1. Introduction
Burns as a result of chemical, thermal, or electrical injury are common, with two million people treated annually in the United States [
1,
2]. Of these, 100,000 people are hospitalized at almost
$75,000 per admission, while nearly 4000 die [
2,
3,
4]. In recent years, better infection control, better surgical techniques, and development of skin substitutes have reduced the death rate by over half from 30 years ago [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. Now, the critical needs are to not only get better in these three areas, but also to have better assessment techniques, faster wound closure times, and to improve the quality of the new tissues formed [
23]. Speed of wound closure can have an effect on cost of treatment, infection rate, and quality of tissue [
23,
24]. The faster a wound closes, the lesser the risk of infection, and in general, the lower the cost [
23,
24]. Further, it has been shown that for many wounds, in particular burn wounds, the faster the healing rate, the less the wound contraction rate, which leads to less scarring [
24,
25,
26]. Scarring in burn wounds can lead to cosmetic and functional issues [
24,
26]. One of the biggest problems is stiffness of the healed burn wound, which tends to scar particularly with meshed skin grafts, and requires over a year of rehabilitation to return the skin stiffness close to the native skin [
24,
26]. The need, therefore, is a strategy to reduce scarring in meshed skin graft healing leading to a better cosmetic result and a reduction in the cost and time of rehabilitation to restore function. The strategy in this paper is to increase the closure rate of a meshed skin graft to reduce scarring, which should also decrease the infection rate.
For full-thickness burns, the standard is to use autologous skin grafts [
5]. For severe burns, however, which cover a high percentage of a patient’s body, allografts or tissue-engineered skin are needed. In most cases, surgeons use autologous meshed grafts, which are typically meshed to increase the area covered [
5]. This allows more body surface area to be covered in a shorter amount of time, thereby reducing the chances of infection and enhancing efforts to restore homeostasis [
5,
6,
7,
8]. Because of the cosmetic and functional issues, unmeshed skin is used if possible on certain areas of the body such as the face or hands [
5].
Synthetic sutures or staples placed at the wound edges are typically used to fix the meshed skin grafts, which are removed after the graft takes [
5,
8,
11]. Furthermore, fixation and survival of skin grafts are related to the re-establishment of an adequate circulation [
5,
7,
8,
24]. This is another reason grafts are meshed; to avoid fluid collection under the graft making it more difficult for blood vessels from the wound bed to grow into or reattach to the old blood vessels in the skin graft [
7,
8,
24].
The two strategies employed here are to use fibrin glue to attach all parts of the graft to the wound bed and add in an angiogenic growth factor to further help the growth of blood vessels from the wound bed into the graft. For skin graft healing, the first phase, occurring within 24 h of transplantation, has been termed “the stage of plasmatic imbibition” where the wound bed attempts to adhere to the graft through formation of a fibrin scaffold [
6,
8,
10,
27]. Subsequently, the graft swells with exudate, thus providing access to nutrients and preserving the patency of existing blood vessels within the graft [
27]. Forty-eight to seventy-two hours after surgery, anastomosis formation occurs, followed by blood vessel budding and neovascularization [
6,
27]. During this period, ingrowth of fibroblasts, fibrocytes, and other cells occurs in attempt to incorporate the graft [
27]. The graft, however, must be securely attached to the wound bed for this to occur [
28]. Full blood and lymphatic circulation are usually restored in 4–7 days [
6,
27]. Sensory nerve regeneration begins 2–4 weeks after transplantation; full function may not return for many months [
6,
27]. Failure to establish a satisfactory blood supply between the graft and vascular bed will lead to graft loss when this fails to occur [
6,
27].
