Facile and Green Fabrication of Carrageenan-Silver Nanoparticles for Colorimetric Determination of Cu2+ and S2−
Abstract
1. Introduction
2. Experimental
2.1. Reagents
2.2. Preparation of Carrageenan-AgNPs
2.3. Characterization
2.4. Colorimetric Detection of Cu2+ and S2−
3. Results and Discussion
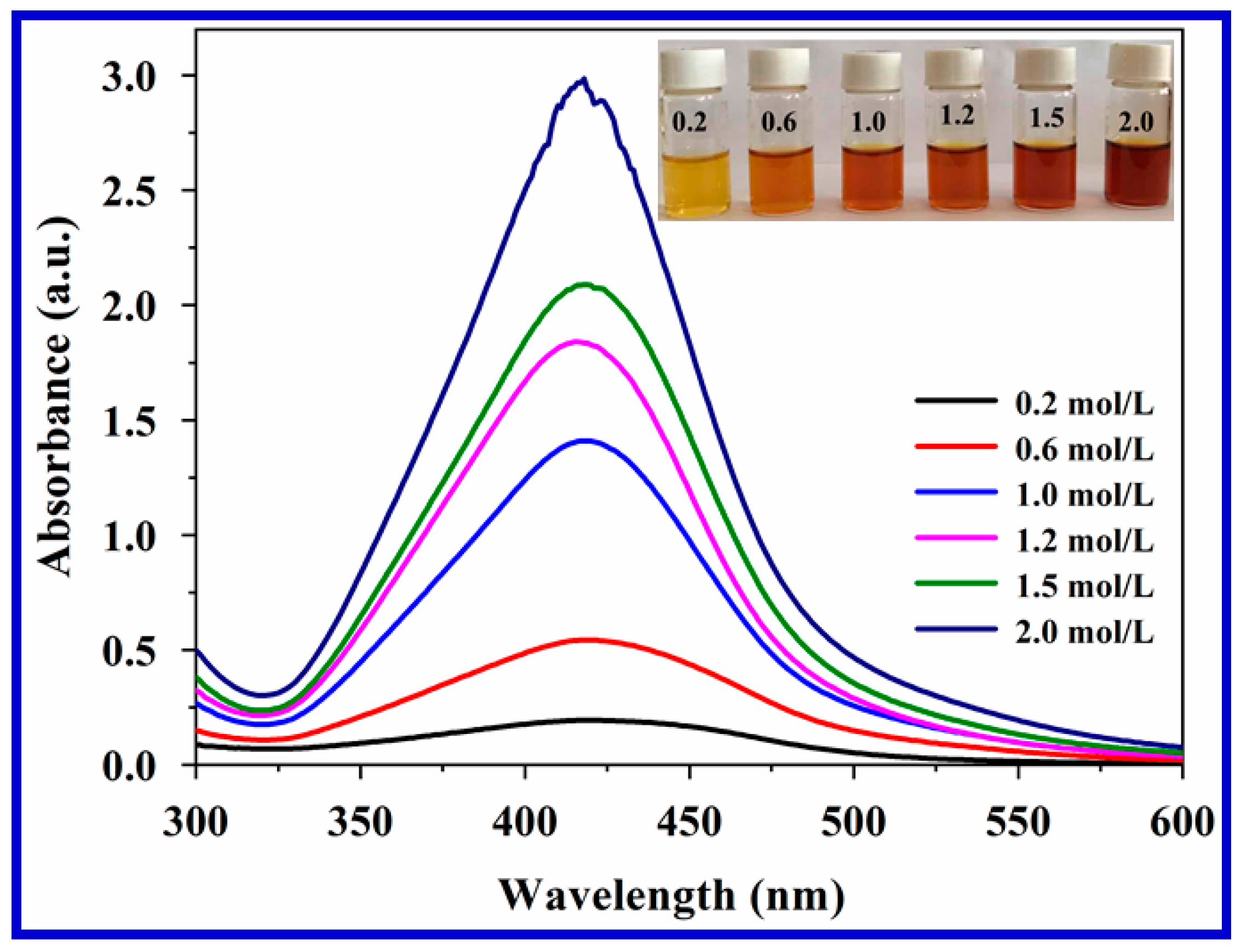
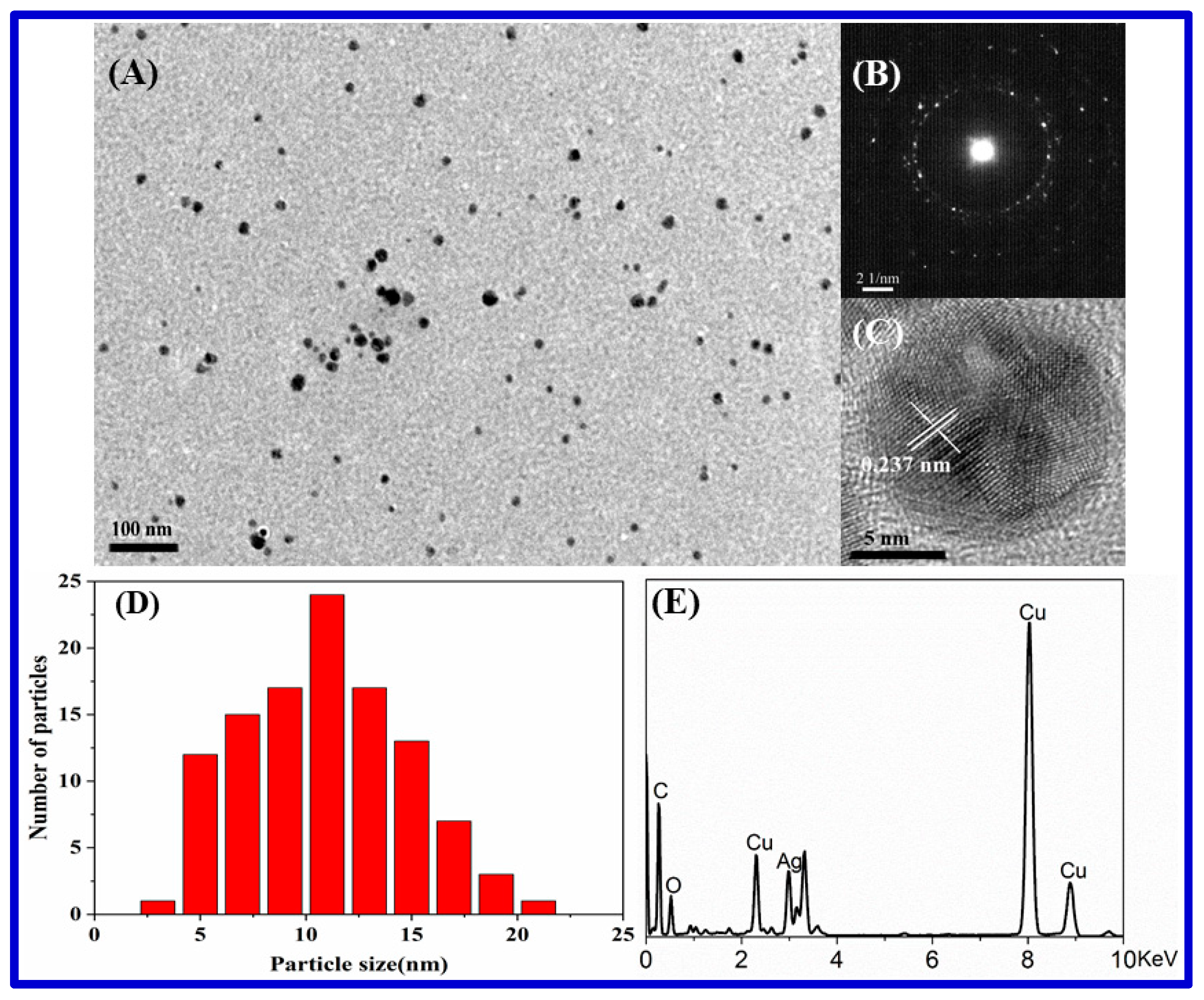
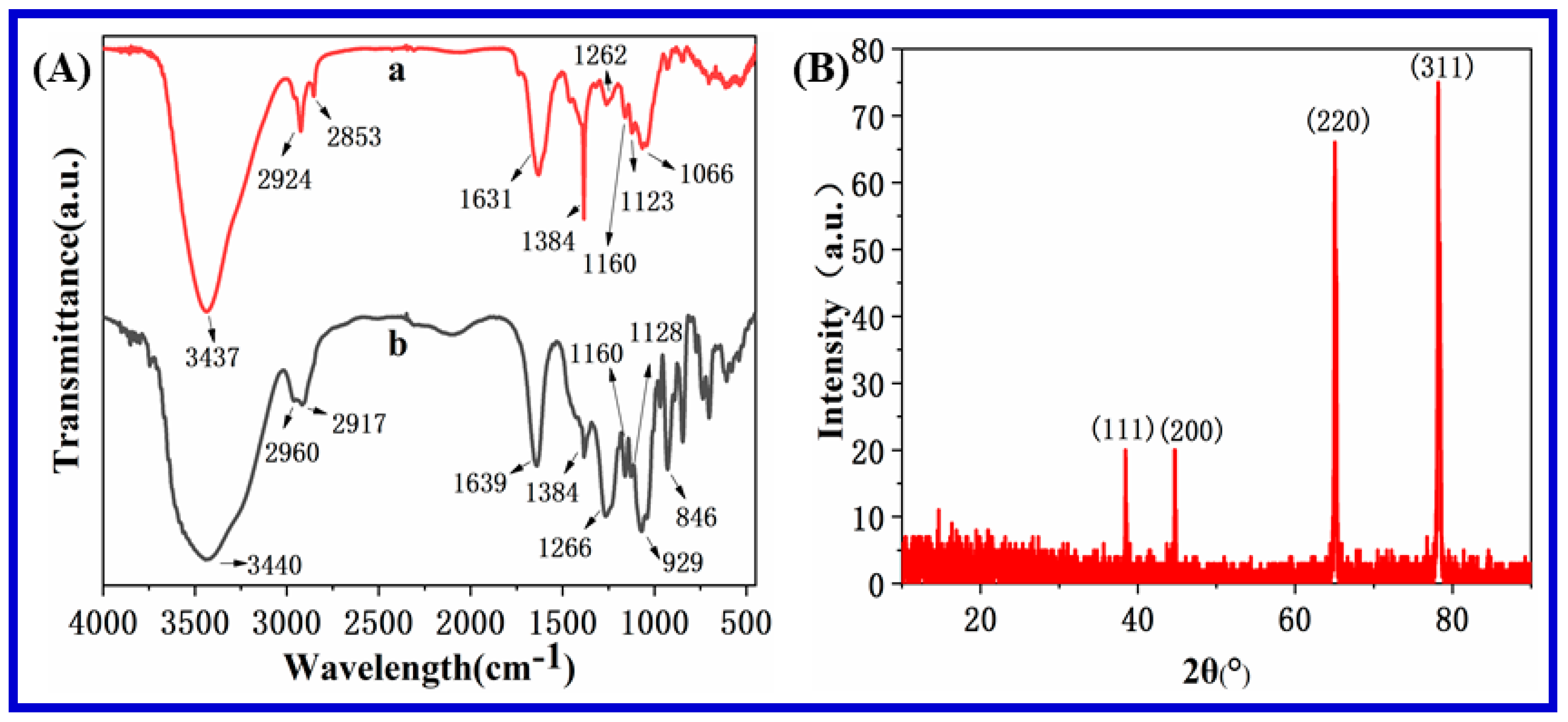
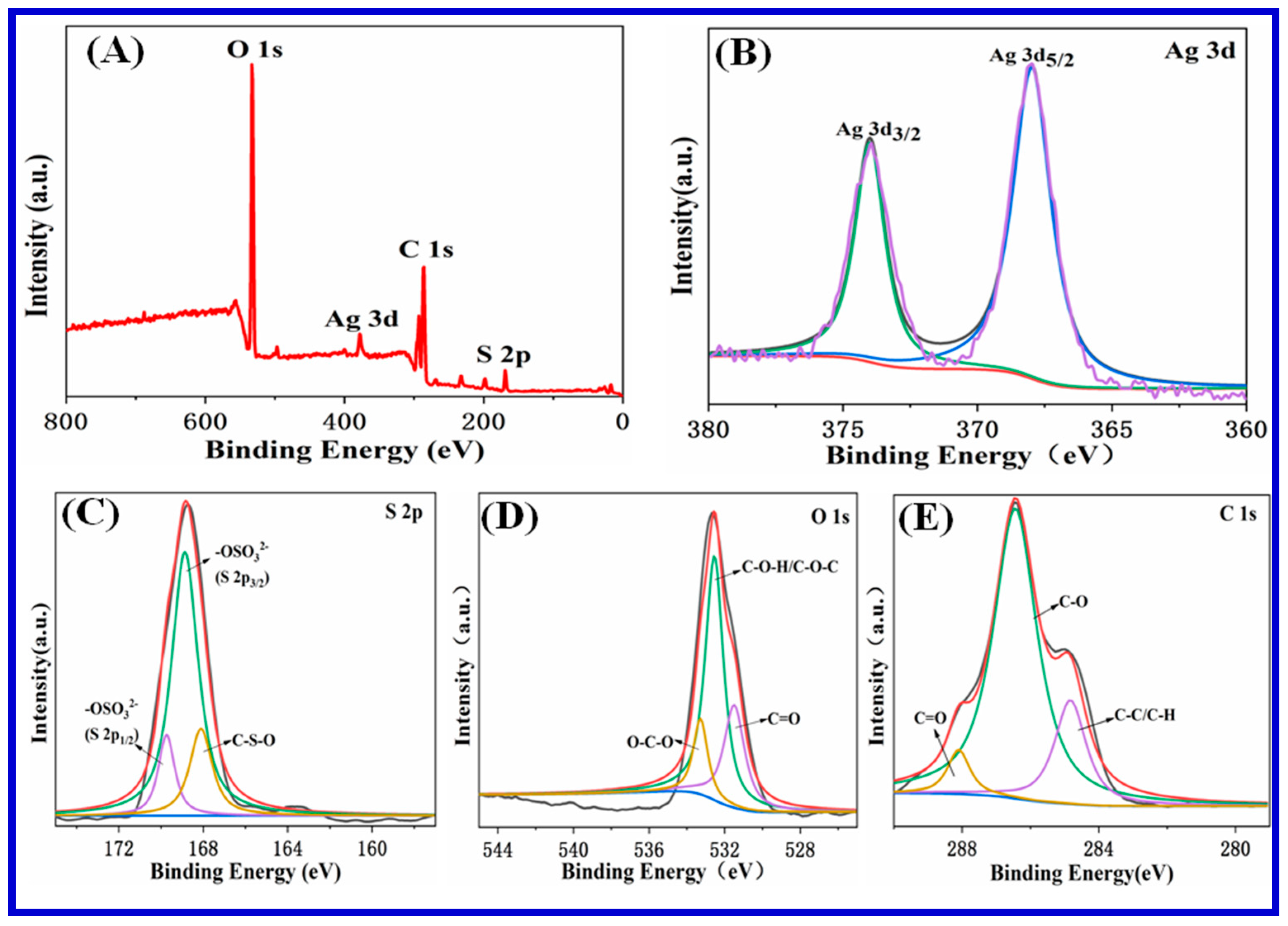
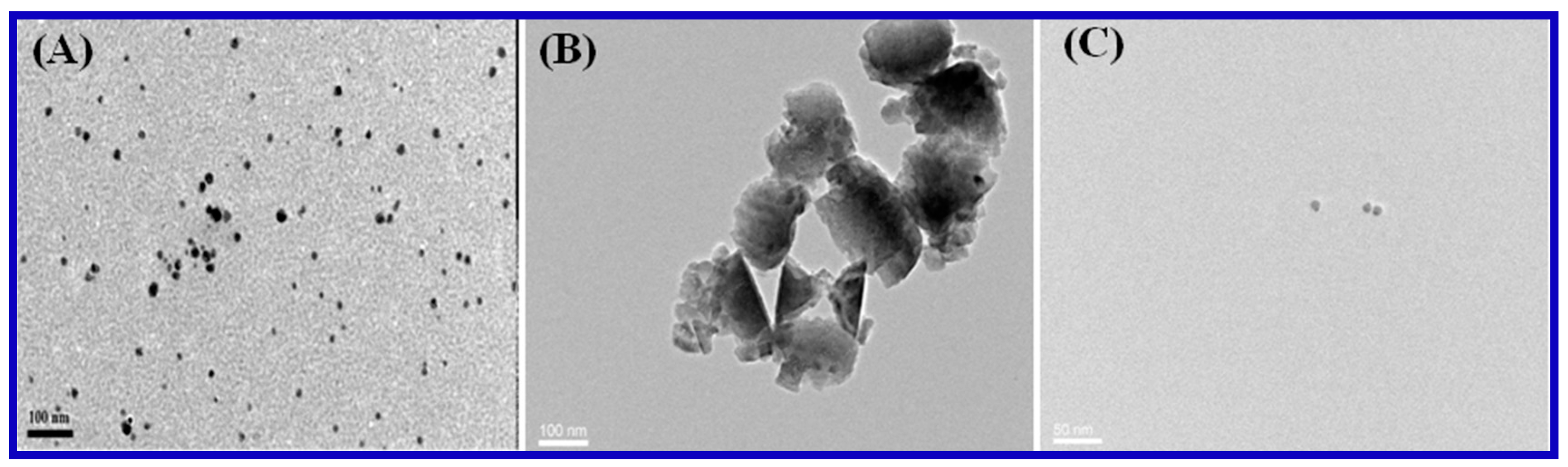
3.1. Characterization of Carrageenan-AgNPs
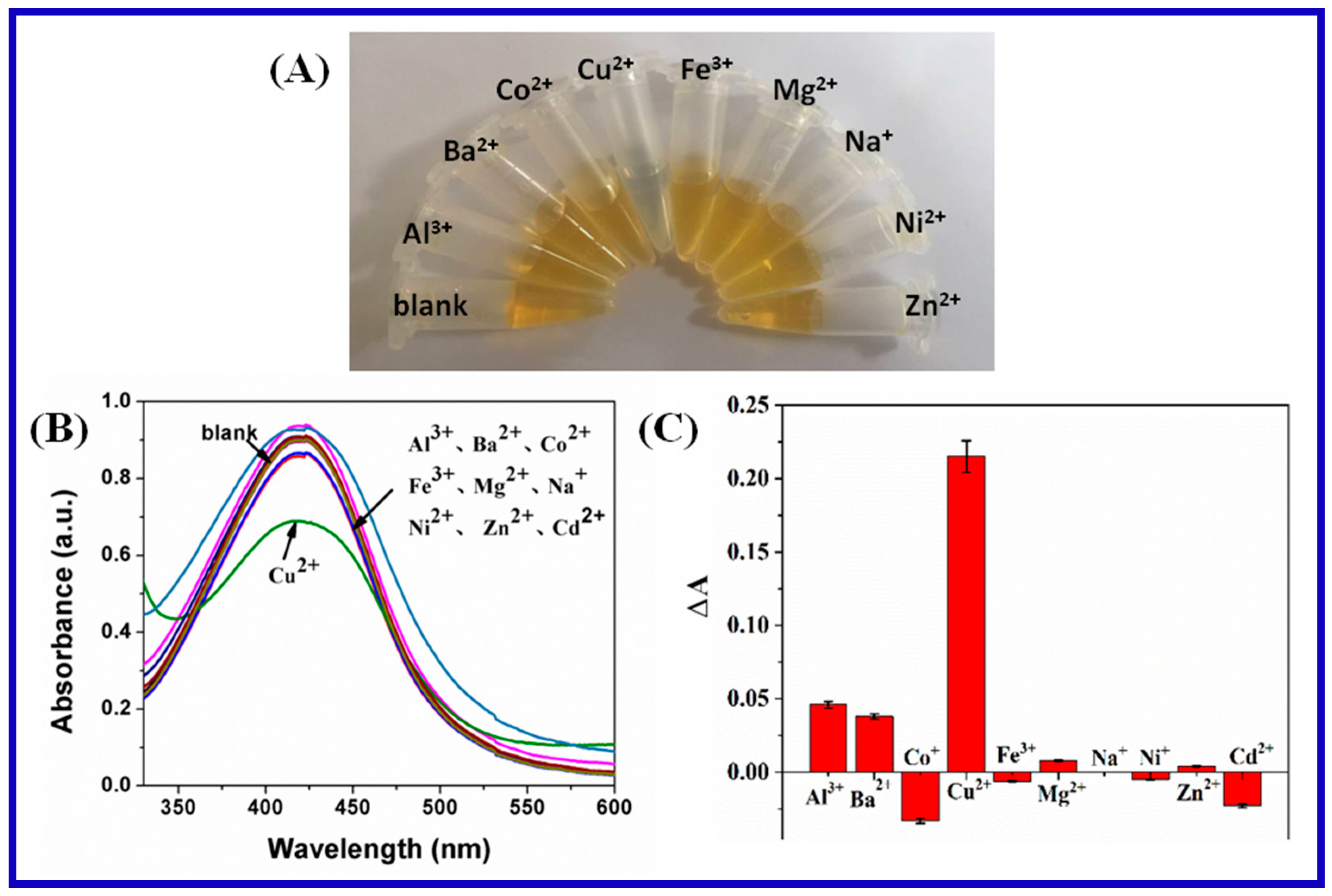
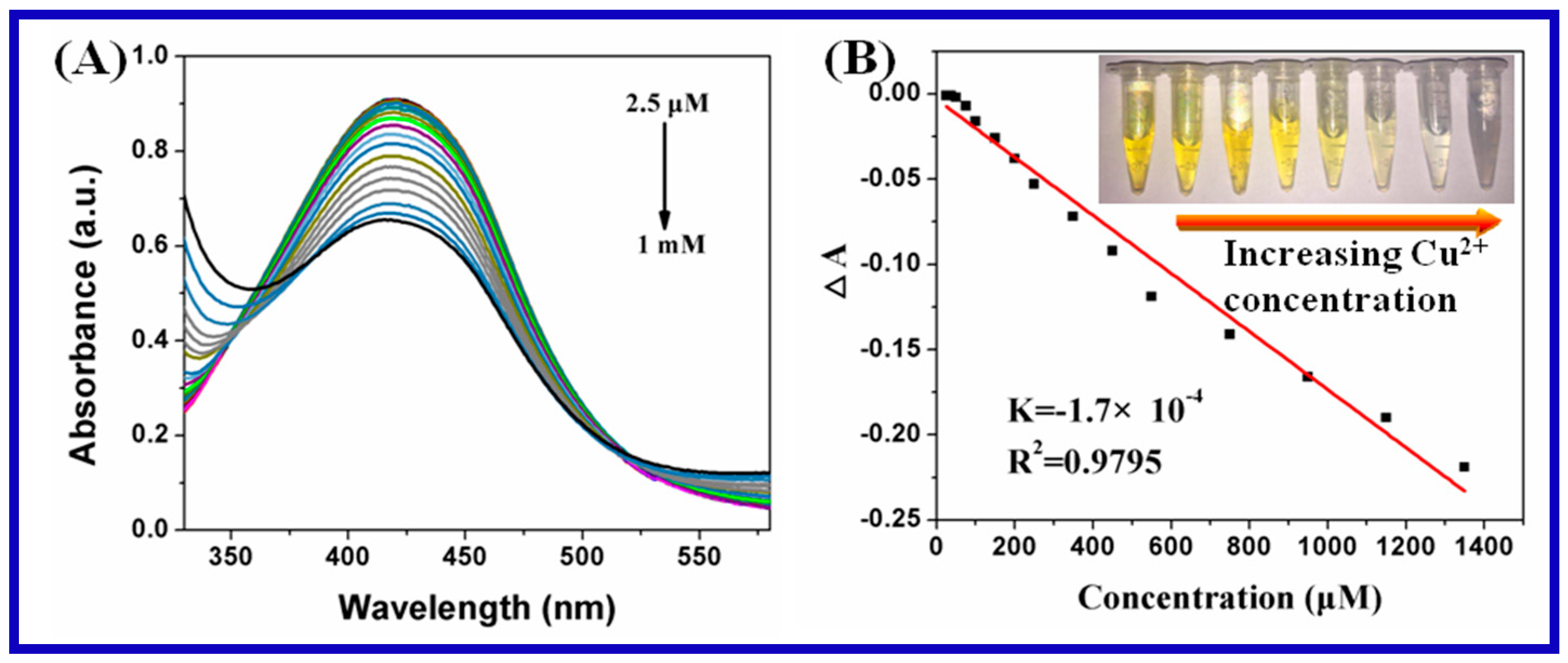
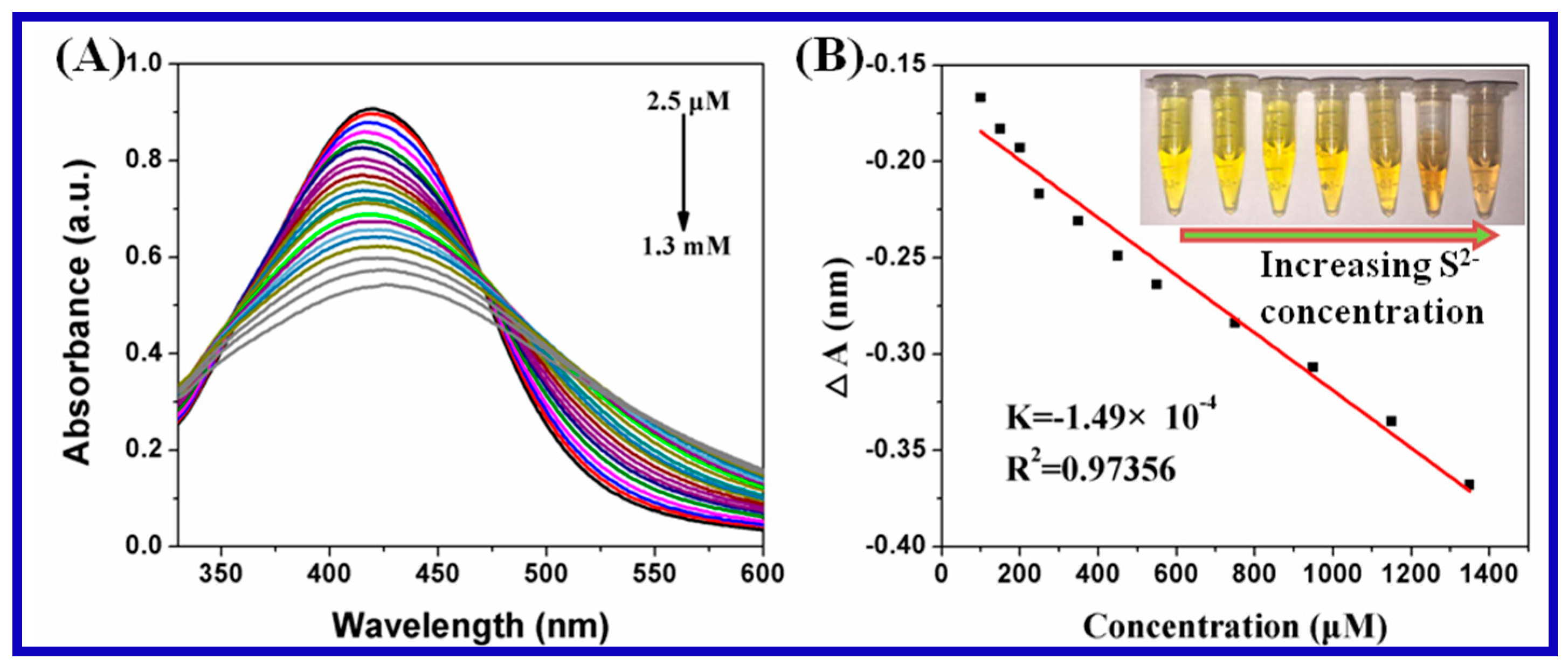
3.2. Ion Detection of Carrageenan-AgNPs
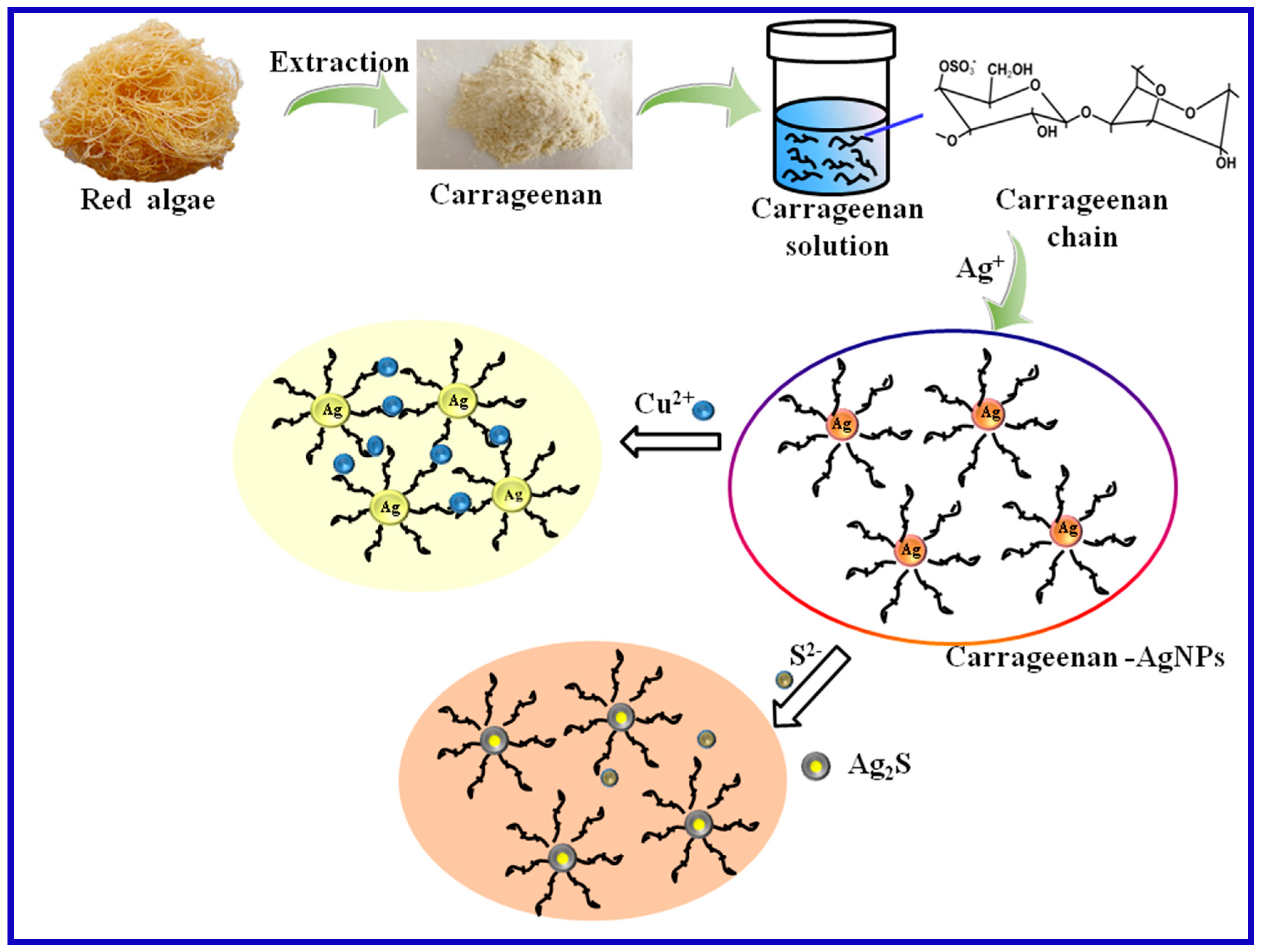
3.3. Mechanism for Carrageenan-AgNPs Formation and Ions Detection
3.4. Determination of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- So, H.; Chae, J.B.; Kim, C. A thiol-containing colorimetric chemosensor for relay recognition of Cu2+ and S2− in aqueous media with a low detection limit. Inorg. Chim. Acta 2019, 492, 83–90. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.Y.; Jung, J.M.; Kim, M.S.; Kim, C. Selective detection of Cu2+ and S2− by a colorimetric chemosensor: Experimental and theoretical calculations. Inorg. Chim. Acta 2018, 471, 709–717. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Li, Y.; Liu, Q.; Xie, M. Fluorescent and colorimetric sensor for Cu2+ ion based on formaldehyde modified hyperbranched polyethylenimine capped gold nanoparticles. Sens. Actuators B Chem. 2018, 255, 78–86. [Google Scholar] [CrossRef]
