Supported Gold Nanoparticles as Catalysts in Peroxidative and Aerobic Oxidation of 1-Phenylethanol under Mild Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Catalytic Results
3.1.1. Peroxidative Oxidation of 1-Phenylethanol
3.1.2. Aerobic Oxidation of 1-Phenylethanol
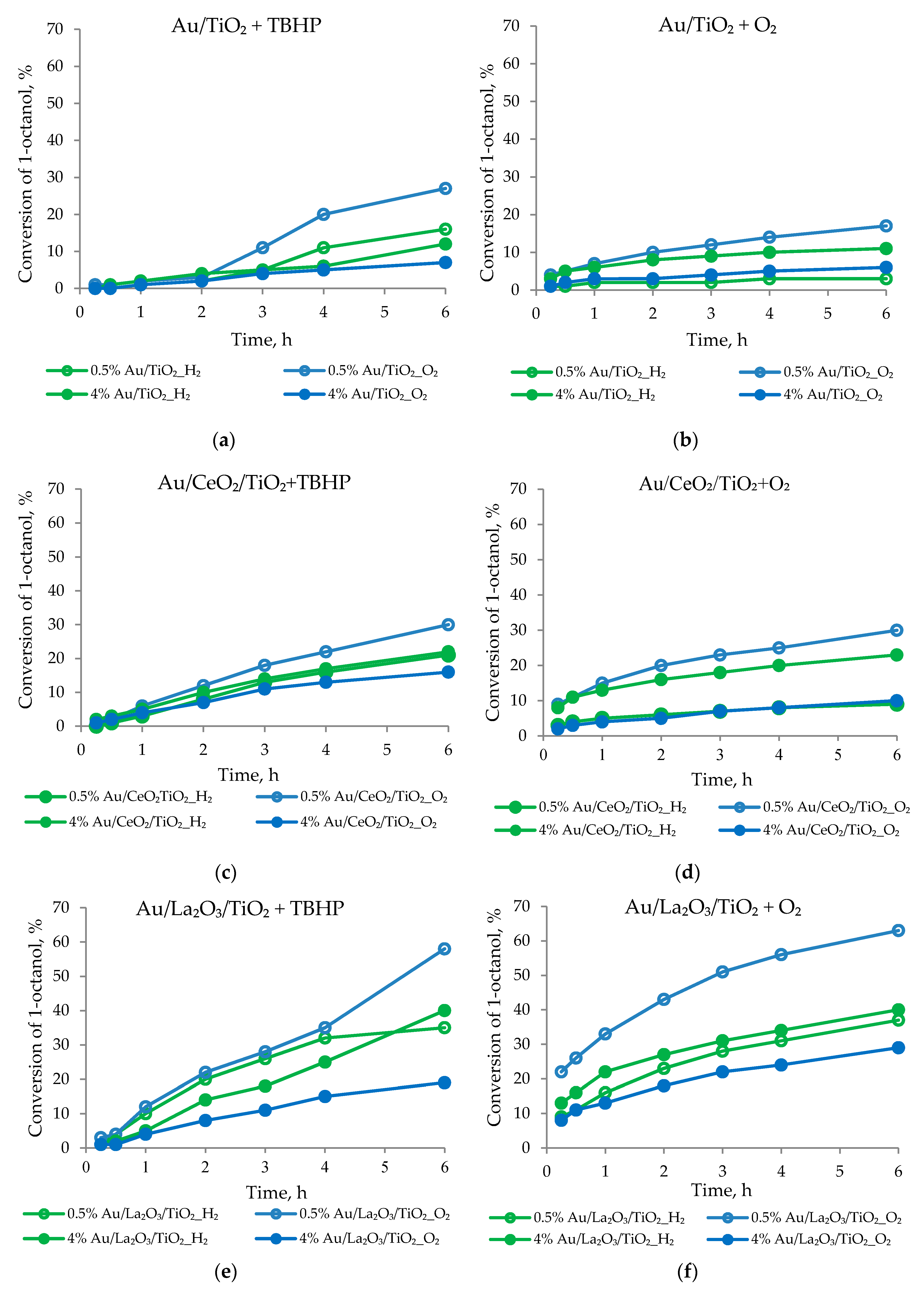
3.1.3. Peroxidative and Aerobic Oxidation of 1-Octanol
