Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vegetal Material
2.3. Preparation of SE Extract
2.4. Determination of Total Polyphenols Content
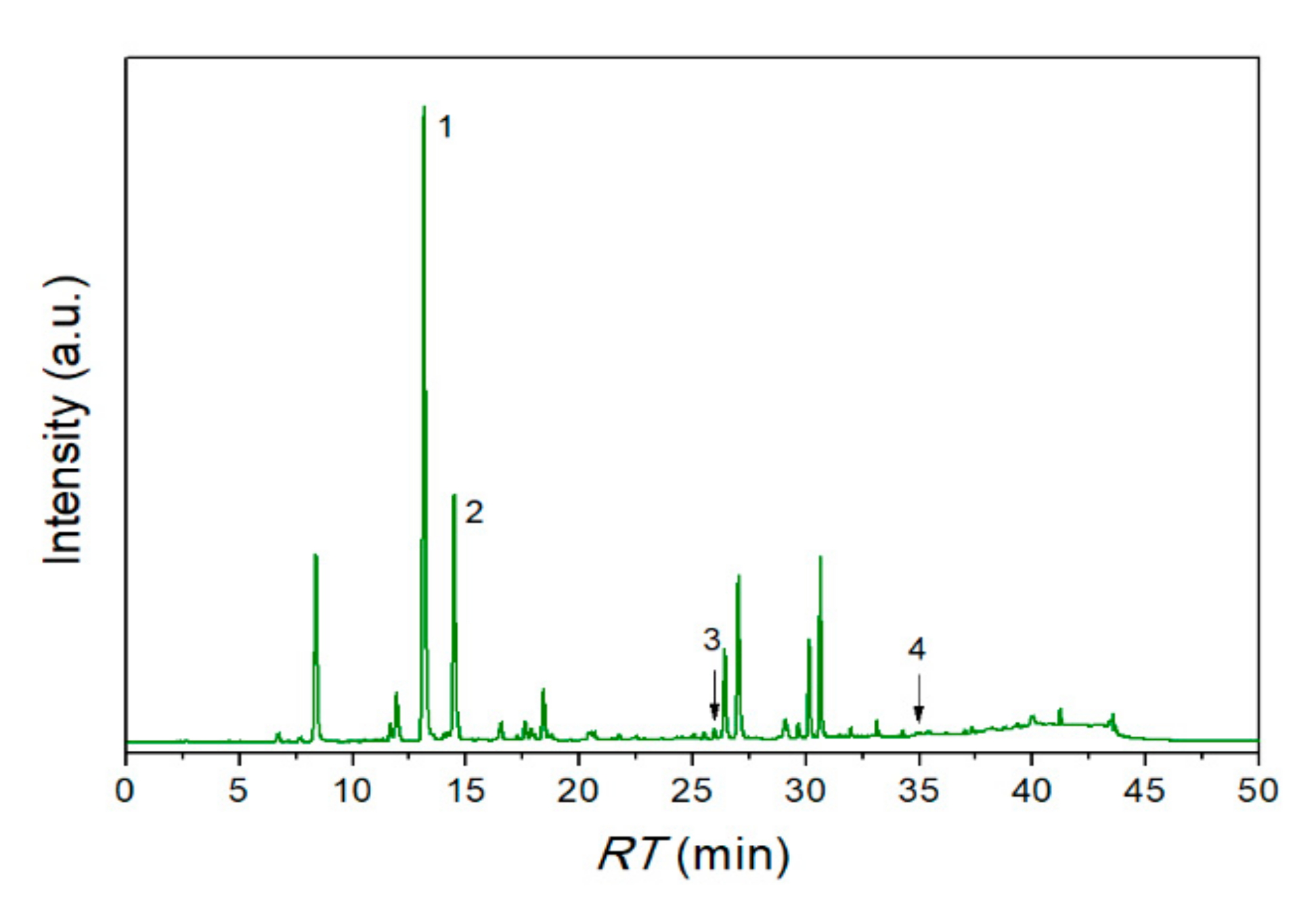
2.5. High-Performance Liquid Chromatography Analysis of SE Extract
2.6. Preparation of SE Extract-Loaded Lipid Vesicles
2.7. Characterization of SE−Loaded Lipid Vesicles
2.8. In Vitro Release Polyphenols from SE Loaded Lipid Vesicles
2.9. Determination of Radical Scavenging Activity
2.10. Induced Oxidative Stress Assay
2.11. Statistical Analysis
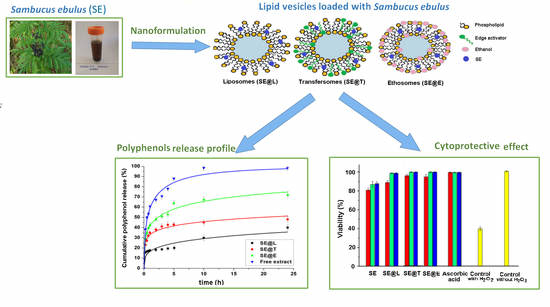
3. Results
3.1. Characterization of SE Extract
3.2. Characterization of SE Extract−Loaded Lipid Vesicles
3.3. In Vitro Polyphenols Release Study
3.4. Effect of Pre−Treatment with SE Extract Free and Loaded into Lipid Vesicles against H2O2−Induced Toxicity on L−929 Fibroblast Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interests
References
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. S1), 69–75. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, J.; Cameron, M.; Chrubasik, S. A systematic review on the sambuci fructus effect and efficacy profiles. Phytother. Res. 2010, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yesilada, E.; Gurbuz, I.; Toker, G. Anti-ulcerogenic activity and isolation of the active principles from Sambucus ebulus L. leaves. J. Ethnopharmacol. 2014, 153, 478–483. [Google Scholar] [CrossRef]
- Bubulica, M.-V.; Chirigiu, L.; Popescu, M. Analysis of sterol compounds from Sambucus ebulus. Chem. Nat. Compd. 2012, 48, 520–521. [Google Scholar] [CrossRef]
- Duymus¸, H.G.; Goger, F.; Baser, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Pieri, V.; Schwaiger, S.; Ellmerer, E.P.; Stuppner, H. Iridoid glycosides from the leaves of Sambucus ebulus. J. Nat. Prod. 2009, 72, 1798–1803. [Google Scholar] [CrossRef]
- Schwaiger, S.; Zeller, I.; Polzelbauer, P.; Frotschnig, S.; Laufer, G.; Messner, B.; Pieri, V.; Stuppner, H.; Bernhard, D. Identification and pharmacological characterization of the anti-inflammatory principal of the leaves of dwarf elder (Sambucus ebulus L.). J. Ethnopharmacol. 2010, 133, 704–709. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.; Kolodziejczyk, P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crop. Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanc, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B. Trends in encapsulation technologies for delivery of food bioactive compounds. J. Food Eng. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Souguir, H.; Salaün, F.; Douillet, P.; Vroman, I.; Chatterjee, S. Nanoencapsulation of curcumin in polyurethane and polyurea shells by an emulsion diffusion method. Chem. Eng. J. 2013, 221, 133–145. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Preparation and antibacterial activity of Litsea cubeba essential oil/dandelion polysaccharide nanofiber. Ind. Crop. Prod. 2019, 140, 1117–1139. [Google Scholar] [CrossRef]
