One Dimensional ZnO Nanostructures: Growth and Chemical Sensing Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization
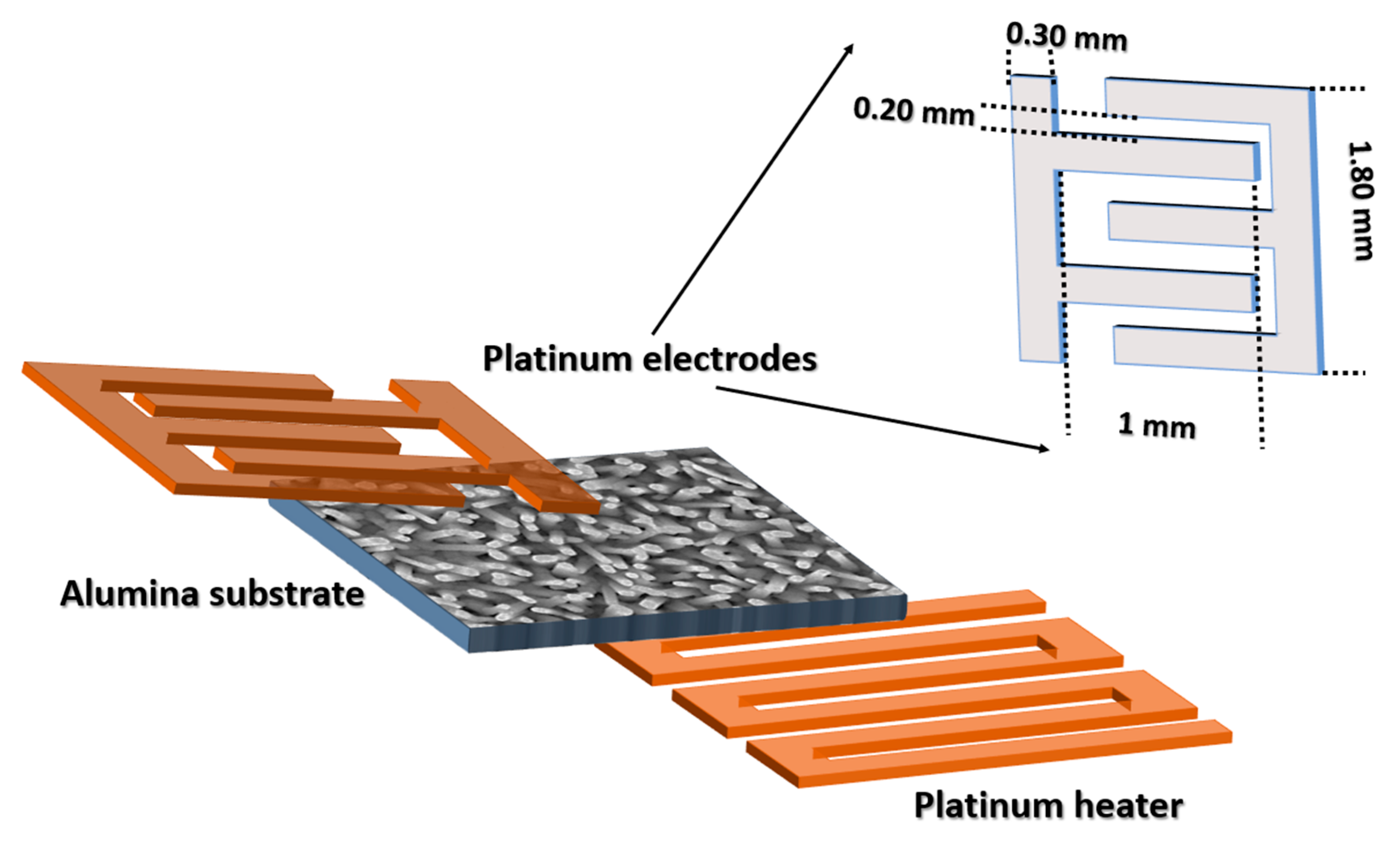
2.3. Device Fabrication
3. Results
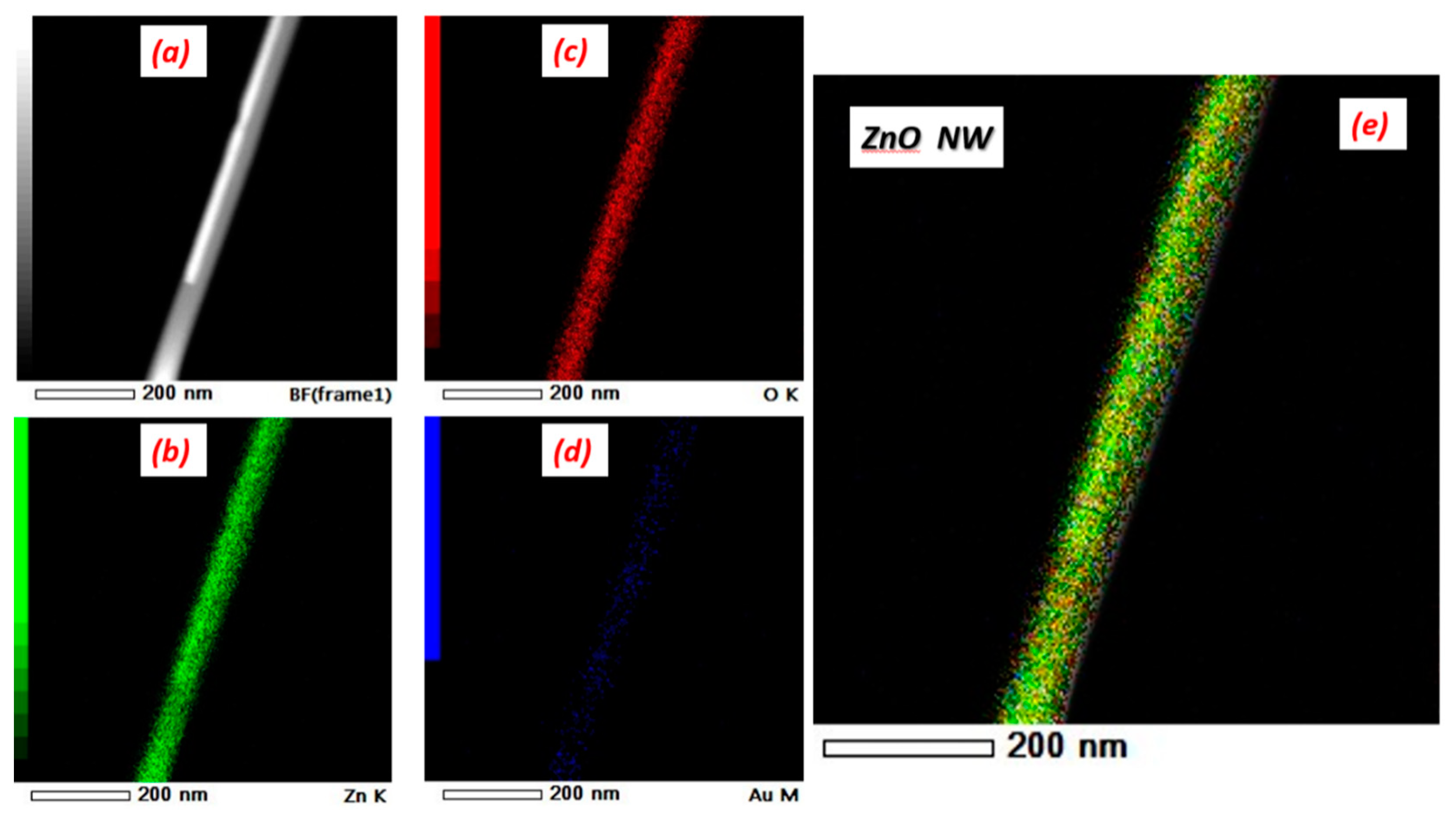
3.1. Surface Morphological Analysis
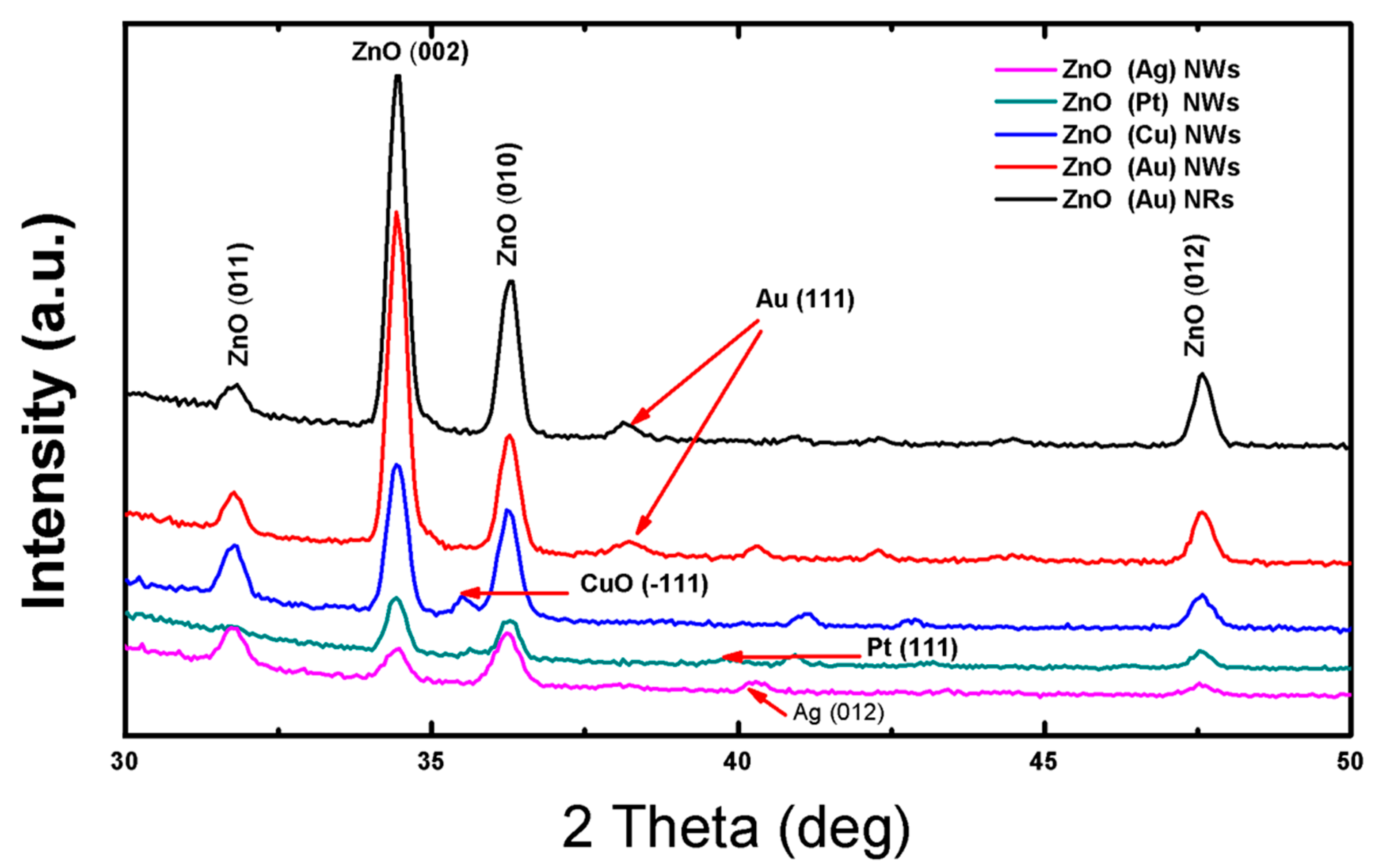
3.2. Structural Properties
3.3. Gas-Sensing Performace
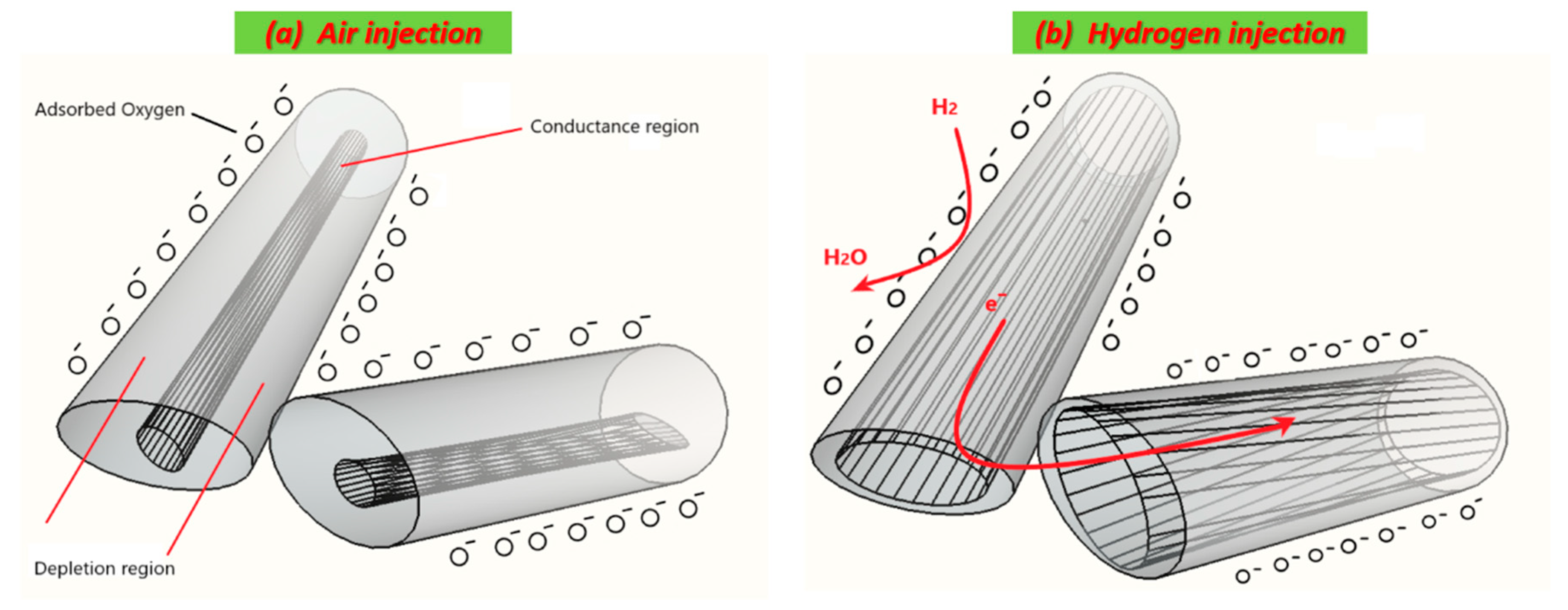
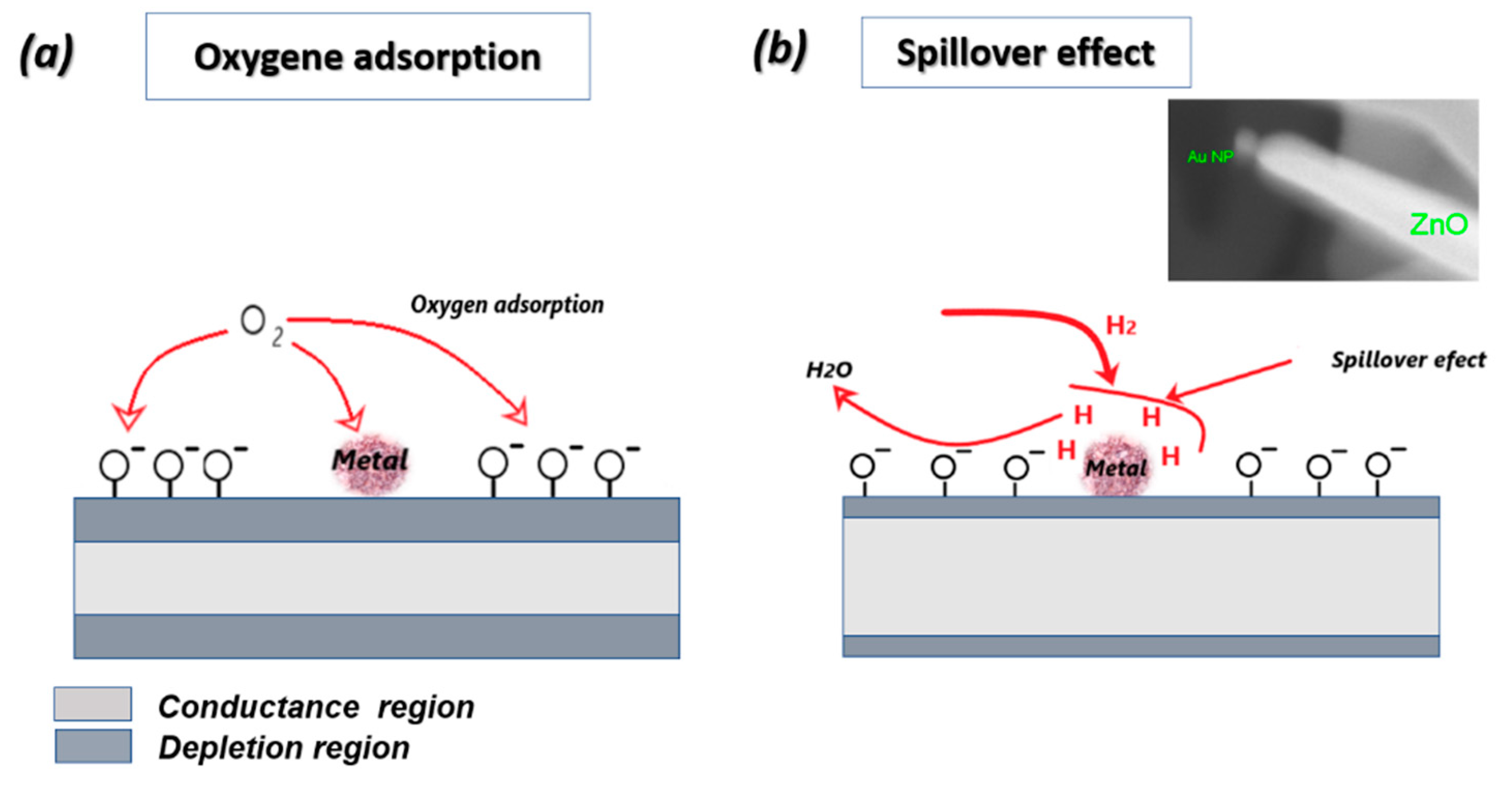
3.3.1. Working Principle
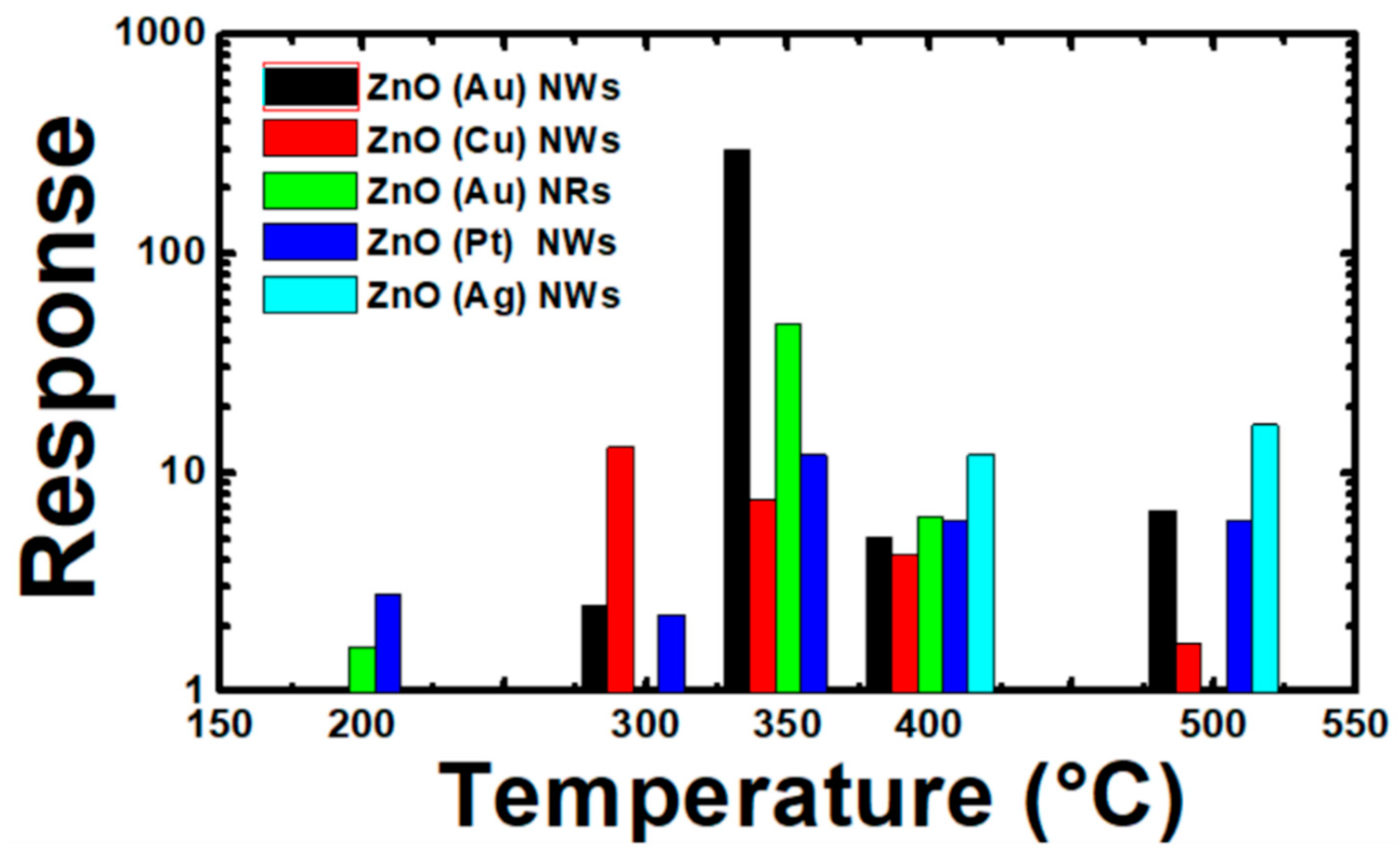
3.3.2. Catalyst Effect on Sensing Characteristics
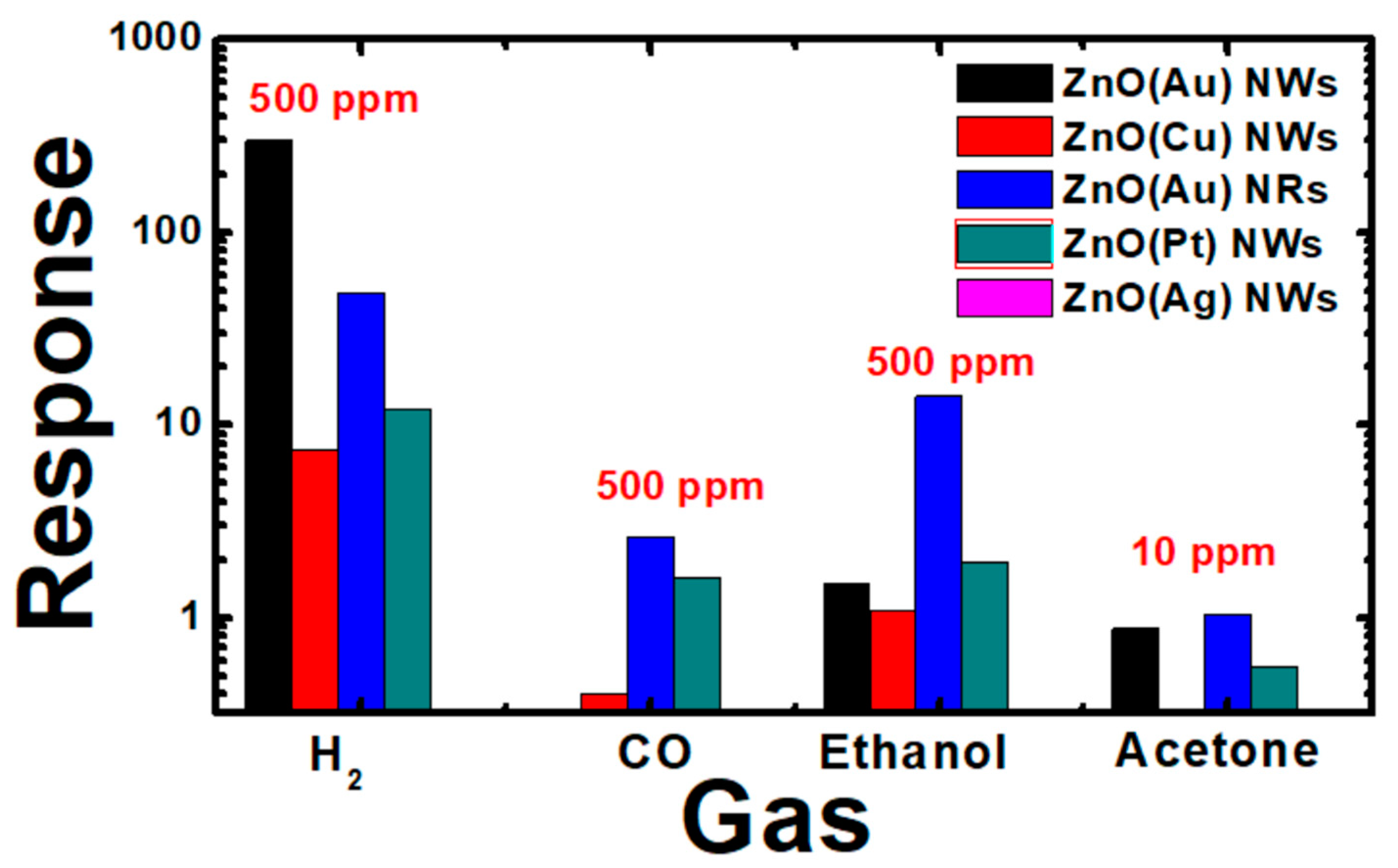
3.3.3. Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [Green Version]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal oxide nanostructures in food applications: Quality control and packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Xie, M.; Luo, J.; Kan, H.; Wei, Q. Nitric oxide sensors using nanospiral ZnO thin film deposited by GLAD for application to exhaled human breath. RSC Adv. 2020, 10, 14877–14884. [Google Scholar] [CrossRef] [Green Version]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- El khalidi, Z.; Comini, E.; Hartiti, B.; Moumen, A.; Munasinghe Arachchige, H.M.M.; Fadili, S.; Thevenin, P.; Kamal, A. Effect of vanadium doping on ZnO sensing properties synthesized by spray pyrolysis. Mater. Des. 2018, 139, 56–64. [Google Scholar] [CrossRef]
- Zappa, D.; Comini, E.; Sberveglieri, G. Thermally oxidized zinc oxide nanowires for use as chemical sensors. Nanotechnology 2013, 24, 444008. [Google Scholar] [CrossRef]
