Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials
Abstract
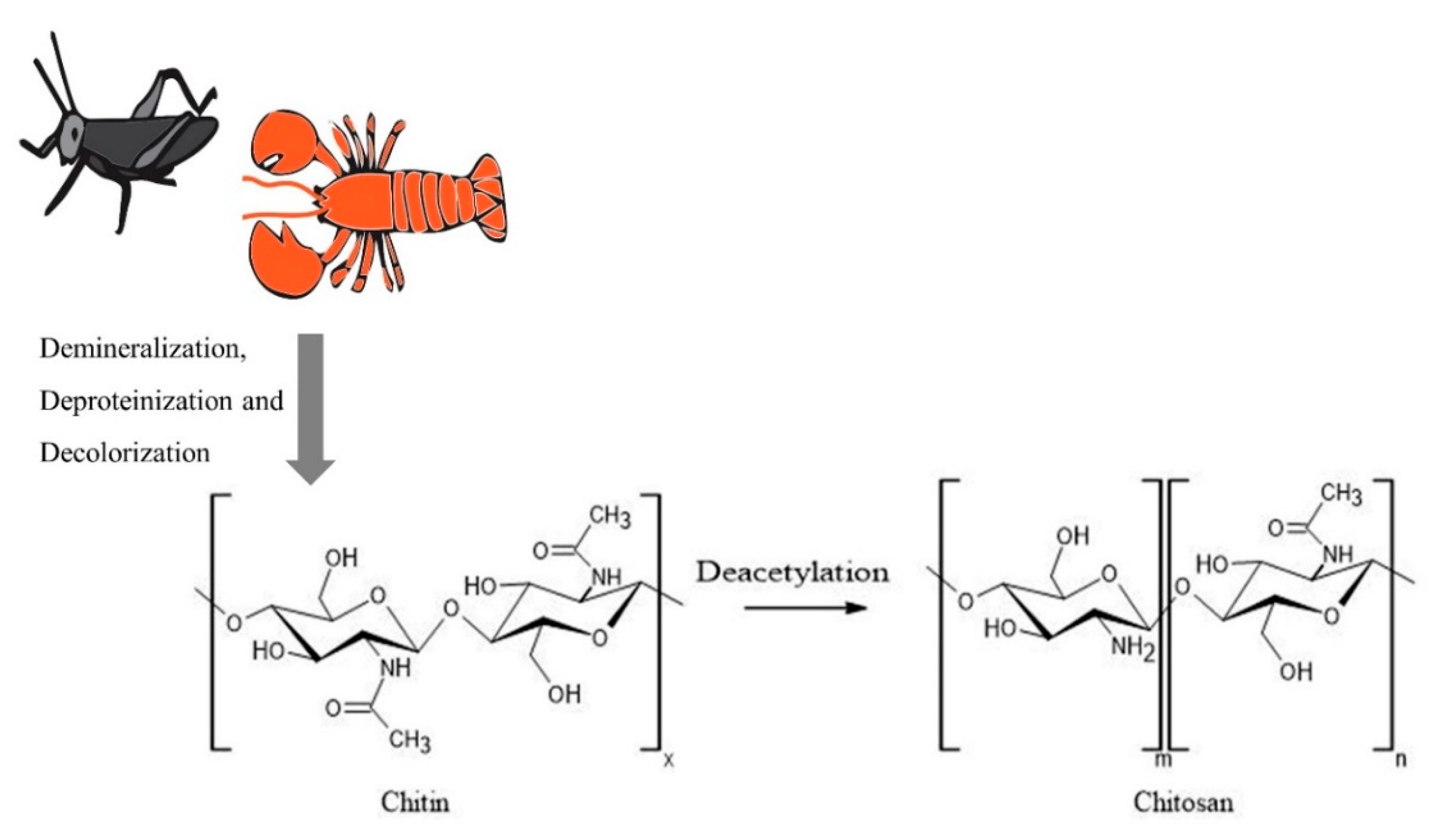
:1. Introduction
2. Applications in Agriculture
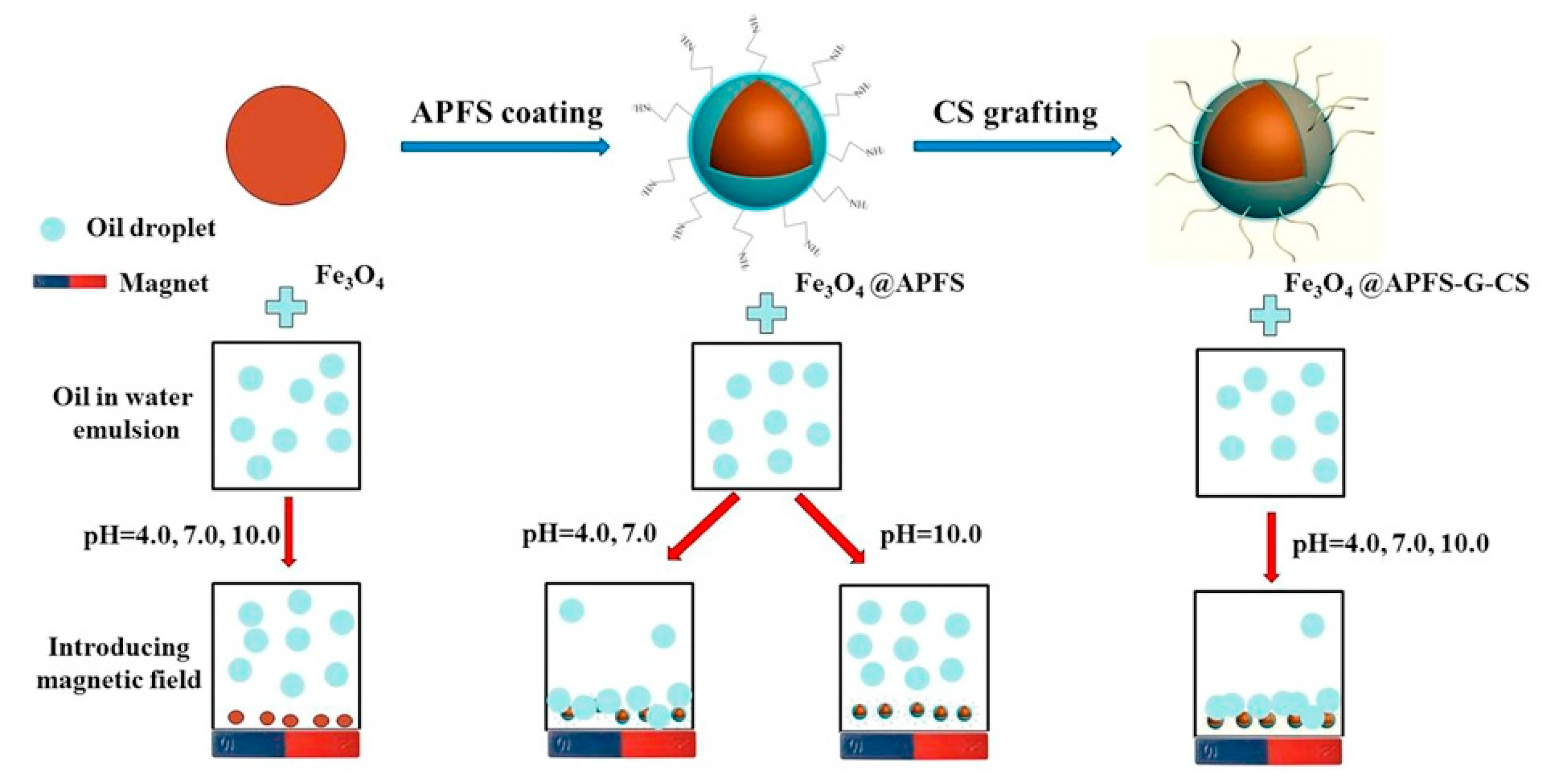
2.1. Water Purification and Sustainable Agriculture
2.2. Applications of Nanochitosan in Regulating Abiotic Stress in Plants
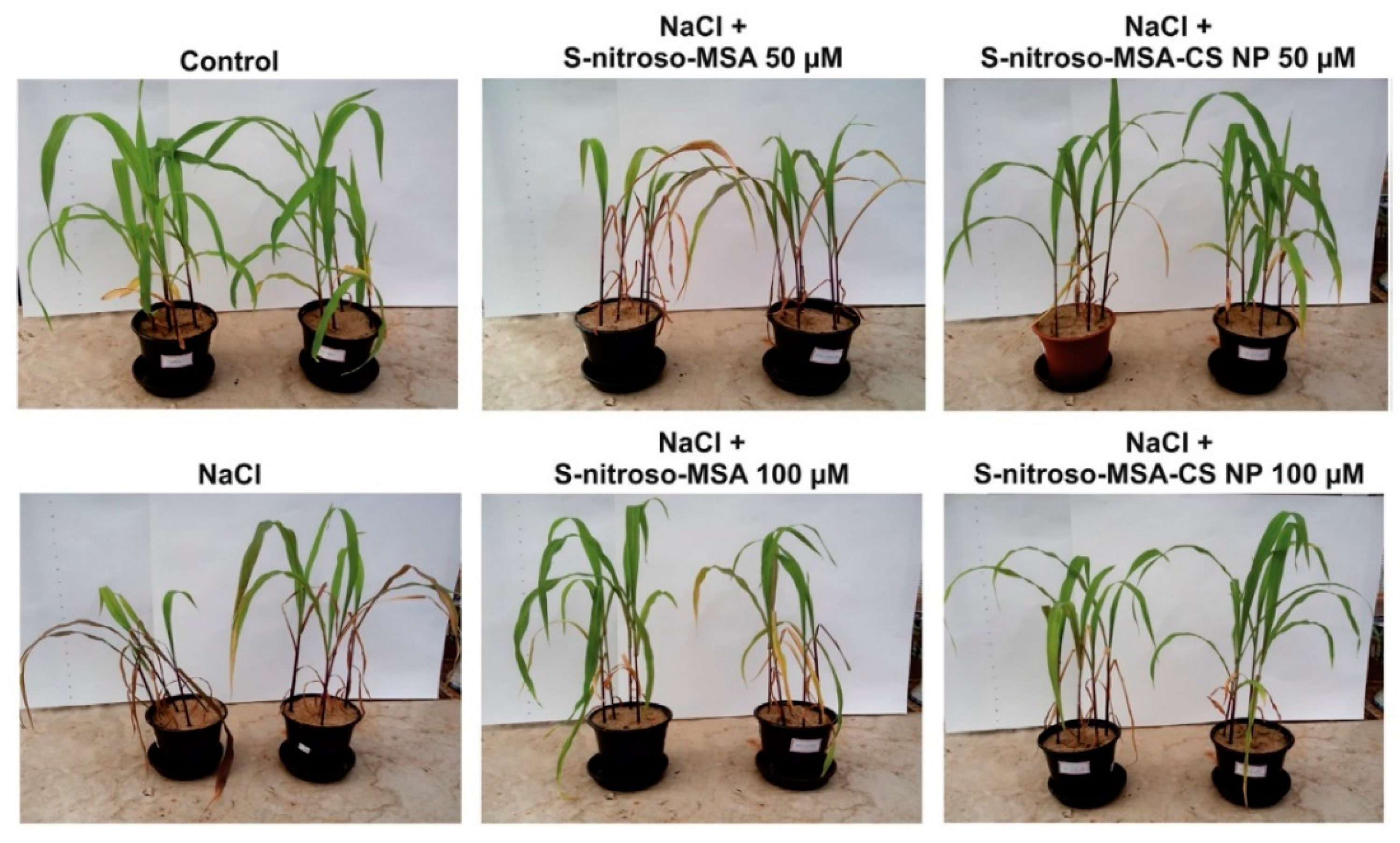
2.2.1. Nanochitosan in Controlling Salinity Stress
2.2.2. Nanochitosan in Controlling Drought Stress
2.2.3. The Potential Use of Nanochitosan under Temperature and Heavy Metal Stress
2.2.4. Mechanism of Action of Chitosan Nanoparticles in Combating Abiotic Stresses
3. Biomedical Applications of Nanochitosan
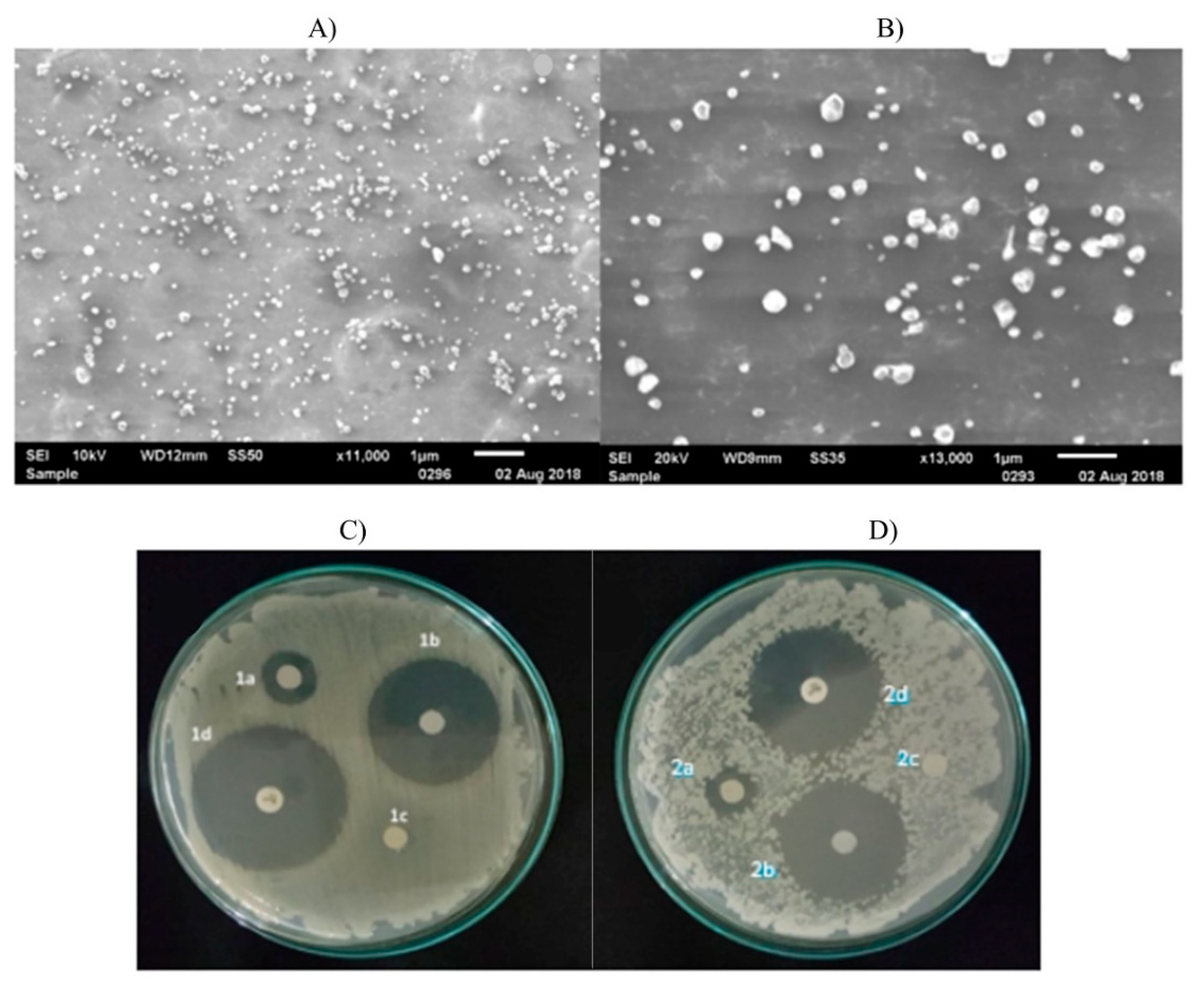
3.1. Chitosan Nanoparticles for Foodborne Pathogens
3.1.1. Direct Use of Chitosan Nanoparticles
3.1.2. Chitosan Nanoparticles with Essential Oils
3.1.3. Nanochitosan with Other Naturally Occurring Antimicrobials
3.1.4. Other Potential Applications of Chitosan Nanoparticles Related to Foodborne Pathogens
3.1.5. Possible Factors Affecting the Antimicrobial Effect of Chitosan
3.2. Role of Chitosan in Cancer Photothermal Therapy
3.2.1. Applications of Nanochitosan in Photothermal Therapy
3.2.2. Compounds Synthesized with Chitosan-Derivatives
3.2.3. The Photothermal Effect
4. Toxicity of Nanochitosan
5. Future Directions and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Gedda, G.; Lee, C.-Y.; Lin, Y.-C.; Wu, H.-F. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens. Actuators B Chem. 2016, 224, 396–403. [Google Scholar] [CrossRef]
- Negm, A.; Hefni, H.H.; Abd-Elaal, A.A.; Badr, E.A.; Kana, M.T.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Boil. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Abu Noqta, O.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chin. J. Phys. 2020, 64, 305–325. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Boil. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Sharma, S.; Kuotsu, K.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Tayel, A.A.; Ibrahim, S.I.; Al-Saman, M.A.; Moussa, S.H. Production of fungal chitosan from date wastes and its application as a biopreservative for minced meat. Int. J. Boil. Macromol. 2014, 69, 471–475. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Edison, T.N.J.I.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A.; Ponnuchamy, K. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Boil. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef]
- Sun, C.; Fu, D.; Jin, L.; Chen, M.; Zheng, X.; Yu, T. Chitin isolated from yeast cell wall induces the resistance of tomato fruit to Botrytis cinerea. Carbohydr. Polym. 2018, 199, 341–352. [Google Scholar] [CrossRef]
- Jones, M.; Weiland, K.; Kujundzic, M.; Theiner, J.; Kählig, H.; Kontturi, E.; John, S.; Bismarck, A.; Mautner, A. Waste-Derived Low-Cost Mycelium Nanopapers with Tunable Mechanical and Surface Properties. Biomacromolecules 2019, 20, 3513–3523. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ramírez, R.; Torres-Castillo, J.A.; Barrientos-Lozano, L.; Almaguer-Sierra, P.; Torres-Acosta, R.I. Schistocerca piceifrons piceifrons (Orthoptera: Acrididae) as a Source of Compounds of Biotechnological and Nutritional Interest. J. Insect Sci. 2019, 19. [Google Scholar] [CrossRef] [Green Version]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Boil. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of Nanochitosan-Based Coating With and Without Copper Loaded on Physicochemical and Bioactive Components of Fresh Strawberry Fruit (Fragaria x ananassa Duchesne) During Storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Ren, Y.; Li, H.; Zhang, X.; Di, J. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012, 113, 408–414. [Google Scholar] [CrossRef]
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Boil. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Corradini, E. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Kanmani, P.; Aravind, J.; Kamaraj, M.; Sureshbabu, P.; Karthikeyan, S.; Jeyaseelan, A.; Sivashanmugam, K. Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresour. Technol. 2017, 242, 295–303. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Sarmiento-Sánchez, J.I.; Ruelas-Leyva, J.P.; Crini, G.; Hermosillo-Ochoa, E.; Gutierrez-Montes, J.A. Environment-Friendly Approach toward the Treatment of Raw Agricultural Wastewater and River Water via Flocculation Using Chitosan and Bean Straw Flour as Bioflocculants. ACS Omega 2020, 5, 3943–3951. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumer, Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Cadogan, E.I.; Lee, C.-H.; Popuri, S.R.; Lin, H.-Y. Efficiencies of chitosan nanoparticles and crab shell particles in europium uptake from aqueous solutions through biosorption: Synthesis and characterization. Int. Biodeterior. Biodegrad. 2014, 95, 232–240. [Google Scholar] [CrossRef]
- Fan, H.; Zhou, S.; Jiao, W.-Z.; Qi, G.-S.; Liu, Y.-Z. Removal of heavy metal ions by magnetic chitosan nanoparticles prepared continuously via high-gravity reactive precipitation method. Carbohydr. Polym. 2017, 174, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, X.; Qiu, T.; Zhou, L.; Li, R.; Deng, Z. A novel and biocompatible Fe3O4 loaded chitosan polyelectrolyte nanoparticles for the removal of Cd2+ ion. Int. J. Boil. Macromol. 2019, 141, 1165–1174. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Fourmentin, M.; Zemmouri, H.; Nascimento, I.O.D.C.; Queiroz, L.M.; Tadza, M.Y.M.; Picos-Corrales, L.A.; Pei, H.; Wilson, L.D.; et al. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019, 17, 1603–1621. [Google Scholar] [CrossRef] [Green Version]
- Lü, T.; Chen, Y.; Qi, D.; Cao, Z.; Zhang, D.; Zhao, H. Treatment of emulsified oil wastewaters by using chitosan grafted magnetic nanoparticles. J. Alloy. Compd. 2017, 696, 1205–1212. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Gangasalam, A. Reduction of chemical oxygen demand and color from the rice mill wastewater by chitosan/2(5H)-furanone-incorporated ultrafiltration membrane system. Sep. Sci. Technol. 2018, 54, 409–425. [Google Scholar] [CrossRef]
