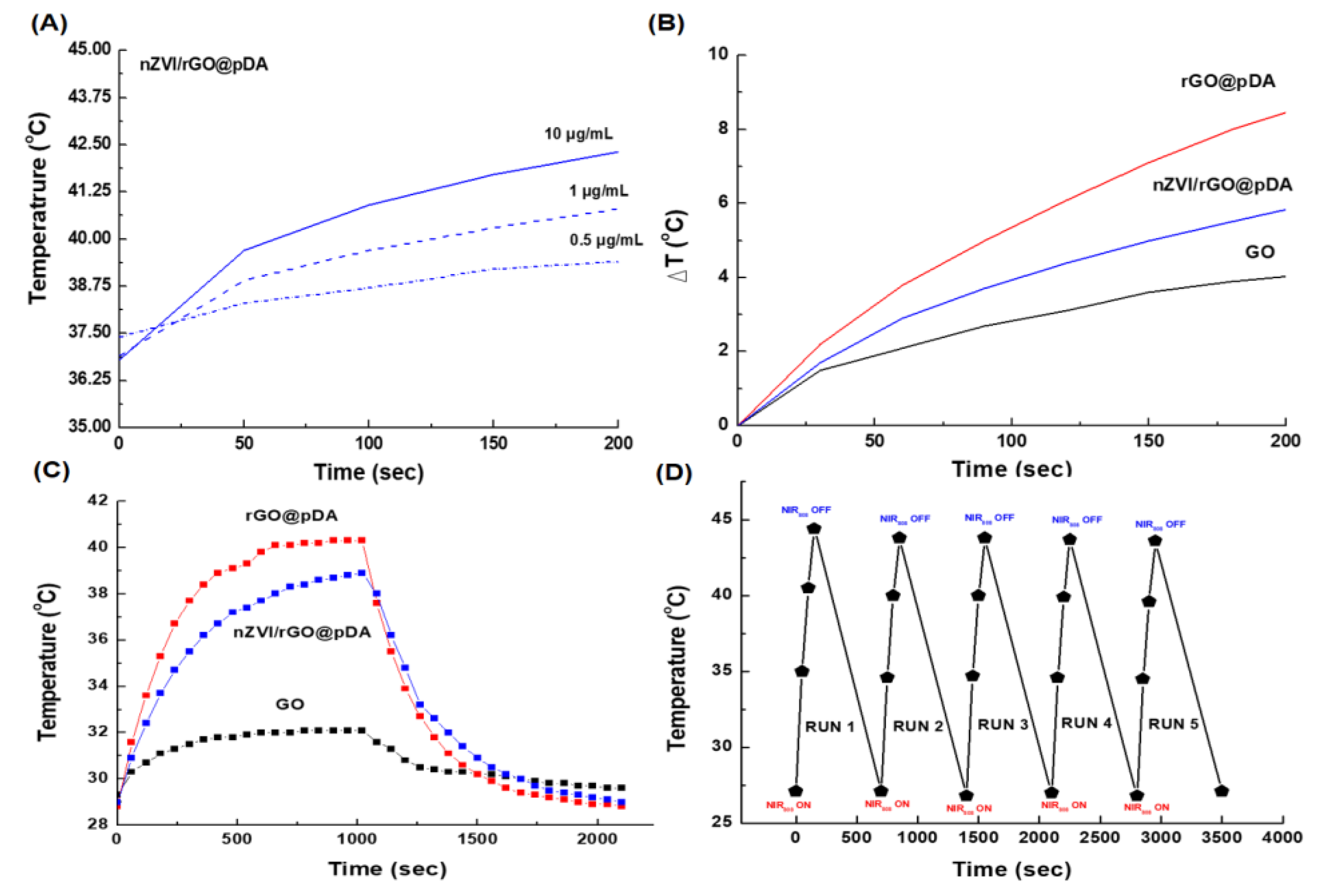
3.2. Photothermal/Photodynamic Effects of nZVI/rGO@pDA
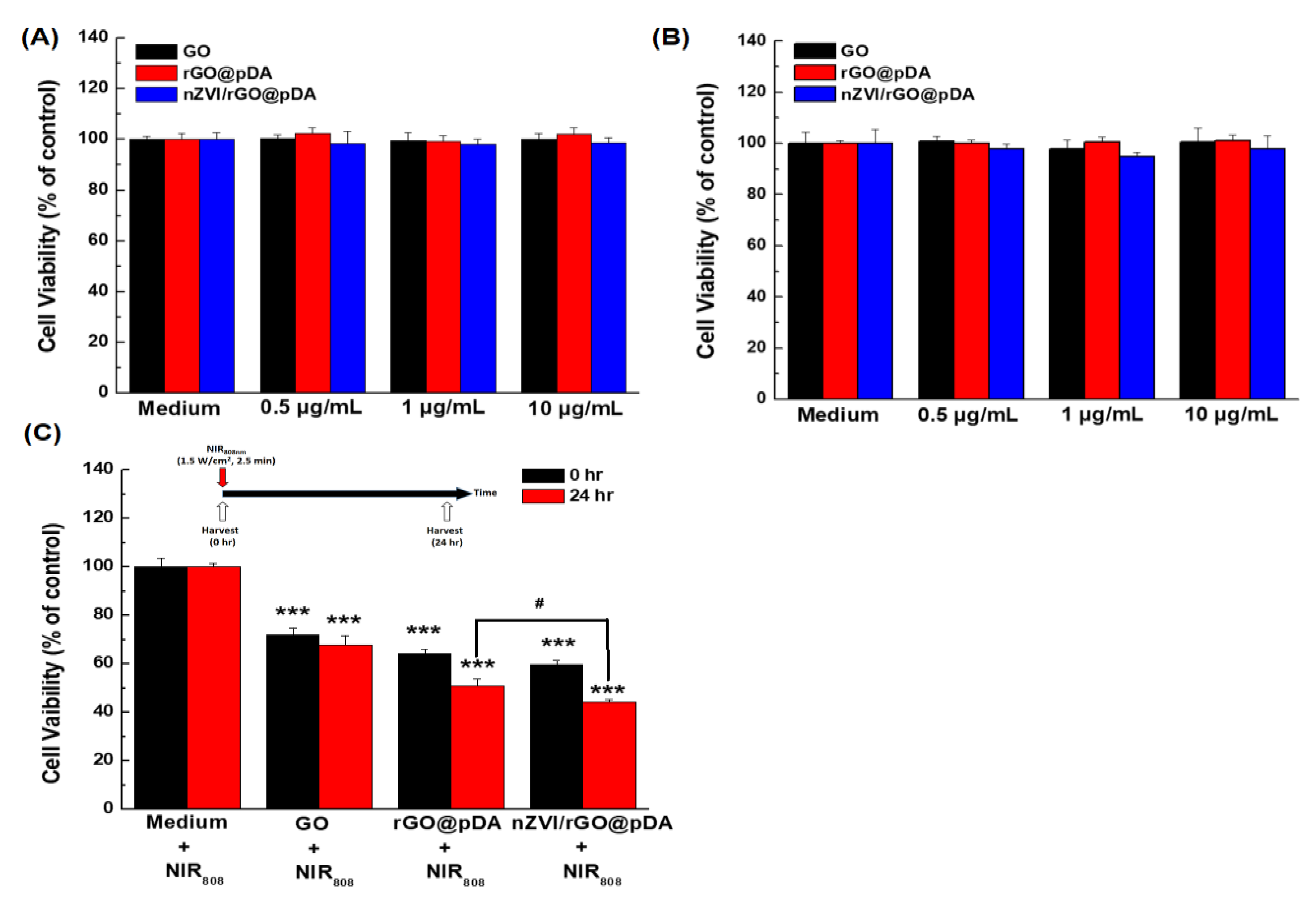
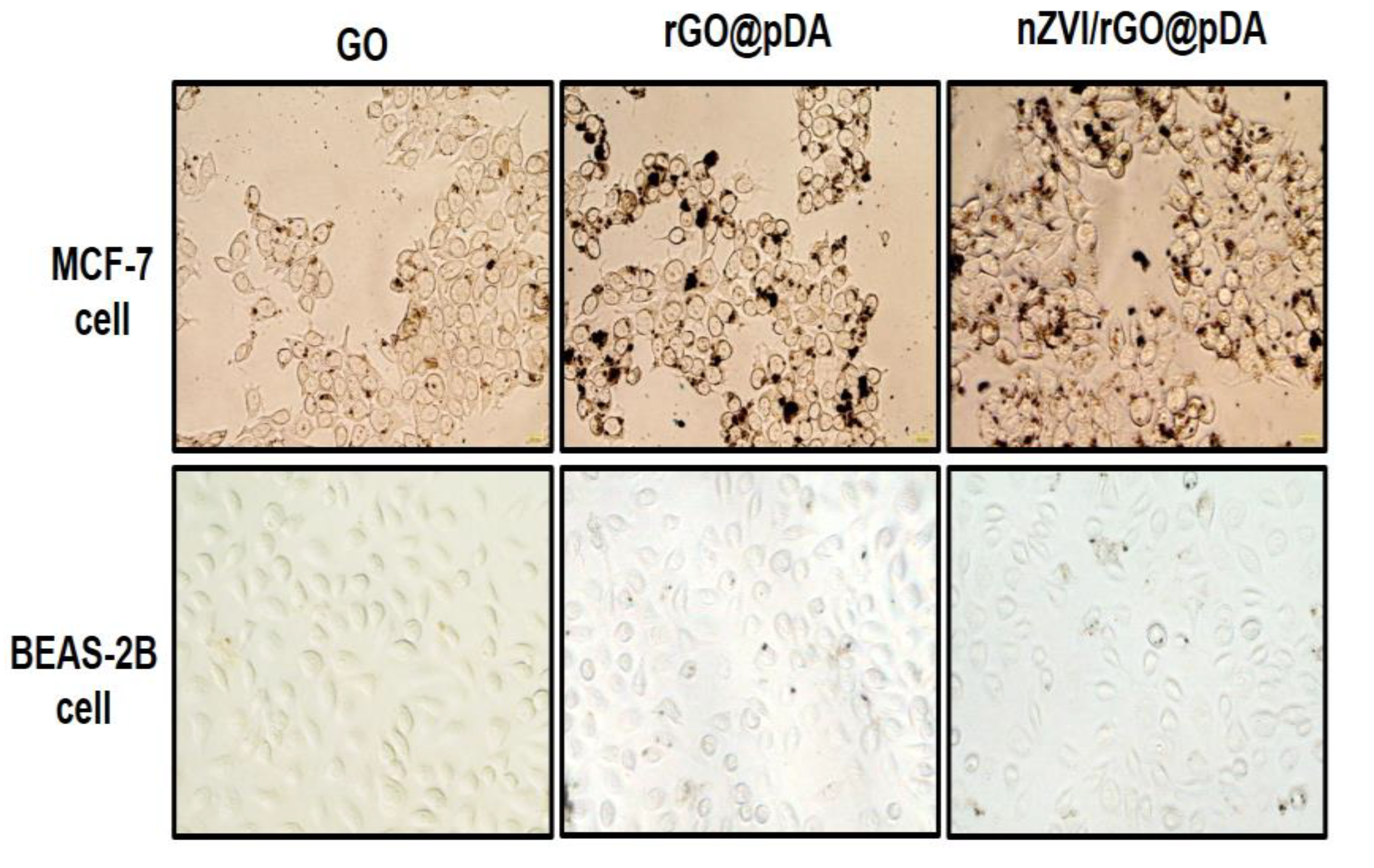
3.3. Bio-Safety, Phototherapy Effect, and Targeting Ability of nZVI/rGO@pDA
3.4. MRI Contrast Effect of nZVI/rGO@pDA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lepock, J.R. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperth. 2003, 19, 252–266. [Google Scholar] [CrossRef]
- Akimoto, J. Photodynamic therapy for malignant brain tumors. Neurol. Med. Chir. 2016, 56, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tseng, Y.T.; Suo, G.; Chen, L.; Yu, J.; Chiu, W.J.; Huang, C.C.; Lin, C.H. Photothermal therapeutic response of cancer cells to aptamer-gold nanoparticle-hybridized graphene oxide under nir illumination. ACS Appl. Mater. Interfaces 2015, 7, 5097–5106. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Van Gemert, M.J.; Welch, A.J.; Pickering, J.W.; Tan, O.T.; Gijsbers, G.H. Wavelengths for laser treatment of port wine stains and telangiectasia. Lasers Surg. Med. 1995, 16, 147–155. [Google Scholar] [CrossRef]
- Gai, S.L.; Yang, G.X.; Yang, P.P.; He, F.; Lin, J.; Jin, D.Y.; Xing, B.G. Recent advances in functional nanomaterials for light-triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.T.; Cui, Y.J.; Zhou, N.L.; Shen, J. Near-infrared light-mediated photodynamic/photothermal therapy nanoplatform by the assembly of fe3o4 carbon dots with graphitic black phosphorus quantum dots. Int. J. Nanomed. 2018, 13, 2803–2819. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Lin, C.H.; Chen, Y.C. Using glucose-bound fe3o4 magnetic nanoparticles as photothermal agents for targeted hyperthermia of cancer cells. J. Nanomed. Nanotechnol. 2015, 6, 264. [Google Scholar]