- Tachapermpon, Y.; Chaneam, S.; Charoenpanich, A.; Sirirak, J.; Wanichacheva, N. Highly Cu2+ sensitive and selective colorimetric and fluorescent probes: Utilizations in batch, flow analysis and living cell imaging. Sens. Actuators B Chem. 2017, 241, 868–878. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, W.; Sun, M.; Liu, X.; Zhang, Q.; Zong, C.; Qu, J.; Gai, H. Colorimetric detection of Cu2+ by surface coordination complexes of polyethyleneimine-capped Au nanoparticles. Sens. Actuators B Chem. 2016, 223, 411–416. [Google Scholar] [CrossRef]
- Fang, H.; Huang, P.C.; Wu, F.Y. A novel jointly colorimetric and fluorescent sensor for Cu2+ recognition and its complex for sensing S2− by a Cu2+ displacement approach in aqueous media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 568–575. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Park, W.H. Green synthesis of silver nanoparticles stabilized with mussel-inspired protein and colorimetric sensing of lead(II) and copper(II) ions. Int. J. Mol. Sci. 2016, 17, 2006. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Shen, J.; Zhang, C.; Wu, Z.; Zhou, H. A colorimetric quinoline-based chemosensor for sequential detection of copper ion and cyanide anions. Tetrahedron 2017, 73, 5715–5719. [Google Scholar] [CrossRef]