- Rahimi, Z.; Zinatizadeh, A.A.; Zinadini, S. Membrane bioreactors troubleshooting through the preparation of a high antifouling PVDF ultrafiltration mixed-matrix membrane blended with O-carboxymethyl chitosan-Fe3O4 nanoparticles. Environ. Technol. 2018, 40, 3523–3533. [Google Scholar] [CrossRef] [PubMed]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Bahrami, K. Energy recovery and hygienic water production from wastewater using an innovative integrated microbial fuel cell–membrane separation process. Energy 2017, 141, 1350–1362. [Google Scholar] [CrossRef]
- Zareie, C.; Eshkalak, S.K.; Najafpour-Darzi, G.; Baei, M.S.; Younesi, H.; Seeram, R. Uptake of Pb(II) Ions from Simulated Aqueous Solution via Nanochitosan. Coatings 2019, 9, 862. [Google Scholar] [CrossRef] [Green Version]
- Eladlani, N.; Dahmane, E.M.; Ouahrouch, A.; Rhazi, M.; Moha, T. Recovery of Chromium(III) from Tannery Wastewater by Nanoparticles and Whiskers of Chitosan. J. Polym. Environ. 2017, 26, 152–157. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Sahebi, H.; Zandavar, H.; Hajimirsadeghi, S. Fabrication of Fe3O4 nanoparticles coated by extracted shrimp peels chitosan as sustainable adsorbents for removal of chromium contaminates from wastewater: The design of experiment. Compos. Part B: Eng. 2019, 175, 107130. [Google Scholar] [CrossRef]
- Khalil, T.E.; Elhusseiny, A.F.; El-Dissouky, A.; Ibrahim, N.M. Functionalized chitosan nanocomposites for removal of toxic Cr (VI) from aqueous solution. React. Funct. Polym. 2020, 146, 104407. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, D.; Lai, C.; Xu, P.; Niu, C.-G.; Wan, J.; Tang, L.; Dong, H.; Huang, B.; Hu, T. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total. Environ. 2018, 644, 1181–1189. [Google Scholar] [CrossRef]
- Altinisik, A.; Yurdakoc, K. Chitosan-/PVA-coated magnetic nanoparticles for Cu (II) ions adsorption. Desalin. Water Treat. 2016, 57, 18463–18474. [Google Scholar] [CrossRef]
- Yu, K.; Ho, J.; McCandlish, E.; Buckley, B.T.; Patel, R.; Li, Z.; Shapley, N.C. Copper ion adsorption by chitosan nanoparticles and alginate microparticles for water purification applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 31–41. [Google Scholar] [CrossRef]
- Bratina, B.; Šorgo, A.; Kramberger, J.; Ajdnik, U.; Zemljič, L.F.; Ekart, J.; Šafarič, R. From municipal/industrial wastewater sludge and FOG to fertilizer: A proposal for economic sustainable sludge management. J. Environ. Manag. 2016, 183, 1009–1025. [Google Scholar] [CrossRef]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2013, 21, 449–461. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Mosber, G.; Gungor, A.A.; Adıguzel, G.; Adiguzel, A. Biodegradation of some azo dyes from wastewater with laccase from Weissella viridescens LB37 immobilized on magnetic chitosan nanoparticles. J. Water Process. Eng. 2019, 31, 100866. [Google Scholar] [CrossRef]
- Sahbaz, D.A.; Yakar, A.; Gündüz, U. Magnetic Fe3O4-chitosan micro- and nanoparticles for wastewater treatment. Part. Sci. Technol. 2018, 37, 732–740. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Shao, Y.; He, F.; Wu, M.; Ni, H.; Zheng, Y.; Sun, Y. Chitosan–silica nanoparticles catalyst (M@CS–SiO2) for the degradation of 1,1-dimethylhydrazine. Res. Chem. Intermed. 2018, 45, 1721–1735. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, X.; Grattieri, M.; Macazo, F.C.; Cai, R.; Minteer, S.D. A sustainable adsorbent for phosphate removal: Modifying multi-walled carbon nanotubes with chitosan. J. Mater. Sci. 2018, 53, 12641–12649. [Google Scholar] [CrossRef]
- Alarcón-Payán, D.A.; Koyani, R.D.; Vazquez-Duhalt, R. Chitosan-based biocatalytic nanoparticles for pollutant removal from wastewater. Enzym. Microb. Technol. 2017, 100, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Riegger, B.R.; Bäurer, B.; Mirzayeva, A.; Tovar, G.E.M.; Bach, M. A systematic approach of chitosan nanoparticle preparation via emulsion crosslinking as potential adsorbent in wastewater treatment. Carbohydr. Polym. 2018, 180, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.V.G.; Petkova, P.; Margalef-Marti, R.; Vives, M.; Aguilar, L.; Gallegos, A.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Mendoza, E.; et al. Hybrid Chitosan–Silver Nanoparticles Enzymatically Embedded on Cork Filter Material for Water Disinfection. Ind. Eng. Chem. Res. 2017, 56, 3599–3606. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Kumar, S.N.; Kamil, T.M.; Ravi, T. Biopolymer-Mediated Coating Influence on Wastewater Treatment Efficacy of Silver Nanoparticles Synthesized from Fungal Consortium. Natl. Acad. Sci. Lett. 2020, 1–5. [Google Scholar] [CrossRef]
- Cho, K.Y.; Yoo, C.H.; Won, Y.-J.; Hong, D.Y.; Chang, J.-S.; Choi, J.W.; Lee, J.-H.; Lee, J.S. Surface-concentrated chitosan-doped MIL-100(Fe) nanofiller-containing PVDF composites for enhanced antibacterial activity. Eur. Polym. J. 2019, 120, 109221. [Google Scholar] [CrossRef]
- Kolangare, I.M.; Isloor, A.M.; Karim, Z.A.; Kulal, A.; Ismail, A.F.; Inamuddin; Asiri, A.M. Antibiofouling hollow-fiber membranes for dye rejection by embedding chitosan and silver-loaded chitosan nanoparticles. Environ. Chem. Lett. 2018, 17, 581–587. [Google Scholar] [CrossRef]
- Zayed, M.; ElKafafi, S.; Zedan, A.; Dawoud, S. Effect of Nano Chitosan on Growth, Physiological and Biochemical Parameters of Phaseolus vulgaris under Salt Stress. J. Plant Prod. 2017, 8, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.K.; Chouhan, D.; Das, D.; Ghosh, R.; Mandal, P. Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. Int. J. Boil. Macromol. 2019, 145, 108–123. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Gomes, B.C.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Sánchez-Aspeytia, D.; González-Morales, S. Chitosan-PVA and Copper Nanoparticles Improve Growth and Overexpress the SOD and JA Genes in Tomato Plants under Salt Stress. Agronomy 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Li, Y.; Yu, C.; Wang, Y.; Li, X.; Li, N.; Chen, Q.; Bu, N. Alleviation of exogenous oligochitosan on wheat seedlings growth under salt stress. Protoplasma 2011, 249, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, B.; Safari, H.; Modarres Sanavy, S.A.M. Effect Of Chitosan Seed Priming On Germination, Ion Relations and Biochemical Characteristics of Chickpea Under Salinity Stress. Plant Prod. Technol. (Agric. Res.) 2015, 15, 163–172. [Google Scholar]