- Kaur, N.; Zappa, D.; Ferroni, M.; Poli, N.; Campanini, M.; Negrea, R.; Comini, E. Branch-like NiO/ZnO heterostructures for VOC sensing. Sens. Actuators B Chem. 2018, 262, 477–485. [Google Scholar] [CrossRef]
- Pargoletti, E.; Hossain, U.H.; Di Bernardo, I.; Chen, H.; Tran-Phu, T.; Lipton-Duffin, J.; Cappelletti, G.; Tricoli, A. Room-temperature photodetectors and VOC sensors based on graphene oxide-ZnO nano-heterojunctions. Nanoscale 2019, 11, 22932–22945. [Google Scholar] [CrossRef]
- Pargoletti, E.; Hossain, U.H.; Di Bernardo, I.; Chen, H.; Tran-Phu, T.; Chiarello, G.L.; Lipton-Duffin, J.; Pifferi, V.; Tricoli, A.; Cappelletti, G. Engineering of SnO2-Graphene Oxide Nano-Heterojunctions for Selective Room-temperature Chemical Sensing and Optoelectronic Devices. ACS Appl. Mater. Interfaces 2020, 12, 39549–39560. [Google Scholar] [CrossRef]
- Le, H.J.; Van Dao, D.; Yu, Y.T. Superfast and efficient hydrogen gas sensor using PdAualloy@ZnO core-shell nanoparticles. J. Mater. Chem. A 2020, 8, 12968–12974. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Bertuna, A.; Faglia, G.; Ferroni, M.; Kaur, N.; Arachchige, H.M.M.M.; Sberveglieri, G.; Comini, E. Metal oxide nanowire preparation and their integration into chemical sensing devices at the SENSOR lab in Brescia. Sensors 2017, 17, 1000. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.J. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002, 12, 323–331. [Google Scholar] [CrossRef]
- Yanagida, T.; Nagashima, K.; Tanaka, H.; Kawai, T. Mechanism of critical catalyst size effect on MgO nanowire growth by pulsed laser deposition. J. Appl. Phys. 2008, 104, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Zappa, D.; Melloni, R.; Maraloiu, V.