Polyol Process Coupled to Cold Plasma as a New and Efficient Nanohydride Processing Method: Nano-Ni2H as a Case Study
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Smeets, F.; Vaes, J.; Zhang, D.; Zeng, K.; Tjarks, G.; Mergel, J.; Stolten, D.; Fritz, D.L.; Carmo, M.; Minh, N.Q. Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Tremel, A.; Wasserscheid, P.; Baldauf, M.; Hammer, T. Techno-economic analysis for the synthesis of liquid and gaseous fuels based on hydrogen production via electrolysis. Int. J. Hydrog. Energy 2015, 40, 11457–11464. [Google Scholar] [CrossRef]
- Wesselbaum, S.; Hintermair, U.; Leitner, W. Continuous-flow hydrogenation of carbon dioxide to pure formic acid using an integrated scCO2 process with immobilized catalyst and base. Angew. Chem. Int. Ed. 2012, 51, 8585–8588. [Google Scholar] [CrossRef]
- Schmidt, I.; Müller, K.; Arlt, W. Evaluation of Formic-Acid-Based Hydrogen Storage Technologies. Energy Fuels 2014, 28, 6540–6544. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Liquid-phase chemical hydrogen storage materials. Energy Environ. Sci. 2012, 5, 9698–9725. [Google Scholar] [CrossRef]
- Kusche, M.; Enzenberger, F.; Bajus, S.; Niedermeyer, H.; Bösmann, A.; Kaftan, A.; Laurin, M.; Libuda, J.; Wasserscheid, P. Highly Effective Pt-Based Water-Gas Shift Catalysts by Surface Modification with Alkali Hydroxide Salts. Angew. Chem. Int. Ed. 2013, 52, 5028–5032. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, Y.Y.; Liu, M.; Cong, H.T.; Cheng, H.M.; Dresselhaus, M.S. Hydrogen storage in single-walled carbon nanotubes at room temperature. Science 1999, 286, 1127–1129. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Roth, S. Hydrogen Adsorption in Different Carbon Nanostructures. Carbon 2005, 43, 2209–2214. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Regli, L.; Chavan, S.; Ricchiardi, G.; Spoto, G.; Dietzel, P.D.C.; Bordiga, S.; Zecchina, A. Role of Exposed Metal Sites in Hydrogen Storage in MOFs. J. Am. Chem. Soc. 2008, 130, 8386–8396. [Google Scholar] [CrossRef] [PubMed]
- Hirscher, M.; Panella, B. Hydrogen storage in metalorganic frameworks. Scr. Mater. 2007, 56, 809–812. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas Storage in Nanoporous Materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—new perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meisner, G.; Meyer, M.; Balogh, M.; Kundrat, M.J. Hydrogen Desorption Exceeding Ten Weight Percent from the New Quaternary Hydride Li3BN2H8. J. Phys. Chem. B 2005, 109, 6–8. [Google Scholar] [CrossRef]
- Aoki, M.; Miwa, K.; Noritake, T.; Kitahara, G.; Nakamori, Y.; Orimo, S.-I.; Towata, S. Destabilization of LiBH4 by mixing with LiNH2. Appl. Phys. A 2005, 80, 1409–1412. [Google Scholar] [CrossRef]
- Chaise, A.; De Rango, P.; Marty, P.; Fruchart, D. Experimental and numerical study of a magnesium hydride tank. Int. J. Hydrog. Energy 2010, 35, 6311–6322. [Google Scholar] [CrossRef]
- Jain, I.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrog. Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Souahlia, A.; Dhaou, H.; Mellouli, S.; Askri, F.; Jemni, A.; Ben Nasrallah, S. Experimental study of metal hydride-based hydrogen storage tank at constant supply pressure. Int. J. Hydrog. Energy 2014, 39, 7365–7372. [Google Scholar] [CrossRef]