- Al-Tawaha, A.R.M.; Al-ghzawi, A.L.A. Effect of chitosan coating on seed germination and salt tolerance of lentil (Lens culinaris L.). Res. Crops 2013, 14, 489–491. [Google Scholar]
- Mahdavi, B.; Rahimi, A. Seed priming with chitosan improves the germination and growth performance of ajowan. Eurasian J. Biosci. 2013, 7, 69–76. [Google Scholar] [CrossRef]
- Masjedi, M.H.; Roozbahani, A.; Baghi, M. Assessment Effect of Chitosan Foliar Application on Total Chlorophyll and Seed Yield of Wheat (Triticum aestivum L.) Under Water Stress Conditions. J. Crop Nutr. Sci. 2017, 3, 14–26. [Google Scholar]
- Khordadi Varamin, J.; Fanoodi, F.; Sinaki, J.M.; Rezvan, S.; Damavandi, A. Physiological response of sesame (Sesamum indicum L.) to application of chitosan and magnesium-nano fertilizers under irrigation cut-off in a sustainable agriculture system. Plant Physiol. 2018, 9, 2629–2639. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A.; Ahmadi, S.B. Evaluation of Chitosan Nanoparticles Effects on Yield and Yield Components of Barley (Hordeum vulgare L.) under Late Season Drought Stress. J. Water Environ. Nanotechnol. 2018, 3, 22–39. [Google Scholar] [CrossRef]
- Priyaadharshini, M.; Sritharan, N.; Senthil, A.; Marimuthu, S. Physiological studies on effect of chitosan nanoemulsion in pearl millet under drought condition. J. Pharmacogn. Phytochem. 2019, 8, 3304–3307. [Google Scholar]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide 2019, 84, 38–44. [Google Scholar] [CrossRef]
- Behboudi, F.; Sarvestani, Z.T.; Kassaee, M.Z.; Modarres-Sanavy, S.A.M.; Sorooshzadeh, A.; Mokhtassi-Bidgoli, A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019, 42, 1439–1451. [Google Scholar] [CrossRef]
- Avestan, S.; Naseri, L.; Barker, A.V. Evaluation of nanosilicon dioxide and chitosan on tissue culture of apple under agar-induced osmotic stress. J. Plant Nutr. 2017, 40, 2797–2807. [Google Scholar] [CrossRef]
- Rabêlo, V.M.; Magalhães, P.C.; Bressanin, L.A.; Carvalho, D.T.; Dos Reis, C.O.; Karam, D.; Doriguetto, A.C.; Dos Santos, M.H.; Filho, P.R.D.S.S.; De Souza, T.C. The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Sci. Rep. 2019, 9, 8164. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A.; Ramadan, W.A. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci. Hortic. 2015, 184, 101–105. [Google Scholar] [CrossRef]
- Guan, Y.-J.; Hu, J.; Wang, X.-J.; Shao, C.-X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Kamari, A.; Pulford, I.; Hargreaves, J.S. Chitosan as a potential amendment to remediate metal contaminated soil—A characterisation study. Colloids Surf. B Biointerfaces 2011, 82, 71–80. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J.-K. Cell Signaling during Cold, Drought, and Salt Stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wollenweber, B.; Jiang, N.; Liu, F.; Zhao, J. Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J. Exp. Bot. 2008, 59, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Khati, P.; Chaudhary, P.; Gangola, S.; Bhatt, P.; Sharma, A. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech 2017, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Ali, A.; Ahmad, M.; Abbas, G.; Akhtar, M.N.; Atif, M. UREA biosensor based on magnetic nano particles (Co3O4, Fe3O4) for the estimation of urea concentration in blood and urine samples. J. Optoelectron. Adv. Mater. 2015, 17, 1515–1521. [Google Scholar]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Oh, M.H.; Lee, M.S.; Nam, Y.S.; Park, T.G.; Jeong, J.H. Stabilized calcium phosphate nano-aggregates using a dopa-chitosan conjugate for gene delivery. Int. J. Pharm. 2013, 445, 196–202. [Google Scholar] [CrossRef]
- Li, J.; Sun, H.; Sun, D.; Yao, Y.; Yao, F.; Yao, K. Biomimetic multicomponent polysaccharide/nano-hydroxyapatite composites for bone tissue engineering. Carbohydr. Polym. 2011, 85, 885–894. [Google Scholar] [CrossRef]
- Razavi, M.; Qiao, Y.; Thakor, A.S. Three-dimensional cryogels for biomedical applications. J. Biomed. Mater. Res. Part A 2019, 107, 2736–2755. [Google Scholar] [CrossRef]
- Jaiswal, A.; Chattopadhyay, A.; Ghosh, S.S. Functional chitosan nanocarriers for potential applications in gene therapy. Mater. Lett. 2012, 68, 261–264. [Google Scholar] [CrossRef]
- Bandara, S.; Carnegie, C.-A.; Johnson, C.; Akindoju, F.; Williams, E.; Swaby, J.M.; Oki, A.; Carson, L. Synthesis and characterization of Zinc/Chitosan-Folic acid complex. Heliyon 2018, 4, e00737. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Burden of Foodborne Illness: Findings. Available online: https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on 1 June 2020).
- World Health Organization. Estimating the Burden of Foodborne Diseases. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 1 June 2020).
- Garrido-Maestu, A.; Ma, Z.; Paik, S.-Y.-R.; Chen, N.; Ko, S.; Tong, Z.; Jeong, K.C. Engineering of chitosan-derived nanoparticles to enhance antimicrobial activity against foodborne pathogen Escherichia coli O157:H7. Carbohydr. Polym. 2018, 197, 623–630. [Google Scholar] [CrossRef]
