Pore- and Core-Scale Insights of Nanoparticle-Stabilized Foam for CO2-Enhanced Oil Recovery
Abstract
1. Introduction
2. Materials and Procedures
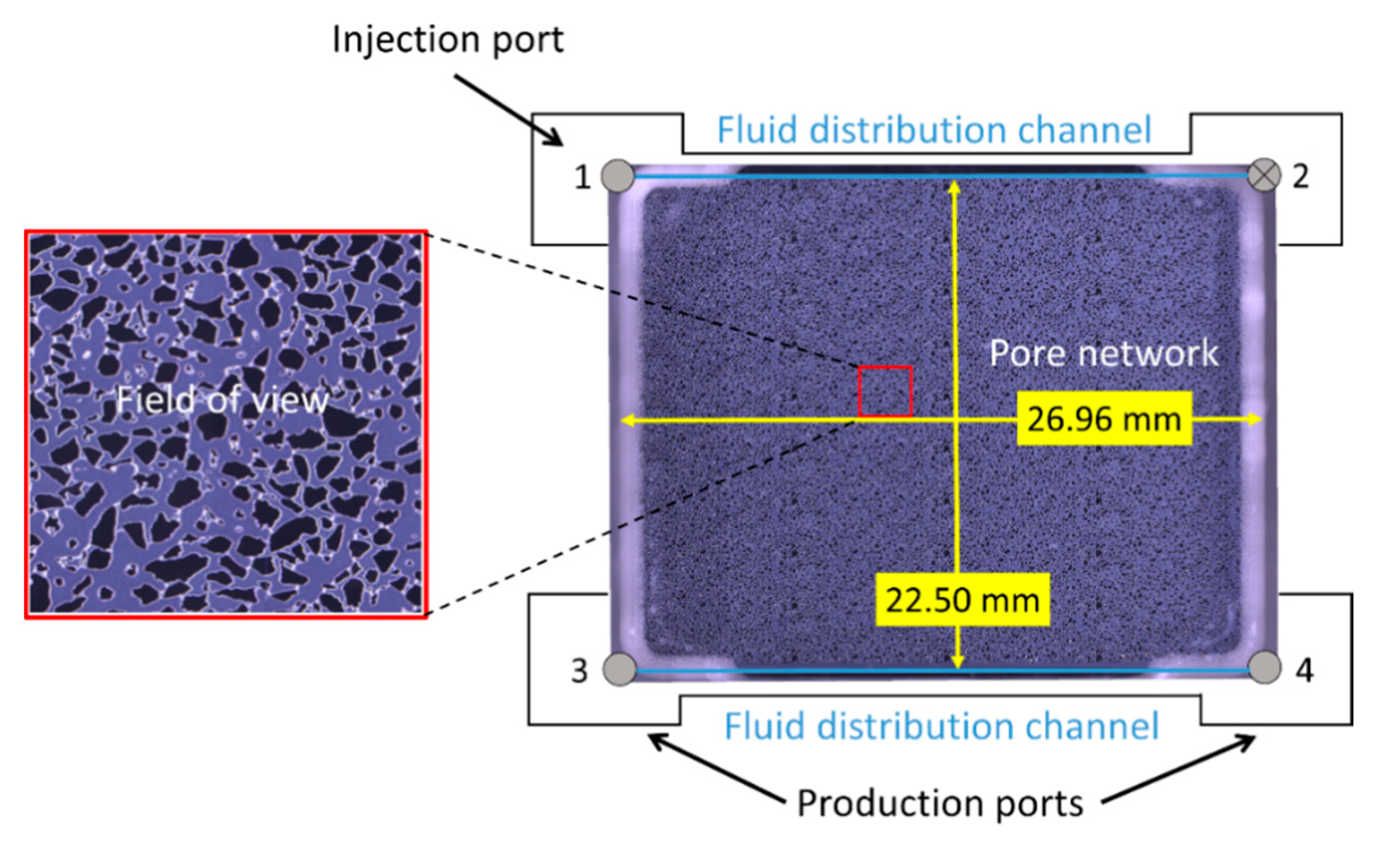
2.1. Pore-Scale System
2.2. Pore-Scale Procedure
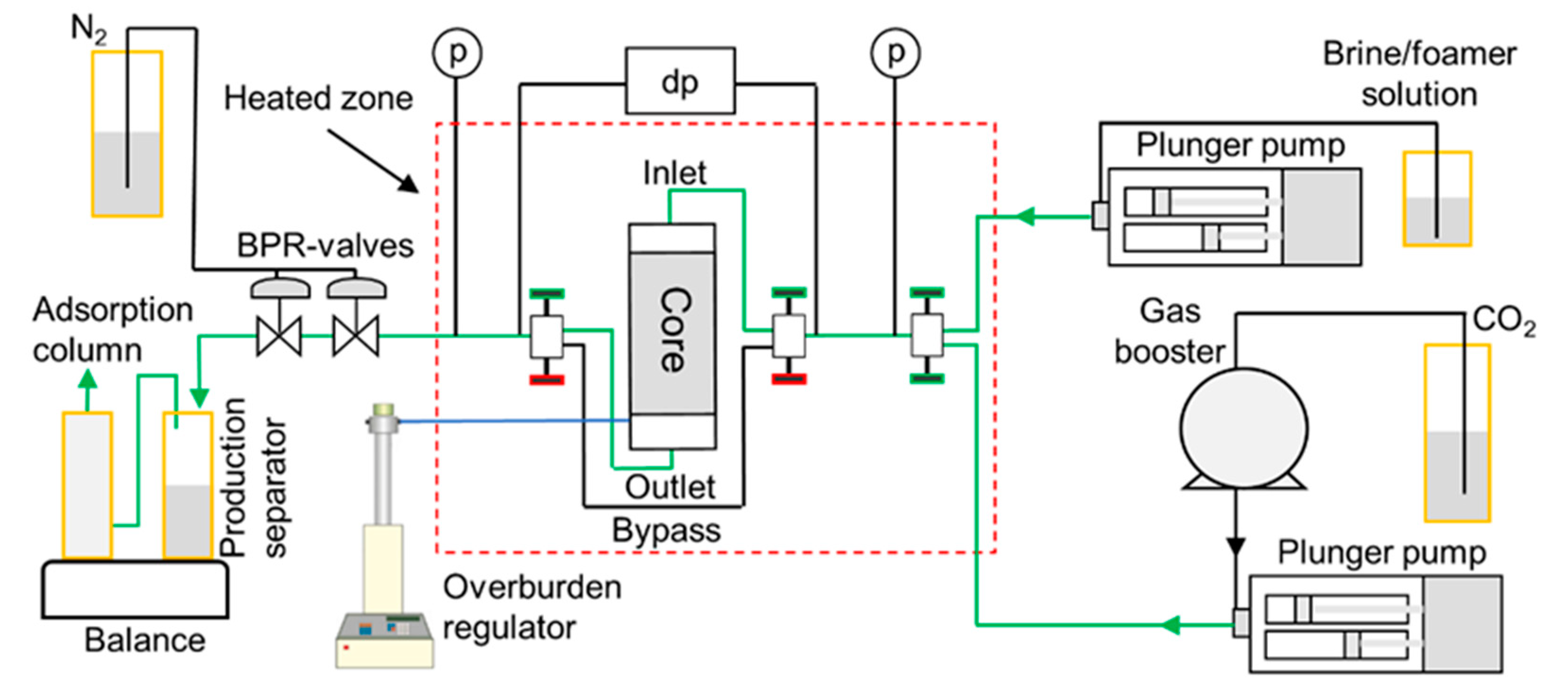
2.3. Core-Scale System
2.4. Core-Scale Procedure
3. Results and Discussion
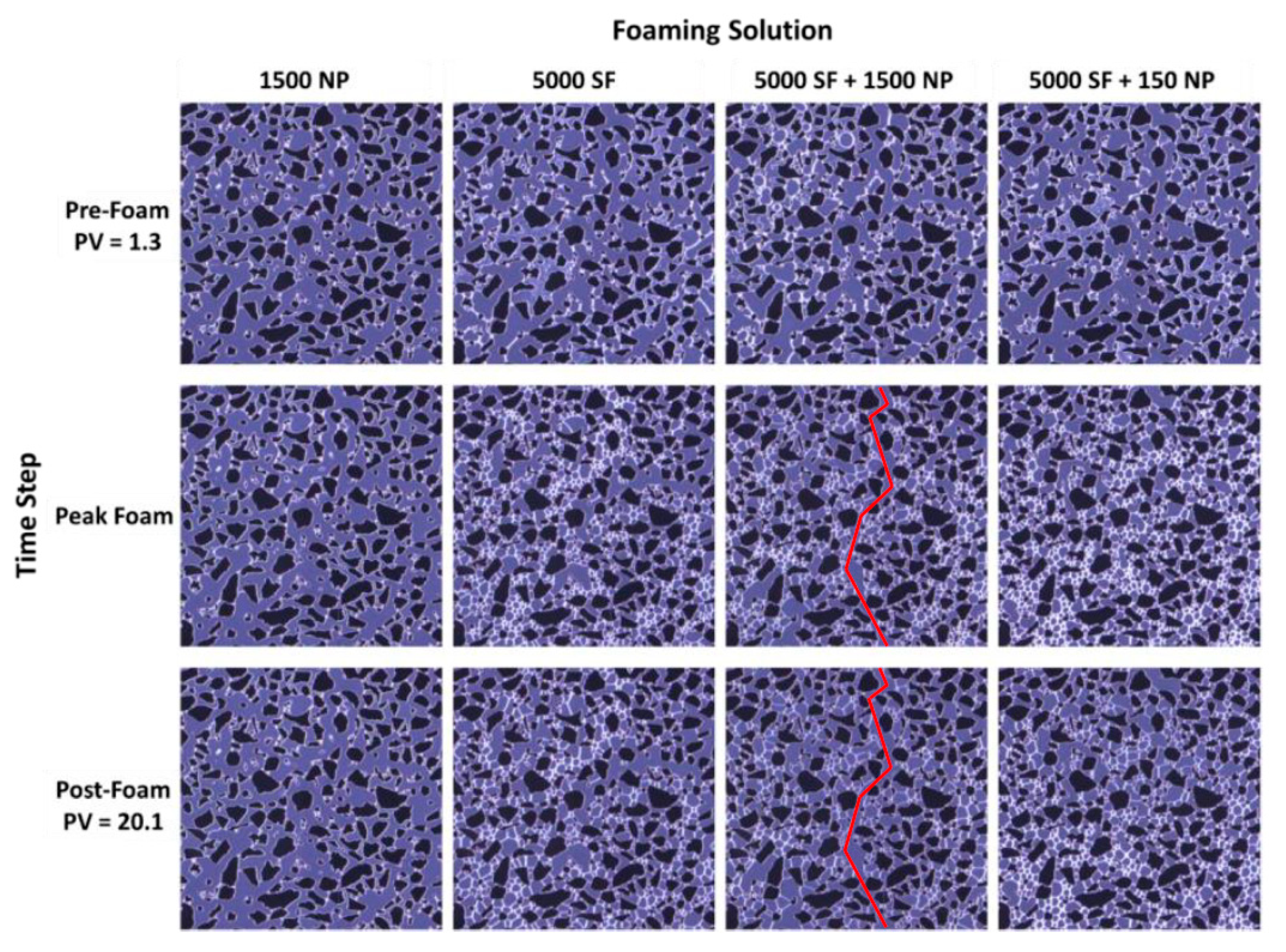
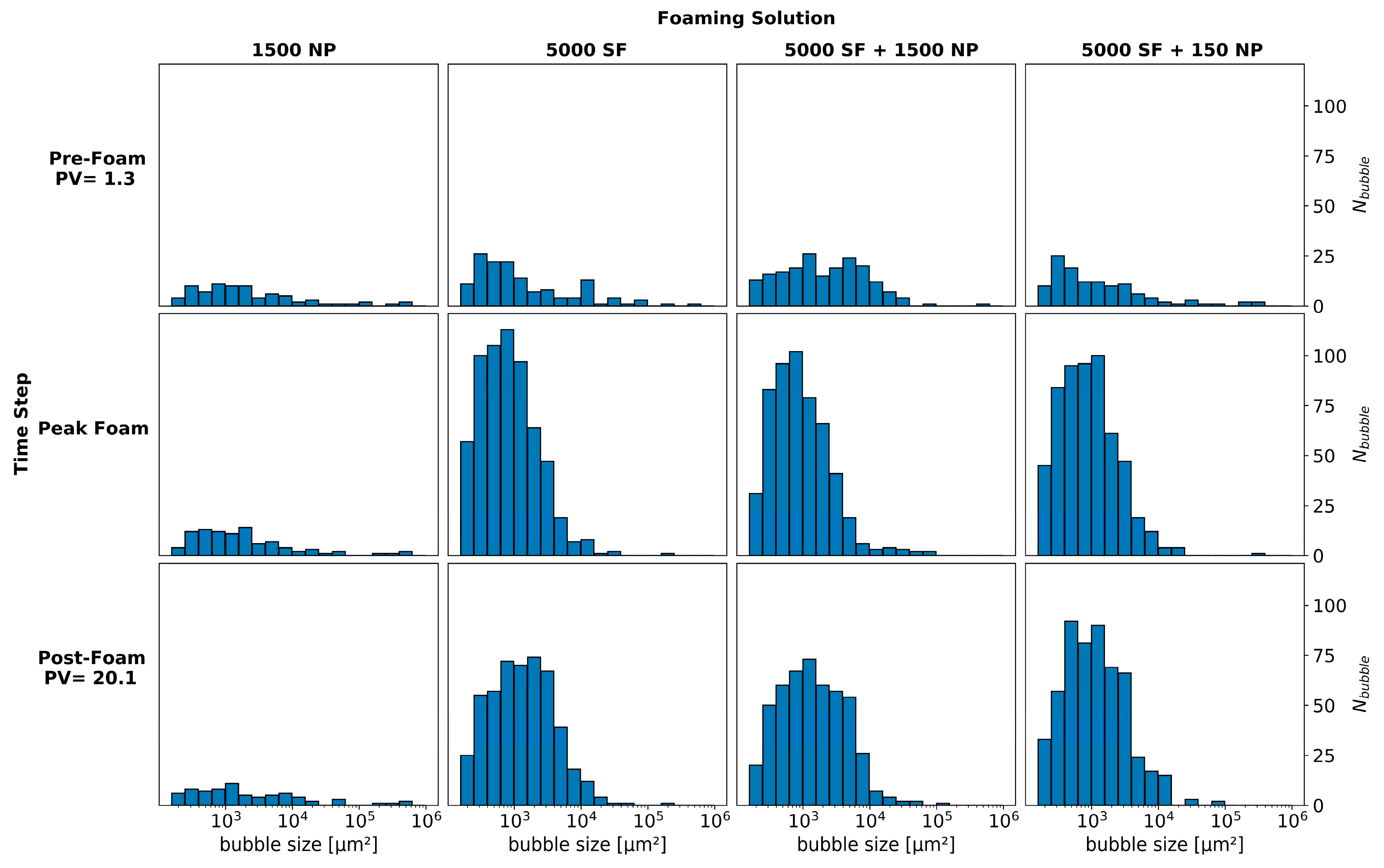
3.1. Pore-Scale: Foam in the Absence of Oil
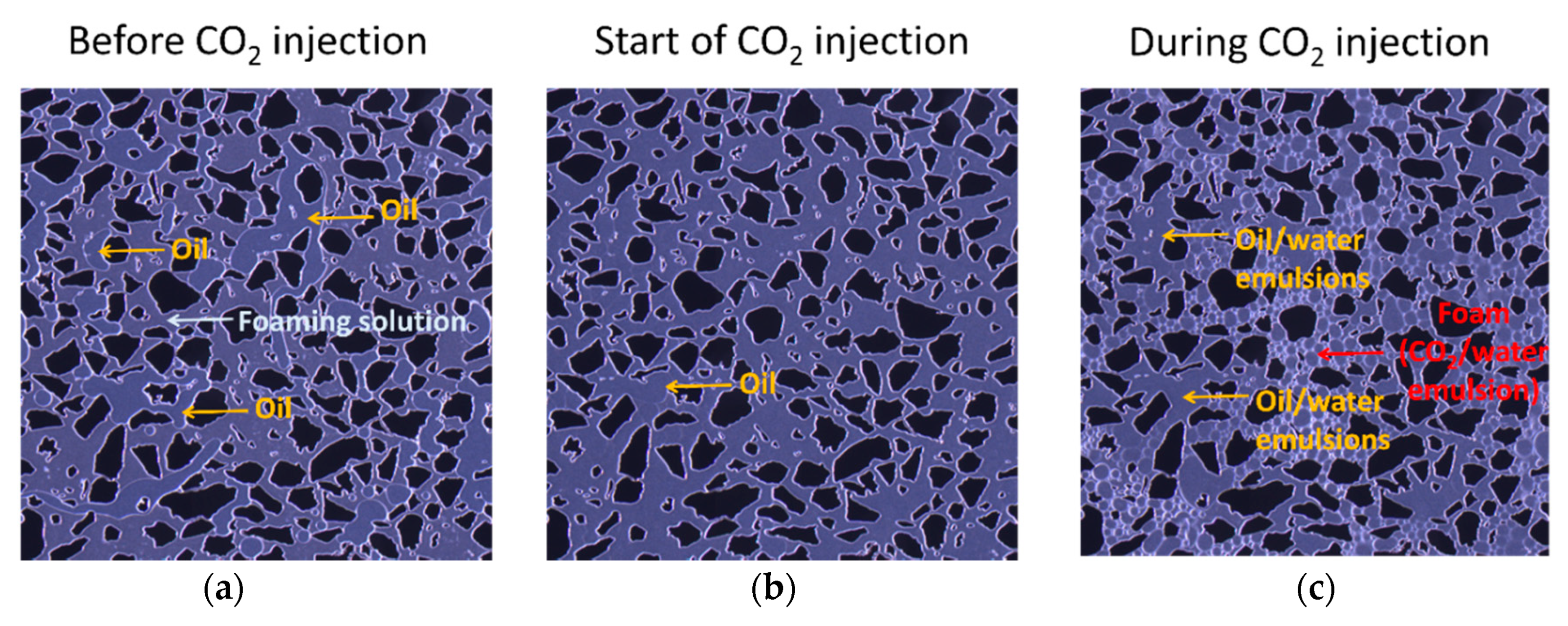
3.2. Pore-Scale: Foam in the Presence of Oil