Fibrin tissue adhesives (FTAs) have become popular over the last twenty years as a method of surgical fixation [
24]. Although the hemostatic properties of fibrin have been known for nearly one hundred years, development into an adhesive has progressed slowly [
24]. Part of the issue was inadequate concentrations of fibrinogen reducing bonding strength, which led to premature repair failures [
24]. It was not until the 1970s that improving technologies allowed for better separation of plasma components and the ability to obtain higher concentrations of fibrinogen [
29]. Subsequently, FTAs have found use in diverse fields such as pediatrics, otorhinolaryngology, and burn care [
24,
28,
29,
30]. The use of FTAs with skin grafts has showed decreased wound contraction and good adhesion in areas difficult to immobilize [
28,
29], since fibrin is the normal mode of graft attachment to the wound bed [
6,
27]. Although studies have shown the ability of fibrin glue to help reduce contraction, these studies were qualitative and did not fully show the mechanisms involved in animal models or clinically.
Fibroblast growth factor-1 (FGF-1) is an angiogenic factor that has previously been incorporated into a degradable fibrin scaffold [
31]. As enzymes degrade the fibrin clot, FGF-1 is released and enhances the angiogenic response [
32]. Porous fibrin systems have been shown to increase angiogenic and fibroblastic response over controls and non-porous systems [
31].
Therefore, the goal of this study was to increase the healing rate of an autologous meshed skin graft in order to make a significant difference in scar formation. This should translate to improvement in both cosmetic and functional clinical performance, as well as reduce the overall cost of the procedure. Specifically, the study was designed to use these systems in both an animal model and clinically. The intent was to not only quantify the benefit for clinical performance, but also quantify the changes in bioprocesses to help determine the mechanisms for any benefits seen. Healing of a graft is both the percentage area of the graft, which survives (graft take), as well as the filling in of the holes of the mesh (healing rate).
In the animal model, the performance measures were tissue stiffness and graft take percent at both three and ten days. To better understand the reason for the differences in performance, the number of cells and blood vessels were determined at each time period from three different regions of the wound.
4. Discussion
Reducing healthcare costs is an important goal. It is complicated by the fact that healthcare costs are driven by multiple factors, as well as that the cost is shared between patients, providers, and insurance companies. Further, it is not always clear how much of a cost reduction is worthwhile, especially if they is a resultant reduction in the quality of care. This study, however, was looking at the feasibility of reducing healthcare costs while still improving the clinical performance.
Specifically, to increase the clinical performance of the standard of care for full-thickness burns (meshed skin graft) by speeding the healing rate in order to make a significant difference in scar formation. This should translate to improvement in both cosmetic and functional clinical performance as well as reduce the rehabilitation time. This would significantly reduce the
$75,000 average per burn patient in the hospital, as well as costs for rehabilitation [
4]. The increased cost of the treatment, however, has to be considered. Adding in fibrin glue would not significantly alter the cost of treatment. Use of a growth factor, however, could significantly increase the cost of the procedure due primarily to the increased development costs of the adhesive system. In this case, therefore, the value added by including the growth factor has to be weighed against the added cost of the treatment.
Although the angiogenic growth factor (FGF-1) added to a FTA (fibrin tissue adhesive) in open wounds, made a significant impact on healing [
31,
32], it did not appear to do so when used with meshed skin grafts. This is probably due to the difference in function of the FTA between the two applications. In open wounds, the FTA is the scaffold system for the entire wound. Formation of tissue requires an angiogenic response to provide the fibroblasts enough oxygen to produce the extracellular matrix to replace the degrading scaffold starting from the edge of the wound [
7,
8,
9,
24]. In a meshed graft, the fibrin is needed to facilitate the attachment and/or ingrowth of blood vessels from the wound bed to the graft [
28,
29]. Therefore, in the meshed skin graft systems evaluated, it appears that the insignificant benefit of using the growth factor would not be worth the additional cost of the treatment.
Specifically, the study was designed to use these systems in both an animal model and clinically. The intent was to not only quantify the benefit for clinical performance, but also quantify the changes in bioprocesses to help determine the mechanisms for any benefits seen. In the animal model both (healing rate and bioprocess rate) can be looked at with only clinical performance (plus angiogenic response) easily studied clinically. Fibrin tissue adhesives have been successfully used in many areas of clinical medicine, including delivering medications as well as growth factors, to obtain clinical benefit [
12,
24,
28,
29]. Previous studies have shown that it is possible to create a porous fibrin scaffold with time-dependent delivery of FGF-1 [
31,
32], but no data exists on its clinical use in conjunction with skin grafting.