- Melo, N.F.C.B.; De Mendonçasoares, B.L.; Diniz, K.M.; Leal, C.F.; Canto, D.; Flores, M.A.; Tavares-Filho, J.H.D.C.; Galembeck, A.; Stamford, T.L.M.; Stamford-Arnaud, T.M.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Boil. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Paomephan, P.; Assavanig, A.; Chaturongakul, S.; Cady, N.C.; Bergkvist, M.; Niamsiri, N. Insight into the antibacterial property of chitosan nanoparticles against Escherichia coli and Salmonella Typhimurium and their application as vegetable wash disinfectant. Food Control. 2018, 86, 294–301. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. LWT-Food Sci. Technol. 2016, 71, 347–355. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Corona-Rangel, M. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Kavaz, D.; Idris, M.; Onyebuchi, C. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Boil. Macromol. 2019, 123, 837–845. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.-S.; Kim, E.S.; Lee, H.G. Preparation, characterization, and food application of rosemary extract-loaded antimicrobial nanoparticle dispersions. LWT 2019, 101, 138–144. [Google Scholar] [CrossRef]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. Int. J. Boil. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of Cardamom Essential Oil in Chitosan Nano-composites: In-vitro Efficacy on Antibiotic-Resistant Bacterial Pathogens and Cytotoxicity Studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.; Bautista-Baños, S.; Ramos-García, M.D.L.; Martínez-González, M.D.C.; Hernández-Romano, J. Physicochemical characterization and antimicrobial activity of edible propolis-chitosan nanoparticle films. Prog. Org. Coat. 2019, 137, 105326. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Esmaiili, M. Simultaneous green synthesis and in-situ impregnation of silver nanoparticles into organic nanofibers by Lythrum salicaria extract: Morphological, thermal, antimicrobial and release properties. Mater. Sci. Eng. C 2019, 105, 110115. [Google Scholar] [CrossRef]
- Lee, E.H.; Khan, I.; Oh, D.-H. Evaluation of the efficacy of nisin-loaded chitosan nanoparticles against foodborne pathogens in orange juice. J. Food Sci. Technol. 2018, 55, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Tango, C.N.; Miskeen, S.; Oh, D.-H. Evaluation of nisin-loaded chitosan-monomethyl fumaric acid nanoparticles as a direct food additive. Carbohydr. Polym. 2018, 184, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Shabbir, S.; Noor, T.; Rahman, A.; Bokhari, H.; Imran, M. Potential of polymer stabilized nano-liposomes to enhance antimicrobial activity of nisin Z against foodborne pathogens. LWT 2018, 96, 98–110. [Google Scholar] [CrossRef]
- Divsalar, E.; Tajik, H.; Moradi, M.; Forough, M.; Lotfi, M.; Kuswandi, B. Characterization of cellulosic paper coated with chitosan-zinc oxide nanocomposite containing nisin and its application in packaging of UF cheese. Int. J. Boil. Macromol. 2018, 109, 1311–1318. [Google Scholar] [CrossRef]
- Lotfi, M.; Tajik, H.; Moradi, M.; Forough, M.; Divsalar, E.; Kuswandi, B. Nanostructured chitosan/ monolaurin film: Preparation, characterization and antimicrobial activity against Listeria monocytogenes on ultrafiltered white cheese. LWT 2018, 92, 576–583. [Google Scholar] [CrossRef]
- Sharaf, O.; Al-Gamal, M.S.; Ibrahim, G.; Dabiza, N.M.; Salem, S.; El-Ssayad, M.F.; Youssef, A. Evaluation and characterization of some protective culture metabolites in free and nano-chitosan-loaded forms against common contaminants of Egyptian cheese. Carbohydr. Polym. 2019, 223, 115094. [Google Scholar] [CrossRef]
- Bernela, M.; Kaur, P.; Chopra, M.; Thakur, R. Synthesis, characterization of nisin loaded alginate–chitosan–pluronic composite nanoparticles and evaluation against microbes. LWT 2014, 59, 1093–1099. [Google Scholar] [CrossRef]
- Cortés-Higareda, M.; Ramos-García, M.D.L.; Correa-Pacheco, Z.N.; García, J.C.D.R.; Bautista-Baños, S. Nanostructured chitosan/propolis formulations: Characterization and effect on the growth of Aspergillus flavus and production of aflatoxins. Heliyon 2019, 5, e01776. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ip, M.; Leung, A.W.; Yang, Z.; Wang, P.; Zhang, B.-T.; Ip, S.; Xu, C. Sonodynamic action of curcumin on foodborne bacteria Bacillus cereus and Escherichia coli. Ultrasonics 2015, 62, 75–79. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials. 2018, 8, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pola, C.C.; Moraes, A.R.; Medeiros, E.A.A.; Teófilo, R.F.; Soares, N.D.F.F.; Gomes, C.L. Development and optimization of pH-responsive PLGA-chitosan nanoparticles for triggered release of antimicrobials. Food Chem. 2019, 295, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Nisaa, K.; Bhattacharyya, S.; Mallick, A.I. Immunogenicity and protective efficacy of mucosal delivery of recombinant hcp of Campylobacter jejuni Type VI secretion system (T6SS) in chickens. Mol. Immunol. 2019, 111, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.-M.; You, J.; Adhikari, M.D.; Hong, S.-G.; Lee, I.; Kim, H.S.; Na Kim, L.; Nam, J.; Kwon, S.J.; Kim, M.I.; et al. Enzyme-Immobilized Chitosan Nanoparticles as Environmentally Friendly and Highly Effective Antimicrobial Agents. Biomacromolecules 2019, 20, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.; Gkogka, E.; Le Guyader, F.S.; Loisy-Hamon, F.; Lee, A.; Van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C.; et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018, 285, 110–128. [Google Scholar] [CrossRef] [PubMed]
- DiCaprio, E.; Ma, Y.; Hughes, J.; Li, J. Epidemiology, Prevention, and Control of the Number One Foodborne Illness. Infect. Dis. Clin. N. Am. 2013, 27, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Inanli, A.G.; Tümerkan, E.T.A.; Abed, N.E.; Regenstein, J.M.; Ozogul, F. The impact of chitosan on seafood quality and human health: A review. Trends Food Sci. Technol. 2020, 97, 404–416. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Murugan, C.; Sharma, V.; Murugan, R.K.; Malaimegu, G.; Sundaramurthy, A. Two-dimensional cancer theranostic nanomaterials: Synthesis, surface functionalization and applications in photothermal therapy. J. Control. Release 2019, 299, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Cui, X. Two-dimensional non-carbonaceous materials-enabled efficient photothermal cancer therapy. Nano Today 2016, 11, 292–308. [Google Scholar] [CrossRef]
- Saneja, A.; Kumar, R.; Arora, D.; Kumar, S.; Panda, A.K.; Jaglan, S. Recent advances in near-infrared light-responsive nanocarriers for cancer therapy. Drug Discov. Today 2018, 23, 1115–1125. [Google Scholar] [CrossRef]