- Chaise, A.; De Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S.; Olives, R.; Garrier, S. Enhancement of hydrogen sorption in magnesium hydride using expanded natural graphite. Int. J. Hydrog. Energy 2009, 34, 8589–8596. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamaridarkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kobayashi, H.; Kitagawa, H. Hydrogen Storage Mediated by Pd and Pt Nanoparticles. Chem. Phys. Chem. 2009, 10, 2566–2576. [Google Scholar] [CrossRef] [PubMed]
- Norberg, N.S.; Arthur, T.S.; Fredrick, S.J.; Prieto, A.L. Size-Dependent Hydrogen Storage Properties of Mg Nanocrystals Prepared from Solution. J. Am. Chem. Soc. 2011, 133, 10679–10681. [Google Scholar] [CrossRef] [PubMed]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen Storage Technologies for Future Energy Systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rzhevskii, A.; Rigos, S.; Xie, W.; Zhang, S.; Lu, T.-M.; Wang, G.-C. A study of Parylene coated Pd/Mg nanoblabes for reversible hydrogen storage. Int. J. Hydrog. Energy 2013, 38, 5019–5029. [Google Scholar] [CrossRef]
- Garrier, S.; Delhomme, B.; De Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S. A new MgH2 tank concept using a phase-change material to store the heat of reaction. Int. J. Hydrog. Energy 2013, 38, 9766–9771. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size-Specific Catalytic Activity of Polymer-Stabilized Gold Nanoclusters for Aerobic Alcohol Oxidation in Water. J. Am. Chem. Soc. 2005, 127, 9374–9375. [Google Scholar] [CrossRef]