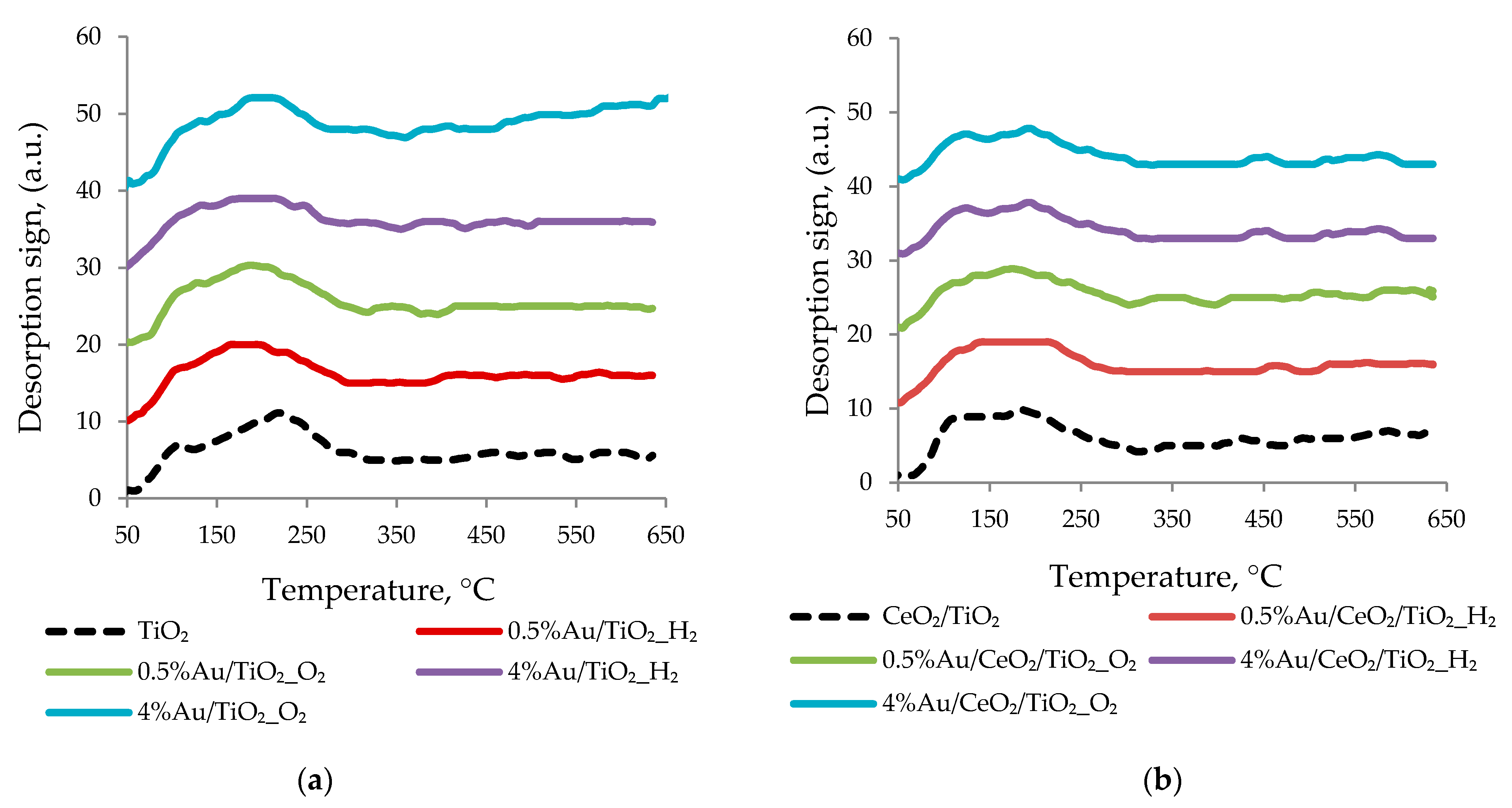
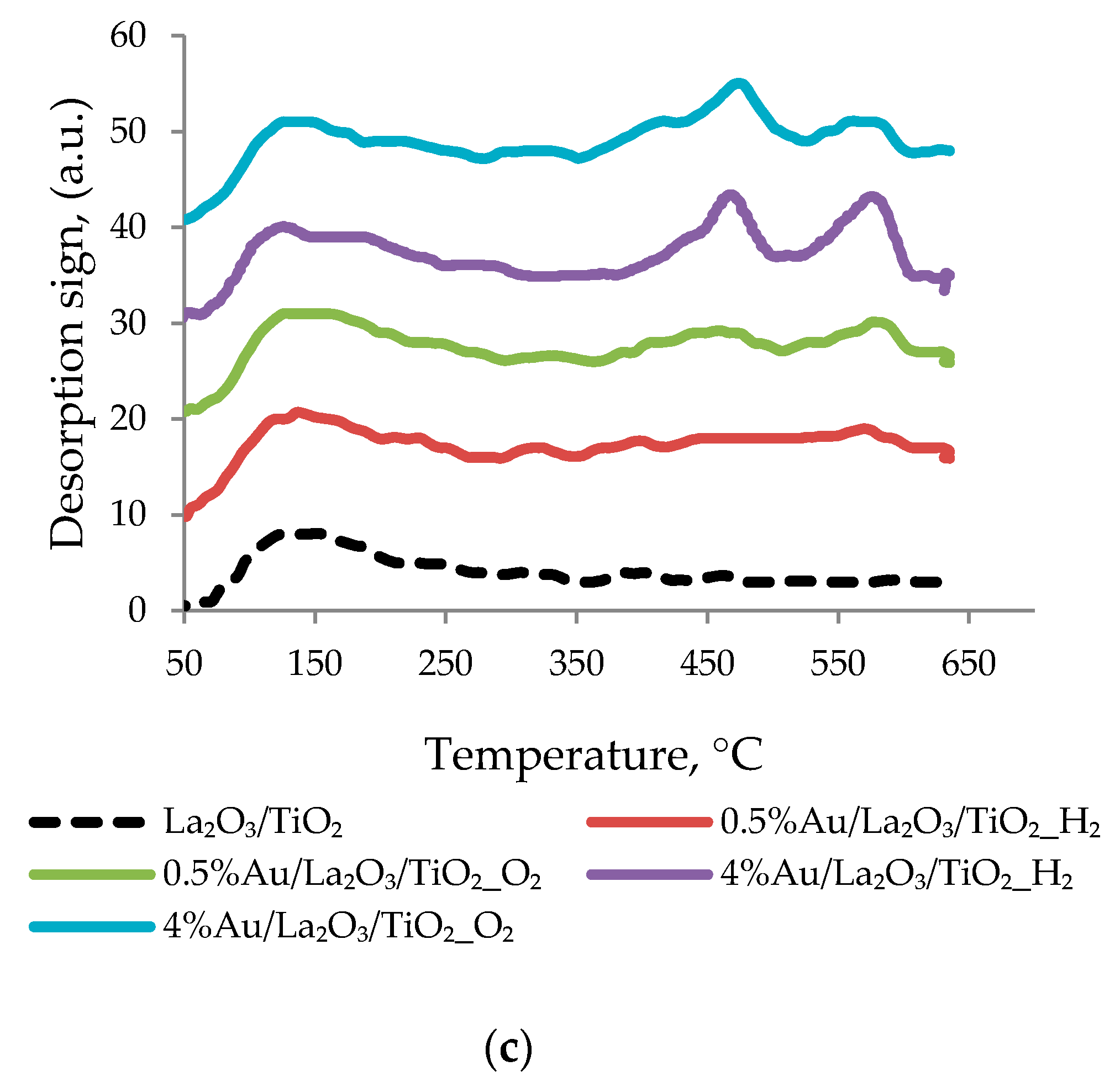
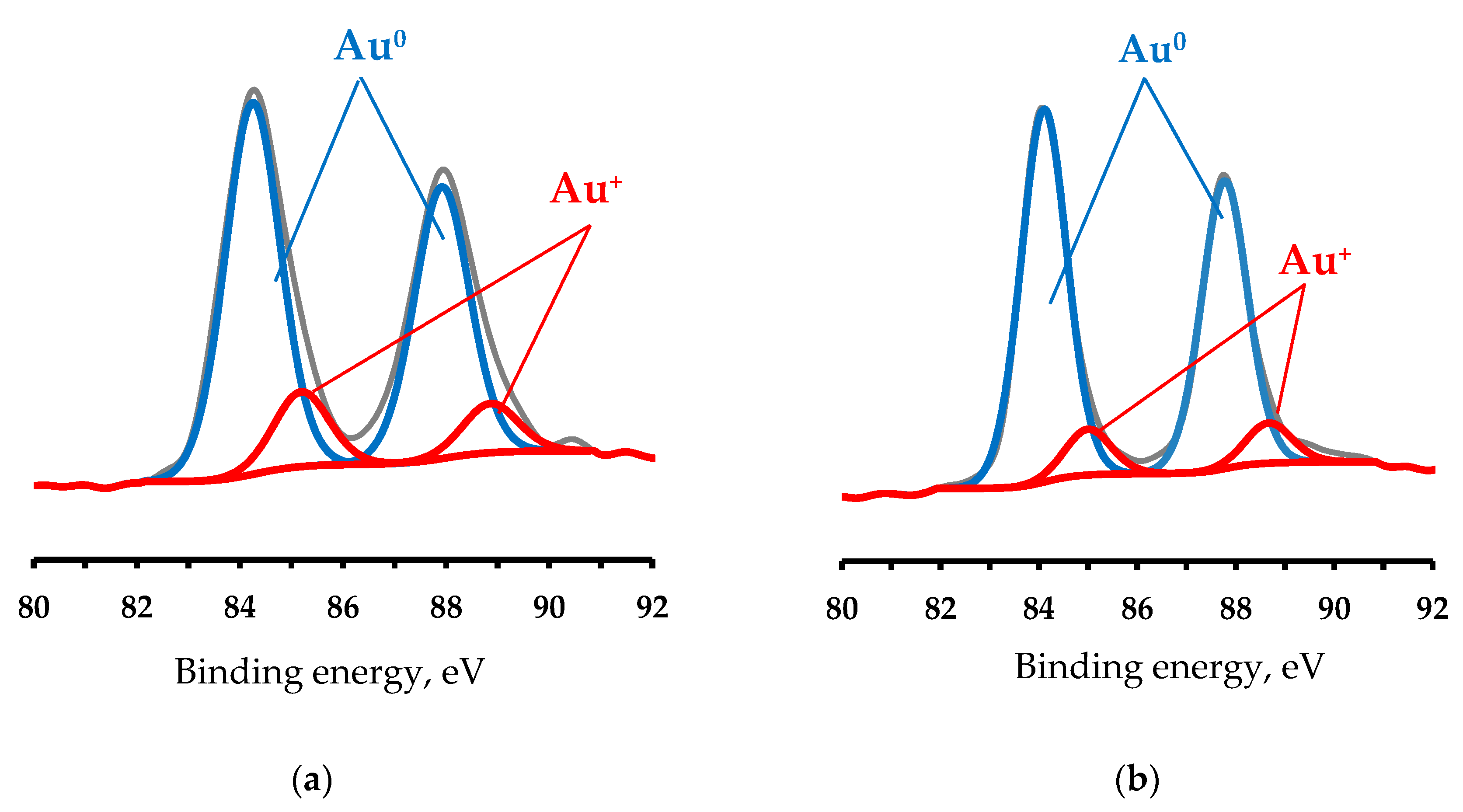
3.2. Catalyst Characterization
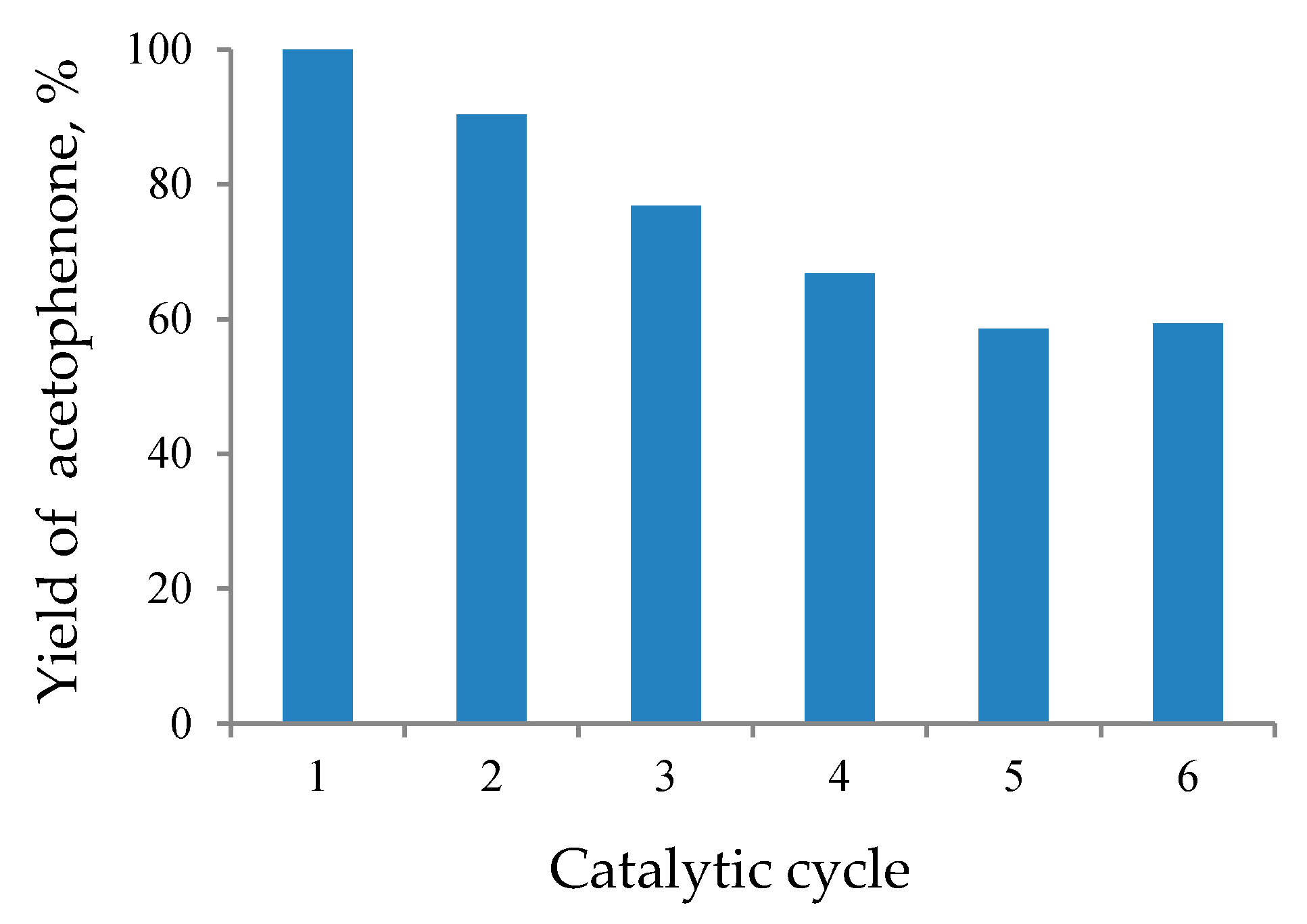
3.3. Catalyst Recycling Tests
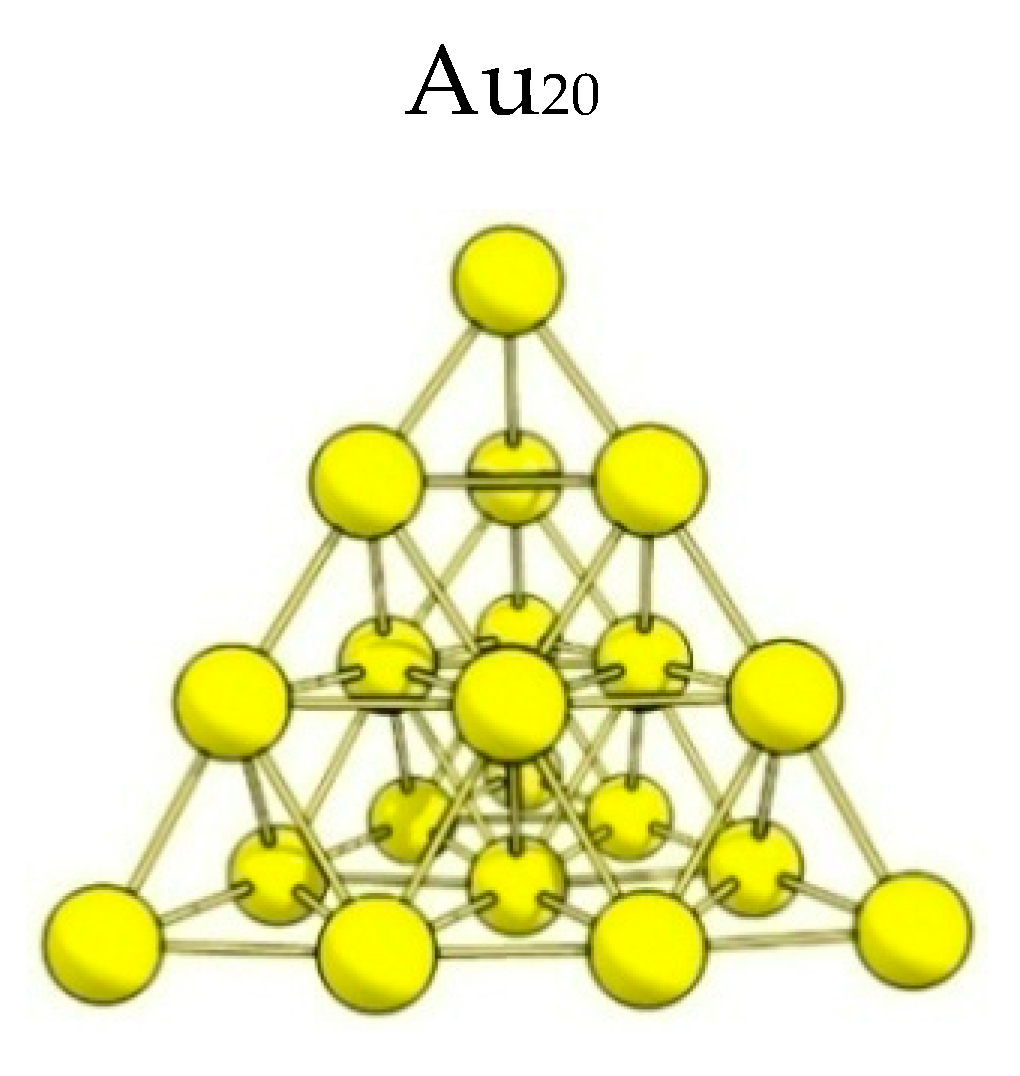
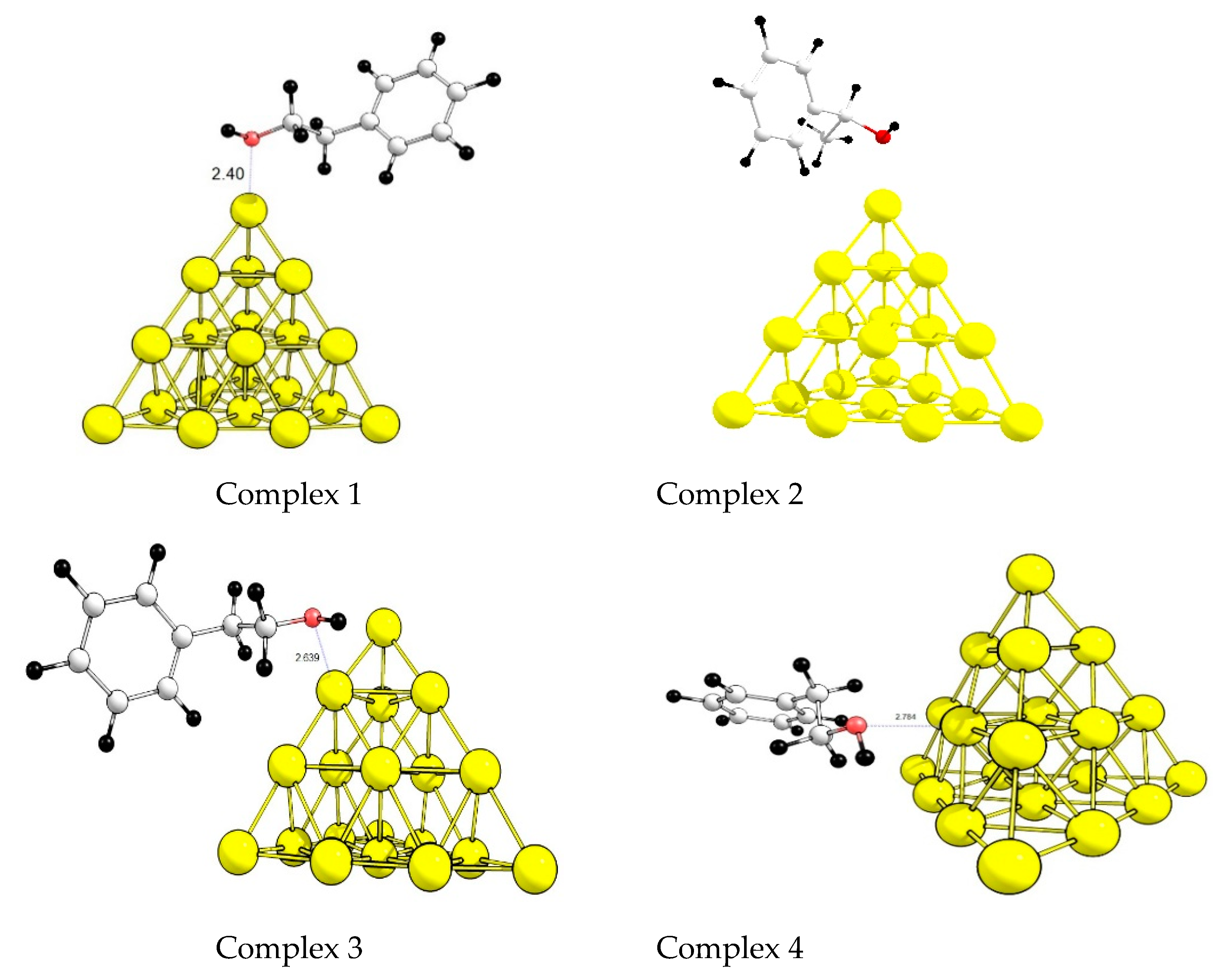
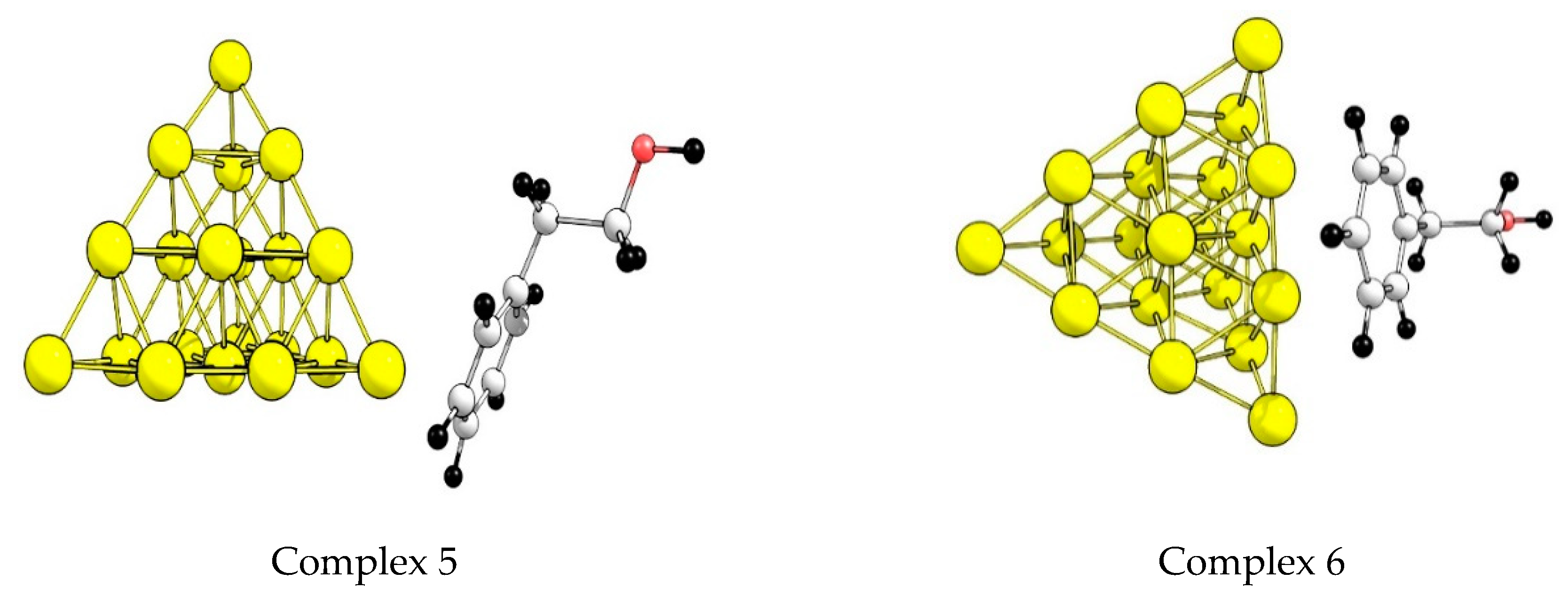
3.4. Quantum Chemical Simulation of the Alcohol Adsorption on a Gold Cluster
- (i)
- Which phenylethanol coordination on a gold cluster is preferred (by OH– or C6H5– groups)?
- (ii)
- How do the structural features of the catalyst surface, including availability of low coordinated gold atoms, affect the adsorption of the alcohol?
- (iii)
- What is the effect of gold cationic sites on alcohol activation?