- Rao, W.; Wang, H.; Han, J.; Zhao, S.; Dumbleton, J.; Agarwal, P.; Zhang, W.; Zhao, G.; Yu, J.; Zynger, D.L.; et al. Chitosan-Decorated Doxorubicin-Encapsulated Nanoparticle Targets and Eliminates Tumor Reinitiating Cancer Stem-like Cells. ACS Nano 2015, 9, 5725–5740. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, F.; Peng, H.; Zhang, Y.; Li, Y.; Xu, Y.; Xie, J. Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy. Colloids Surf. B Biointerfaces 2019, 176, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ruan, L.; Zheng, T.; Wang, D.; Zhou, M.; Lu, H.; Gao, J.; Chen, J.; Hu, Y. A surface convertible nanoplatform with enhanced mitochondrial targeting for tumor photothermal therapy. Colloids Surf. B Biointerfaces 2020, 189, 110854. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Han, P.; Liu, Y.; Li, W.; Zhu, F.; Fu, K.; Chu, M. Biocompatible chitosan-carbon nanocage hybrids for sustained drug release and highly efficient laser and microwave co-irradiation induced cancer therapy. Acta Biomater. 2020, 103, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.; Potara, M.; Gabudean, A.-M.; Juhem, A.; Baldeck, P.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, T.; Xie, Y.; Sun, Z.; Liu, H.; Lin, J.; Liu, C.; Mao, Z.-W.; Nie, S. Chitosan layered gold nanorods as synergistic therapeutics for photothermal ablation and gene silencing in triple-negative breast cancer. Acta Biomater. 2015, 25, 194–204. [Google Scholar] [CrossRef]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
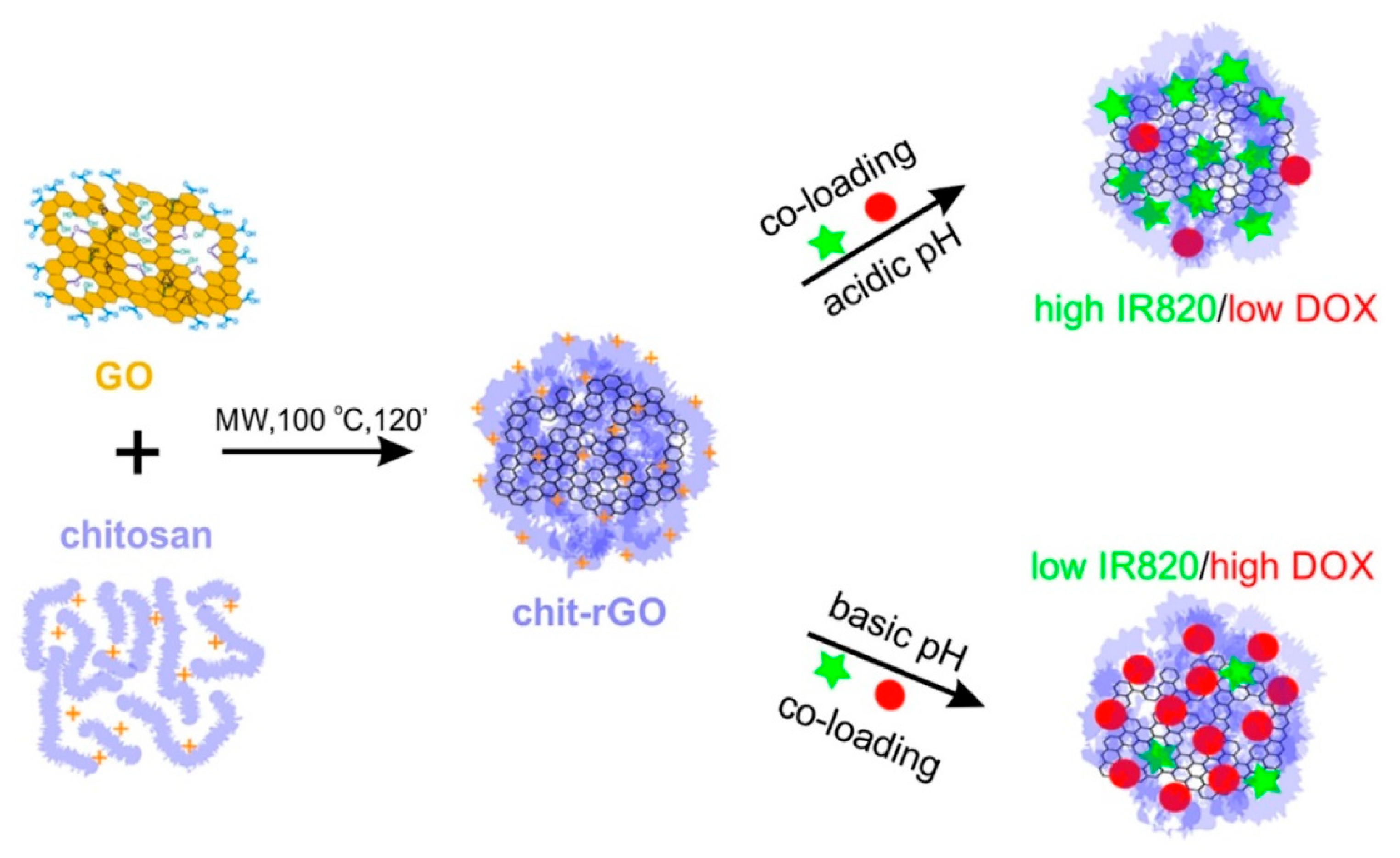
- Zaharie-Butucel, D.; Potara, M.; Suarasan, S.; Licarete, E.; Astilean, S. Efficient combined near-infrared-triggered therapy: Phototherapy over chemotherapy in chitosan-reduced graphene oxide-IR820 dye-doxorubicin nanoplatforms. J. Colloid Interface Sci. 2019, 552, 218–229. [Google Scholar] [CrossRef]
- Yana, T.; Heb, J.; Liua, R.; Liua, Z.; Chengac, J. Chitosan capped pH-responsive hollow mesoporous silica nanoparticles for targeted chemo-photo combination therapy. Carbohydr. Polym. 2020, 231, 115706. [Google Scholar] [CrossRef]
- Rajasekar, S.; Martin, E.M.; Kuppusamy, S.; Vetrivel, C.; Monisha, E. Chitosan coated molybdenum sulphide nanosheet incorporated with tantalum oxide nanomaterials for improving cancer photothermal therapy. Arab. J. Chem. 2020, 13, 4741–4750. [Google Scholar] [CrossRef]
- Duab, K.; Leia, P.; Dongab, L.; Zhangab, M.; Gaoab, X.; Yaoa, S.; Feng, J.; Zhang, H. In situ decorating of ultrasmall Ag2Se on upconversion nanoparticles as novel nanotheranostic agent for multimodal imaging-guided cancer photothermal therapy. Appl. Mater. Today 2020, 18, 100497. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Zhou, N.; Yuan, P.; Su, Y.; Shao, M.; Chi, C.; Pan, F. Near-infrared light triggered photo-therapy, in combination with chemotherapy using magnetofluorescent carbon quantum dots for effective cancer treating. Carbon 2017, 118, 752–764. [Google Scholar] [CrossRef]
- Li, R.; Du, Y.; Guo, W.; Su, Y.; Meng, Y.; Shan, Z.; Feng, Y.; Meng, S. Methotrexate coated AZA-BODIPY nanoparticles for chemotherapy, photothermal and photodynamic synergistic therapy. Dye. Pigment. 2020, 179, 108351. [Google Scholar] [CrossRef]
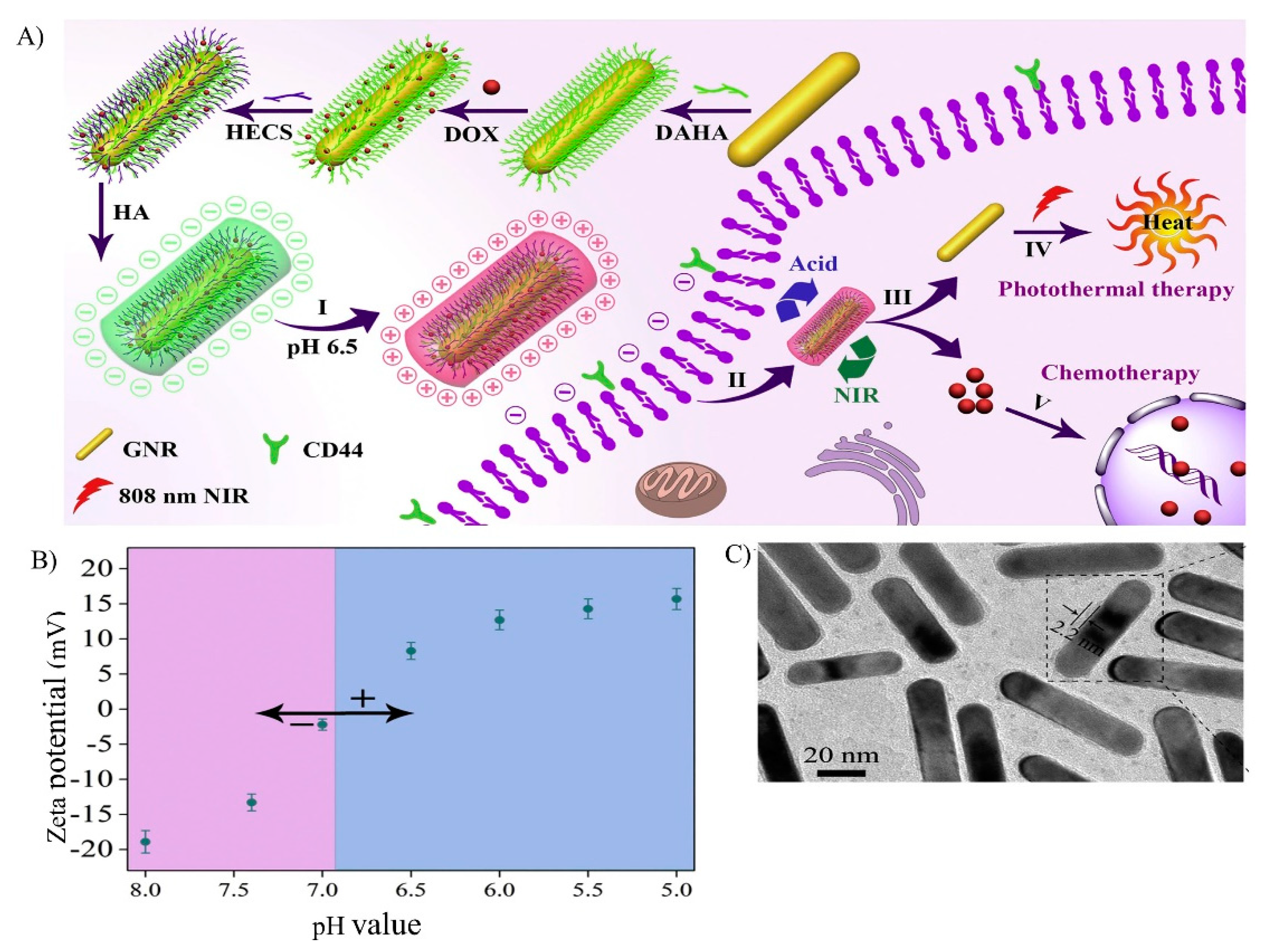
- Xu, W.; Wang, J.; Qian, J.; Hou, G.; Wang, Y.; Ji, L.; Suo, A. NIR/pH dual-responsive polysaccharide-encapsulated gold nanorods for enhanced chemo-photothermal therapy of breast cancer. Mater. Sci. Eng. C 2019, 103, 109854. [Google Scholar] [CrossRef]
- Hou, G.; Qian, J.; Xu, W.; Sun, T.; Wang, Y.; Wang, J.; Ji, L.; Suo, A. A novel pH-sensitive targeting polysaccharide-gold nanorod conjugate for combined photothermal-chemotherapy of breast cancer. Carbohydr. Polym. 2019, 212, 334–344. [Google Scholar] [CrossRef]
- Huang, X.; Xu, C.; Li, Y.; Cheng, H.-B.; Wang, X.; Sun, R. Quaternized chitosan-stabilized copper sulfide nanoparticles for cancer therapy. Mater. Sci. Eng. C 2019, 96, 129–137. [Google Scholar] [CrossRef]
- Almada, M.; Leal-Martínez, B.; Hassan, N.; Kogan, M.; Burboa, M.; Topete, A.; Valdez, M.; Juárez, J. Photothermal conversion efficiency and cytotoxic effect of gold nanorods stabilized with chitosan, alginate and poly(vinyl alcohol). Mater. Sci. Eng. C 2017, 77, 583–593. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Zhou, L.; Shang, L.; Su, Z. Reduced graphene oxide (rGO) hybridized hydrogel as a near-infrared (NIR)/pH dual-responsive platform for combined chemo-photothermal therapy. J. Colloid Interface Sci. 2018, 536, 160–170. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Kumawat, M.K.; Prasad, R.; Reddy, P.K.; Dhanka, M.; Mishra, S.K.; Bahadur, R.; Neekhra, S.; De, A.; Srivastava, R. Plasmonic carbon nanohybrids for repetitive and highly localized photothermal cancer therapy. Colloids Surf. B Biointerfaces 2018, 172, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Nguyen, V.T.; Jun, S.W.; Hoang, G.; Mondal, S.; Kim, H.; Doan, V.H.M.; Kim, J.; Kim, C.-S.; Oh, J. Anti-EGFR antibody conjugated thiol chitosan-layered gold nanoshells for dual-modal imaging-guided cancer combination therapy. J. Control. Release 2019, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Shanavas, A.; Rengan, A.K.; Chauhan, D.S.; George, L.; Vats, M.; Kaur, N.; Yadav, P.; Mathur, P.; Chakraborty, S.; Tejaswini, A.; et al. Glycol chitosan assisted in situ reduction of gold on polymeric template for anti-cancer theranostics. Int. J. Boil. Macromol. 2018, 110, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Choi, W.-I.; Kim, M.; Tae, G. Tumor-targeting nanogel that can function independently for both photodynamic and photothermal therapy and its synergy from the procedure of PDT followed by PTT. J. Control. Release 2013, 171, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Choi, W.-I.; Kim, Y.H.; Tae, G.; Lee, S.Y.; Kim, K.; Kwon, I.C. In-vivo tumor targeting of pluronic-based nano-carriers. J. Control. Release 2010, 147, 109–117. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Li, M.; Luo, Z.; Zhang, J.; Guo, X.; Cai, K. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials. 2018, 157, 107–124. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chiou, S.-H.; Chou, C.-P.; Chen, Y.-C.; Huang, Y.-J.; Peng, C.-A. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomed. Nanotechnol. Boil. Med. 2011, 7, 69–79. [Google Scholar] [CrossRef]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial Photothermal and Immuno Cancer Therapy Using Chitosan-Coated Hollow Copper Sulfide Nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef]