- Teranishi, T.; Miyake, M. Size Control of Palladium Nanoparticles and Their Crystal Structures. Chem. Mater. 1998, 10, 594–600. [Google Scholar] [CrossRef]
- Teranishi, T.; Hori, H.; Miyake, M. ESR Study on Palladium Nanoparticles. J. Phys. Chem. B 1997, 101, 5774–5776. [Google Scholar] [CrossRef]
- Teranishi, T.; Hosoe, M.; Tanaka, T.; Miyake, M. Size Control of Monodispersed Pt Nanoparticles and Their 2D Organization by Electrophoretic Deposition. J. Phys. Chem. B 1999, 103, 3818–3827. [Google Scholar] [CrossRef]
- Myers, S.M.; Richards, P.M.; Follstaedt, D.M.; Schirber, J.E. Superstoichiometry, accelerated diffusion, and nuclear reactions in deuterium-implanted palladium. Phys. Rev. B 1991, 43, 9503–9510. [Google Scholar] [CrossRef] [PubMed]
- Tavares, S.; Miraglia, S.; Fruchart, D.; Dos Santos, D.; Ortega, L.; Lacoste, A. Evidence for a superstructure in hydrogen-implanted palladium. J. Alloy Compd. 2004, 372, L6–L8. [Google Scholar] [CrossRef]
- Wulff, H.; Quaas, M.; Deutsch, H.; Ahrens, H.; Fröhlich, M.; Helm, C.A. Formation of palladium hydrides in low temperature Ar/H2 plasma. Thin Solid Films 2015, 596, 185–189. [Google Scholar] [CrossRef]
- Ayadi, S.; Charles, Y.; Gaspérini, M.; Lemaire, I.C.; Botelho, T.D.S. Effect of loading mode on blistering in iron submitted to plastic prestrain before hydrogen cathodic charging. Int. J. Hydrog. Energy 2017, 42, 10555–10567. [Google Scholar] [CrossRef]
- Ouaras, K.; Redolfi, M.; Vrel, D.; Quirós, C.; Lombardi, G.; Bonnin, X.; Hassouni, K. Tungsten Blister Formation Kinetic as a Function of Fluence, Ion Energy and Grain Orientation Dependence Under Hydrogen Plasma Environment. J. Fusion Energy 2018, 37, 144–153. [Google Scholar] [CrossRef]