- Mohammadi, A.; Yaghoubi, S. Development of a highly selective and colorimetric probe for simultaneous detection of Cu2+ and CN− based on an azo chromophore. Sens. Actuators B Chem. 2017, 251, 264–271. [Google Scholar] [CrossRef]
- Mohammadi, A.; Yaghoubi, S. A new dual colorimetric chemosensor based on quinazolinone for CN−, AcO− and Cu2+ ions. Sens. Actuators B Chem. 2017, 241, 1069–1075. [Google Scholar] [CrossRef]
- More, A.B.; Mula, S.; Thakare, S.; Chakraborty, S.; Ray, A.K.; Sekar, N.; Chattopadhyay, S. An acac-BODIPY dye as a reversible “ON-OFF-ON” fluorescent sensor for Cu2+ and S2− ions based on displacement approach. J. Lumin. 2017, 190, 476–484. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. β-cyclodextrin functionalised silver nanoparticles as a duel colorimetric probe for ultrasensitive detection of Hg2+ and S2− ions in environmental water samples. Mater. Today Commun. 2018, 15, 61–69. [Google Scholar] [CrossRef]
- Ryu, K.Y.; Lee, S.Y.; Park, D.Y.; Kim, S.Y.; Kim, C. A novel colorimetric chemosensor for detection of Co2+ and S2− in an aqueous environment. Sens. Actuators B Chem. 2017, 242, 792–800. [Google Scholar] [CrossRef]