- Wang, K.K.; Zhang, Y.F.; Wang, J.; Yuan, A.; Sun, M.J.; Wu, J.H.; Hu, Y.Q. Self-assembled ir780-loaded transferrin nanoparticles as an imaging, targeting and pdt/ptt agent for cancer therapy. Sci. Rep. 2016, 6, 27421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Bhattarai, P.; Dai, Z.F.; Chen, X.Y. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.A.; Zhang, G.X.; Sun, X.M.; Lee, S.T.; Liu, Z.A. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Cabizza, R.; Bianco, A.; Delogu, L.G. Graphene as cancer theranostic tool: Progress and future challenges. Theranostics 2015, 5, 710–723. [Google Scholar] [CrossRef]
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. Acs Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef]
- Song, X.; Liang, C.; Gong, H.; Chen, Q.; Wang, C.; Liu, Z. Photosensitizer-conjugated albumin-polypyrrole nanoparticles for imaging-guided in vivo photodynamic/photothermal therapy. Small 2015, 11, 3932–3941. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Liu, C.H.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Designing multi-branched gold nanoechinus for nir light activated dual modal photodynamic and photothermal therapy in the second biological window. Adv. Mater. 2014, 26, 6689–6695. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Yu, J.T.; Lin, Y.H.; Yang, L.Y.; Huang, C.C.; Chen, L.L.; Wang, W.C.; Chen, G.W.; Yan, J.Y.; Sawettanun, S.; Lin, C.H. Improved anticancer photothermal therapy using the bystander effect enhanced by antiarrhythmic peptide conjugated dopamine-modified reduced graphene oxide nanocomposite. Adv. Healthc Mater. 2017, 6. [Google Scholar] [CrossRef]
- Hou, L.; Feng, Q.; Wang, Y.; Yang, X.; Ren, J.; Shi, Y.; Shan, X.; Yuan, Y.; Wang, Y.; Zhang, Z. Multifunctional hyaluronic acid modified graphene oxide loaded with mitoxantrone for overcoming drug resistance in cancer. Nanotechnology 2016, 27, 015701. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Zhao, C.; Qin, G.; Xi, G.; Li, T.; Wang, X.; Chen, T. One-step reduction and pegylation of graphene oxide for photothermally controlled drug delivery. Biomaterials 2014, 35, 4986–4995. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lyv, Z.; Li, Y.; Liu, H.; Wang, J.; Zhan, W.; Chen, H.; Chen, H.; Li, X. A theranostic prodrug delivery system based on pt(iv) conjugated nano-graphene oxide with synergistic effect to enhance the therapeutic efficacy of pt drug. Biomaterials 2015, 51, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H.; Shiao, N.H.; Lu, P.Z. Cdse quantum dots induce apoptosis in human neuroblastoma cells via mitochondrial-dependent pathways and inhibition of survival signals. Toxicol. Lett. 2006, 167, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Maysinger, D.; Jain, M.; Roder, B.; Hackbarth, S.; Winnik, F.M. Long-term exposure to cdte quantum dots causes functional impairments in live cells. Langmuir 2007, 23, 1974–1980. [Google Scholar] [CrossRef]
- Choi, A.O.; Cho, S.J.; Desbarats, J.; Lovric, J.; Maysinger, D. Quantum dot-induced cell death involves fas upregulation and lipid peroxidation in human neuroblastoma cells. J. Nanobiotechnology 2007, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Lovric, J.; Cho, S.J.; Winnik, F.M.; Maysinger, D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem. Biol. 2005, 12, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Li, G.Q.; Cheng, C.; Liu, P.; Shi, L.; Ma, Z.; Zheng, X. Removal of bacteriophage f2 in water by nanoscale zero-valent iron and parameters optimization using response surface methodology. Chem. Eng. J. 2014, 252, 150–158. [Google Scholar] [CrossRef]
- Yousefzadeh, S.; Matin, A.R.; Ahmadi, E.; Sabeti, Z.; Alimohammadi, M.; Aslani, H.; Nabizadeh, R. Response surface methodology as a tool for modeling and optimization of bacillus subtilis spores inactivation by uv/nano-fe-0 process for safe water production. Food Chem. Toxicol. 2018, 114, 334–345. [Google Scholar] [CrossRef]