-A.; Poli, N.; Rizzoni, M.; Sberveglieri, V.; Sisman, O.; Soprani, M.; Comini, E. Influence of Metal Catalyst on SnO2 Nanowires Growth and Gas Sensing Performance. Proceedings 2017, 1, 460. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, S.J.; Yu, T.; Wu, T. Controlling the growth mechanism of ZnO nanowires by selecting catalysts. J. Phys. Chem. C 2007, 111, 17500–17505. [Google Scholar] [CrossRef]
- Bruncko, J.; Michalka, M.; Kovac, J.; Andrej, J. A low-temperature limit for growth of ZnO nanowires by using of laser ablation processes. Appl. Phys. A 2020, 126, 1–7. [Google Scholar] [CrossRef]
- El khalidi, Z.; Hartiti, B.; Siadat, M.; Comini, E.; Arachchige, H.M.M.M.; Fadili, S.; Thevenin, P. Acetone sensor based on Ni doped ZnO nanostructues: Growth and sensing capability. J. Mater. Sci. Mater. Electron. 2019, 30, 7681–7690. [Google Scholar] [CrossRef]
- Moumen, A.; Hartiti, B.; Comini, E.; El khalidi, Z.; Arachchige, H.M.M.M.; Fadili, S.; Thevenin, P. Preparation and characterization of nanostructured CuO thin films using spray pyrolysis technique. Superlattices Microstruct. 2019, 127, 2–10. [Google Scholar] [CrossRef]
- Sangpour, P.; Roozbehi, M.; Akhavan, O.; Moshfegh, A. ZnO Nanowires from Nanopillars: Influence of Growth Time. Curr. Nanosci. 2012, 5, 479–484. [Google Scholar] [CrossRef]
- Labidi, A.; Lambert-Mauriat, C.; Jacolin, C.; Bendahan, M.; Maaref, M.; Aguir, K. dc and ac characterizations of WO3 sensors under ethanol vapors. Sens. Actuators B Chem. 2006, 119, 374–379. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta. 2006, 568, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Sett, D.; Basak, D. Highly enhanced H2 gas sensing characteristics of Co:ZnO nanorods and its mechanism. Sens. Actuators B Chem. 2017, 243, 475–483. [Google Scholar] [CrossRef]