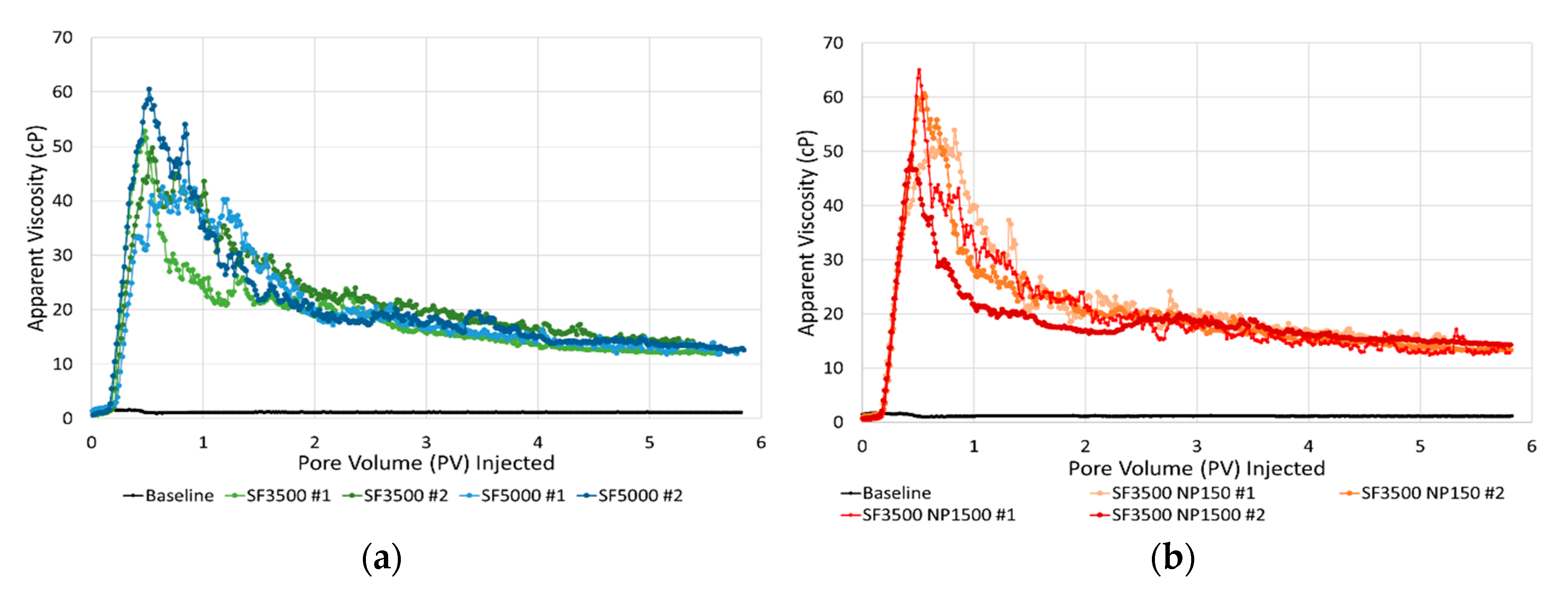
3.3. Core-Scale: Foam in the Absence of Oil
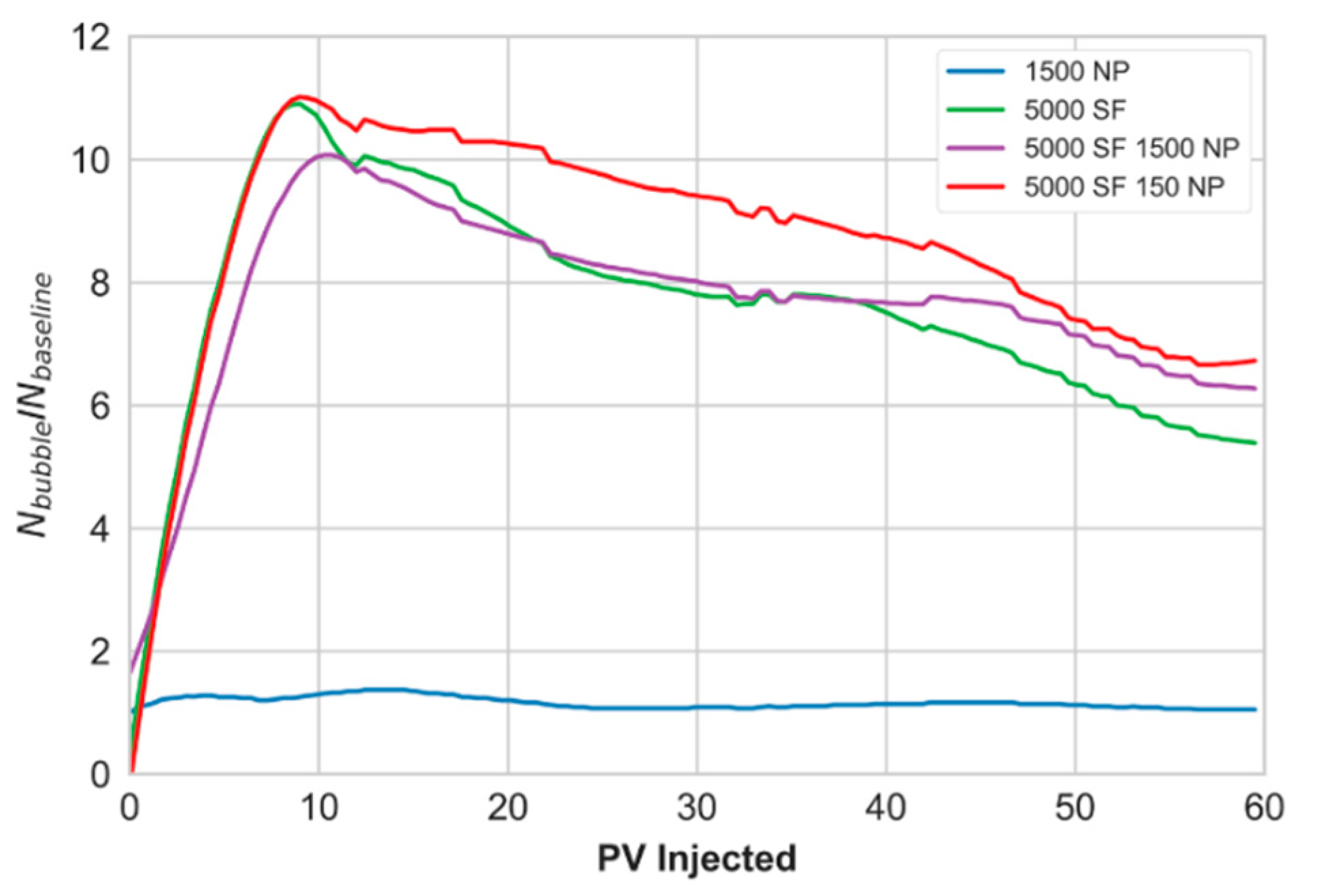
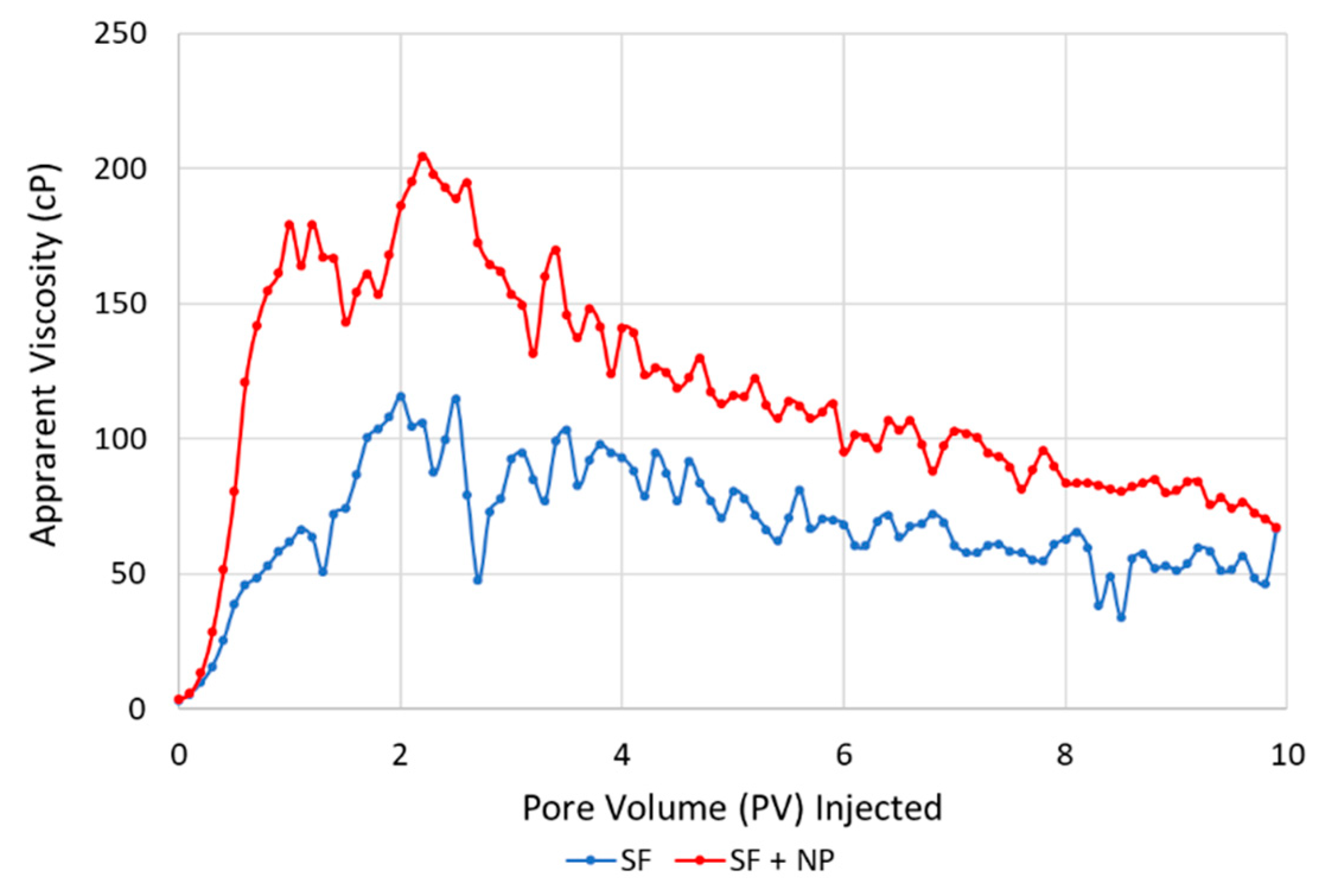
3.4. Core-Scale: Foam in the Presence of Oil
3.5. From Pore- to Core-Scale
4. Conclusions
- Direct pore-scale observations of dense phase CO2 injection into a micromodel saturated with foaming solutions containing only surfactant or a hybrid nanoparticle–surfactant foaming solution revealed snap-off as the primary foam generation mechanism and leave-behind as a secondary foam generation mechanism.