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

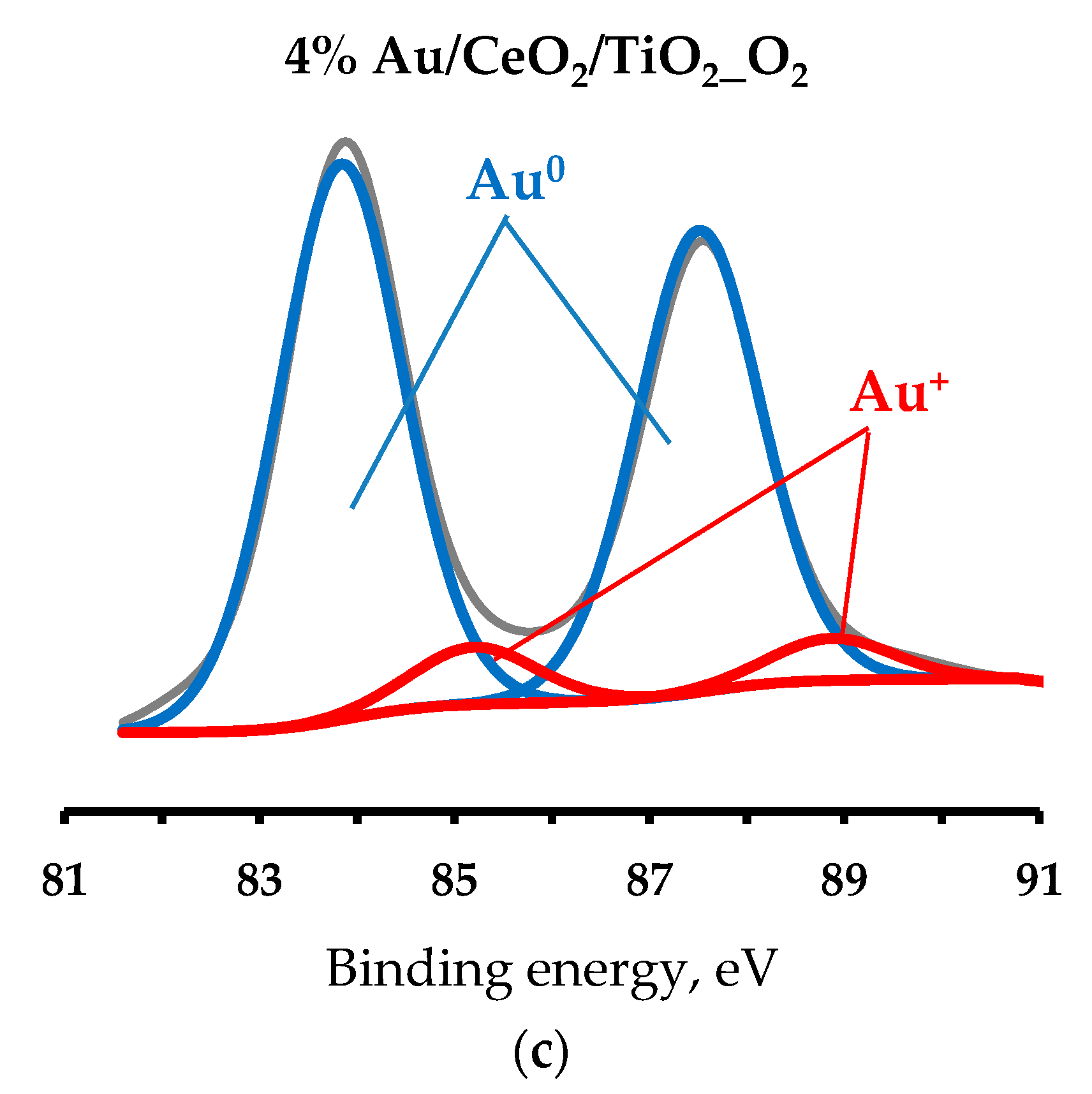
Appendix B
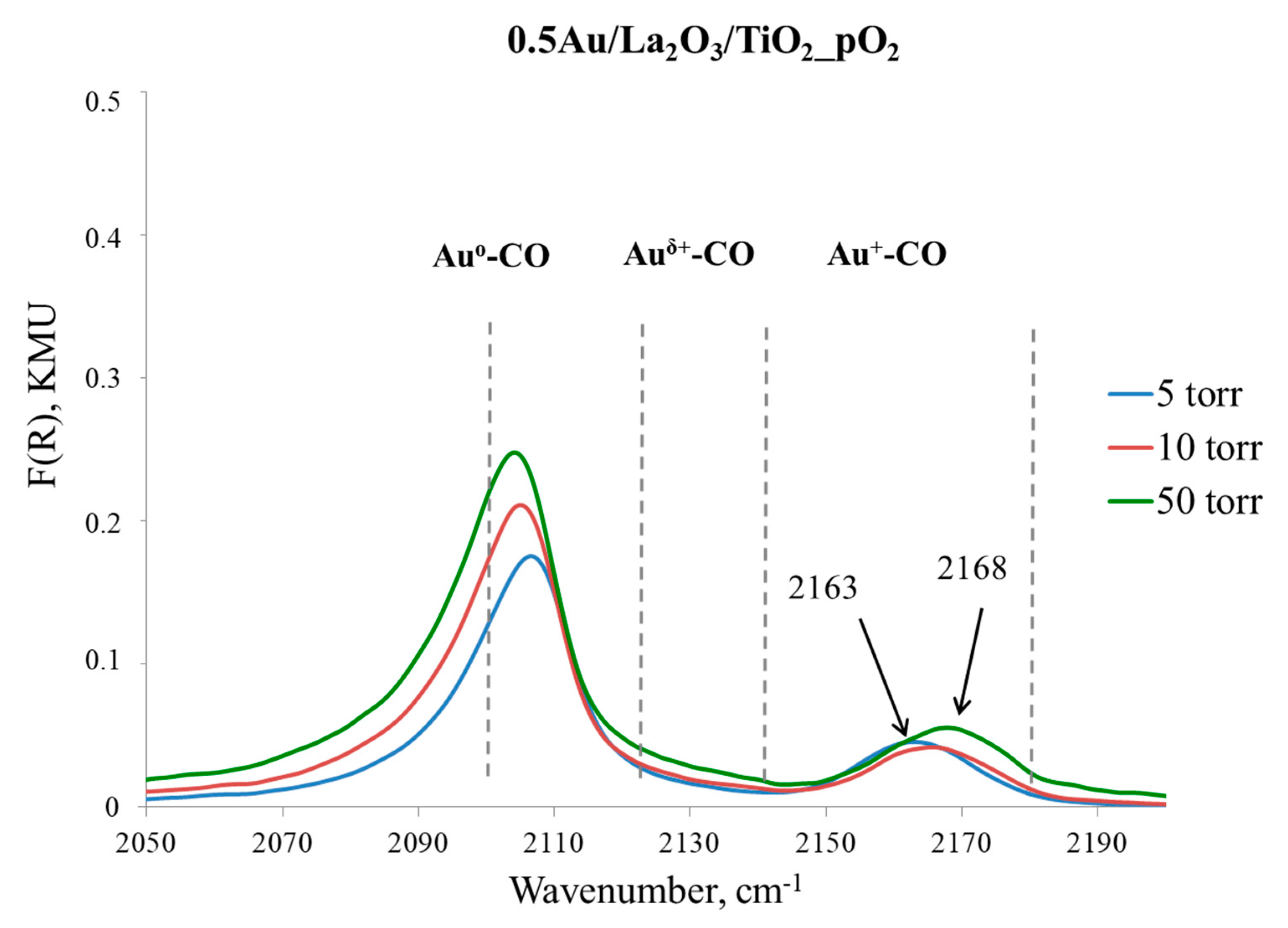
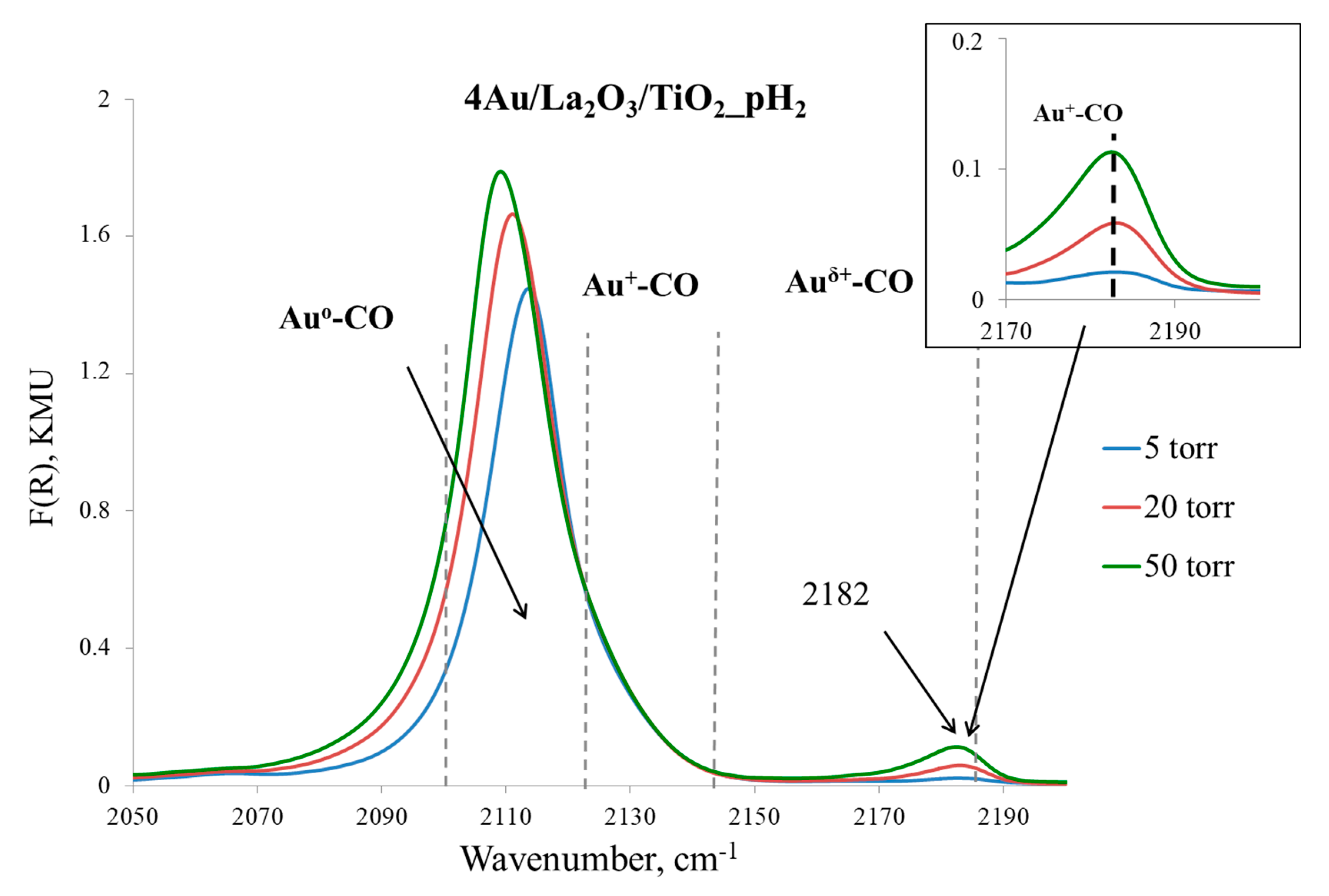
Appendix C
| Entry | Sample | Conversion of 1-Octanol After 6 h, ca.% | Relative Au Content, % | |
|---|---|---|---|---|
| Au0 | Au+ | |||
| 1 | 0.5% Au/La2O3/TiO2_O₂ 1 | 63 | 65 | 35 |
| 2 | 0.5% Au/La2O3/TiO2_O₂ 2,a | 31 | 87 | 13 |
| 3 | 0.5% Au/La2O3/TiO2_H₂ 1,b | 34 | 87 | 13 |
References
- Barati, B.; Moghadam, M.; Rahmati, A.; Mirkhani, V.; Tangestaninejad, S.; Mohammadpoor-Baltork, I. Direct oxidation of alcohols to carboxylic acids over ruthenium hydride catalyst with diphenylsulfoxide oxidant. Inorg. Chem. Commun. 2013, 29, 114–117. [Google Scholar] [CrossRef]
- Corberán, V.C.; González-Pérez, M.E.; Martínez-González, S.; Gómez-Avilés, A. Green oxidation of fatty alcohols: Challenges and opportunities. Appl. Catal. A Gen. 2014, 474, 211–223. [Google Scholar] [CrossRef]
- Tojo, G.; Fernández, M. Oxidation of Primary Alcohols to Carboxylic Acids: A Guide to Current Common Practice; Springer: Berlin, Germany, 2006. [Google Scholar]
- Zhao, M.; Li, J.; Song, Z.; Desmond, R.; Tschaen, D.M.; Grabowski, E.J.J.; Reider, P.J. A novel chromium trioxide catalyzed oxidation of primary alcohols to the carboxylic acids. Tetrahedron Lett. 1998, 39, 5323–5326. [Google Scholar] [CrossRef]
- Anbu, S.; Alegria, E.C.B.A.; Pombeiro, A.J.L. Catalytic activity of a benzoyl hydrazone based dimericdicopper(II) complex in catechol and alcohol oxidation reactions. Inorg. Chim. Acta 2015, 431, 139–144. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Nesterov, D.S.; Krogul-Sobczak, A.; Guedes da Silva, M.C.; Pombeiro, A.J.L. Synthesis, crystal structures and catalytic activity of Cu(II) and Mn(III)Schiff base complexes: Influence of additives on the oxidation catalysis of cyclohexane and 1-phenylehanol. J. Mol. Catal. A Chem. 2017, 426, 506–515. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Roy Barman, T.; Scorcelletti, F.; Guedes da Silva, M.C.; Pombeiro, A.J.L. Microwave-assisted peroxidative oxidation of toluene and 1-phenylethanol with monomeric keto and polymeric enolaroylhydrazone Cu(II) complexes. Mol. Catal. 2017, 439, 224–232. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Fontolan, E.; Alegria, E.C.B.A.; Kopylovich, M.N.; Bertani, R.; Pombeiro, A.J.L. The influence of multiwalled carbon nanotubes and graphene oxide additives on the catalytic activity of 3d metal catalysts towards 1-phenylethanol oxidation. J. Mol. Catal. A Chem. 2017, 426, 557–563. [Google Scholar] [CrossRef]
- Karmakar, A.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Hazra, S.; Pombeiro, A.J.L. Solvent-free microwave-assisted peroxidative oxidation of alcohols catalyzed by iron(III)-TEMPO catalytic systems. Catal. Lett. 2015, 145, 2066–2076. [Google Scholar] [CrossRef]
- Cozzi, I.S.; Crotti, C.; Farnetti, E. Microwave-assisted green oxidation of alcohols with hydrogen peroxide catalyzed by iron complexes with nitrogen ligands. J. Organomet. Chem. 2018, 878, 38–47. [Google Scholar] [CrossRef]
- Du, Z.; Ma, J.; Ma, H.; Gao, J.; Xu, J. Synergistic effect of vanadium–phosphorus promoted oxidation of benzylic alcohols with molecular oxygen in water. Green Chem. 2010, 12, 590–592. [Google Scholar] [CrossRef]
- Sasaki, T.; Ichikuni, N.; Hara, T.; Shimazu, S. Study on the promoting effect of nickel silicate for 1-phenylethanol oxidation on supported NiO nanocluster catalysts. Catal. Today 2018, 307, 29–34. [Google Scholar] [CrossRef]
- Bhaumik, C.; Stein, D.; Vincendeau, S.; Poli, R.; Manoury, E. Oxidation of alcohols by TBHP in the presence of sub-stoichiometric amounts of MnO2. C. R. Chim. 2016, 19, 566–570. [Google Scholar] [CrossRef]
- Reis, M.C.; Barros, S.D.T.; Lachter, E.R.; San Gil, R.A.S.; Floresc, J.H.; Pais da Silva, M.I.; Onfroyd, T. Synthesis, characterization and catalytic activity of meso-niobium phosphate in the oxidation of benzyl alcohols. Catal. Today 2012, 192, 117–122. [Google Scholar] [CrossRef]
- Burange, A.S.; Jayaram, R.V.; Shukla, R.; Tyagi, A.K. Oxidation of benzylic alcohols to carbonyls using tert-butyl hydroperoxide over pure phase nanocrystalline CeCrO3. Catal. Commun. 2013, 40, 27–31. [Google Scholar] [CrossRef]
- Yadav, G.D.; Yadav, A.R. Selective liquid phase oxidation of secondary alcohols into ketones by tert-butyl hydroperoxide on nano-fibrous Ag-OMS-2 catalyst. J. Mol. Catal. A Chem. 2013, M380, 70–77. [Google Scholar] [CrossRef]
- Antonetti, C.; Toniolo, L.; Cavinato, G.; Forte, C.; Ghignoli, C.; Ishake, R.; Cavani, F.; Raspolli Galletti, A.M. A hybrid polyketone–SiO2 support for palladium catalysts and their applications in cinnamaldehyde hydrogenation and in 1-phenylethanol oxidation. Appl. Catal. A Gen. 2015, 496, 40–50. [Google Scholar] [CrossRef]
- Yasueda, T.; Seike, R.; Ikenaga, N.; Miyake, T.; Suzuki, T. Palladium-loaded oxidized diamond catalysis for the selective oxidation of alcohols. J. Mol. Catal. A Chem. 2009, 306, 136–142. [Google Scholar] [CrossRef]
- Abad, A.; Almela, C.; Corma, A.; Garcıa, H. Efficient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts. Tetrahedron 2006, 62, 6666–6672. [Google Scholar] [CrossRef]
