Low Working Temperature of ZnO-MoS2 Nanocomposites for Delaying Aging with Good Acetylene Gas-Sensing Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Materials Synthesis
2.3. Characterization
2.4. Fabrication of Gas Sensor
2.5. Gas-Sensing Measurements
3. Results and Discussion
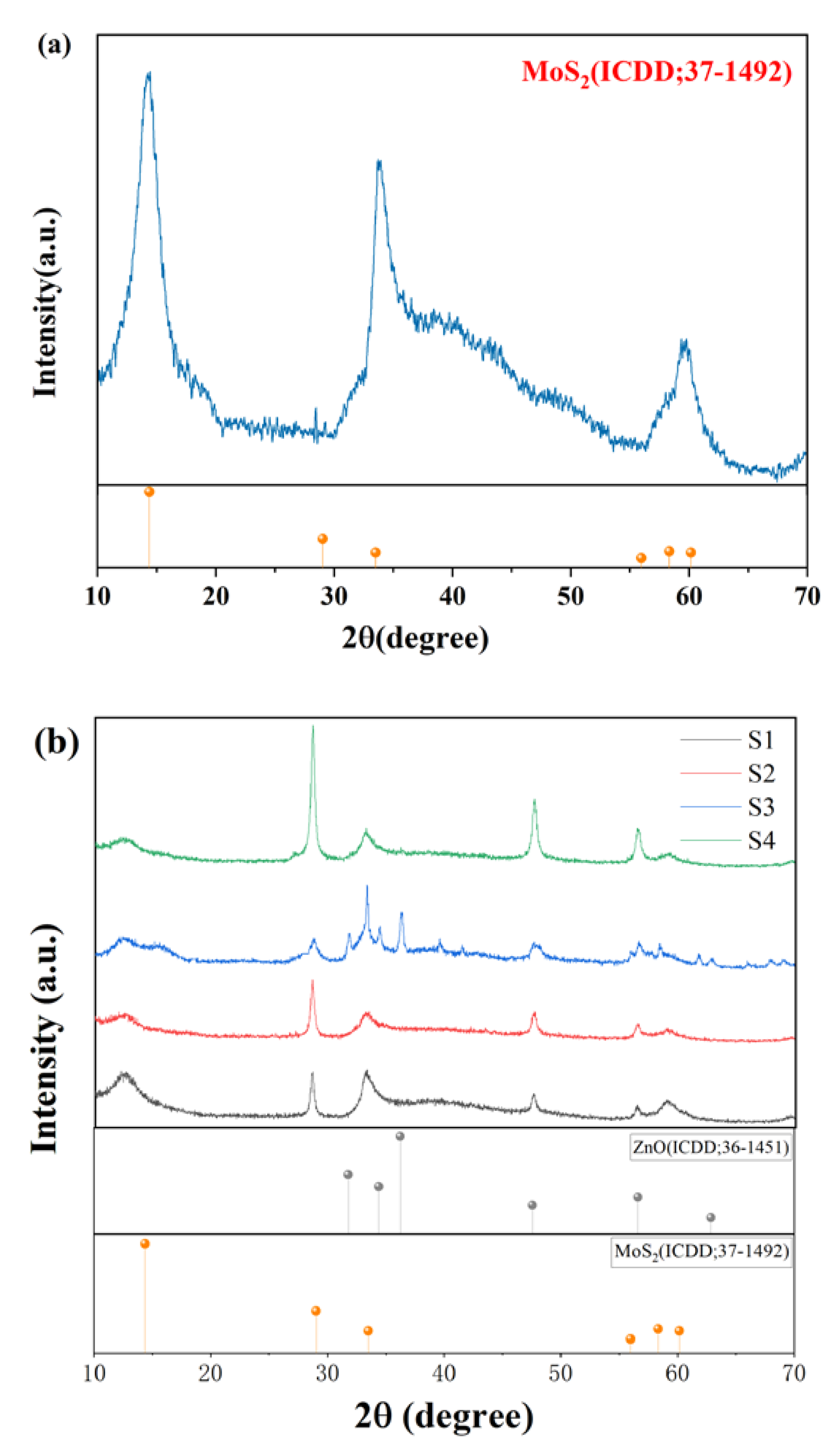
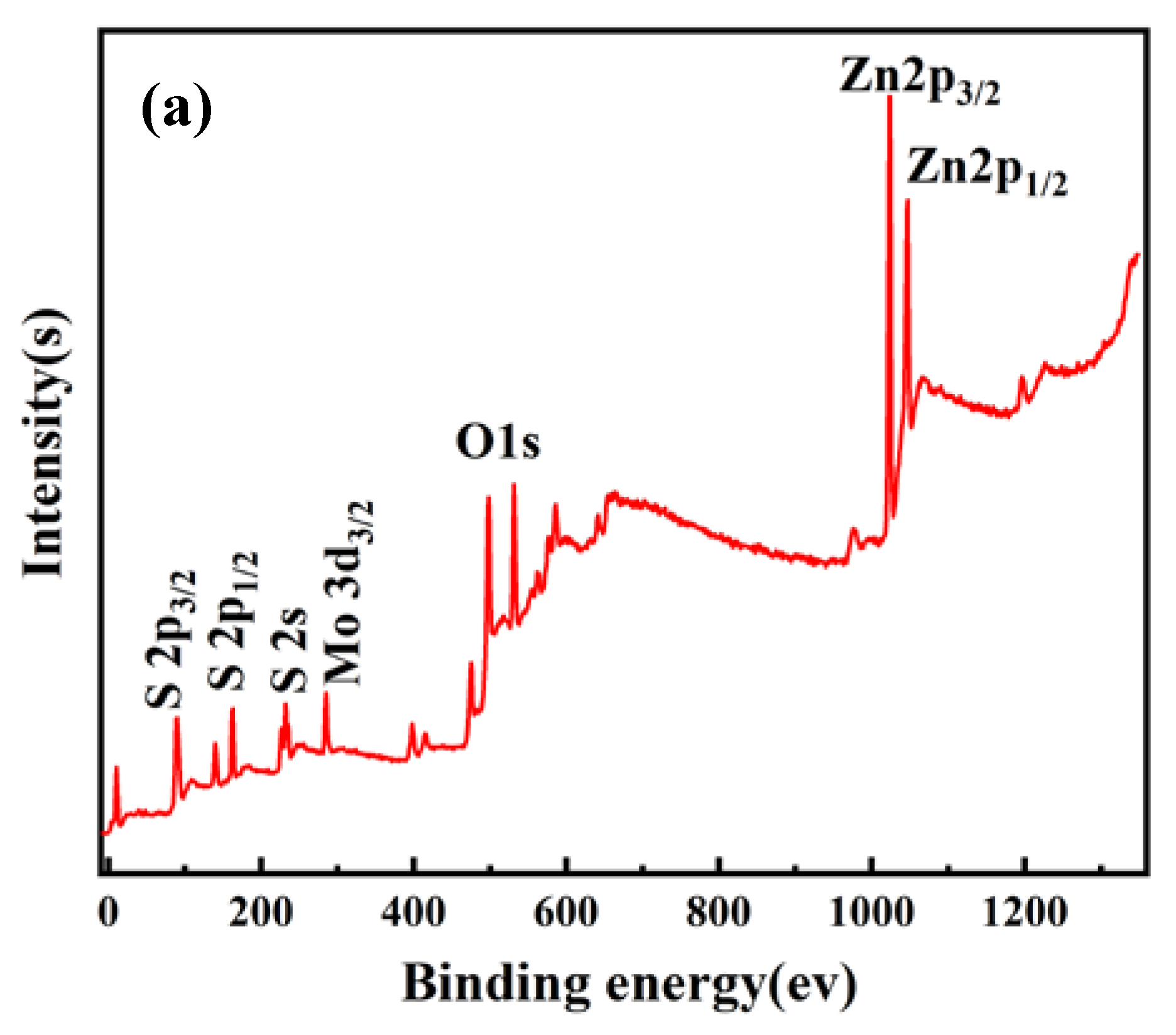
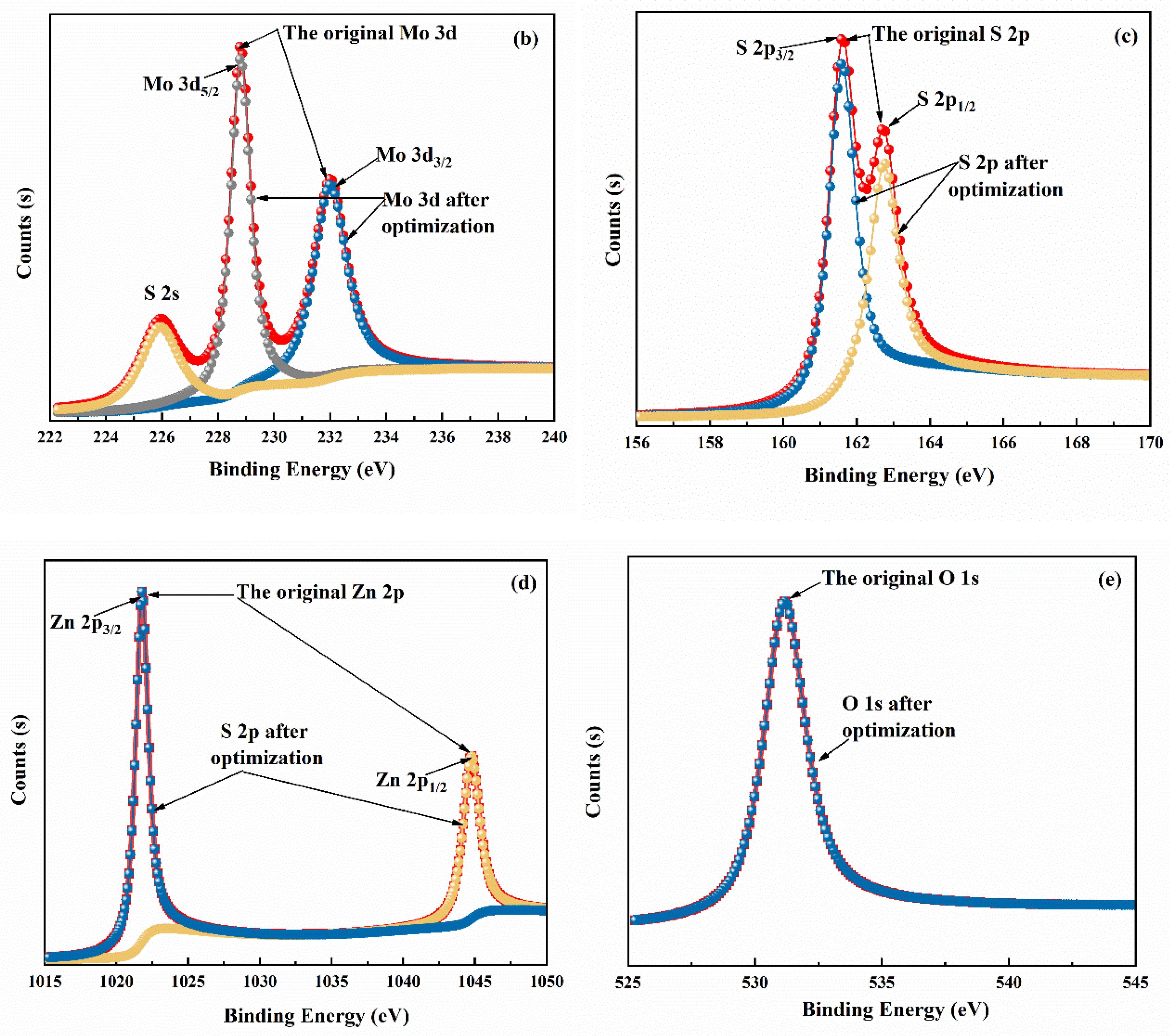
3.1. Morphology and Structure
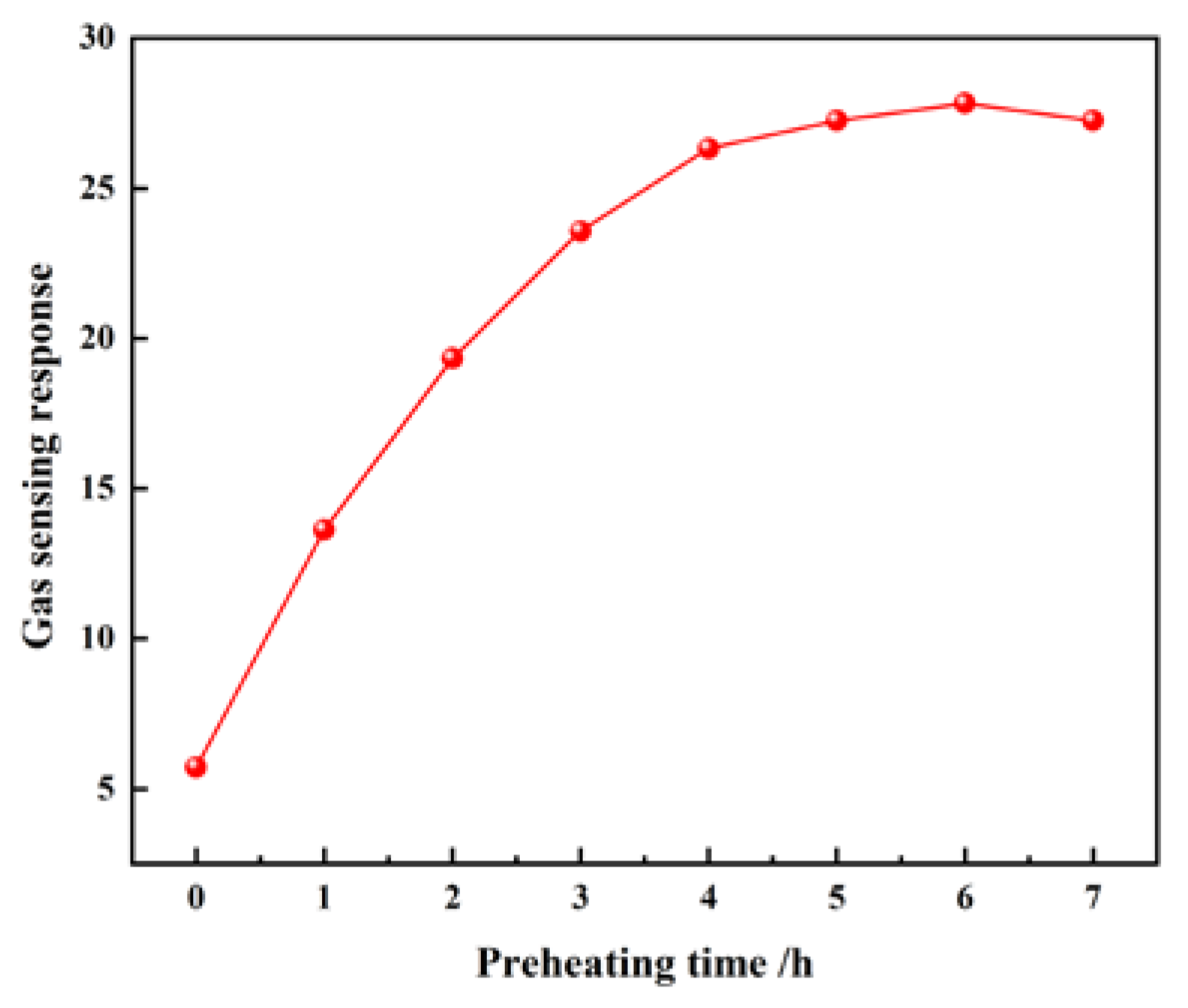
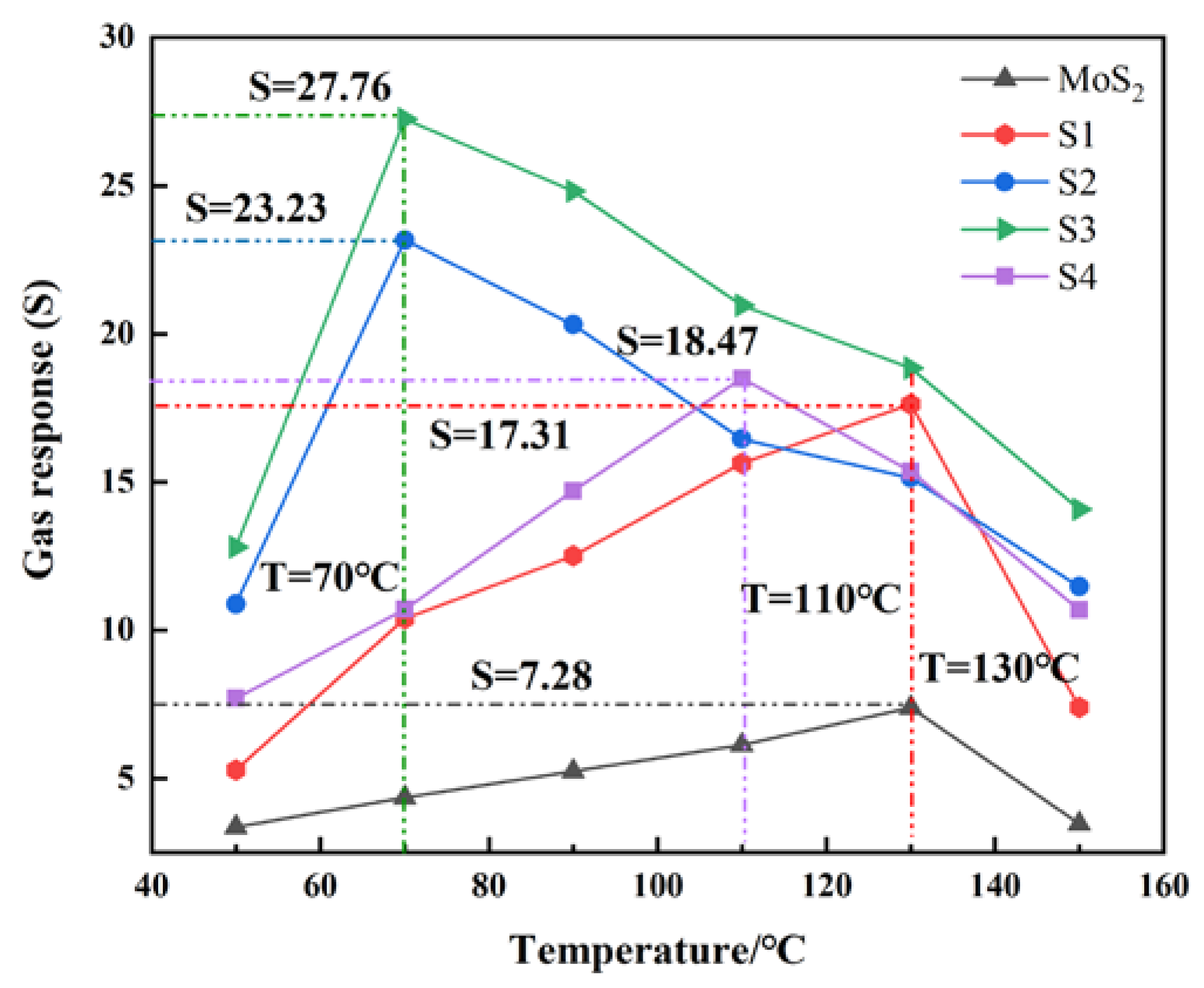
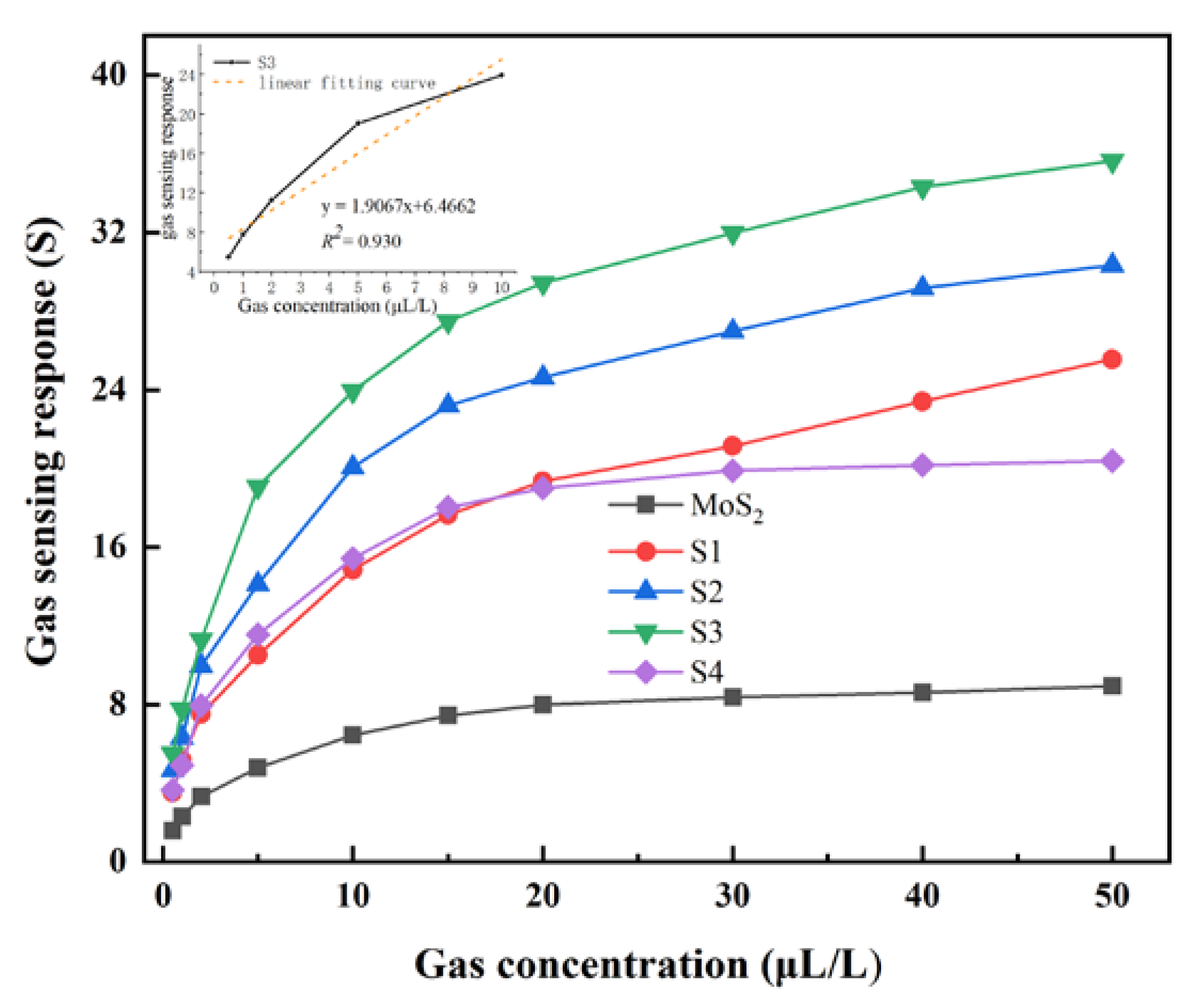
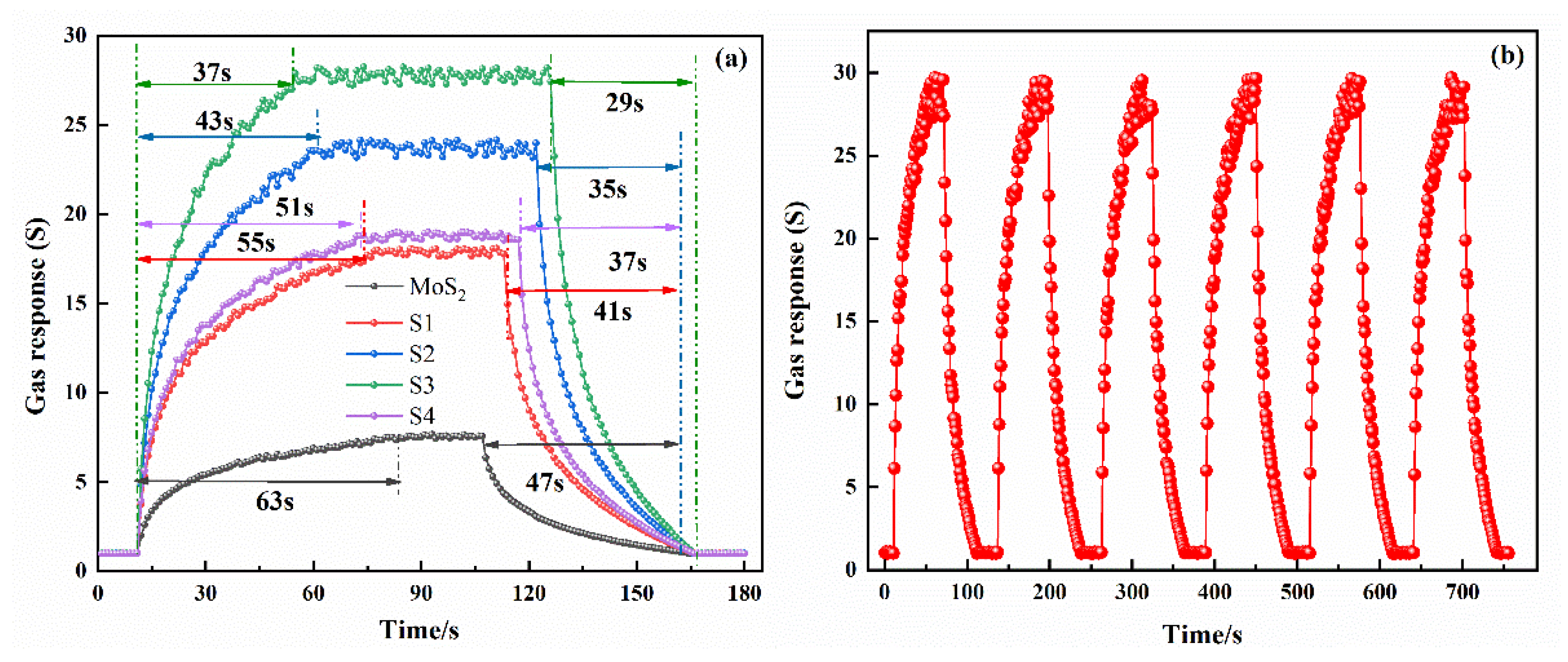
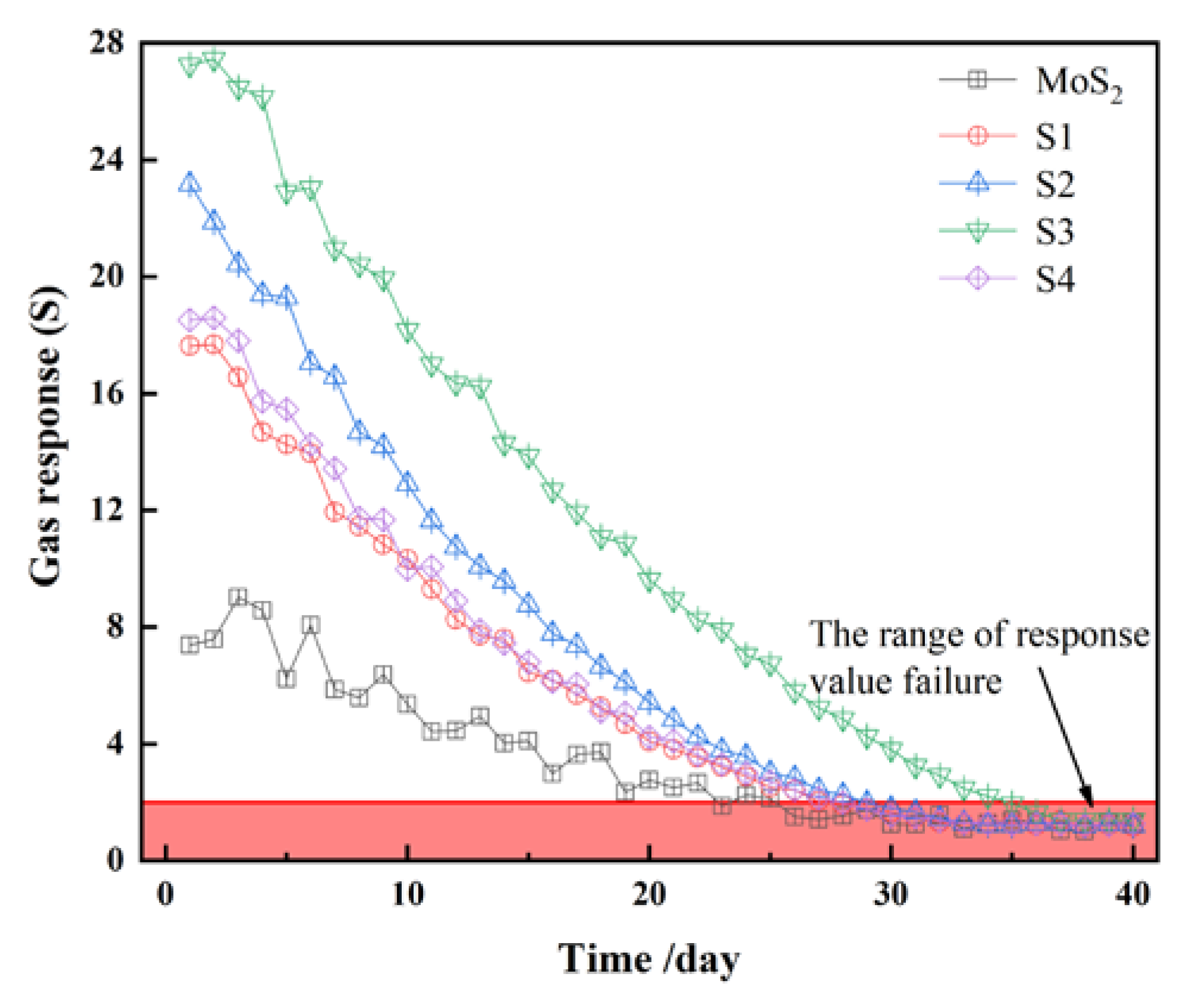
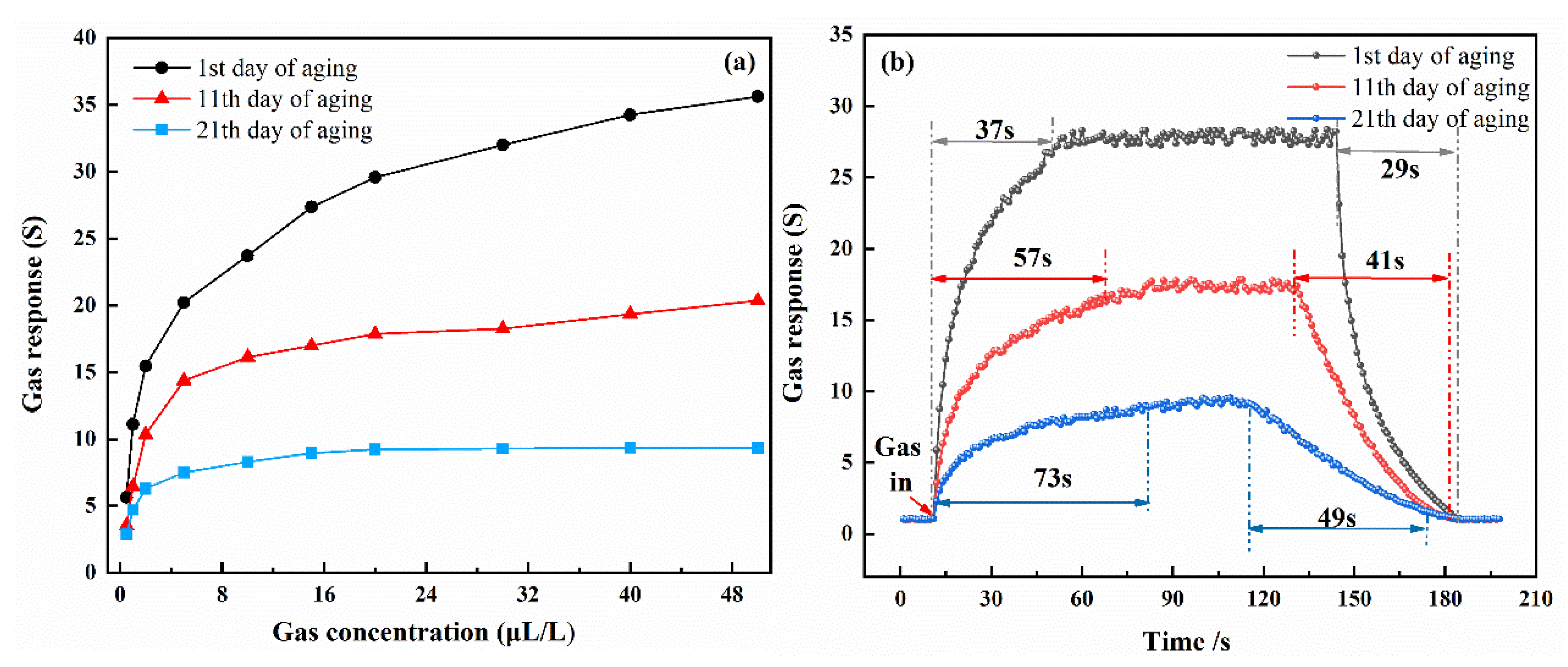
3.2. Gas-Sensing and Aging Characteristics
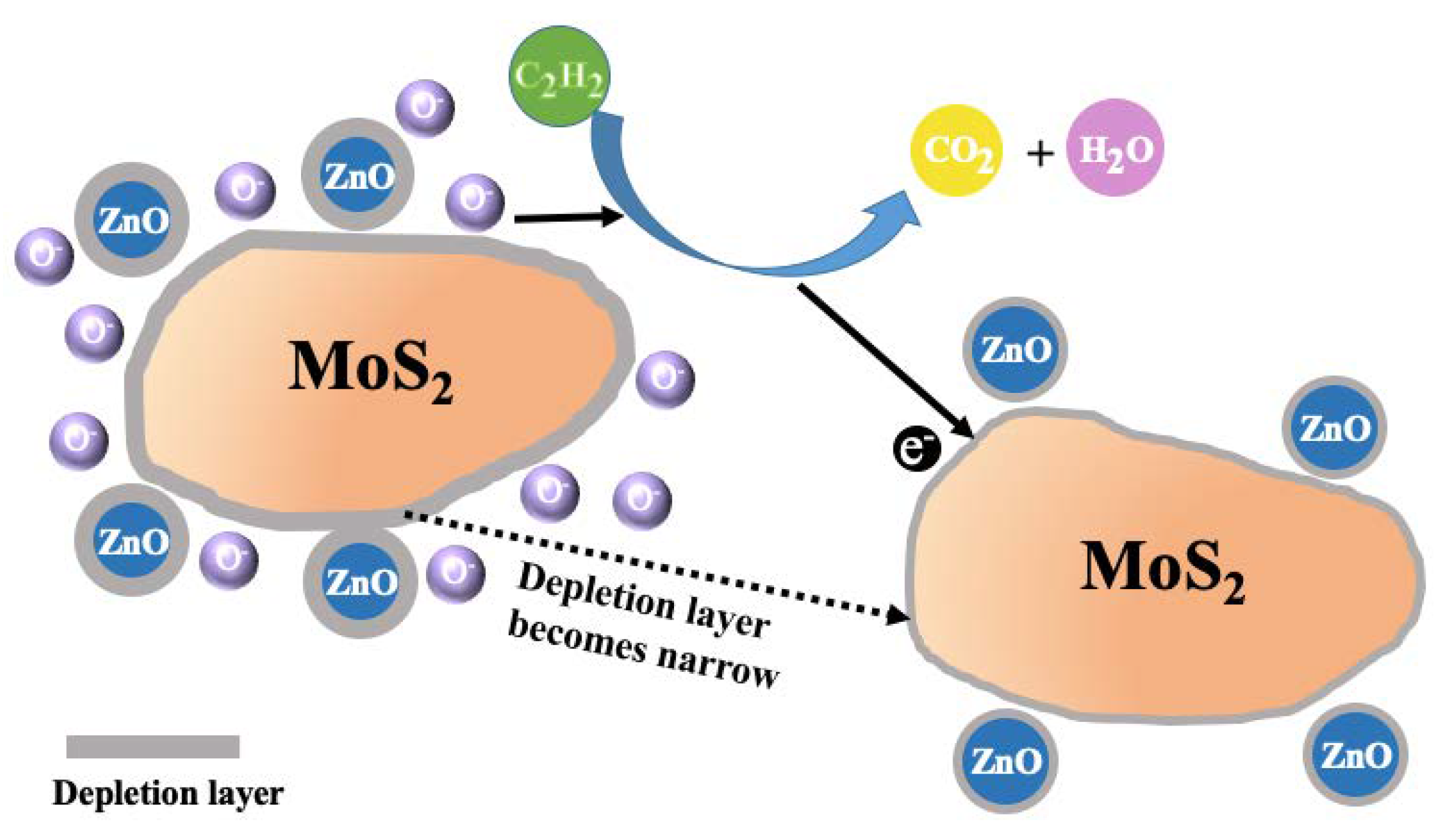
3.3. Gas-Sensing and Aging Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Regnima, G.O.; Betié, A.; Koffi, T. Monitoring power transformers oils deterioration using structured laser illumination planar imaging. Measurement 2017, 113, 38–45. [Google Scholar]
- Arabul, A.Y.; Kumru, C.F.; Arabul, F.K. Experimental investigation on creep characteristic of the spacer between winding turns of power transformers. Trans. Inst. Meas. Control 2019, 153, 1167–1175. [Google Scholar]