- Zadegan, S.; Nourmohammadi, J.; Vahidi, B.; Haghighipour, N. An investigation into osteogenic differentiation effects of silk fibroin-nettle (Urtica dioica L.) nanofibers. Int. J. Biol. Macromol. 2019, 133, 795–803. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Assadpour, E.; Mahdi Jafari, S. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.F.; Jafari, S.M.; Assadpour, E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chem. 2017, 221, 1962–1969. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Aditya, N.P.; Macedo, A.S.; Doktorovova, S.; Souto, E.B.; Kim, S.; Chang, P.S.; Ko, S. Development and evaluation of lipid nanocarriers for quercetin delivery: A comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). Food Sci. Technol. 2014, 59, 115–121. [Google Scholar] [CrossRef]
- Athmouni, K.; Belhaj, D.; Gammoudi, S.; El Feki, A.; Ayadi, H. Nano-encapsulation using macrocyclic carbohydrate polymers (β-cyclodextrins) of Periploca angustifolia extract: Physical stability and protective effect against cadmium-induced alterations in HepG2 cells. Int. J. Biol. Macromol. 2019, 125, 711–720. [Google Scholar] [CrossRef]
- Simionato, I.; Domingues, F.; Nerín, C.; Silva, F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem. Toxicol. 2019, 132, 1–9. [Google Scholar] [CrossRef]
- Trinh, L.H.; Takzare, A.; Ghafoor, D.; Siddiqi, A.F.; Ravali, S.; Shalbaf, M.; Bakhtiar, M. Trachyspermum copticum essential oil incorporated niosome for cancer treatment. J. Drug Deliv. Sci. Technol. 2019, 52, 818–824. [Google Scholar] [CrossRef]
- Fidan-Yardimci, M.; Akay, S.; Sharifi, F.; Sevimli-Gur, C.; Ongen, G.; Yesil-Celiktas, O. A novel niosome formulation for encapsulation of anthocyanins and modelling intestinal transport. Food Chem. 2019, 293, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, J.M.; Lignell, A.; Pettersson, A.; Hoglund, P. Oral bioavailability of the antioxidant astaxanthin on humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Gowtham, R.; Gopalkrishna, B. Phytosomes: A potential phyto-phospholipid carriers for herbal drug delivery. J. Pharm. Res. 2009, 2, 1267–1270. [Google Scholar]