- Kantam, M.L.; Reddy, R.S.; Pal, U.; Sudhakar, M.; Venugopal, A.; Jeeva Ratnam, K.; Figueras, F.; Reddy Chintareddy, V.; Nishinad, Y. Ruthenium/magnesium–lanthanum mixed oxide: An efficient reusable catalyst for oxidation of alcohols by using molecular oxygen. J. Mol. Catal. A Chem. 2012, 359, 1–7. [Google Scholar] [CrossRef]
- Hosseini-Monfared, H.; Meyer, H.; Janiak, C. Dioxygen oxidation of 1-phenylethanol with gold nanoparticles and N-hydroxyphthalimide in ionic liquid. J. Mol. Catal. A Chem. 2013, 372, 72–78. [Google Scholar] [CrossRef]
- Restrepo, J.; Lozano, P.; Burguete, M.I.; García-Verdugo, E.; Luis, S.V. Gold nanoparticles immobilized onto supported ionic liquid-like phases for microwave phenylethanol oxidation in water. Catal. Today 2015, 255, 97–101. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Ribeiro, A.P.C.; Buijnsters, J.G.; Avalos-Borja, M.; Pombeiro, A.J.L.; Figueiredo, J.L.; Martins, L.M.D.R.S. Solvent-free oxidation of 1-phenylethanol catalysed by gold nanoparticles supported on carbon powder materials. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Efficient aerobic oxidation of alcohols using a hydrotalcite-supported gold nanoparticle catalyst. Adv. Synth. Catal. 2009, 351, 1890–1896. [Google Scholar] [CrossRef]
- Liang, W.; Xiangju, M.; Fengshou, X. Au nanoparticles supported on a layered double hydroxide with excellent catalytic properties for the aerobic oxidation of alcohols. Chin. J. Catal. 2010, 31, 943–947. [Google Scholar]
- Haider, P.; Grunwaldt, J.D.; Baiker, A. Gold supported on Mg, Al and Cu containing mixed oxides: Relation between surface properties and behavior in catalytic aerobic oxidation of 1-phenylethanol. Catal. Today 2009, 141, 349–354. [Google Scholar] [CrossRef]
- Shanahan, A.E.; McNamara, J.A.; Sullivan, H.J.; Byrne, M. An insight into the superior performance of a gold nanocatalyst on single wall carbon nanotubes to that on titanium dioxide and amorphous carbon for the green aerobic oxidation of aromatic alcohols. New Carbon Mater. 2017, 32, 242–251. [Google Scholar] [CrossRef]
- Wang, L.; He, L.; Liu, Q.; Liu, Y.; Chen, M.; Cao, Y.; He, H.; Fan, K. Solvent-free selective oxidation of alcohols by molecular oxygen over gold nanoparticles supported on β-MnO2 nanorods. Appl. Catal. A Gen. 2008, 344, 150–157. [Google Scholar] [CrossRef]
- Nepak, D.; Darbha, S. Selective aerobic oxidation of alcohols over Au–Pd/sodium titanate nanotubes. Catal. Commun. 2015, 58, 149–153. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Liang, C.; Su, W.; Wang, C.; Feng, Z.; Li, C.; Qiu, J. Aerobic oxidation of alcohols over Au/TiO2: An insight on the promotion effect of water on the catalytic activity of Au/TiO2. Catal. Commun. 2008, 9, 2278–2281. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Z.; Cao, M.; Cao, R. Stable gold nanoparticle encapsulated in silica-dendrimers organic–inorganic hybrid composite as recyclable catalyst for oxidation of alcohol. Microporous Mesoporous Mater. 2010, 136, 42–49. [Google Scholar] [CrossRef]
- Mertens, P.G.N.; Corthals, S.L.F.; Yeb, X.; Poelman, H.; Jacobs, P.A.; Sels, B.F.; Vankelecom, I.F.J.; De Vos, D.E. Selective alcohol oxidation to aldehydes and ketones over base-promoted gold–palladium clusters as recyclable quasi homogeneous and heterogeneous metal catalysts. J. Mol. Catal. A Chem. 2009, 313, 14–21. [Google Scholar] [CrossRef]
- Abad, A.; Concepcion, P.; Corma, A.; Garcia, H. A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew. Chem. Int. Ed. 2005, 44, 4066–4069. [Google Scholar] [CrossRef] [PubMed]
- Daliran, S.; Santiago-Portillo, A.; Navalón, S.; Oveisi, A.; Álvaro, M.; Ghorbani-Vaghei, R.; Azarifar, D.; García, H. Cu(II)-Schiff base covalently anchored to MIL-125(Ti)-NH2 as heterogeneous catalyst for oxidation reactions. J. Coll. Interface Sci. 2018, 532, 700–710. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Ma, X.; Liu, P.; Gu, C.; Dai, B. Metal-free oxidation of secondary benzylic alcohols using aqueous TBHP. Synth. Commun. 2006, 46, 1747–1758. [Google Scholar] [CrossRef]
- Kotolevich, Y.; Kolobova, E.; Khramov, E.; Farıas, M.H.; Zubavichus, Y.; Tiznado, H.; González-Pérez, M.E.; Corberán, V.C.; Mota-Morales, J.D.; Pestryakov, A.N.; et al. n-octanol oxidation on Au/TiO2 catalysts promoted with La and Ce oxides. J. Mol. Catal. 2017, 427, 1–10. [Google Scholar] [CrossRef]
- Kotolevich, Y.; Kolobova, E.; Mamontov, G.; Khramov, E.; Cabrera Ortega, J.E.; Tiznado, H.; Farias, M.H.; Bogdanchikova, N.; Zubavichus, Y.; Mota-Morales, J.D.; et al. Au/TiO2 catalysts promoted with Fe and Mg for n-octanol oxidation under mild conditions. Catal. Today 2016, 278, 104–112. [Google Scholar] [CrossRef]
- Kolobova, E.; Pakrieva, E.; Pascual, L.; Cortés Corberán, V.; Bogdanchikova, N.; Farias, M.; Pestryakov, M. Selective oxidation of n-octanol on unmodified and La-modified nanogold catalysts: Effect of metal content. Catal. Today 2019, 333, 127–132. [Google Scholar] [CrossRef]
- Pakrieva, E.; Kolobova, E.; Mamontov, G.; Bogdanchikova, N.; Farias, M.H.; Pascual, L.; Cortés Corberán, V.; Martinez Gonzalez, S.; Carabineiro, S.A.C.; Pestryakov, A. Green oxidation of n-octanol on supported nanogold catalysts: Formation of gold active sites under combined effect of gold content, additive nature and redox pretreatment. ChemCatChem 2019, 11, 1615–1624. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Henry, C.R.; Louis, C. Alternative methods for the preparation of gold nanoparticles supported on TiO2. J. Phys. Chem. B. 2002, 106, 7634–7642. [Google Scholar] [CrossRef]