- Wang, C.; Mallela, J.; Garapati, U.S.; Ravi, S.; Chinnasamy, V.; Girard, Y.; Howell, M.; Mohapatra, S. A chitosan-modified graphene nanogel for noninvasive controlled drug release. Nanomed. Nanotechnol. Boil. Med. 2013, 9, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Chen, J.; Bi, Y.; Xu, X.; Zhou, H.; Gao, J.; Hu, Y.; Zhao, Y.; Chai, Z. Near-infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 2015, 17, 201–209. [Google Scholar] [CrossRef]
- Jacinto, T.A.; Rodrigues, C.F.; Moreira, A.F.; Miguel, S.P.; Costa, E.C.; Ferreira, P.; Correia, I.J. Hyaluronic acid and vitamin E polyethylene glycol succinate functionalized gold-core silica shell nanorods for cancer targeted photothermal therapy. Colloids Surf. B Biointerfaces 2020, 188, 110778. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, X.-C.; Luo, Y.-L.; Chang, Y.-C.; Hsieh, Y.-Z.; Hsu, H.-Y. Non-metallic nanomaterials in cancer theranostics: A review of silica- and carbon-based drug delivery systems. Sci. Technol. Adv. Mater. 2013, 14, 044407. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Wang, Z.; Chen, L.; Wang, X.; Huang, C.; Cui, M.; Yang, D.-P.; Jia, N. Combining Magnetic Resonance Imaging with Photothermal Therapy of CuS@BSA Nanoparticles for Cancer Theranostics. ACS Appl. Nano Mater. 2018, 1, 2332–2340. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, X.; Liu, Y.; Liu, H.; Zhao, K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J. Pharm. Sci. 2016, 11, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the Fundamental Mechanisms of Cell Death Triggered by Photothermal Therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Asgari-Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018, 127, 393–402. [Google Scholar] [CrossRef]
- Abdel-Aziz, H. Effect of Priming with Chitosan Nanoparticles on Germination, Seedling Growth and Antioxidant Enzymes of Broad Beans. Catrina Int. J. Environ. Sci. 2019, 18, 81–86. [Google Scholar] [CrossRef]
- Murugan, K.; Anitha, J.; Dinesh, D.; Suresh, U.; Rajaganesh, R.; Chandramohan, B.; Subramaniam, J.; Paulpandi, M.; Vadivalagan, C.; Amuthavalli, P.; et al. Fabrication of nano-mosquitocides using chitosan from crab shells: Impact on non-target organisms in the aquatic environment. Ecotoxicol. Environ. Saf. 2016, 132, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Darwesh, O.M.; Sultan, Y.Y.; Seif, M.M.; Marrez, D.A. Bio-evaluation of crustacean and fungal nano-chitosan for applying as food ingredient. Toxicol. Rep. 2018, 5, 348–356. [Google Scholar] [CrossRef]
- Gao, J.-Q.; Hu, Y.-L.; Han, F.; Qi, W.; Shao, J.-Z. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351–3359. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Dai, W.; Gao, J.; Li, H.; Tan, W.; Wang, J.; Deng, L.; Kong, Y. pH-sensitive drug delivery based on chitosan wrapped graphene quantum dots with enhanced fluorescent stability. Mater. Sci. Eng. C 2020, 112, 110888. [Google Scholar] [CrossRef]
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gündüz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 2014, 62, 243–250. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticles | Targeted Pollutants | Effectiveness and/or Efficiency | Reference |
|---|---|---|---|
| Nanochitosan | Pb(II) in water | Adsorption capacity: 32.26 mg/g at pH 6 | [30] |
| Magnetic chitosan nanoparticles | Pb(II) and Cd(II) in wastewater | Adsorption capacity: 79.24 mg/g for Pb(II) and 36.42 mg/g for Cd(II) | [23] |
| Magnetic chitosan polyelectrolyte nanoparticles | Cd(II) in industrial wastewater | 97.5 removal from the original 100 mg/L concentration | [24] |
| Chitosan nanoparticle | Cr(III) in tannery wastewater | 70% removal of chromium in 24 h | [31] |
| chitosan magnetite nanoparticles | Cr(VI) in wastewater | 75–88% removal from the standard 500 mg/L K2Cr2O7 solution | [32] |
| Magnetic chitosan nanoparticles | Cr(VI) in wastewater | Adsorption capacity: 58.14 mg/g at pH 3.0 | [33] |
| chitosan-stabilized Fe/Cu bimetallic nanoparticles | Cr(VI) in different types of water | Removal efficiency: 90% (river water), 85%(tannery water), and 80% (smelting water) | [34] |
| Chitosan-/PVA-coated magnetic nanoparticles | Cu(II) in wastewater | Adsorption capacity: up to 500 mg/g at pH 5.0 | [35] |
| Chitosan gel nanoparticles | Cu(II) in wastewater | Adsorption capacity: 78–112 mg/L | [36] |
| Chitosan magnetite nanoparticles | Heavy metals in the water part of the sludge | Adsorption: 20–50% more heavy metals than magnetite | [37] |
| Chitosan nanoparticles | Eu(III) in water | Adsorption capacity: 114 mg/g, >30 times compared to crab shell particles | [22] |
| Chitin nanocrystals | Ag(I) in water | 27% removal from the original 107.8 mg/L concentration | [38] |
| Magnetic chitosan nanoparticles | Azo dyes in wastewater | 94–96% removal at pH 6.0 in 1 h | [39] |
| Magnetic chitosan nanoparticles | Dyes in wastewater | Adsorption capacity: 82.2 mg/g for removing Bromothymol Blue | [40] |
| Chitosan-silica nanoparticles with immobilized Cu(II) ions | 1,1–dimethyl hydrazine in wastewater | 100% degradation of 1,1–dimethyl hydrazine in 10 min | [41] |
| Chitosan modified multi-wall carbon nanotubes | Phosphate in wastewater | Adsorption capacity: 36.1 mg P/g, and 94–98% of the original efficiency after 5 cycles | [42] |
| Enzymatic chitosan nanoparticles | Phenols in wastewater | Higher thermostability than free enzyme and same activity | [43] |
| Highly deacetylated chitosan nanoparticles | Diclofenac and carbamazepine in wastewater | Adsorption capacity: up to 351.8 mg g−1 for diclofenac | [44] |
| Chitosan−silver Nanoparticles | Bacteria in drinking water | 99.99% removal of bacteria in 15 min, and complete removal in 8 h | [45] |
| Chitosan-coated silver nanoparticles | Various toxic contaminants | Inhibition of biofilm formation | [46] |
| 2(5H)–furanone loaded chitosan nanoparticles | COD* and color in Rice mill wastewater | Better foulant rejection, better removal of COD, and color | [27] |