- Choo, T.F.; Saidin, N.U.; Kok, K.Y. Hydrogen sensing enhancement of zinc oxide nanorods via voltage biasing. R. Soc. Open Sci. 2018, 5, 172372. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Y.; Wu, S.H.; Yin, Y.T. Catalyst-free growth of vertical alignment ZnO nanowire arrays by a Two-Stage Process. J. Phys. Chem. C 2009, 113, 21572–21576. [Google Scholar] [CrossRef]
- Hannon, J.B.; Kodambaka, S.; Ross, F.M.; Tromp, R.M. The influence of the surface migration of gold on the growth of silicon nanowires. Nature 2006, 440, 69–71. [Google Scholar] [CrossRef]
- Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Phanichphant, S. Sensing characteristics of flame-spray-made Pt/ZnO thick films as H2 gas sensor. Sensors 2009, 9, 6652–6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Bahariqushchi, R.; Cosentino, S.; Scuderi, M.; Dumons, E.; Tran-huu-hue, L.P. Nanoscale Free carrier enhanced depletion in ZnO nanorods decorated with bimetallic AuPt nanoclusters. Nanoscale 2020, 16. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Zhou, P.; Zhong, X.; Li, G.; Han, C.; Wei, D.; Li, S. Bimetallic Au/Pd nanoparticles decorated ZnO nanowires for NO2 detection. Sens. Actuators B Chem. 2019, 289, 160–168. [Google Scholar] [CrossRef]
- Liao, T.W.; Verbruggen, S.W.; Claes, N.; Yadav, A.; Grandjean, D.; Bals, S.; Lievens, P. TiO2 films modified with au nanoclusters as self-cleaning surfaces under visible light. Nanomaterials 2018, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Najim, S.A.; Jamil, N.Y.; Muhammed, K.M. Effect of Au dopant on the structural and optical properties of ZnO thin films prepared by CVD. J. Nano Electron. Phys. 2019, 11. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Fan, J.; Ma, X.; Wei, Z.; Zou, Y.; Xu, Y. Study of solid solubility of gold doped in ZnO films and its effect on optical properties. In Proceedings of the 2015 International Conference on Optoelectronics and Microelectronics (ICOM), Changchun, China, 16–18 July 2015; pp. 407–410. [Google Scholar]
- Katoch, A.; Choi, S.W.; Kim, H.W.; Kim, S.S. Highly sensitive and selective H2 sensing by ZnO nanofibers and the underlying sensing mechanism. J. Hazard. Mater. 2015, 286, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Drmosh, Q.A.; Yamani, Z.H. Synthesis, characterization, and hydrogen gas sensing properties of AuNs-catalyzed ZnO sputtered thin films. Appl. Surf. Sci. 2016, 375, 57–64. [Google Scholar] [CrossRef]
- Kim, H.W.; Na, H.G.; Kwak, D.S.; Cho, H.Y.; Kwon, Y.J. Enhanced gas sensing characteristics of Ag2O-functionalized networked In2O3 nanowires. Jpn. J. Appl. Phys. 2013, 52, 10MD01. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, J.; Yao, P.; Li, J.; Bi, H.; Wang, X. Synthesis of ZnO-Ag Hybrids and Their Gas-Sensing Performance toward Ethanol. Ind. Eng. Chem. Res. 2015, 54, 8947–8953. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; MacCato, C.; Sberveglieri, G.; Tondello, E. 1D ZnO nano-assemblies by Plasma-CVD as chemical sensors for flammable and toxic gases. Sens. Actuators B Chem. 2010, 149, 1–7. [Google Scholar] [CrossRef]