- Liu, Y.; Li, H.; Yu, Z. Reliability evaluation method for AC/DC hybrid distribution power network considering cascaded multiport power electronic transformer. IET Gener. Transm. Distrib. 2019, 13, 5357–5364. [Google Scholar]
- Ding, J.; Li, X.; Cao, J. New sensor for gases dissolved in transformer oil based on solid oxide fuel cell. Sens. Actuators B Chem. 2014, 202, 232–239. [Google Scholar]
- Kunicki, M.; Wotzka, D. A classification method for select defects in power transformers based on the acoustic signals. Sensors 2019, 19, 564577. [Google Scholar]
- Fei, S.W.; Wang, M.J.; Miao, Y.B. Particle swarm optimization-based support vector machine for forecasting dissolved gases content in power transformer oil. Energy Convers. Manag. 2009, 50, 1604–1609. [Google Scholar]
- Chatterjee, A.; Bhattacharjee, P.; Roy, N.K.; Kumbhakar, P. Usage of nanotechnology based gas sensor for health assessment and maintenance of transformers by DGA method. Int. J. Electr. Power Energy Syst. 2013, 45, 137–141. [Google Scholar]
- Meira, M.; Ruschetti, C.; Alvarez, R. Dissolved gas analysis differences between natural esters and mineral oils used in power transformers: A review. IET Gener. Transm. Distrib. 2019, 13, 5441–5448. [Google Scholar]