| chitosan-doped MIL-100(Fe) nanoparticles | Bacteria in wastewater | higher biofouling resistance of 85% comparedto the original 51% | [47] |
| Silver-loaded chitosan nanoparticles | Foulants on hollow fiber membranes | Optimal rejection of 89.27 and 86.04% for Reactive Black 5 and Reactive Orange 16 | [48] |
| O-carboxymethyl chitosan-Fe3O4 nanoparticles | Foulants on membranes | Achieving the lowest irreversible fouling resistance of 4.2% at 0.05 wt.% | [28] |
| chitosan-grafted magnetic nanoparticles | Oil drops in emulsified wastewater | Best flocculation performance at pH 4.0, and reuse up to 7 times | [26] |
| Compoxund | Foodborne Pathogens | Major Method of Analysis | Values from Analysis | Particle Size (nm) | Reference |
|---|---|---|---|---|---|
| CSNPs | Escherichia coli O157:H7 | Log reduction (units not given) | 0.4–9.7 | <300 | [81] |
| Edible coating of CSNPs on grapes | Salmonella spp. | MIC (g/L) vs. MBC (g/L) | 3.0 vs. 6.0 | 128.3 | [82] |
| E. coli | 3.0 vs. 3.0 | ||||
| S. aureus | 2.0 vs. 6.0 | ||||
| P. aeruginosa | 3.0 vs. 4.0 | ||||
| L. monocytogenes | 3.0 vs. 6.0 | ||||
| Vegetable wash with CSNPs and 1% citric acid | E. coli | Reduction of viable bacteria (log CFU/g) | 1.63 | 352.7 ± 2.8 (most effective size) | [83] |
| S. Typhimurium | 1.16 | 865.9 ± 15.3 (most effective size) | |||
| Low molecular weight CSNPs | E. coli | MIC(%w/v) vs. MBC (%w/v) | 0.018 vs. 0.037 | 60 ± 5.48 | [84] |
| Medium molecular weight CSNPs | 0.037 vs. 0.075 | 78.50 ± 6.77 | |||
| Middle-viscous CSNPs (crab shell CS) | 0.037 vs. 0.075 | 105.20 ± 8.58 |
| Compound | Foodborne Pathogens | Major Method of Analysis | Values | Particle Size (nm) | Reference |
|---|---|---|---|---|---|
| CSNPs- lime essential oil (LEO) | S. aureus | Minimum Inhibitory Volume (µL) for CSNPs-LEO:CSNPs | 1.25:2.5 | 4.7 ± 1.2 (CSNPs) 6.1 ± 0.4 (CSNPs-LEO) | [85] |
| L. monocytogenes | 1.25:1.25 | ||||
| S. dysenteriae | 1.25:1.25 | ||||
| E. coli | 2.5:5 | ||||
| CSnanocapsules(CSNC) – lime essential oil (LEO) | S. aureus | Minimum Inhibitory Volume (µL) for CSNC-LEO:CSNC | 5:10 | 5.8 ± 1.6 (CSNC) 6.1 ± 0.6 (CSNC-LEO) | |
| L. monocytogenes | 5:20 | ||||
| S. dysenteriae | 5:no inhibition | ||||
| E. coli | 10:no inhibition | ||||
| Fish gelatin/CSNPs-oregano essential oil bio-nanofilm | S. aureus | Agar diffusion Method (highest effect observed at 1.2 (% w/v) OEO) | 26.33 ± 0.57 | 40–80 | [86] |
| L. monocytogenes | 26.66 ± 1.52 | ||||
| S. enteritidis | 30.33 ± 1.15 | ||||
| E. coli | 33.00 ± 1.00 | ||||
| CSNPs-Cyperus articulatus Essential oil (CPEO) (1: 0.25) | E. coli | MIC (mg/L) vs. MBC (mg/L) | 5 vs. 10 (CSNP-CPEO) 40 vs. 80 (CSNPs) 10 vs. 20 (CPEO) | 119 (CSNP-CPEO) | [87] |
| S. aureus | 10 vs. 15 (CSNP-CPEO) 80 vs.160 (CSNPs) 20 vs. 25 (CPEO) | ||||
| Rosemary extract loaded NPs with CS and ɣ-PGA | B. subtilis | Log reduction of growth in Barley tea (log CFU/mL) | More than 0.5–3.6 | 200–600 | [88] |
| Rosemary essential oil encapsulated in CS-Benzoic acid nanogel | S. aureus | MIC (µg/mL) | 40 | Less than 100 | [89] |
| Cardamom oil (CDEO) loaded CSNPs | E. coli (ESBL positive) | OD based micro-dilution broth assays | CDEO –CSNPs Maintained antimicrobial effect for 7 days against both pathogens. CSNPs alone was effective only for 48 h. | 50–100 | [90] |
| S. aureus (Methicillin- resistant) | |||||
| Clove essential oil (CEO) loaded CSNPs | L. monocytogenes | Minimum inhibitory volume (µL) CEO-CSNPs: CEO: CSNPs | 2:2:8 | 223–444 | [91] |
| E. coli | 2:4:8 | ||||
| S. aureus | 2:2:8 | ||||
| S. typhi | 2:2:8 |
| Compound | Foodborne Pathogens | Major Method of Analysis | Values | Particle Size (nm) | Reference |
|---|---|---|---|---|---|
| Edible film with CSNPs and 10% EEP | E. coli | CFU on Agar plates in 24 h vs. 48 h | 0 vs. 5.67 | 28.42 ± 7.43 (for CSNPs) | [92] |
| L. monocytogenes | 0 vs. 43.33 | ||||
| S. enteritidis | 11.33 vs. 13.33 | ||||
| Chitosan nanofiber-AgNPs | E. coli O157:H7 | Inhibition zone observed by Agar well diffusion method (mm) | 14.54 ± 0.23 | 40 (nanofibers) 45–60 (AgNPs) | [93] |
| S. aureus | 17.62 ± 0.205 | ||||
| Nisin-CSNPs (against pathogens inoculated in orange juice) | S. aureus | Log reduction of growth (CFU/mL) for Nisin-CSNPs:CSNPs | 3.82 ± 0.03: 2.21 ± 0.01 | 147.93 ± 2.9 (Nisin-CSNPs) 64.34 ± 2.12 (CSNPs) | [94] |
| L. monocytogenes | 3.61 ± 0.05:2.15 ± 0.04 | ||||
| E. coli O157:H7 | 3.49 ± 0.01:2.03 ± 0.03 | ||||
| S. Typhimurium | 2.88 ± 0.03:1.96 ± 0.01 | ||||
| Nisin-chitosan-fumaric acid | S. aureus | Log reduction of growth in 24 h (CFU/mL) | 3.43 | 207.93 ± 4.72 | [95] |
| L. monocytogenes | 3.30 | ||||
| E. coli O157:H7 | 3.33 | ||||
| Chitosomes with nisin | S. aureus | Minimum Inhibitory Concentration for nisin (µg/mL) | 5 | 50–108 | [96] |
| L. monocytogenes | 50 | ||||
| Enterococcus faecalis | 200 | ||||
| Edible Nisin loaded bilayer film with cellulose and chitosan-zinc oxide nanocomposite | L. monocytogenes | Log reduction of growth in UF cheese after 14 days (log CFU/g) | 2.7 (500 ppm nisin film) 5 (1000 ppm nisin film) | Not given | [97] |
| Monolaurin incorporated nanostructured chitosan-zinc oxide-cellulose films | L. monocytogenes | Log reduction of growth in UF cheese after 14 days (log CFU/g) | 2.4 (0.5% Monolaurin film) 2.3 (1% Monolaurin film) | Not given | [98] |
| Cell-free LAB culture supernatant loaded on CSNPs | Staphylococcus sciuri | Minimum Inhibitory Concentration (mg/mL) | 46.7 ± 2.77 | 5–10 (size was reported only for natamycin control loaded with CSNPs) | [99] |
| Bacillus cereus | 43.3 ± 1.39 | ||||
| Salmonella enterica | 40 ± 0.00 | ||||
| Escherichia coli | 80 ± 0.00 | ||||
| Pseudomonas aeruginosa | 80 ± 0.00 | ||||
| Penicillium chrysogenum | 175 ± 0.00 | ||||
| Candida parapsilosis | 550 ± 0.00 | ||||
| Nisin loaded alginate-chitosan-pluronic F68 nanoparticles | P. aeruginosa | Absorbance of inoculated samples at 600 nm | Inhibited microbial growth at least 20 days in nutrient media and up to 6 months in tomato juice | 208.2–831.9 | [100] |
| S. enterica | |||||
| E. aerogenes | |||||