- Baratto, C.; Rigoni, F.; Faglia, G.; Comini, E.; Zappa, D.; Sberveglieri, G. ZnO and SnO2 one-dimensional sensors for detection of hazardous gases. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Qurashi, A.; Tabet, N.; Faiz, M.; Yamzaki, T. Ultra-fast microwave synthesis of ZnO nanowires and their dynamic response toward hydrogen gas. Nanoscale Res. Lett. 2009, 4, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wong, M.H.; Man, H.C.; Ong, C.W. Resistive hydrogen sensing response of Pd-decorated ZnO “nanosponge” film. Sens. Actuators B Chem. 2017, 249, 624–631. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, S.S.; Nayak, M.; Maity, A.; Majumder, S.B.; Bhattacharya, S. Hydrogen sensing based on nanoporous silica-embedded ultra dense ZnO nanobundles. RSC Adv. 2014, 4, 7476–7482. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Int. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- Tonezzer, M.; Iannotta, S. H2 sensing properties of two-dimensional zinc oxide nanostructures. Talanta 2014, 122, 201–208. [Google Scholar] [CrossRef]
- Bie, L.J.; Yan, X.N.; Yin, J.; Duan, Y.Q.; Yuan, Z.H. Nanopillar ZnO gas sensor for hydrogen and ethanol. Sens. Actuators B Chem. 2007, 126, 604–608. [Google Scholar] [CrossRef]
- Ren, S.; Fan, G.; Qu, S.; Wang, Q. Enhanced H2 sensitivity at room temperature of ZnO nanowires functionalized by Pd nanoparticles. J. Appl. Phys. 2011, 110, 084312. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Hazra, S.K.; Basu, S. Hydrogen sensitivity of ZnO p-n homojunctions. Sens. Actuators B Chem. 2006, 117, 177–182. [Google Scholar] [CrossRef]
- Al-Salman, H.S.; Abdullah, M.J.; Al-Hardan, N. ZnO thin film nanostructures for hydrogen gas sensing applications. Ceram. Int. 2013, 39, S447–S450. [Google Scholar] [CrossRef]