- Memmel, E.; Peters, D.; Voelker, R. Simulation of vertical power flow at MV/HV transformers for quantification of curtailed renewable power. IET Renew. Power Gener. 2019, 13, 3071–3079. [Google Scholar]
- Song, L.; Yue, H.; Li, H. Hierarchical porous ZnO microflowers with ultra-high ethanol gas-sensing at low concentration. Chem. Phys. Lett. 2018, 699, 1–7. [Google Scholar]
- Lu, G.; Xu, J.; Sun, J. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B Chem. 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yu, Y. UV-activated room temperature metal oxide based gas sensor attached with reflector. Sens. Actuators B Chem. 2012, 169, 291–296. [Google Scholar] [CrossRef]
- Fan, S.W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Matsuura, Y.; Takahata, K. Stabilization of SnO2 sintered gas sensors. Sens. Actuators B 1991, 5, 205–209. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cornet, A.; Rossinyol, E.; Arbiol, J.; Brinzari, V.; Blinov, Y. Faceting characterization of tin dioxide nanocrystals deposited by spray pyrolysis from stannic chloride water solution. Thin Solid Films 2004, 471, 310–319. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Smulko, J.M.; Trawka, M.; Granqvist, C.G. New approaches for improving selectivity and sensitivity of resistive gas sensors: A review. Sens. Rev. 2015, 35, 340–347. [Google Scholar]