- Baomiao, D.; Xiaoming, Z.; Khizar, H.; Shiqin, X.; Chengsheng, J.; Mingyong, X.; Chengmei, L. Preparation, characterization and the stability of ferrous glycinate nanoliposomes. J. Food Eng. 2011, 102, 202–208. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiao, J.; Fan, H.X.; Yu, Y.; He, R.-R.; Feng, X.-L.; Kurihara, H.; So, K.-F.; Yao, X.-S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2016, 214. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, Transfersomes. BBA-Biomembr. 2001, 1514, 191–205. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes-novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Stanciuc, N.; Oancea, A.M.; Aprodu, I.; Turturica, M.; Barbu, V.; Ionita, E.; Rapeanu, G.; Bahrim, G. Investigations on binding mechanism of bioactives from elderberry (Sambucus nigra L.) by whey proteins for efficient microencapsulation. J. Food Eng. 2017, 223, 197–207. [Google Scholar] [CrossRef]
- Sobieralska, M.; Kurek, M.A. Beta-Glucan as Wall Material in Encapsulation of Elderberry (Sambucus nigra) Extract. Plant Foods Hum. Nutr. 2019, 74, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Bryła, A.; Lewandowicz, G.; Juzwa, W. Encapsulation of elderberry extract into phospholipid nanoparticles. J. Food Eng. 2015, 167, 189–195. [Google Scholar] [CrossRef]
- Jabbari, M.; Daneshfard, B.; Emtiazy, M.; Khiveh, A.; Hashempur, M.H. Biological Effects and Clinical Applications of Dwarf Elder (Sambucus ebulus L.): A Review. J. Evid.-Based Complement. Altern. Med. 2017, 22, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Nicoletti, I.; Bello, C.; De Rosii, A.; Corradini, D. Identification and quatification of phenolic compounds in grapes by HPLC-PDA-ESI-MS on a semimicro separation scale. J. Agric. Food Chem. 2008, 56, 8801–8808. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Agric. Food Chem. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Feizbakhsh, A.; Pazoki, H.; Ebrahimzadeh, M.A. Sambucus ebulus, introduction to mechanism of action; a chemical viewpoint. Pharmacologyonline 2010, 1, 16–22. [Google Scholar]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Catalan Latorre, A.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef]
- Gharib, R.; Amal Najjar, A.; Auezova, L.; Catherine Charcosset, C.; Greige-Gerges, H. Interaction of selected phenylpropenes with dipalmitoylphosphatidylcholine membrane and their relevance to antibacterial activity. J. Membr. Biol. 2017, 250, 259–271. [Google Scholar] [CrossRef]
- Gibis, M.; Vogt, E.; Weiss, J. Encapsulation of polyphenolic grape seed extract in polymer-coated liposomes. Food Funct. 2012, 3, 246–254. [Google Scholar] [CrossRef]