- Quirós, C.; Mougenot, J.; Lombardi, G.; Redolfi, M.; Brinza, O.; Charles, Y.; Michau, A.; Hassouni, K. Blister formation and hydrogen retention in aluminium and beryllium: A modeling and experimental approach. Nucl. Mater. Energy 2017, 12, 1178–1183. [Google Scholar] [CrossRef]
- Ouaras, K.; Dine, S.; Vrel, D.; Bonnin, X.; Redolfi, M.; Lombardi, G.; Hassouni, K. Synthesis and hydrogen plasma interaction of model mixed materials for fusion. Int. J. Hydrog. Energy 2014, 39, 17422–17428. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Shizuku, Y.; Yamamoto, S.; Fukai, Y. Phase diagram of the Ni–H system at high hydrogen pressures. J. Alloys Compd. 2002, 336, 159–162. [Google Scholar] [CrossRef]
- Quaas, M.; Wulff, H.; Ivanova, O.; Helm, C.A. Formation of nickel hydrides in reactive plasmas. Zeitschrift für Kristallographie Suppl. 2009, 2009, 241–246. [Google Scholar] [CrossRef]
- Le Bail, A. Monte Carlo indexing with McMaille. Powder Diffr. 2004, 19, 249–254. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A friendly Java program for material analysis using diffraction. IUCr Newslett. CPD 1999, 21, 14–15. [Google Scholar]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Legrand, D.; Bancroft, G.M. Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner. 2000, 27, 357–366. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Dornheim, M. Thermodynamics of Metal Hydrides: Tailoring Reaction Enthalpies of Hydrogen Storage Materials; IntechOpen: London, UK, 2011. [Google Scholar]
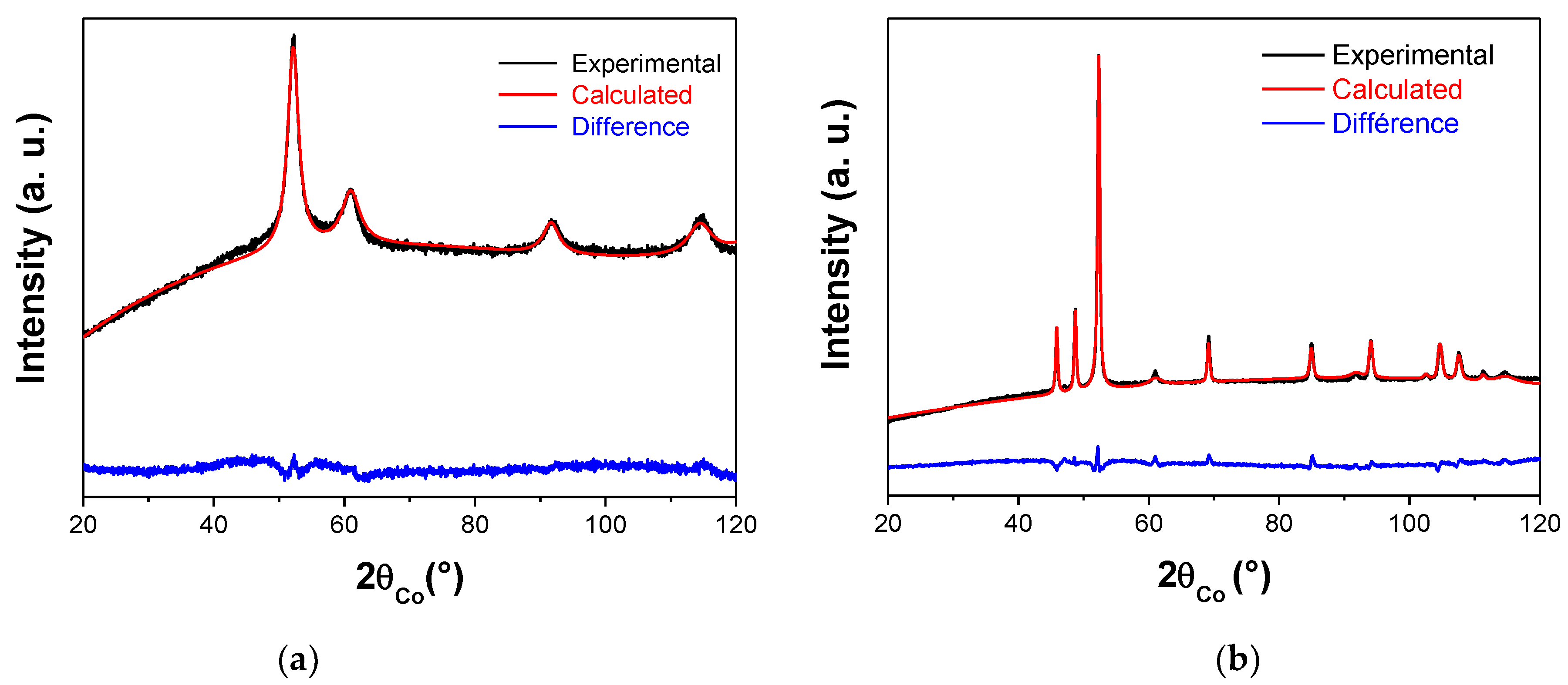
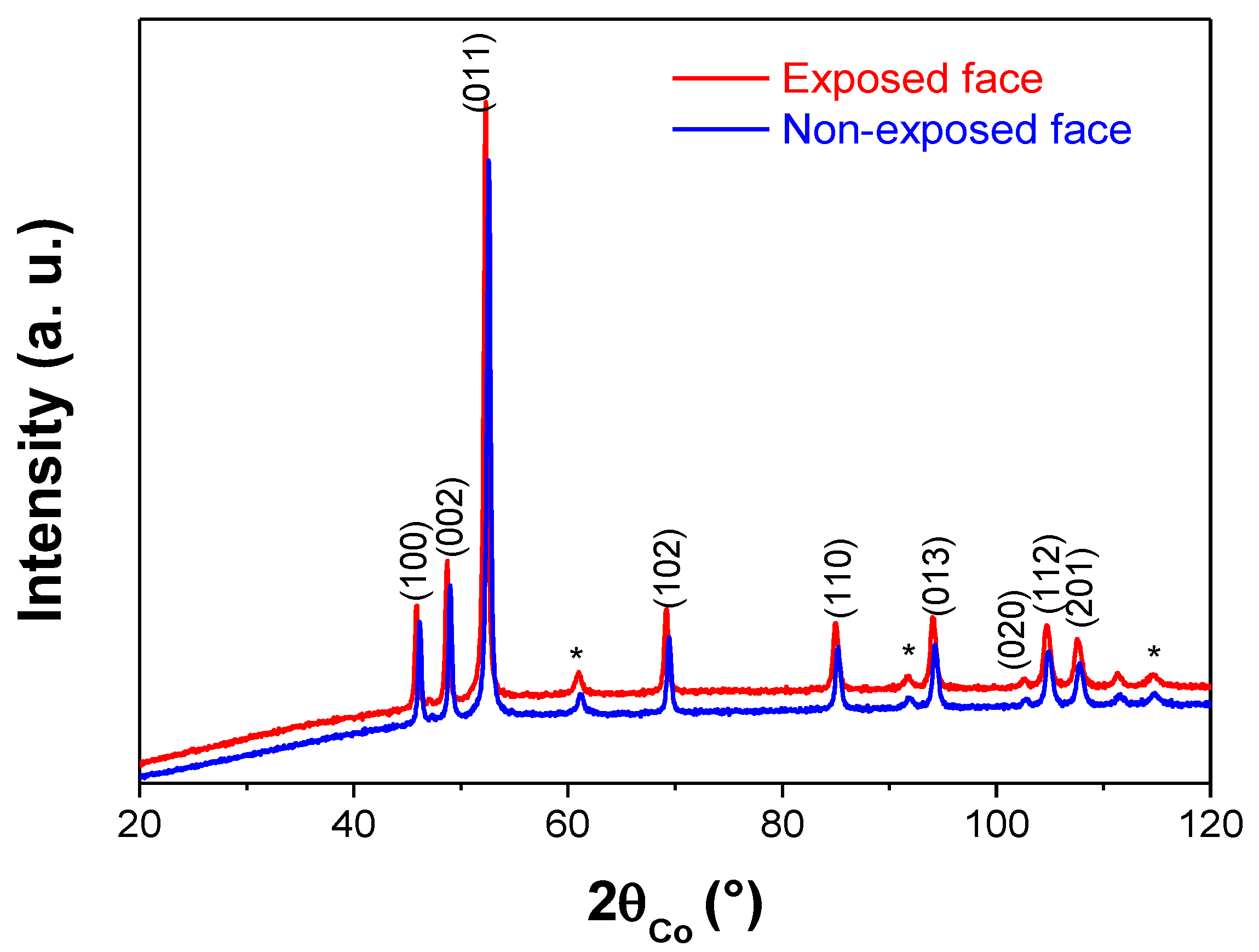