The cellular data, in this study, showed that although no statistically significant difference was noted in Tv (total cell volume fraction), several individual cell populations showed differences for certain treatment types at different time periods. Both the PF and PFW (porous fibrin with and without FGF-1) groups attracted neutrophils in greater numbers than other treatments, predominantly in the T1 region, where the FTA was applied. Although the PEO, used to make pores, hydrolyzes to a chemically inert compound and is water soluble, the large molecular weight of the polymer may interfere with rapid hydrolysis to smaller components. It is therefore possible that the PEO affects the wound as an irritant and disrupts wound healing. As with any traumatic wound, it must be free of debris for rapid tissue ingrowth. This was corroborated by the fact that PF and PFW treated wounds tended to have decreased graft take as well as an increase in the macrophage response. This again could be due to the macrophages required to phagocytize the PEO particles. Although this increase in PF and PFW treated wounds occurred at 3 days, the 10-day data revealed no significant differences between treatments in Nv or Mv as well as an overall decrease in cells compared to the 3-day data. This suggests that even though the PEO may act as an irritant early in wound healing, by day 10 this may have subsided along with a reduction in the overall inflammatory response.
For Fv (fibroblast volume fraction), as expected, there was a more vigorous response with FGF-1 particularly in T1 at 3 Days, consistent with previous studies involving FGF-1 and fibroblast activity [
24,
31]. The porous systems, with FGF-1 (PFW), however, did not show an increase in fibroblast response; probably due to the increased infiltration of inflammatory cells and the delay of wound healing.
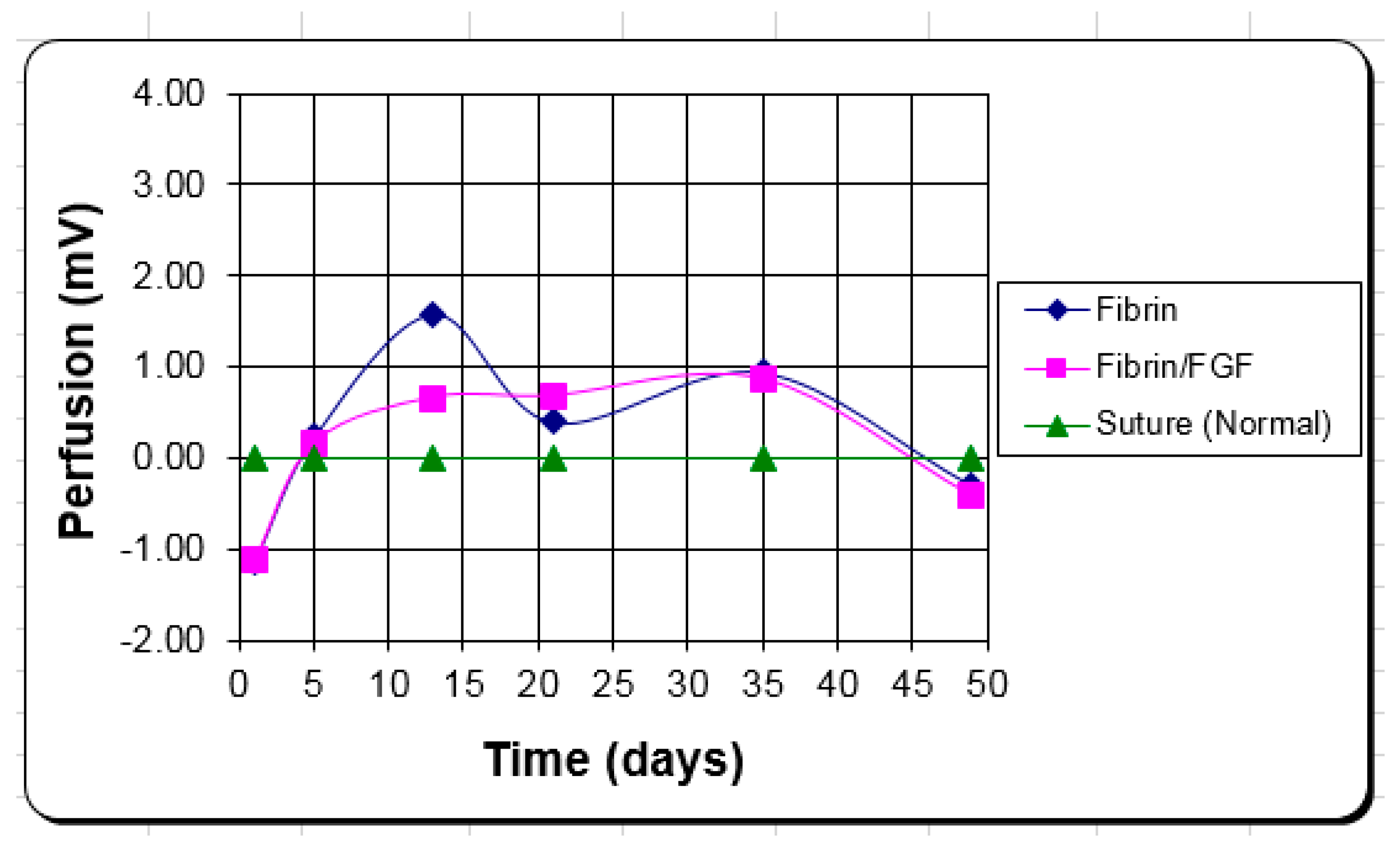
The function of the FGF-1 added to the FTA was to provide additional angiogenic stimulus for more rapid and successful graft take. Analysis of the data revealed no statistical difference in the number of blood vessels or volume fraction of blood vessels between any of the treatments as well as no clinical difference in the angiogenic response (blood perfusion). There were differences between time points in the same region (number of vessels) and between regions at the same time period (volume fraction of vessels) but no statistical differences between with or with FGF-1, or even consistent trends.