- At the pore-scale, foam readily generated in areas where oil was not present and oil/water emulsions initially occupied pores without foam present.
- All foaming solutions containing surfactant generated foam in the presence and absence of oil, whereas foaming solution only containing nanoparticles did not. Thus, surfactant was the main foam generator and nanoparticles may be more important for foam stabilization.
- Foam strength was not sensitive to nanoparticle concentration when used together with surfactant in the tested foaming solutions.
- At the core-scale, all foaming solutions rapidly generated foam in the presence of residual oil.
- Foam apparent viscosity values with the hybrid foaming solution, in the presence of oil, were nearly three times as high as the experiments without oil. This was related to the development of oil/water emulsions, which were likely stabilized by the foaming agents.
- A link is proposed between direct pore-scale visual observations and quantitative core-scale measurements. The combined influence of stable oil/water emulsions and foam (CO2/water emulsions) may be beneficial for increasing the capillary number by achieving higher apparent viscosity and lower interfacial tension.
- The experiments in this work were characterized by a period of rapid foam generation during drainage-like CO2 injection and a period of foam coalescence during prolonged CO2 injection. The decline in foam strength is related to the development of open CO2 flow paths through the generated foam.
- Increased apparent viscosities with foam reduced CO2 mobility at multiple length scales, which can improve volumetric sweep efficiency in field-scale CO2 EOR and CO2 storage processes.
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2014: Chapter 1: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Victor, D.G., Zhou, D., Ahmed, E.H.M., Dadhich, P.K., Olivier, J.G.J., Rogner, H.-H., Sheikho, K., Yamaguchi, M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Enick, R.M.; Olsen, D.K.; Ammer, J.R.; Schuller, W. Mobility and Conformance Control for CO2 EOR via Thickeners, Foams, and Gels—A Detailed Literature Review of 40 Years of Research; DOE/NETL-2012/1540; USDOE, National Energy Technology Laboratory: Washington, DC, USA, 2012.