- Chen, Y.H.; Kirankumar, R.; Kao, C.L.; Chen, P.Y. Electrodeposited Ag, Au, and AuAg nanoparticles on graphene oxide-modified screen-printed carbon electrodes for the voltammetric determination of free sulfide in alkaline solutions. Electrochim. Acta 2016, 205, 124–131. [Google Scholar] [CrossRef]
- Zhao, Y.; Gui, L.; Chen, Z. Colorimetric detection of Hg2+ based on target-mediated growth of gold nanoparticles. Sens. Actuators B Chem. 2017, 241, 262–267. [Google Scholar] [CrossRef]
- Mehta, V.N.; Basu, H.; Kumar, R.; Kumar, S. Sensors and Actuators B: Chemical Simple and sensitive colorimetric sensing of Cd2+ ion using chitosan dithiocarbamate functionalized gold nanoparticles as a probe. Sens. Actuators B. Chem. 2015, 220, 850–858. [Google Scholar] [CrossRef]
- Ratnarathorn, N.; Chailapakul, O.; Dungchai, W. Highly sensitive colorimetric detection of lead using maleic acid functionalized gold nanoparticles. Talanta 2015, 132, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Dong, J.X.; Liu, S.G.; Li, N.; Lin, S.M.; Fan, Y.Z.; Lei, J.L.; Luo, H.Q.; Li, N.B. Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of Ag/CQDs composite for Hg2+ ions detection. J. Hazard. Mater. 2017, 322, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Azeman, N.H.; Mobarak, N.N.; Zan, M.S.D.; Ahmad, A.A. Sensitivity enhancement of localized SPR sensor towards Pb(II) ion detection using natural bio-polymer based carrageenan. Optik 2018, 168, 784–793. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, D.K.; Mohan, S.; Bano, D.; Gundampati, R.K.; Hasan, S.H. Green synthesis of silver nanoparticle for the selective and sensitive colorimetric detection of mercury (II) ion. J. Photochem. Photobiol. B Biol. 2017, 168, 67–77. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, Y.; Chen, L.; Lu, A.; Zhang, L. One-step synthesis of size-tunable gold nanoparticles immobilized on chitin nanofibrils via green pathway and their potential applications. Chem. Eng. J. 2017, 315, 573–582. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Geng, C.; Xue, Z.; Xia, Y.; Qin, Y. Study on the preparation and fl ame retardant properties of an eco-friendly potassium-calcium carrageenan fi ber. Carbohydr. Polym. 2019, 206, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Meher, M.K.; Gupta, P.; Gulati, K.; Pruthi, V.; Poluri, K.M. Microwave assisted κ-carrageenan capped silver nanocomposites for eradication of bacterial biofilms. Carbohydr. Polym. 2019, 206, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J. Food Hydrocolloids Carrageenan-based antimicrobial bionanocomposite fi lms incorporated with ZnO nanoparticles stabilized by melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Bui, V.T.N.T.; Nguyen, B.T.; Renou, F.; Nicolai, T. Rheology and microstructure of mixtures of iota and kappa-carrageenan. Food Hydrocoll. 2019, 89, 180–187. [Google Scholar] [CrossRef]