- Teimoori, F.; Khojier, K.; Dehnavi, N.Z. On the Dependence of H2 Gas Sensitivity of ZnO thin Films on Film Thickness. Procedia Mater. Sci. 2015, 11, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Bhati, V.S.; Ranwa, S.; Fanetti, M.; Valant, M.; Kumar, M. Efficient hydrogen sensor based on Ni-doped ZnO nanostructures by RF sputtering. Sens. Actuators B Chem. 2018, 255, 588–597. [Google Scholar] [CrossRef]



| Catalyst | Ar Flow (SCCM) | Pressure (10−3 mbar) | Magnetron Power (W) | Deposition Time (s) |
|---|---|---|---|---|
| Gold (Au) | 7 | 5 | 75 | 5 |
| Platinum (Pt) | 7 | 5 | 75 | 2 |
| Silver (Ag) | 7 | 5 | 50 | 5 |
| Cooper (Cu) | 7 | 5 | 50 | 15 |
| Sample | Crystallite Size (nm) | Average ZnO Length (nm) |
|---|---|---|
| ZnO (Au) NWs | 22 | 772 ± 47 |
| ZnO (Au) NRs | 22 | 442 ± 11 |
| ZnO (Pt) NWs | 30 | 840 ± 30 |
| ZnO (Ag) NWs | 31 | 342.1 ± 9.8 |
| ZnO (Cu) NWs | 24 | ≈4000 |
| Sample | Diffraction Peaks | ||
|---|---|---|---|
| (010) | (002) | (011) | |
| ZnO (Au) NWs | 0.41 | 2.18 | 0.39 |
| ZnO (Au) NRs | 0.67 | 1.78 | 0.53 |
| ZnO (Pt) NWs | 0.80 | 1.53 | - |
| ZnO (Ag) NWs | 1.18 | 1.17 | 0.64 |
| ZnO (Cu) NWs | 0.75 | 1.67 | 0.57 |
| Material | Technique | Temperature (°C) | Response/H2 (ppm) | Ref. |
|---|---|---|---|---|
| 1D ZnO nano-assemblies | PE-CVD | 400 | 13/5000 | [40] |
| 1D ZnO NWs | VLS process | 400 | 90/300 | [41] |
| ZnO Nanowires | Ultra-fast Microwave | 250 | 0.95/500 | [42] |
| Pd-decorated ZnO “nanosponge” | Supersonic cluster beam deposition (SCBD) | UV illumination, 20 °C | 85/2% | [43] |
| ZnO nanobundles | nano-templating technique | 350 | 20%/- | [44] |
| ZnO nanowires | electrochemical anodization | 400 | 11.26/1000 | [45] |