- Hoefner, M.L.; Evans, E.M. CO2 Foam: Results from Four Developmental Field Trials. SPE Reserv. Eng. 1995, 10, 273–281. [Google Scholar] [CrossRef]
- Martin, F.D.; Stevens, J.E.; Harpole, K.J. CO2-Foam Field Test at the East Vacuum Grayburg/San Andres Unit. SPE Reserv. Eng. 1995, 10, 266–272. [Google Scholar] [CrossRef]
- Rossen, W.R. Foams in Enhanced Oil Recovery. In Foams Theory, Measurements, and Applications; Prud’homme, R.K., Khan, S.A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; Volume 57, Chapter 11, pp. 414–464. [Google Scholar]
- David, A.; Marsden, S.S. The Rheology of Foam. In Proceedings of the 44th Annual Fall Meeting of SPE of AIME, Denver, CO, USA, 28 September–1 October 1969. [Google Scholar] [CrossRef]
- Espinosa, D.; Caldelas, F.; Johnston, K.; Bryant, S.L.; Huh, C. Nanoparticle-Stabilized Supercritical CO2 Foams for Potential Mobility Control Applications. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Rognmo, A.U.; Al-Khayyat, N.; Heldal, S.; Vikingstad, I.; Eide, Ø.; Fredriksen, S.B.; Alcorn, Z.P.; Graue, A.; Bryant, S.L.; Kovscek, A.R.; et al. Performance of Silica Nanoparticles in CO2-Foam for EOR and CCUS at Tough Reservoir Conditions. In Proceedings of the SPE Norway One-Day Seminar, Bergen, Norway, 18 April 2019. [Google Scholar]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced Oil Recovery Using Nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar]
- Yu, J.; Wang, S.; Liu, N.; Lee, R. Study of Particle Structure and Hydrophobicity Effects on the Flow Behavior of Nanoparticle-Stabilized CO2 Foam in Porous Media. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Bennetzen, M.; Mogensen, K. Novel Applications of Nanoparticles for Future Enhanced Oil Recovery. In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 10–12 December 2014. [Google Scholar]
- Ettinger, R.A.; Radke, C.J. Influence of Texture on Steady Foam Flow in Berea Sandstone. SPE Reserv. Eng. 1992, 7, 83–90. [Google Scholar] [CrossRef]
- Chen, M.; Yortos, Y.C.; Rossen, W.R. A Pore-Network Study of the Mechanisms of Foam Generation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. SPE-90939. [Google Scholar]
- Farajzadeh, R.; Andrianov, A.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam–oil interaction in porous media: Implications for foam assisted enhanced oil recovery. Adv. Colloid Interface Sci. 2012, 183, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Harkins, W.D.; Feldman, A. Films: The spreading of liquids and the spreading coefficient. J. Am. Chem. Soc. 1922, 44, 2665–2685. [Google Scholar] [CrossRef]
- Schramm, L.L.; Novosad, J.J. Micro-visualization of Foam Interactions with Crude Oil. Colloids Surf. 1990, 46, 21–43. [Google Scholar] [CrossRef]
- Marsden, S.S. Foams in Porous Media—SUPRI TR-49; U.S. Department of Energy Topical Report; 1986. Available online: https://www.osti.gov/biblio/5866567 (accessed on 27 June 2020).
- Talebian, S.H.; Masoudi, R.; Tan, I.M.; Zitha, P.L.J. Foam Assisted CO2 EOR; Concepts, Challenges and Applications. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013. [Google Scholar]
- Farajzadeh, R. Enhanced Transport Phenomena in CO2 Sequestration and CO2 EOR. Ph.D. Thesis, Faculty of Civil Engineering and Geosciences, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Kristiansand, T.S.; Holt, T. Properties of Flowing Foam in Porous media Containing Oil. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 22–24 April 1992. [Google Scholar]
- Kovscek, A.R.; Radke, C.J. Fundamentals of Foam Transport in Porous Media. In Foams: Fundamentals and Applications in the Petroleum Industry; American Chemical Society: Washington, DC, USA, 1994; pp. 115–163. [Google Scholar]
- Saint-Jalmes, A. Physical chemistry in foam drainage and coarsening. Soft Matter 2006, 2, 836–849. [Google Scholar] [CrossRef]
- Marchalot, J.; Lambert, J.; Cantat, I.; Tabeling, P.; Jullien, M.-C. 2D foam coarsening in a microfluidic system. EPL (Europhys. Lett.) 2008, 83, 64006. [Google Scholar] [CrossRef]
- Falls, A.; Musters, J.; Ratulowski, J. The Apparent Viscosity of Foams in Homogeneous Bead Packs. SPE Reserv. Eng. 1989, 4, 155–164. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Lotfollahi, M.; Eftekhari, A.A.; Rossen, W.R.; Hirasaki, G.J.H. Effect of Permeability on Implicit-Texture Foam Model Parameters and the Limiting Capillary Pressure. Energy Fuels 2015, 29, 3011–3018. [Google Scholar] [CrossRef]
- Buchgraber, M.; Al-Dossary, M.; Ross, C.M.; Kovscek, A.R. Creation of a dual-porosity micromodel for pore-level visualization of multiphase flow. J. Pet. Sci. Eng. 2012, 86, 27–38. [Google Scholar] [CrossRef]
- Hornbrook, J.W.; Castanier, L.M.; Pettit, P.A. Observation of Foam/Oil Interactions in a New, High-Resolution Micromodel. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 6–9 October 1991; Society of Petroleum Engineers: Houston, TX, USA, 1991. [Google Scholar]
- Benali, B. Quantitative Pore-Scale Analysis of CO2 Foam for CCUS. Master’s Thesis, University of Bergen, Bergen, Norway, December 2019. Available online: http://hdl.handle.net/1956/21300 (accessed on 27 June 2020).