- Zanella, R.; Louis, C. Influence of the conditions of thermal treatments and of storage on the size of the gold particles in Au/TiO2 samples. Catal. Today 2005, 107, 768–777. [Google Scholar] [CrossRef]
- Kolobova, E.; Maki-Arvela, P.; Pestryakov, A.; Pakrieva, E.; Pascual, L.; Smeds, A.; Rahkila, J.; Sandberg, T.; Peltonen, J.; Murzin, D.Y. Reductive amination of ketones with benzylamine over gold supported on different oxides. Catal. Lett. 2019, 149, 3432–3446. [Google Scholar] [CrossRef]
- Kolobova, E.N.; Pakrieva, E.G.; Carabineiro, S.; Bogdanchikova, N.; Kharlanov, A.; Kazantsev, S.O.; Hemming, J.; Mäki-Arvela, P.; Pestryakov, A.N.; Murzin, D. Oxidation of a wood extractive betulin to biologically active oxo-derivatives using supported gold catalysts. Green Chem. 2019, 21, 3370–3382. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Tollberg, B.; Hu, X.; Wang, L. Structural study of gold clusters. J. Chem. Phys. 2006, 124, 114309. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedzyanova, D.F.; Ratmanova, N.K.; Pichugina, D.A.; Kuzmenko, E.N. A structural and stability evaluation of Au12 from an isolated cluster to the deposited material. J. Phys. Chem. C. 2012, 116, 11507–11518. [Google Scholar] [CrossRef]
- Beletskaya, A.V.; Pichugina, D.A.; Shestakov, A.F.; Kuz’menko, N.E. Formation of H2O2 on Au20 and Au19Pd Clusters: Understanding the Structure Effect on the Atomic Level. J. Phys. Chem. A. 2013, 117, 6817–6826. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A Quantum-ChemicalProgram Suite. New Possibilities in the Study of Molecular Systems with the Application of Parallel Computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Sadovnichy, V.; Tikhonravov, A.; Voevodin, V.; Opanasenko, V. Contemporary High Performance Computing: From Petascale toward Exascale; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Yu, J.; Wu, G.; Mao, D. Effect of La2O3 on catalytic performance of Au/TiO2 for CO oxidation. Acta Phys.-Chim. Sin. 2008, 24, 1751–1755. [Google Scholar] [CrossRef]
- Lee, K.J.; Kumar, P.A.; Maqbool, M.S.; Rao, K.N.; Song, K.H.; Ha, H.P. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR: Physico-chemical properties and catalytic activity. Appl. Catal. B 2013, 142, 705–717. [Google Scholar] [CrossRef]
- Carja, G.; Kameshima, Y.; Okada, K.; Madhusoodana, C.D. Mn–Ce/ZSM5 as a new superior catalyst for NO reduction with NH3. Appl. Catal. B 2007, 73, 60–64. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Costa, V.V.; Estrada, M.; Demidova, Y.; Prosvirin, I.; Kriventsov, V.; Cotta, R.F.; Fuentes, S.; Simakov, A.; Gusevskaya, E. Gold nanoparticles supported on magnesium oxide as catalysts for the aerobic oxidation of alcohols under alkali-free conditions. J. Catal. 2012, 292, 148–156. [Google Scholar] [CrossRef]
- Feng, R.; Li, M.; Liu, J. Synthesis of core–shell Au@Pt nanoparticles supported on Vulcan XC-72 carbon and their electrocatalytic activities for methanol oxidation. Colloids Surf. A 2012, 406, 6–12. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Bogdanchikova, N.; Temkin, O.N.; Smolentseva, E. Active states of gold in small and big metal particles in CO and methanol selective oxidation. Fuel 2013, 110, 48–53. [Google Scholar] [CrossRef]
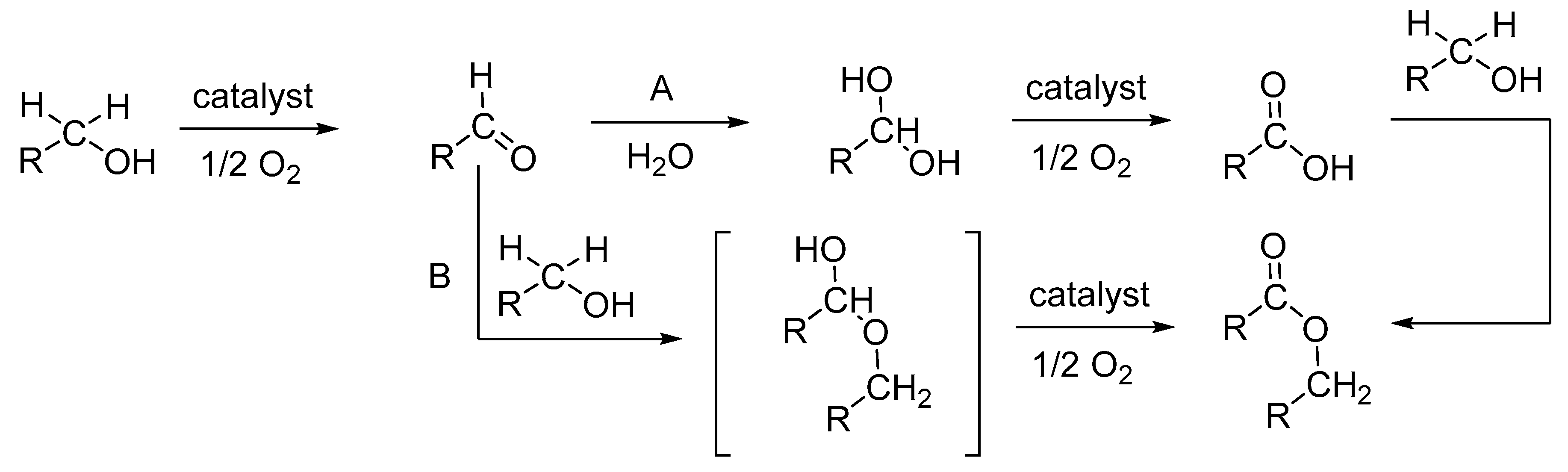
| Catalyst | Oxidant | P, Atm | Solvent | T, °C | Reaction Time, h | cR | dYac, % | Ref. |
|---|---|---|---|---|---|---|---|---|
| [Cu2(R)(CH3O)(NO3)]2(CH3O)2 | TBHP | 1 | - | 80 | b 1MW | 71 | 54 | [5] |
| g [Cu(κONN’HL)(NO3) (N,N-dimethylformamide(DMF)](NO3)∙H2 | TBHP | 1 | - | 80 | 2 | 1000 | 12 | [6] |
| g [Cu(κONN’HL)(NO3)(DMF)] (NO3)∙H2+K2CO3 | TBHP | 1 | - | 80 | 2 | 1000 | 62 | [6] |
| h [Cu(1κNOO’,2κO’,3κO”L)]n | TBHP | 1 | - | 120 | b 1MW | 250 | 66 | [7] |
| h [Cu(1κNOO’,2κO’,3κO”L)]n +2,2,6,6-Tetramethylpiperidin-1-yl)oxyl(TEMPO) | TBHP | 1 | - | 120 | b 1MW | 250 | 81 | [7] |
| h [Cu(κNOO’HL)Cl(CH3OH)] | TBHP | 1 | - | 120 | b 1MW | 250 | 82 | [7] |
| h [Cu(κNOO’HL)Cl(CH3OH)] +TEMPO | TBHP | 1 | - | 120 | b 1MW | 250 | 92 | [7] |
| Carbon nanotubes (CNTs) | TBHP | 1 | - | 80 | b 1MW | 50 | 8 | [8] |
| Graphene oxide (GO) | TBHP | 1 | - | 80 | b 1MW | 50 | 14 | [8] |
| CoCl2 | TBHP | 1 | - | 80 | b 1MW | 50 | 28 | [8] |
| CoCl2–5%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 85 | [8] |
| CoCl2–5% GO | TBHP | 1 | - | 80 | b 1MW | 50 | 72 | [8] |
| CuO | TBHP | 1 | - | 80 | b 1MW | 50 | 16 | [8] |
| CuO–1%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 59 | [8] |
| Fe2O3 | TBHP | 1 | - | 80 | b 1MW | 50 | 10 | [8] |
| Fe2O3–1%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 32 | [8] |
| Fe2O3–CoCl2–5%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 73 | [8] |
| V2O5 | TBHP | 1 | - | 80 | b 1MW | 50 | 45 | [8] |
| CoCl2–V2O5–5%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 54 | [8] |
| [FeCl2(L)(2,20bipy)] | TBHP | 1 | - | 150 | b 1MW | 333 | 99 | [9] |
| [FeCl2(L)(2,20bipy)] | TBHP | 1 | - | 150 | 46 | 333 | 99 | [9] |
| [Fe(bipy)3](CF3SO3)2 | H2O2 | 1 | CH3CN | 100 | 0.5 | 100 | 62 | [10] |
| [Fe(bipy)3](CF3SO3)2)+2-pyridinecarboxylic acid | H2O2 | 1 | CH3CN | 100 | 0.5 | 100 | 93 | [10] |