- Arya, S.K.; Subramanian, K.; Hayde, S. Advances in materials for room temperature hydrogen sensors. Analyst 2012, 137, 2743–2756. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Akinwande, D.; Huyghebaert, C.; Wang, C.H. Graphene and two-dimensional materials for silicon technology. Nature 2019, 573, 507–518. [Google Scholar] [CrossRef]
- Gong, S.H.; Alpeggiani, F.; Sciacca, B. Nanoscale chiral valley-photon interface through optical spin-orbit coupling. Science 2018, 359, 443–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, D.; Cai, S.; Paudel, T.R. Freestanding crystalline oxide perovskites down to the monolayer limit. Nature 2019, 570, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, Z.; Hang, Y. Electron ptychography of 2D materials to deep sub-angstrom resolution. Nature 2018, 559, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Kim, J.; Utama, M.I.B. Imaging of pure spin-valley diffusion current in WS2-WSe2 heterostructures. Science 2018, 360, 893–896. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, S.; Wang, Y. Synthesis of hierarchical SnO2 nanostructures assembled with nanosheets and their improved gas sensing properties. Sens. Actuators B Chem. 2013, 188, 85–93. [Google Scholar] [CrossRef]
- Yan, A.; Xie, C.; Zeng, D. Synthesis, formation mechanism and sensing properties of WO3 hydrate nanowire netted-spheres. Mater. Res. Bull. 2010, 45, 1541–1547. [Google Scholar] [CrossRef]
- Wu, W.; Tang, S.; Gu, J. Realizing semiconductor to metal transition in graphitic ZnO and MoS2 nanocomposite with external electric field. Rsc. Adv. 2015, 5, 99153–99163. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Mishra, M. Growth of MoS2-MoO3 Hybrid Microflowers via Controlled Vapor Transport Process for Efficient Gas Sensing at Room Temperature. Adv. Mater. Interfaces 2018, 5, 1356–1365. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Gu, C.; Huang, J.; Wu, Y. Preparation of porous flower-like ZnO nanostructures and their gas-sensing property. J. Alloys Compd. 2011, 509, 4499–4504. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.G.; Li, X.X. UV activated hollow ZnO microspheres for selective ethanol sensors at low temperatures. Sens. Actuators B Chem. 2016, 232, 158–164. [Google Scholar] [CrossRef]
- Kelly, A.G.; Hallam, T.; Backes, C. All-printed thin-film transistors from networks of liquid-exfoliated nanosheets. Science 2017, 356, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Lien, D.H.; Uddin, S.Z.; Yeh, M. Device technology electrical