Again, the difference in the fibrin function from an open wound scaffold to an adhesive for a skin graft could help explain why FGF-1 appeared to be effective in one case but not the other. It is probable that the activity level over time of the FGF-1 is different between the two applications due to different release profiles or different half-lives between the two environments; but most likely not enough to make a significant difference. It is more likely that the thinner profile of the fibrin as a tissue adhesive vs. an open wound scaffold makes the difference. For skin grafts, the proximity of the graft to the wound bed is important [
4,
8,
24], and the fibrin alone seems to have a sufficient angiogenic response over the short distance to make the FGF-1 unnecessary.
The clinical blood perfusion level is closer to the in vivo volume fraction of blood vessels measure than the number per area. Again, the better attachment to the wound bed by the fibrin is what appears to provide the clinical advantage vs. the angiogenic response of FGF-1. The fibrin alone keeps the blood perfusion level up to improve healing with little to no added benefit from the FGF-1, in this case.
Inadequate mechanical strength, however, may have affected the ability for angiogenesis to lead to skin graft take, in this study, making the porous systems not as successful as anticipated. Testing revealed, as expected, an inverse relationship between porosity and strength (decreasing strength with increasing porosity). The differences (30–40% decreases). however, were not statistically different most likely due to the small sample (
n = 3) and high standard deviation (25–45% of the mean). If the strength becomes a clinical issue (graft stabilization), the strength of the FTA can be increased by increasing the fibrinogen concentration or slowing the degradation with an anti-fibrolytic agent, like aprotinin, or a cross-liking agent like Factor XIII [
29,
38,
39].