| VOPO4+TEMPO | O2 | 4 | H2O | 80 | 6 | 20 | a 38.5(89) | [11] |
| NiO/SiO2 | O2 | 1 | p-xylen | 100 | 6 | 12 | 51 | [12] |
| MnO2 commercial | TBHP | 1 | e ACN:tol | RT | 7 | 1 | 84 | [13] |
| MnO2 commercial | TBHP | 1 | e ACN:tol | 40 | 7 | 20 | 34 | [13] |
| MnO2 commercial | H2O2 | 1 | e ACN:tol | 40 | 5 | 20 | 0 | [13] |
| MnO2 commercial | TBHP | 1 | e ACN:tol | 80 | 7 | 10 | 67 | [13] |
| MnO2 commercial | - | 1 | e ACN:tol | 80 | 24 | 1 | 30 | [13] |
| NbP–C | H2O2 | 1 | CH3CN | 90 | 24 | 11 | a 72 | [14] |
| CeCrO3 | TBHP | 1 | DMSO | 90 | 6 | 10 | a 100 | [15] |
| 15wt.% Ag- Octahedral molecular sieve-2 | TBHP | 1 | CH3CN | 75 | 4 | 625 | a 71.5 | [16] |
| 0.9wt.% Pd/Aerosil380 | O2 | 10 | H2O | 100 | 6 | 262 | 44.9 | [17] |
| 0.9wt.% Pd/Aerosil380 | O2 | 10 | H2O | 100 | 12 | 262 | 75.1 | [17] |
| 1.0wt.% Pd/60wt.% Polyketone (PK)–SiO2 | O2 | 10 | H2O | 100 | 6 | 262 | 62.2 | [17] |
| 1.0wt.% Pd/60wt.% PK–SiO2 | O2 | 10 | H2O | 100 | 12 | 262 | 100 | [17] |
| 1.0wt.% Pd/76wt.% PK–SiO2 | O2 | 10 | H2O | 100 | 6 | 262 | 58.3 | [17] |
| 1.0wt.% Pd/76wt.% PK–SiO2 | O2 | 10 | H2O | 100 | 12 | 262 | 94.8 | [17] |
| 3wt.%Pd/O-Diamonds(Dia) | O2 | 1 | o-xylen | 100 | 4 | 1428 | a 27.9 | [18] |
| 3wt.%Pd/CeO2/O-Dia | O2 | 1 | o-xylen | 100 | 4 | 1428 | a 72.5 | [18] |
| 1.57wt.% Pd/CeO2 | O2 | 1 | - | 120 | 2 | 649 | a 91 | [19] |
| 1.44wt.% Pd/apatite | O2 | 1 | - | 120 | 2 | 649 | a 90 | [19] |
| 10.10wt.% Ru/Mg–LaO | O2 | 1 | toluene | 80 | 4 | 10 | 96 | [20] |
| 10.10wt.% Ru/SiO2 | O2 | 1 | toluene | 80 | 4 | 10 | 45 | [20] |
| 10.10wt.% Ru/Al2O3 | O2 | 1 | toluene | 80 | 4 | 10 | 40 | [20] |
| 10.10wt.% Ru/MgO | O2 | 1 | toluene | 80 | 4 | 10 | 36 | [20] |
| 10.10wt.% Ru/TiO2 | O2 | 1 | toluene | 80 | 4 | 10 | 36 | [20] |
| 1.54wt.% Au/CeO2 | O2 | 1 | - | 120 | 2 | 649 | 95 | [19] |
| 1wt.% gold nanoparticles (Au NPs)/Ionic liquid(IL)/N-hydroxyphthalimide(NHPI) | O2 | 4 | - | 100 | 24 | 1356 | a 60(47) | [21] |
| 1wt.% Au NPs/IL/NHPI | O2 | 4 | - | 160 | 24 | 6780 | a 77 (58) | [21] |
| 1wt.%Au NPs– supported ionic liquid-like phases | H2O2 | 1 | H2O | 150 | 0.25 | 8 | >90 | [22] |
| 1wt.% Au/Active carbon | TBHP | 1 | - | 150 | b 2 MW | 500 | 55 | [23] |
| 1wt.% Au/carbon xerogel | TBHP | 1 | - | 150 | b 2 MW | 500 | 90 | [23] |
| 1wt.% Au/Graphite | TBHP | 1 | - | 150 | b 2 MW | 500 | 63 | [23] |
| 1wt.% Au/Microdiamonds | TBHP | 1 | - | 150 | b 2 MW | 500 | 100 | [23] |
| 1wt.% Au/Nanodiamonds for liquid dispersion | TBHP | 1 | - | 150 | b 2 MW | 500 | 83 | [23] |
| 1wt.%Au/Silicone carbide | TBHP | 1 | - | 150 | b 2 MW | 500 | 73 | [23] |
| 0.89wt.% Au/Hydrotalcite (Ht) | Air | 1 | toluene | 80 | 0.33 | 222 | 99 | [24] |
| 0.89wt.% Au/Ht | Air | 1 | toluene | 40 | 3 | 222 | 99 | [24] |
| 0.89wt.% Au/Ht | Air | 1 | toluene | 27 | 6 | 222 | 99 | [24] |
| 0.89wt.% Au/Al2O3 | Air | 1 | toluene | 27 | 3 | 222 | 71 | [24] |
| 0.89wt.% Au/MgO | Air | 1 | toluene | 27 | 3 | 222 | 71 | [24] |
| 0.89wt.% Au/TiO2 | Air | 1 | toluene | 27 | 3 | 222 | 14 | [24] |
| 0.89wt.% Au/TiO2+Na2CO3 | Air | 1 | toluene | 27 | 3 | 222 | 65 | [24] |
| 0.89wt.% Au/SiO2 | Air | 1 | toluene | 27 | 3 | 222 | <1 | [24] |
| 1.8wt.% Au/Layered double hydroxide | O2 | 1 | toluene | 80 | 2 | 200 | 99 | [25] |
| 1.0wt.% Au/CuaMgbAlcOx | O2 | 1 | f mes. | 90 | 1 | 1181 | 85.1 | [26] |
| 5wt.% Au/TiO2 | O2 | 1 | - | 120 | 6 | 500 | 99 | [27] |
| 5wt.% Au/Carbon black | O2 | 1 | - | 120 | 4 | 500 | 65 | [27] |
| 5wt.% Au/Single wall carbon nanotubes | O2 | 1 | - | 120 | 3 | 500 | 99 | [27] |
| 5wt.% Au/MnO2-R | O2 | 4 | - | 120 | 8 | 40,000 | 81 | [28] |
| Au–Pd (2 wt.%, 1:1)/Sodium titanate nanotubes | Air | 1 | - | 120 | 10 | 10,000 | a 84(86) | [29] |
| 7.8wt.% Au/TiO2 | O2 | 10 | H2O | 100 | 8 | 100 | 100 | [30] |
| 7.8wt.% Au/TiO2+ K2CO3 | O2 | 10 | H2O | 100 | 2 | 100 | 93 | [30] |
| 10.83wt.% Au-dendrimers/ Mesoporous SiO2SBA-15+ 3 eq. K3PO4 | O2 | 1 | CH2Cl2/H2O | RT | 24 | 33 | 99.1 | [31] |
| 0.5 wt.%(Au0–Pd0)/high surface area-BaAl2O4 | O2 | 20 | - | 140 | 0.83 | 50,000 | a 97 | [32] |
| 4wt.% Au/La2O3/TiO2 | TBHP | 1 | - | 80 | 1 | 5000 | 98 | This |
| 4wt.% Au/La2O3/TiO2 | O2 | 1 | f mes. | 80 | 1 | 100 | 99 | work |
| Entry | Sample | Yield of Acetophenone (mol%) at Time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | ||
| 1 | - | 0 | 0 | 1 | 2 | 3 | 3 | 4 |
| 2 | TiO2 | 2 | 2 | 2 | 2 | 2 | 4 | 6 |
| 3 | CeO2/TiO2 | 3 | 3 | 3 | 4 | 6 | 8 | 14 |
| 4 | La2O3/TiO2 | 3 | 3 | 3 | 6 | 8 | 10 | 17 |
| 5 | 0.5% Au/TiO2_H2 | 25 | 40 | 55 | 80 | 100 | 100 | 100 |
| 6 | 0.5% Au/TiO2_O₂ | 18 | 30 | 43 | 69 | 81 | 98 | 100 |
| 7 | 0.5% Au/TiO2_as | 1 | 1 | 3 | 9 | 16 | 28 | 51 |
| 8 | 4% Au/TiO2_H₂ | 33 | 46 | 75 | 100 | 100 | 100 | 100 |
| 9 | 4% Au/TiO2_O₂ | 30 | 49 | 65 | 99 | 100 | 100 | 100 |
| 10 | 4% Au/TiO2_as | 1 | 2 | 6 | 19 | 28 | 45 | 82 |
| 11 | 0.5% Au/CeO2/TiO2_H₂ | 27 | 37 | 60 | 85 | 100 | 100 | 100 |
| 12 | 0.5% Au/CeO2/TiO2_O₂ | 28 | 36 | 72 | 90 | 100 | 100 | 100 |
| 13 | 0.5% Au/CeO2/TiO2_as | 2 | 3 | 3 | 7 | 14 | 28 | 61 |
| 14 | 4% Au/CeO2/TiO2_H₂ | 36 | 49 | 85 | 100 | 100 | 100 | 100 |
| 15 | 4% Au/CeO2/TiO2_O₂ | 22 | 41 | 75 | 100 | 100 | 100 | 100 |
| 16 | 4% Au/CeO2/TiO2_as | 3 | 4 | 10 | 25 | 46 | 67 | 100 |
| 17 | 0.5% Au/La2O3/TiO2_H₂ | 31 | 45 | 55 | 88 | 100 | 100 | 100 |
| 18 | 0.5% Au/La2O3/TiO2_O₂ | 38 | 49 | 61 | 96 | 100 | 100 | 100 |