suppression of all nonradiative recombination pathways in monolayer semiconductors. Science 2019, 364, 468–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, R.; Inoue, T.; Zheng, Y. One-dimensional van der Waals heterostructures. Science 2020, 367, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Tammanoon, N.; Wisitsoraat, A.; Sriprachuabwong, C. Ultra-Sensitive NO2 Sensor Based on Ohmic Metal-Semiconductor Interfaces of Electrolytically Exfoliated Graphene/Flame-Spray-Made SnO2 Nanoparticles Composite Operating at Low Temperatures. ACS Appl. Mater. Interface 2015, 7, 24338–24352. [Google Scholar] [CrossRef]
- Garg, R.; Dutta, N.; Choudhury, N. Work Function Engineering of Graphene. Nanomaterials 2014, 4, 267–300. [Google Scholar] [CrossRef] [Green Version]
- Quang, P.L.; Cuong, N.D.; Hoa, T.T. Simple post-synthesis of mesoporous p-type Co3O4 nanochains for enhanced H2S gas sensing performance. Sens. Actuators B Chem. 2018, 270, 158–166. [Google Scholar] [CrossRef]
- Urasinskawojcik, B.; Vincent, T.A.; Chowdhury, M.F. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Boris, I.; Brinzari, V.; Han, S.H. The role of doping effect on the response of SnO2-based thin film gas sensors: Analysis based on the results obtained for Co-doped SnO2 films deposited by spray pyrolysis. Sens. Actuators B Chem. 2013, 182, 112–124. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, A.; Chang, H. Room-temperature high-performance acetone gas sensor based on hydrothermal synthesized SnO2-reduced graphene oxide hybrid composite. RSC Adv. 2015, 83, 3016–3022. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yin, J.; Li, L. Enhanced ethanol gas-sensing properties of flower-like p-CuO/n-ZnO heterojunction nanorods. Sens. Actuators B Chem. 2014, 202, 500–507. [Google Scholar] [CrossRef]
- Acharya, S.; Bangera, K.V.; Shivakumar, G.K. Conduction Mechanism in n-CdSe/p-ZnTe Heterojunction. J. Electron. Mater. 2016, 45, 3324–3331. [Google Scholar] [CrossRef]
- Bai, S.; Liu, J.; Guo, J. Facile preparation of SnO2/NiO composites and enhancement of sensing performance to NO2. Sens. Actuators B Chem. 2017, 249, 22–29. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Wang, W.; Xue, Y.; Sun, Y.; Li, P.; Lian, K.; Zhang, W.; Chen, L.; Shi, J.; et al. Synthesis and gas sensing properties of NiO/SnO2 hierarchical structures toward ppb-level acetone detection. Mater. Res. Bull. 2018, 102, 294–303. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Xu, Q. High triethylamine-sensing properties of NiO/SnO2 hollow sphere P–N heterojunction sensors. Sens. Actuators B Chem. 2015, 215, 39–44. [Google Scholar] [CrossRef]