- Gibis, M.; Rahn, N.; Weiss, J. Physical and oxidative stability of uncoated and chitosan-coated liposomes containing grape seed extract. Pharmaceutics 2013, 5, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Soon, S.K.; Sun, Y.K.; Bong, J.K.; Kyeong, J.K.; Geun, Y.N.; Na, R.I.; Ji, W.L.; Ji, H.H.; Junoh, K.; Soo, N.P. Cell penetrating peptide conjugated liposomes as transdermal delivery system of Polygonum aviculare L. extract. Int. J. Pharm. 2015, 483, 26–37. [Google Scholar] [CrossRef]
- Maitani, Y.; Soeda, H.; Junping, W.; Takayama, K. Modified ethanol injection method for liposomes containing beta-sitosterol beta-D-glucoside. J. Liposome Res. 2001, 11, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Detoni, C.B.; Cabral-Albuquerque, E.C.; Hohlemweger, S.V.; Sampaio, C.; Barros, T.F.; Velozo, E.S. Essential oil from Zanthoxylum tingoassuiba loaded in to multilamellar liposomes useful as antimicrobial agents. J. Microencapsul. 2009, 26, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Detoni, C.B.; de Oliveira, D.M.; Santo, I.E.; Santo, I.E.; Pedro, A.S.; El-Bacha, R.; da Silva Velozo, E.; Ferreira, D.; Sarmento, B.; de Magalhães Cabral-Albuquerque, E.C. Evaluation of thermal-oxidative stability and antiglioma activity of Zanthoxylum tingoassuiba essential oil entrapped into multi- and unilamellar liposomes. J. Liposome Res. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Makino, K.; Yamada, T.; Kimura, M.; Oka, T.; Oshima, H. Temperature- and ionic strength-induced conformational changes in the lipid head group region of liposomes as suggested by zeta potential data. Biophys. Chem. 1991, 41, 175–183. [Google Scholar] [CrossRef]
- Sinico, C.; De Logu, A.; Lai, F.; Valenti, D.; Manconi, M.; Loy, G.; Bonsignore, L.; Fadda, A.M. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur. J. Pharm. Biopharm. 2005, 59, 161–168. [Google Scholar] [CrossRef]
- Fang, J.Y.; Hong, C.T.; Chiu, W.T.; Wang, Y.Y. Effect of liposomes and niosomes on skin permeation of enoxacin. Int. J. Pharm. 2001, 219, 61–72. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Gupta, A.; Aggarwal, G.; Singla, S.; Arora, R. Transfersomes: A Novel Vesicular Carrier for Enhanced Transdermal Delivery of Sertraline: Development, Characterization, and Performance Evaluation. Sci. Pharm. 2012, 80, 1061–1080. [Google Scholar] [CrossRef]
- Ignacio, M.; Chubynsky, M.V.; Slater, G.W. Interpreting the Weibull fitting parameters for diffusion-controlled release data. Phys. A 2017, 486, 486–496. [Google Scholar] [CrossRef]
- Martínez, E.; Villalobos, R.; Sánchez, M.; Cruz, J.; Ganem, A.; Melgoza, L.M. Monte Carlo simulations for the study of drug release from cylindrical matrix systems with an inert nucleus. Int. J. Pharm. 2009, 369, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Gibis, M.; Zeeb, B.; Weiss, J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocoll. 2014, 38, 28–39. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valento, P.; Pereira, J.A.; Andrad, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 1529. [Google Scholar] [CrossRef]