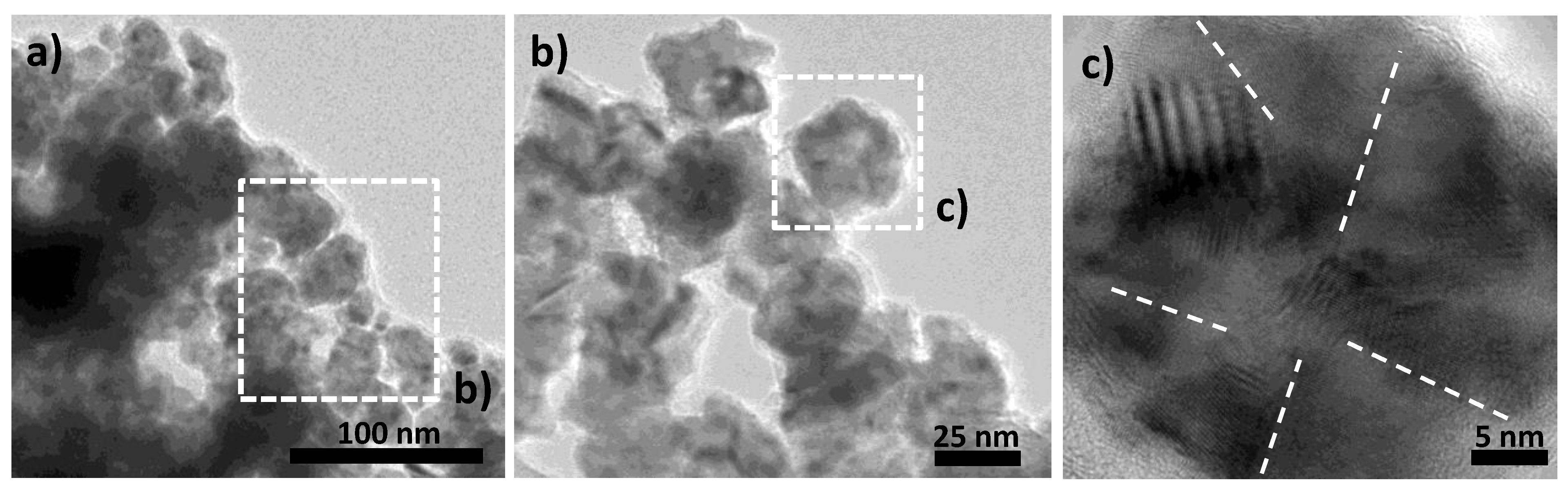
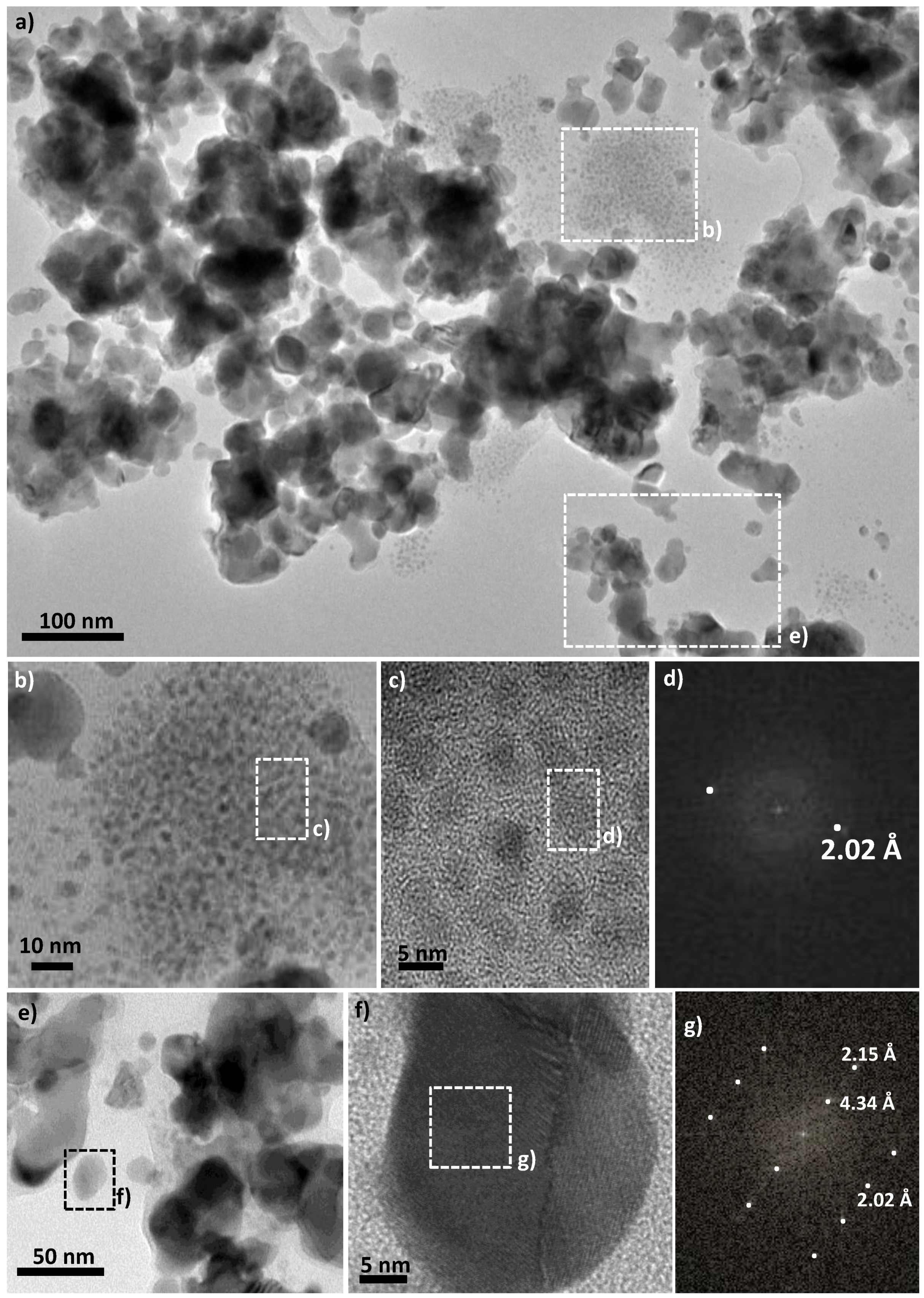
| face Centered Cubic Structure: fcc-Ni | Compact Hexagonal Structure: hc-Ni2H | |||||
|---|---|---|---|---|---|---|
| a (Å) | <L> (nm) | Ratio (wt.%) | a and c (Å) | <L> (nm) | Ratio (wt.%) | |
| Polyol-made Ni powder | 3.535(5) | 4(1) | 100 | - | - | - |
| Plasma-treated Ni powder | 3.535(5) | 7(1) | 14 | 2.651(5) 4.338(5) | 35(5) | 86 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haj-Khlifa, S.; Nowak, S.; Beaunier, P.; De Rango, P.; Redolfi, M.; Ammar-Merah, S. Polyol Process Coupled to Cold Plasma as a New and Efficient Nanohydride Processing Method: Nano-Ni2H as a Case Study. Nanomaterials 2020, 10, 136. https://doi.org/10.3390/nano10010136
Haj-Khlifa S, Nowak S, Beaunier P, De Rango P, Redolfi M, Ammar-Merah S. Polyol Process Coupled to Cold Plasma as a New and Efficient Nanohydride Processing Method: Nano-Ni2H as a Case Study. Nanomaterials. 2020; 10(1):136. https://doi.org/10.3390/nano10010136
Chicago/Turabian StyleHaj-Khlifa, Sonia, Sophie Nowak, Patricia Beaunier, Patricia De Rango, Michaël Redolfi, and Souad Ammar-Merah. 2020. "Polyol Process Coupled to Cold Plasma as a New and Efficient Nanohydride Processing Method: Nano-Ni2H as a Case Study" Nanomaterials 10, no. 1: 136. https://doi.org/10.3390/nano10010136
APA StyleHaj-Khlifa, S., Nowak, S., Beaunier, P., De Rango, P., Redolfi, M., & Ammar-Merah, S. (2020). Polyol Process Coupled to Cold Plasma as a New and Efficient Nanohydride Processing Method: Nano-Ni2H as a Case Study. Nanomaterials, 10(1), 136. https://doi.org/10.3390/nano10010136