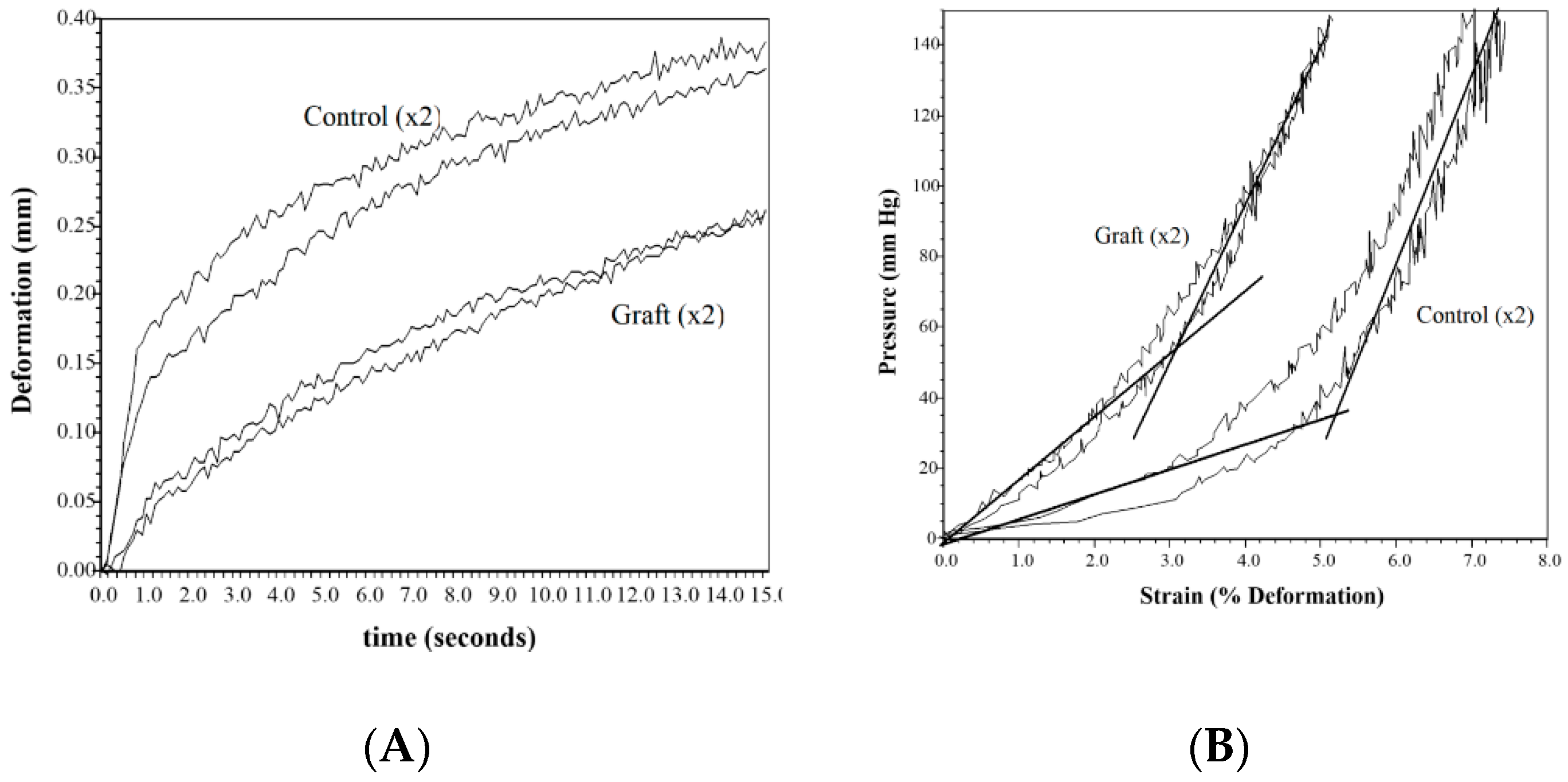
Stiffness and scarring of the wound are a concern for both the patent and the physician. The elastic modulus measures the distensibility of the tissue and is the critical clinical measure that is worked on to get close to normal skin. Although not statistically different from the normal control, the sutured wound showed a trend towards increased modulus at both 3 days and 10 days (80–110% increase). The differences also were not statistically different most likely due to the small sample (
n = 5) and high standard deviation (40% of the mean at 3 days and over 100% at 10 days). The treated wounds were close to the normal skin values versus twice the values for the sutured control. Only the FW at day 3 was statistically different. The clinical data showed this same trend with the controls about twice the stiffness of the treated samples even after a month of healing, supported by other studies [
24,
26,
29,
37]. This clinically manifests itself as the anticipated scar tissue, which can complicate function and appearance [
24,
29]. If the treated wounds have a stiffness comparable to normal skin vs. the twice as stiff controls (
Figure 3), the one- to two-year rehabilitation time to get flexibility back to normal can be reduced if not eliminated, leading to both a reduction in cost and a clinical improvement [
24].