| CS+ 40% Propolis NPs | Aspergillus flavus | % mycelial inhibition: % germination inhibition: Aflatoxins (µg/L) | 28.9:12.3:2.7 | 3.0 (CSNPs) 2.33 (PropolisNPs) | [101] |
| CS+ CSNPs | 21.2:8.0:1.5 | ||||
| CS+ Propolis extract | 15.3:4.5:2.0 | ||||
| CS+ 20% CSNPs+ 20% propolis Nps | 30.4:1.3:2.8 | ||||
| CS+ 20% CSNPs+ 20% propolis Nps+ Propolis extract | 33.0:96.6:2.8 | ||||
| CS+ 40% propolis Nps + Propolis extract | 18.1:55.5:2.6 | ||||
| CS+ 40% CSNPs + Propolis extract | 19.0: 6.5:2.5 | ||||
| CS-protamine nanoparticles | E.coli | MIC vs. MBC (µg/mL) | 31.25 vs. 31.25–62.5 | 27.67–32.23 | [103] |
| B. cereus | 31.25 to >250 vs. >250 | ||||
| PLGA-chitosan-TCIN nanoparticles | S. Typhimurium | MIC (µg/mL) vs. MBC (µg/mL) | ~16 vs. >64 | 277.3–295.0 | [104] |
| S. aureus | ~16 vs. >64 |
| Type of Chitosan Nano Polymer | Photothermal Agent | Tumor Models Used | Wavelength of Laser Source (nm) | Reference |
|---|---|---|---|---|
| Layer by layer modification with chitosan and sodium alginate | Graphene oxide | A549 human lung cancer cells | 808, 1 W/cm2 | [116] |
| Glutaldehyde-crosslinked chitosan layer | Single-walled carbon nanotubes | MB49 Murine bladder cancer cells | 808, 2 W/cm2 | [117] |
| Chitosan coating | Graphite carbon nanocages | CNE human nasopharyngeal cells and in vivo studies with tumor-bearing BALB/c nude mice | 808, 0.25 W/cm2 co-irradiated with microwave radiation (2–10 W, 2450 MHz) | [118] |
| Chitosan coating | Silver nanotriangles | NCI-H460 human non-small lung cancer cells | 800, 12–55 W/cm2 | [119] |
| Chitosan coating | Gold nanorods | MDA-MB-231 human breast cancer cells and in vivo studies with tumor-bearing thymic nude mice | 808, 0.5 W | [120] |
| Chitosan scaffolds | Graphene oxide | Human osteosarcoma cells and MC3T3-E1 pre-osteoblastic cells or human bone mesenchymal stem cells, in vivo studies for antitumor therapy, was conducted with male tumor-bearing mice | 808, 2.5 and 1.2 W/cm2 | [121] |
| Chitosan layer | Reduced Graphene oxide nanoflakes and IR820 dye | C26 murine colon carcinoma cells | 785, 9.62 W/cm2 | [122] |
| Chitosan layer | Pheophorbide | KB human oral squamous cell carcinoma cells and in vivo studies with tumor-bearing female BALB/c nude mice | 680, 0.5 W/cm2 | [123] |
| Chitosan coated nanosheet with tantalum oxide (TaO2) deposition | Molybdenum disulfide | MCF-7 human breast cancer cells | 808, 0.5 W/cm2 | [124] |
| Chitosan layer coating upconversion nanoparticles | Ag2Se | A549 human lung cancer cells and in vivo studies with tumor-bearing Kunming mice | 808, 1.3 W/cm2 | [125] |
| Iron crosslinked chitosan complexes | Carbon quantum dots | HeLa human cervix adenocarcinoma cells and HepG2 human hepatocellular carcinoma cells. | 671, 2W/cm2 | [126] |
| Type of Chitosan Nano Polymer | Photothermal Agent | Tumor Models Used | Wavelength of Laser Source (nm) | Reference |
|---|---|---|---|---|
| O-Carboxymethyl chitosan-based nanoshell carrier | Photosensitizer (ABDP-SI) based on AZA-boron dipyrrolide | HeLa human cervical cancer cells | 808, 1.2 W/cm2 | [127] |
| Hydroxyethyl chitosan coating | Gold nanorods | MCF-7 human breast cancer cells | 808, 2W/cm2 | [128] |
| Dihydrophenyl/hydrazide bifunctionalized Hydroxyethyl chitosan and oxidized hyaluronic acid coating | Gold nanorods | MCF-7 human breast cancer cells | 808, 2 W/cm2 | [129] |
| 2-hydroxypropyltrimethyl ammonium chloride chitosan coating | Copper sulfide | 4T1 mammary tumor cells (mouse) and in vivo studies with Balb/c mice | 808, 1.5 W/cm2 | [130] |
| Thiolated chitosan coating | Gold nanorods | MDA-MB-231 human breast cancer cells | 808, (1–2 W) | [131] |
| Carboxymethyl chitosan grafting | Reduced graphene oxide | L-929 mouse connective tissue fibroblasts | 808, 1 W/cm2 | [132] |
| Glycol chitosan coating | Graphene oxide-IR780 (NIR dye) and gold deposited plasmonic polylactic-co-glycolic acid nanoshells with graphene oxide | MDA-MB-231 and MCF-7 human breast cancer cells | 808, 500 mW | [133] |
| Thiol chitosan coating | Gold nanoshells | HeLa human cervix adenocarcinoma cells and MDA-MB-231 human breast cancer cells. in vivo studies with tumor-bearing female BALB/c nude mice | 808, 1.2 W/cm2 | [134] |
| Glycol chitosan coating on PLGA nanoparticles | Gold nanoshells | MCF-7 human breast cancer cells. | 808, 500 mW | [135] |
| Glycidyl methacrylate-conjugated chitosan functionalized nanogel | Gold nanorods | SCC7 mouse tumor cells, NIH/3T3 fibroblast cells and in vivo studies with male athymic nude mice | 808, 4 W/cm2 | [136] |
| 2,3-dimethylmaleic anhydride-modified chitosan oligosaccharide-block-poly (ethylene glycol) polymer coating | Gold nanorods | MCF-7 human breast cancer cells and in vivo studies with tumor-bearing female nude mice | 808, 1.5 to 2 W/cm2 | [138] |
| Water soluble chitosan functionalization | Single-walled carbon nanotubes | Glioblastoma CD133+ and CD133- cells, in vivo studies with tumor bearing BALB/c strain immunicompromised nude mice | 808, 2 W/cm2 | [139] |
| Thiolated chitosan surface coating | Hollow CuS nanoparticles | BALB/c mice bearing EMT6 tumor | 900 nm, 2W/cm2 | [140] |
| Acrylated chitosan coating | Chemically reduced graphene oxide | TRAMP-C1 mouse prostate cancer cells and Lewis lung cancer cells | 808, 900 mW/cm2 | [141] |
| Chitosan grafted oleic acid copolymer coating | Single-walled carbon nanotubes | HeLa human cervix adenocarcinoma cells | 808, 1 W/cm2 | [142] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials. Nanomaterials 2020, 10, 1903. https://doi.org/10.3390/nano10101903
Bandara S, Du H, Carson L, Bradford D, Kommalapati R. Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials. Nanomaterials. 2020; 10(10):1903. https://doi.org/10.3390/nano10101903
Chicago/Turabian StyleBandara, Subhani, Hongbo Du, Laura Carson, Debra Bradford, and Raghava Kommalapati. 2020. "Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials" Nanomaterials 10, no. 10: 1903. https://doi.org/10.3390/nano10101903
APA StyleBandara, S., Du, H., Carson, L., Bradford, D., & Kommalapati, R. (2020). Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials. Nanomaterials, 10(10), 1903. https://doi.org/10.3390/nano10101903