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Roman, F. Antimicrobial nanomaterials as water disinfectant: Applications, limitations and future perspectives. Sci. Total Environ. 2014, 466, 1047–1059. [Google Scholar] [CrossRef]
- Keenan, C.R.; Goth-Goldstein, R.; Lucas, D.; Sedlak, D.L. Oxidative stress induced by zero-valent iron nanoparticles and fe(ii) in human bronchial epithelial cells. Environ. Sci. Technol. 2009, 43, 4555–4560. [Google Scholar] [CrossRef]
- Phenrat, T.; Long, T.C.; Lowry, G.V.; Veronesi, B. Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ. Sci. Technol. 2009, 43, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Otero-Gonzalez, L.; Sierra-Alvarez, R.; Boitano, S.; Field, J.A. Application and validation of an impedance-based real time cell analyzer to measure the toxicity of nanoparticles impacting human bronchial epithelial cells. Environ. Sci. Technol. 2012, 46, 10271–10278. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Lin, L.S.; Cong, Z.X.; Cao, J.B.; Ke, K.M.; Peng, Q.L.; Gao, J.; Yang, H.H.; Liu, G.; Chen, X. Multifunctional fe(3)o(4)@polydopamine core-shell nanocomposites for intracellular mrna detection and imaging-guided photothermal therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.H.; Wang, H.; Yang, H.; Li, Z.L.; Zhen, L.; Xu, C.Y. Intrinsically mn2+-chelated polydopamine nanoparticles for simultaneous magnetic resonance imaging and photothermal ablation of cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 16946–16952. [Google Scholar] [CrossRef]
- Yin, T.; He, S.; Shen, G.; Ye, T.; Guo, F.; Wang, Y. Dopamine receptor antagonist thioridazine inhibits tumor growth in a murine breast cancer model. Mol. Med. Rep. 2015, 12, 4103–4108. [Google Scholar] [CrossRef] [Green Version]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Maeng, J.H.; Lee, D.H.; Jung, K.H.; Bae, Y.H.; Park, I.S.; Jeong, S.; Jeon, Y.S.; Shim, C.K.; Kim, W.; Kim, J.; et al. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 2010, 31, 4995–5006. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Liang, C.; Liu, Y.M.; Li, Z.W.; Yang, G.B.; Cheng, H.; Li, Y.G.; Liu, Z. Iron oxide @ polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano 2013, 7, 6782–6795. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339-1339. [Google Scholar] [CrossRef]
- Sun, Z.L.; Yang, L.Y.; Chen, K.F.; Chen, G.W.; Peng, Y.P.; Chen, J.K.; Suo, G.L.; Yu, J.T.; Wang, W.C.; Lin, C.H. Nano zerovalent iron particles induce pulmonary and cardiovascular toxicity in an in vitro human co-culture model. Nanotoxicology 2016, 10, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Yeh, T.Y.; Kao, C.M.; Sung, W.P.; Lin, C.C. Application of nanoscale zero-valent iron (nzvi) to enhance microbial reductive dechlorination of tce: A feasibility study. Curr. Nanosci. 2012, 8, 55–59. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Nyangiwe, N.N.; Khenfouch, M.; Thema, F.T.; Nukwa, K.; Kotsedi, L.; Maaza, M. Free-green synthesis and dynamics of reduced graphene sheets via sun light irradiation. Graphene 2015, 4, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Rattana; Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Çiplak, Z.; Yildiz, N.; Çalimli, A. Investigation of graphene/ag nanocomposites synthesis parameters for two different synthesis methods. Fuller. Nanotub. Carbon Nanostructures 2015, 23, 361–370. [Google Scholar] [CrossRef]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical property and structure of covalent functionalised graphene/epoxy nanocomposites. Sci. Rep. 2014, 4, 4375. [Google Scholar] [CrossRef] [Green Version]