The animal study looked at graft take as the healing performance measure, with the clinical study looking at epithelialization rate. The short-term in vivo study showed that the porous fibrin systems seemed to perform the worst, probably due to the increased inflammatory response and mechanical instability negating any benefit of using a porous system.
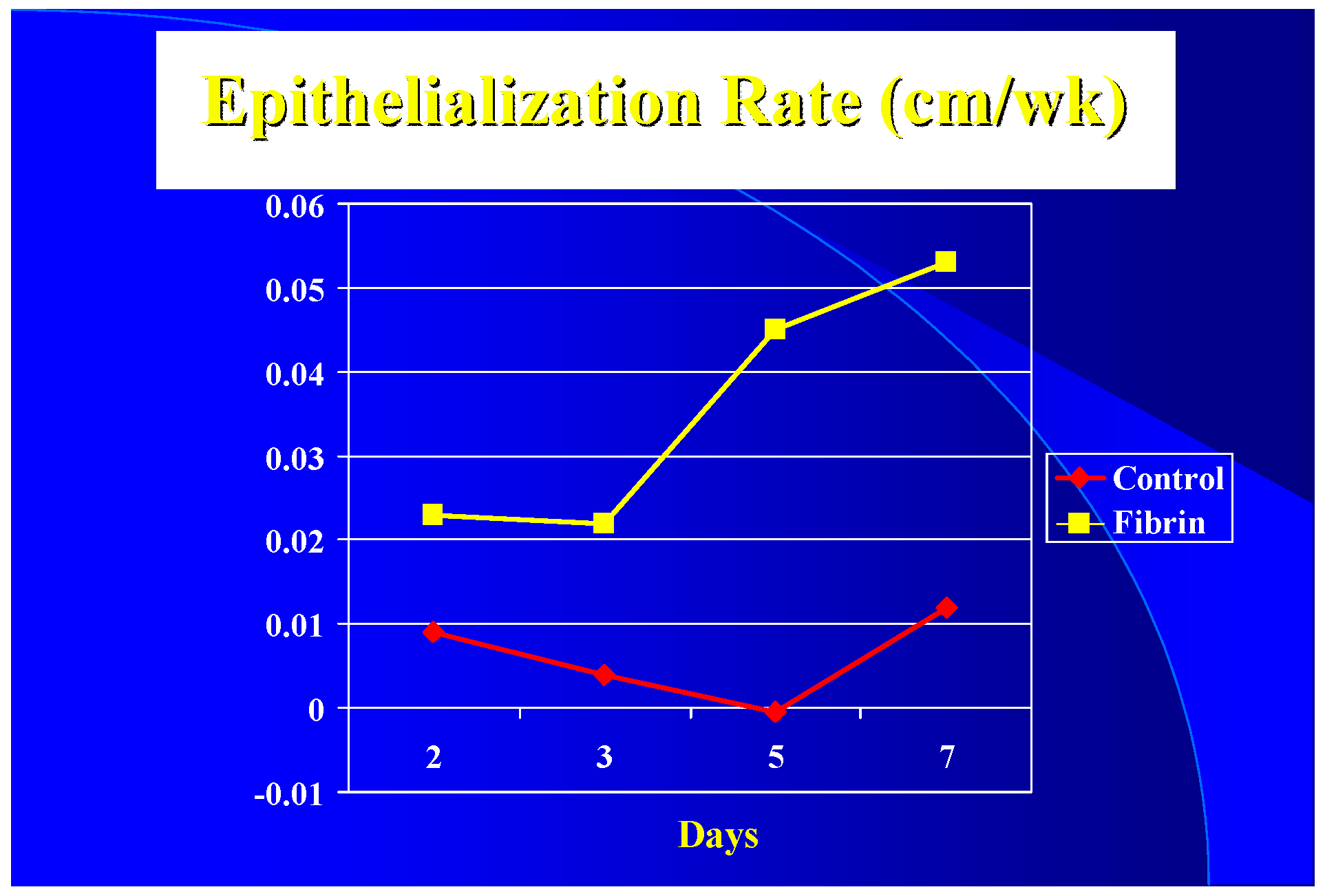
Clinically the epithelialization rate was used vs. graft take. It appears that with a 3 to 1 mesh graft that the controls (sutured or stapled) had at least a five-day lag in epithelialization over the fibrin treated grafts (
Figure 2) resulting in a five-day difference in closure time (16 versus 21 days). Due to treatment protocols, it was not possible to get epithelialization rates after seven days. It appears, however, once started that the rate increases until it peaks at the 0.1–0.2 cm/wk level seen by others [
40].