- Loukotová, L.; Bogomolova, A.; Konefal, R.; Špírková, M.; Štěpánek, P.; Hrubý, M. Hybrid κ-carrageenan-based polymers showing “schizophrenic” lower and upper critical solution temperatures and potassium responsiveness. Carbohydr. Polym. 2019, 210, 26–37. [Google Scholar] [CrossRef]
- Elsupikhe, R.F.; Shameli, K.; Ahmad, M.B.; Ibrahim, N.A.; Zainudin, N. Green sonochemical synthesis of silver nanoparticles at varying concentrations of κ-carrageenan. Nanoscale Res. Lett. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Lobregas, M.O.S.; Bantang, J.P.O.; Camacho, D.H. Carrageenan-stabilized silver nanoparticle gel probe kit for colorimetric sensing of mercury (II) using digital image analysis. Sens. Bio-Sens. Res. 2019, 26, 100303. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Li, Z.; Deng, Y.; Zhao, X.; Xia, Y. Facile synthesis of chitosan-gold nanocomposite and its application for exclusively sensitive detection of Ag+ ions. Carbohydr. Polym. 2019, 226, 115290. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Ma, X.; Quan, F.; Wang, J.; Xia, Y. The preparation of alginate—AgNPs composite fiber with green approach and its antibacterial activity. J. Ind. Eng. Chem. 2015, 24, 188–195. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Deng, Y.; Zhao, Z.; Li, X.; Xia, Y. Facile synthesis of gold nanoparticles with alginate and its catalytic activity for reduction of 4-nitrophenol and H2O2 detection. Materials (Basel) 2017, 10, 557. [Google Scholar] [CrossRef]