- Banga, A.; Witzmann, F.A.; Petrache, H.I.; Blazer-Yost, B.L. Functional effects of nanoparticle exposure on calu-3 airway epithelial cells. Cell. Physiol. Biochem. 2012, 29, 197–212. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, D.; Song, S.; Wang, X.; Zhang, H. Graphene oxide covalently grafted upconversion nanoparticles for combined nir mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials 2013, 34, 7715–7724. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.C.; Guo, Z.Y.; Liu, Z.M.; Zhang, W.; Wan, M.M.; Yang, B.W. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sawettanun, S.; Chen, K.F.; Lee, C.Y.; Yan, J.Y.; Chen, H.H.; Chen, G.W.; Lin, C.H. Enhanced efficient nir photothermal therapy using pleurocidin nrc-03 peptide-conjugated dopamine-modified reduced graphene oxide nanocomposite. ACS Omega 2019, 4, 3298–3305. [Google Scholar] [CrossRef] [Green Version]
- Lebel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, S.H.; Teng, G.J.; Zhang, Y.; Ma, M.; Chen, F.; Ni, Y.C. In vitro labeling and mri of mesenchymal stem cells from human umbilical cord blood. Magn. Reson. Imaging 2006, 24, 611–617. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Chen, Y.-C.; Huang, P.-I. Preparation of Multifunctional Dopamine-Coated Zerovalent Iron/Reduced Graphene Oxide for Targeted Phototheragnosis in Breast Cancer. Nanomaterials 2020, 10, 1957. https://doi.org/10.3390/nano10101957
Lin C-H, Chen Y-C, Huang P-I. Preparation of Multifunctional Dopamine-Coated Zerovalent Iron/Reduced Graphene Oxide for Targeted Phototheragnosis in Breast Cancer. Nanomaterials. 2020; 10(10):1957. https://doi.org/10.3390/nano10101957
Chicago/Turabian StyleLin, Chia-Hua, Yi-Chun Chen, and Pin-I. Huang. 2020. "Preparation of Multifunctional Dopamine-Coated Zerovalent Iron/Reduced Graphene Oxide for Targeted Phototheragnosis in Breast Cancer" Nanomaterials 10, no. 10: 1957. https://doi.org/10.3390/nano10101957
APA StyleLin, C.-H., Chen, Y.-C., & Huang, P.-I. (2020). Preparation of Multifunctional Dopamine-Coated Zerovalent Iron/Reduced Graphene Oxide for Targeted Phototheragnosis in Breast Cancer. Nanomaterials, 10(10), 1957. https://doi.org/10.3390/nano10101957