- Turkez, H.; Sozio, P.; Geyikoglu, F.; Tatar, A.; Hacimuftuoglu, A.; Di Stefano, A. Cell Neuroprotective effects of farnesene against hydrogen peroxide-induced neurotoxicity in vitro. Mol. Neurobiol. 2014, 34, 101–111. [Google Scholar] [CrossRef] [PubMed]




| Sample | Formulation | EE (%) | Size (nm) | PDI | ξ (mV) |
|---|---|---|---|---|---|
| SE@L | PC:Cholesterol:SE = 10:1:2 (w/w) | 80.05 ± 0.51 | 123 ± 2.50 | 0.182 ± 0.01 | −43 ± 1.03 |
| SE@T | PC:Sodium cholate:SE = 8:2:2 (w/w) | 75.10 ± 1.12 | 155 ± 3.31 | 0.161 ± 0.02 | −39 ± 0.50 |
| SE@E | PC:SE = 8:2.5 (w/w); EtOH:H2O = 7:3 (v/v) | 85.10 ± 1.50 | 190 ± 2.53 | 0.209 ± 0.01 | −37 ± 0.23 |
| L | PC:Cholesterol = 10:1 (w/w) | - | 49 ± 1.52 | 0.440 ± 0.01 | - |
| T | PC:Sodium cholate = 8:2 (w/w) | - | 105 ± 0.23 | 0.379 ± 0.02 | - |
| Sample | Weibull | Korsmayer-Peppas | Higuchi | |||||
|---|---|---|---|---|---|---|---|---|
| a | b | R2 | n | kKP | R2 | kH | R2 | |
| Free extract | 0.885 | 0.455 | 0.918 | - | - | - | - | - |
| SE@L | 0.181 | 0.280 | 0.983 | 0.076 | 17.390 | 0.991 | 2.363 | 0.973 |
| SE@T | 0.400 | 0.184 | 0.984 | 0.221 | 32.568 | 0.963 | 12.507 | 0.922 |
| SE@E | 0.538 | 0.297 | 0.970 | 0.216 | 40.640 | 0.978 | 16.980 | 0.949 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păvăloiu, R.-D.; Sha’at, F.; Bubueanu, C.; Deaconu, M.; Neagu, G.; Sha’at, M.; Anastasescu, M.; Mihailescu, M.; Matei, C.; Nechifor, G.; et al. Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles. Nanomaterials 2020, 10, 56. https://doi.org/10.3390/nano10010056
Păvăloiu R-D, Sha’at F, Bubueanu C, Deaconu M, Neagu G, Sha’at M, Anastasescu M, Mihailescu M, Matei C, Nechifor G, et al. Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles. Nanomaterials. 2020; 10(1):56. https://doi.org/10.3390/nano10010056
Chicago/Turabian StylePăvăloiu, Ramona-Daniela, Fawzia Sha’at, Corina Bubueanu, Mihaela Deaconu, Georgeta Neagu, Mousa Sha’at, Mihai Anastasescu, Mona Mihailescu, Cristian Matei, Gheorghe Nechifor, and et al. 2020. "Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles" Nanomaterials 10, no. 1: 56. https://doi.org/10.3390/nano10010056
APA StylePăvăloiu, R.-D., Sha’at, F., Bubueanu, C., Deaconu, M., Neagu, G., Sha’at, M., Anastasescu, M., Mihailescu, M., Matei, C., Nechifor, G., & Berger, D. (2020). Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles. Nanomaterials, 10(1), 56. https://doi.org/10.3390/nano10010056