- Bradski, G. The OpenCV Library. Dr. Dobb’s J. Softw. Tools 2000, 25, 120–125. [Google Scholar]
- Føyen, T.; Alcorn, Z.P.; Fernø, M.A.; Holt, T. 2020 CO2 Mobility Reduction Using Foam Stabilized by CO2- and Water-Soluble Surfactants. J. Pet. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Lawson, J.B. Mechanisms of Foam Flow in Porous Media: Apparent Viscosity in Smooth Capillaries. SPE J. 1985, 25, 176–190. [Google Scholar] [CrossRef]
- Jones, S.A.; Laskaris, G.; Vincent-Bonnieu, S.; Farajzadeh, R. Surfactant Effect on Foam: From Core Flood Experiments to Implicit-Texture Foam- Model Parameters. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. SPE-179637. [Google Scholar] [CrossRef]
- Føyen, T.; Brattekås, B.; Fernø, M.; Barrabino, A.; Holt, T. Increased CO2 storage capacity using CO2-foam. Int. J. Greenh. Gas Control 2020, 96, 103016. [Google Scholar] [CrossRef]
- Chambers, K.T.; Radke, C.J. Interfacial Phenomena is Oil Recovery; Morrow, N.R., Ed.; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Ranshoff, T.C.; Radke, C.J. Mechanics of Foam Generation in Glass Bead Packs. SPE Reserv. Eng. 1988, 3, 573–585. [Google Scholar] [CrossRef]
- Rossen, W.R. Theory of mobilization pressure gradient of flowing foams in porous media: III. Asymmetric lamella shapes. J. Colloids Interface Sci. 1990, 136, 38–53. [Google Scholar] [CrossRef]
- Falls, A.H.; Hirasaki, G.J.; Patzek, T.W.; Gauglitz, D.A.; Millerand, D.D.; Ratulowski, T. Development of a Mechanistic Foam Simulator: The Population Balance and Generation by Snap-Off. SPE Reserv. Eng. 1988, 3, 884–892. [Google Scholar] [CrossRef]
- Kovscek, A.R.; Patzek, T.W.; Radke, C.J. A Mechanistic Population Balance Model for Transient and Steady-State Foam Flow in Boise Sandstone. Chem. Eng. Sci. 1995, 50, 3783–3799. [Google Scholar] [CrossRef]
- Simjoo, M.; Zitha, P.L.J. Modeling and Experimental Validation of Rheological Transition during Foam Flow in Porous Media. Transp. Porous Media 2020, 131, 315–332. [Google Scholar] [CrossRef]
- Ma, K.; Ren, G.; Mateen, K.; Moreland, D.; Cordelier, P. Modeling techniques for foam flow through porous media. SPE J. 2015, 20, 453–470. [Google Scholar] [CrossRef]
- Gauteplass, J.; Follesø, H.N.; Graue, A.; Kovscek, A.R.; Fernø, M.A. Visualization of pore-level displacement mechanisms during CO2 injection and EOR processes. In Proceedings of the EAGE IOR—17th European Symposium on Improved Oil Recovery, St. Petersburg, Russia, 16–18 April 2013. [Google Scholar]
- Simjoo, M.; Dong, Y.; Andrianov, A.; Talanana, M.; Zitha, P.L.J. A CT Scan Study of Immiscible Foam Flow in Porous Media for EOR. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar]
| Foaming Agents | Concentration, Component | Scale |
|---|---|---|
| Nanoparticle (NP) | 1500 ppm, Levasil CC301 | Pore |
| Surfactant (SF) | 3500 ppm, Surfonic L24-22 | Core |
| 5000 ppm, Surfonic L24-22 | Pore and Core | |
| Hybrid (SF + NP) | 3500 ppm, Surfonic L24-22 + 1500 ppm, Levasil CC301 | |
| 5000 ppm, Surfonic L24-22 + 1500 ppm, Levasil CC301 | Pore | |
| 5000 ppm, Surfonic L24-22 + 150 ppm, Levasil CC301 | ||
| 3500 ppm, Surfonic L24-22 + 150 ppm, Levasil CC301 | Core |
| Core Properties | Value |
|---|---|
| Length (cm) | 24.6 ± 0.01 |
| Diameter (cm) | 3.64 ± 0.01 |
| Pore Volume (mL) | 68.23 |
| Porosity | 0.24 |
| Permeability (mD) | 1400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcorn, Z.P.; Føyen, T.; Gauteplass, J.; Benali, B.; Soyke, A.; Fernø, M. Pore- and Core-Scale Insights of Nanoparticle-Stabilized Foam for CO2-Enhanced Oil Recovery. Nanomaterials 2020, 10, 1917. https://doi.org/10.3390/nano10101917
Alcorn ZP, Føyen T, Gauteplass J, Benali B, Soyke A, Fernø M. Pore- and Core-Scale Insights of Nanoparticle-Stabilized Foam for CO2-Enhanced Oil Recovery. Nanomaterials. 2020; 10(10):1917. https://doi.org/10.3390/nano10101917
Chicago/Turabian StyleAlcorn, Zachary Paul, Tore Føyen, Jarand Gauteplass, Benyamine Benali, Aleksandra Soyke, and Martin Fernø. 2020. "Pore- and Core-Scale Insights of Nanoparticle-Stabilized Foam for CO2-Enhanced Oil Recovery" Nanomaterials 10, no. 10: 1917. https://doi.org/10.3390/nano10101917
APA StyleAlcorn, Z. P., Føyen, T., Gauteplass, J., Benali, B., Soyke, A., & Fernø, M. (2020). Pore- and Core-Scale Insights of Nanoparticle-Stabilized Foam for CO2-Enhanced Oil Recovery. Nanomaterials, 10(10), 1917. https://doi.org/10.3390/nano10101917