| Vanadium- doped ZnO thin film | Spray pyrolysis | 300 | 55/500 | [6] |
| ZnO two-dimensional nanostructures | thermal oxidation | 175 | 5.37/200 | [46] |
| Nanopillar ZnO | Two-step solution approach | 350 | 28/2500 | [47] |
| NPs-decorated networked ZnO NWs | Chemical vapor deposition (CVD) | Room temperature | 4,6 (460%)/1000 | [48] |
| ZnO NWs @ZIF-8 | Vapor phase growth + Solvothermal | 300 | 1.44/50 | [49] |
| ZnO nanorods | facile one-pot galvanic-assisted technique | Room temperature | 33/2000 | [26] |
| p–n junction of ZnO thin films | D.C. sputtering technique + CVD | 400 | 1.2/1000 | [50] |
| ZnO thin films | Magnetron sputtering | 350 | 98%/200 | [51] |
| ZnO thin films | e-beam evaporation | 400 | 59/40 | [52] |
| Ni-doped ZnO thin film | RF sputtering | 150 | ∼69%/10,000 | [53] |
| Co:ZnO nanorods | hydrothermal method | 150 | 53.7%/3000 | [25] |
| ZnO nanowires | VLS | 350 | 300 (30,000%)/500 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moumen, A.; Kaur, N.; Poli, N.; Zappa, D.; Comini, E. One Dimensional ZnO Nanostructures: Growth and Chemical Sensing Performances. Nanomaterials 2020, 10, 1940. https://doi.org/10.3390/nano10101940
Moumen A, Kaur N, Poli N, Zappa D, Comini E. One Dimensional ZnO Nanostructures: Growth and Chemical Sensing Performances. Nanomaterials. 2020; 10(10):1940. https://doi.org/10.3390/nano10101940
Chicago/Turabian StyleMoumen, Abderrahim, Navpreet Kaur, Nicola Poli, Dario Zappa, and Elisabetta Comini. 2020. "One Dimensional ZnO Nanostructures: Growth and Chemical Sensing Performances" Nanomaterials 10, no. 10: 1940. https://doi.org/10.3390/nano10101940