- He, J.; Jiao, W.; Zhang, L. Preparation and gas-sensing performance of SnO2/NiO composite oxide synthesized by microwave-assisted liquid phase deposition. Particuology 2018, 41, 118–125. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zeng, W. Enhanced ethanol sensing and mechanism of Cr-doped ZnO nanorods: Experimental and computational study. Ceram. Int. 2017, 43, 14873–14879. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, W.; Li, J.; Song, Z.; Zhang, H.; Zeng, W. Low Working Temperature of ZnO-MoS2 Nanocomposites for Delaying Aging with Good Acetylene Gas-Sensing Properties. Nanomaterials 2020, 10, 1902. https://doi.org/10.3390/nano10101902
Wang S, Chen W, Li J, Song Z, Zhang H, Zeng W. Low Working Temperature of ZnO-MoS2 Nanocomposites for Delaying Aging with Good Acetylene Gas-Sensing Properties. Nanomaterials. 2020; 10(10):1902. https://doi.org/10.3390/nano10101902
Chicago/Turabian StyleWang, Sijie, Weigen Chen, Jian Li, Zihao Song, He Zhang, and Wen Zeng. 2020. "Low Working Temperature of ZnO-MoS2 Nanocomposites for Delaying Aging with Good Acetylene Gas-Sensing Properties" Nanomaterials 10, no. 10: 1902. https://doi.org/10.3390/nano10101902