- Roy, S.; Shankar, S.; Rhim, J.W. Melanin-mediated synthesis of silver nanoparticle and its use for the preparation of carrageenan-based antibacterial films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Modolon, K.; Souza, M.; Marques, M.; Dal, F.; Morisso, P.; Alberto, L. International Journal of Biological Macromolecules Facile, green and scalable method to produce carrageenan-based hydrogel containing in situ synthesized AgNPs for application as wound dressing. Int. J. Biol. Macromol. 2018, 113, 51–58. [Google Scholar]
- Chettri, P.; Vendamani, V.S.; Tripathi, A.; Singh, M.K.; Pathak, A.P.; Tiwari, A. Green synthesis of silver nanoparticle-reduced graphene oxide using Psidium guajava and its application in SERS for the detection of methylene blue. Appl. Surf. Sci. 2017, 406, 312–318. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Latorre-Sanchez, M.; Asiri, A.M.; Primo, A.; Garcia, H. Sulphur-doped graphene as metal-free carbocatalysts for the solventless aerobic oxidation of styrenes. Catal. Commun. 2015, 65, 10–13. [Google Scholar] [CrossRef]
- Ramin, B.B.S.; Rufato, K.B.; Sabino, R.M.; Popat, K.C.; Kipper, M.J.; Martins, A.F.; Muniz, E.C. Chitosan/iota-carrageenan/curcumin-based materials performed by precipitating miscible solutions prepared in ionic liquid. J. Mol. Liq. 2019, 290, 1–10. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Lerbret, A.; Assifaoui, A. Specific binding of trivalent metal ions to λ-carrageenan. Int. J. Biol. Macromol. 2018, 109, 350–356. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Ilanchelian, M. Colorimetric determination of sulfide using chitosan-capped silver nanoparticles. Microchim. Acta 2016, 183, 1721–1728. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dong, X.; Zhao, L.; Xue, Y.; Zhao, X.; Li, Q.; Xia, Y. Facile and Green Fabrication of Carrageenan-Silver Nanoparticles for Colorimetric Determination of Cu2+ and S2−. Nanomaterials 2020, 10, 83. https://doi.org/10.3390/nano10010083
Wang Y, Dong X, Zhao L, Xue Y, Zhao X, Li Q, Xia Y. Facile and Green Fabrication of Carrageenan-Silver Nanoparticles for Colorimetric Determination of Cu2+ and S2−. Nanomaterials. 2020; 10(1):83. https://doi.org/10.3390/nano10010083
Chicago/Turabian StyleWang, Yesheng, Xueyi Dong, Li Zhao, Yun Xue, Xihui Zhao, Qun Li, and Yanzhi Xia. 2020. "Facile and Green Fabrication of Carrageenan-Silver Nanoparticles for Colorimetric Determination of Cu2+ and S2−" Nanomaterials 10, no. 1: 83. https://doi.org/10.3390/nano10010083
APA StyleWang, Y., Dong, X., Zhao, L., Xue, Y., Zhao, X., Li, Q., & Xia, Y. (2020). Facile and Green Fabrication of Carrageenan-Silver Nanoparticles for Colorimetric Determination of Cu2+ and S2−. Nanomaterials, 10(1), 83. https://doi.org/10.3390/nano10010083