In both the animal and clinical studies, the “n” number was too low and standard deviations too high to allow the tests to be sensitive enough to pick up all the differences that would make a clinical difference. It normally required an increase of over 100% to be detected. In some cases, however, ANOVA was able to pick up these differences not statistically significant in the multiple comparisons tests. These include Fv in S1 at 10 days, Mv in T1 at 3 days, and Nv within PF at 3 days. For the t-tests, increasing the sample size most likely would result in more of the clinically significant differences being statistically different.
Although not all differences were statistically significant, the results suggest the following:
The use of FTAs on a meshed skin graft appears to speed up the regenerative healing rate leading to less scarring in the holes in the mesh. It appears to shorten the healing time by five days and keeps the tissue stiffness close to normal levels vs. the doubling of the stiffness by the controls. Although making the fibrin porous and adding an angiogenic agent were both supposed to increase the healing rate further, neither seemed to make a significant clinical difference and the porous systems tested had reduced graft take.
It appears that the FTA performed better than the controls, serving as an adhesive and a scaffold. The fibrin allows closer and more stable attachment of the graft to the wound bed, as well as serves as a scaffold for the blood vessels to grow through to reattach to the graft. Histologically the controls had more of an inflammatory response (macrophage volume fraction) and less of a healing response (fibroblast volume fraction) compared to the FTAs in the first ten days. Although there were not statistically significant differences, among treatments, for the volume fraction of blood vessels during the first 10 days, the Laser Doppler scanner showed that for all time points after five days, the blood perfusion was greater in the FTAs than the controls.
It appears that although graft take was not much different by ten days, the healing in the spaces of the mesh started earlier in the FTAs. Epithelialization seemed to have a five-day lag in the controls compared to the FTAs. It is possible that for the first five days the blood perfusion response in the controls was more due to inflammation than healing; after that, the elevated blood flow in the FTAs probably helped keep the healing rate higher than the controls.
Healing does not usually start until after the inflammatory phase starts to resolve. Both better attachment of the mesh to the wound base and the use of the fibrin as a dermal scaffold could have led to a faster resolution of the inflammatory stage as well as the observed quicker start to re-epithelialization. The quicker start to healing led to a faster closure of the wound, and therefore, less scarring. Therefore, the FTAs can allow wound closure about five days earlier than the controls leading to a better cosmetic outcome. The earlier wound closure would reduce the time for wound dressings, as well as reduce the chance of infection. Having the resultant wound less stiff would lead to a shortened rehabilitation time. Therefore, using fibrin alone appears to meet the goals of the study: reducing scarring in meshed skin graft healing leading to a better cosmetic result and reducing the cost and time of rehabilitation to restore function. The fibrin appears to do this by increasing the closure rate of a meshed skin graft to reduce scarring, which should also decrease the infection rate.
Although it appears that the fibrin system provides a better result clinically from both a cost and performance perspective, the sample size needs to be increased to prove this benefit as well as the mechanism for this improvement. A five-day decrease in closure time and a reduction in stiffness close to normal values (vs. twice the stiffness for controls) both are significant clinical improvements. Eliminating the need to look further at porous systems or FGF-1 delivery to get the desired clinical result, will streamline future in vivo and clinical studies. This will also make it easier to determine if the proposed mechanisms are further supported in larger in vivo and clinical studies.