| 19 | 0.5% Au/La2O3/TiO2_as | 1 | 1 | 2 | 8 | 18 | 42 | 84 |
| 20 | 4% Au/La2O3/TiO2_H₂ | 41 | 55 | 98 | 100 | 100 | 100 | 100 |
| 21 | 4% Au/La2O3/TiO2_O₂ | 22 | 40 | 67 | 100 | 100 | 100 | 100 |
| 22 | 4% Au/La2O3/TiO2_as | 1 | 2 | 6 | 29 | 54 | 100 | 100 |
| Au Amount (µmol) | Yield of Acetophenone (mol%) at Time (min) | |||||
|---|---|---|---|---|---|---|
| 5 | 15 | 30 | 60 | 120 | 180 | |
| 1.27 | 15 | 22 | 40 | 67 | 100 | 100 |
| 5 | 33 | 40 | 50 | 81 | 100 | 100 |
| 10 | 80 | 100 | 100 | 100 | 100 | 100 |
| 20 | 100 | 100 | 100 | 100 | 100 | 100 |
| Catalyst | R | Yield of Acetophenone, % |
|---|---|---|
| 4% Au/La2O3/TiO2_H₂ | 5000 | 0 2 |
| 500 | 50 2 | |
| 100 | 98 3 |
| Entry | Catalyst | Yield of Acetophenone (mol%) at Time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | ||
| 1 | 0.5% Au/TiO2_H₂ | 21 | 25 | 31 | 40 | 45 | 47 | 50 |
| 2 | 0.5% Au/TiO2_O₂ | 26 | 32 | 38 | 44 | 49 | 52 | 58 |
| 3 | 4% Au/TiO2_H₂ | 32 | 36 | 35 | 41 | 42 | 43 | 44 |
| 4 | 4% Au/TiO2_O₂ | 19 | 21 | 25 | 30 | 35 | 38 | 40 |
| 5 | 0.5% Au/CeO2/TiO2_H₂ | 45 | 50 | 60 | 65 | 68 | 70 | 72 |
| 6 | 0.5% Au/CeO2/TiO2_O₂ | 70 | 86 | 96 | 99 | 100 | 100 | 100 |
| 7 | 4% Au/CeO2/TiO2_H₂ | 53 | 59 | 65 | 69 | 71 | 73 | 78 |
| 8 | 4% Au/CeO2/TiO2_O₂ | 40 | 44 | 50 | 55 | 59 | 65 | 70 |
| 9 | 0.5% Au/La2O3/TiO2_H₂ | 90 | 95 | 97 | 100 | 100 | 100 | 100 |
| 10 | 0.5% Au/La2O3/TiO2_O₂ | 95 | 98 | 100 | 100 | 100 | 100 | 100 |
| 11 | 4% Au/La2O3/TiO2_H₂ | 88 | 96 | 99 | 100 | 100 | 100 | 100 |
| 12 | 4% Au/La2O3/TiO2_O₂ | 79 | 88 | 93 | 97 | 98 | 99 | 100 |
| Sample | SBET, m2/g | EDX Au Content, wt.% | Au Average Particle Size, nm |
|---|---|---|---|
| TiO2 | 55 | - | - |
| La2O3/TiO2 | 48 | - | - |
| CeO2/TiO2 | 48 | - | - |
| 0.5% Au/TiO2_H2 | 54 | 0.4 | 4.4 |
| 0.5% Au/TiO2_O2 | 54 | 0.4 | 4.2 |
| 4% Au/TiO2_H2 | 50 | 4.0 | 2.9 |
| 4% Au/TiO2_O2 | 50 | 4.0 | 3.3 |
| 0.5% Au/CeO2/TiO2_H2 | 47 | 0.3 | 3.4 |
| 0.5% Au/CeO2/TiO2_O2 | 47 | 0.3 | 3.8 |
| 4% Au/CeO2/TiO2_H2 | 46 | 4.1 | 2.8 |
| 4% Au/CeO2/TiO2_O2 | 46 | 4.1 | 2.4 |
| 0.5% Au/La2O3/TiO2_H2 | 47 | 0.5 | 2.8 |
| 0.5% Au/La2O3/TiO2_O2 | 47 | 0.5 | 2.4 |
| 4% Au/La2O3/TiO2_H2 | 43 | 3.3 | 2.6 |
| 4% Au/La2O3/TiO2_O2 | 43 | 3.3 | 2.7 |
| Catalyst | Au(0, 1+ or 3+) Relative Content, % | ||
|---|---|---|---|
| Au0 | Au1+ | Au3+ | |
| 1 0.5% Au/TiO2_H2 | 91 | 9 | 0 |
| 1 0.5% Au/TiO2_O2 | 84 | 16 | 0 |
| 1 4% Au/TiO2_H2 | 73 | 14 | 11 |
| 1 4% Au/TiO2_O2 | 89 | 11 | 0 |
| 3 0.5% Au/CeO2/TiO2_H2 | 91 | 9 | 0 |
| 3 0.5% Au/CeO2/TiO2_O2 | 85 | 15 | 0 |
| 2 4% Au/CeO2/TiO2_H2 | 68 | 20 | 12 |
| 3 4% Au/CeO2/TiO2_O2 | 90 | 10 | 0 |
| 1 0.5% Au/La2O3/TiO2_H2 | 80 | 20 | 0 |
| 1 0.5% Au/La2O3/TiO2_O2 | 65 | 35 | 0 |
| 1 4% Au/La2O3/TiO2_H2 | 81 | 19 | 0 |
| 1 4% Au/La2O3/TiO2_O2 | 83 | 17 | 0 |
| Entry | Sample | Yield of Acetophenone in 2 h, mol % | Relative Au Content, % | |
|---|---|---|---|---|
| Au0 | Au+ | |||
| 1 | 4% Au/La2O3/TiO2_H₂ 1,a | 100 | 81 | 19 |
| 2 | 0.5% Au/La2O3/TiO2_O₂ 2,a | 100 | 65 | 35 |
| 3 | 4% Au/La2O3/TiO2_H₂_1c 1,b | 90 | 83 | 17 |
| 4 | 4% Au/La2O3/TiO2_H₂_6c 1,c | 59 | 89 | 11 |
| z | Complex | Isomer | Type of Coordination | ΔE | ΔH | |
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | OH– | top | −57 | −54 |
| 0 | 2 | 1 | OH– | top | −60 | −57 |
| 0 | 3 | 2 | OH– | edge | −33 | −29 |
| 0 | 4 | 2 | OH– | facet | −30 | −31 |
| 0 | 5 | 2 | C6H5– | top | −49 | −45 |
| 0 | 6 | 2 | C6H5– | edge | −23 | −21 |
| +1 | 1 | 2 | OH– | top | −97 | −92 |
| +1 | 2 | 1 | OH– | top | −112 | −108 |
| +1 | 3 | 2 | OH– | edge | −75 | −70 |
| +1 | 4 | 2 | OH– | facet | −56 | −54 |
| +1 | 5 | 2 | C6H5– | top | −102 | −100 |
| +1 | 6 | 2 | C6H5– | edge | −75 | −72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakrieva, E.; P. C. Ribeiro, A.; Kolobova, E.; M. D. R. S. Martins, L.; A. C. Carabineiro, S.; German, D.; Pichugina, D.; Jiang, C.; J. L. Pombeiro, A.; Bogdanchikova, N.; et al. Supported Gold Nanoparticles as Catalysts in Peroxidative and Aerobic Oxidation of 1-Phenylethanol under Mild Conditions. Nanomaterials 2020, 10, 151. https://doi.org/10.3390/nano10010151
Pakrieva E, P. C. Ribeiro A, Kolobova E, M. D. R. S. Martins L, A. C. Carabineiro S, German D, Pichugina D, Jiang C, J. L. Pombeiro A, Bogdanchikova N, et al. Supported Gold Nanoparticles as Catalysts in Peroxidative and Aerobic Oxidation of 1-Phenylethanol under Mild Conditions. Nanomaterials. 2020; 10(1):151. https://doi.org/10.3390/nano10010151
Chicago/Turabian StylePakrieva, Ekaterina, Ana P. C. Ribeiro, Ekaterina Kolobova, Luísa M. D. R. S. Martins, Sónia A. C. Carabineiro, Dmitrii German, Daria Pichugina, Ce Jiang, Armando J. L. Pombeiro, Nina Bogdanchikova, and et al. 2020. "Supported Gold Nanoparticles as Catalysts in Peroxidative and Aerobic Oxidation of 1-Phenylethanol under Mild Conditions" Nanomaterials 10, no. 1: 151. https://doi.org/10.3390/nano10010151
APA StylePakrieva, E., P. C. Ribeiro, A., Kolobova, E., M. D. R. S. Martins, L., A. C. Carabineiro, S., German, D., Pichugina, D., Jiang, C., J. L. Pombeiro, A., Bogdanchikova, N., Cortés Corberán, V., & Pestryakov, A. (2020). Supported Gold Nanoparticles as Catalysts in Peroxidative and Aerobic Oxidation of 1-Phenylethanol under Mild Conditions. Nanomaterials, 10(1), 151. https://doi.org/10.3390/nano10010151