Development of Phosphatized Calcium Carbonate Biominerals as Bioactive Bone Graft Substitute Materials, Part II: Functionalization with Antibacterial Silver Ions
Abstract
1. Introduction
2. Results and Discussion
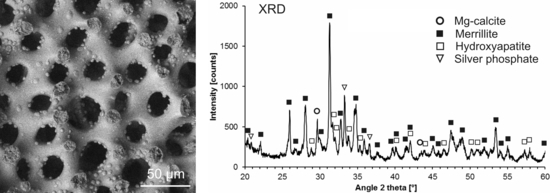
2.1. Modification with Silver Ions
2.2. Release of Silver Ions upon Dissolution
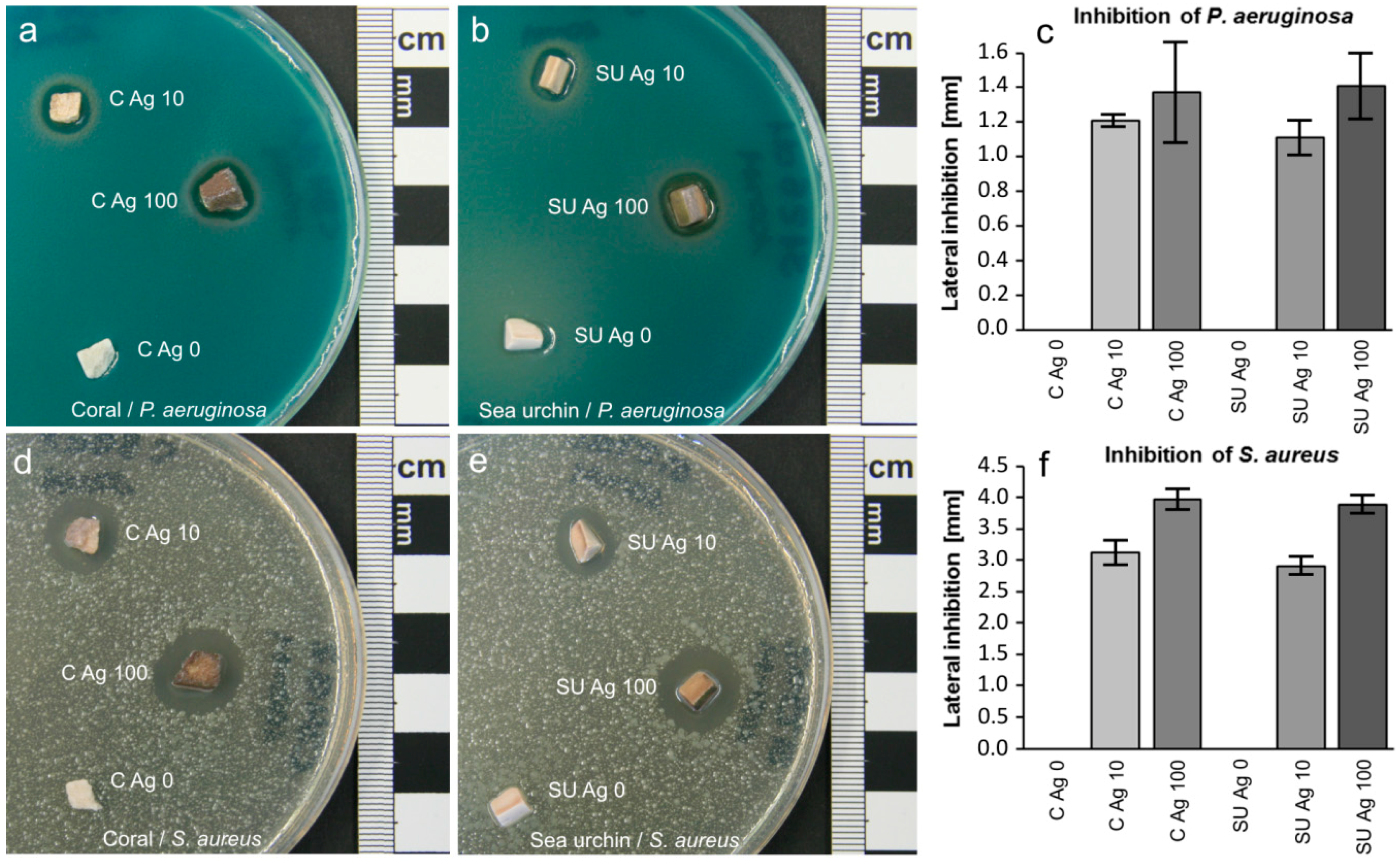
2.3. Antibacterial Tests
3. Materials and Methods
3.1. Starting Materials
3.2. Treatment with Silver Ions
3.3. Sample Characterization
3.4. Cation Release upon Dissolution
3.5. Antibacterial Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lewandrowski, K.U.; Gresser, J.D.; Wise, D.L.; Trantolo, D.J. Bioresorbable bone graft substitutes of different osteoconductivities: A histologic evaluation of osteointegration of poly(propylene glycol-co-fumaric acid)-based cement implants in rats. Biomaterials 2000, 21, 757–764. [Google Scholar] [CrossRef]
- Seiler, J.G., 3rd; Johnson, J. Iliac crest autogenous bone grafting: Donor site complications. J. South. Orthop. Assoc. 2000, 9, 91–97. [Google Scholar] [PubMed]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M. Autogenous iliac crest bone graft—Complications and functional assessment. Clin. Orthop. Rel. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef]
- Banwart, J.C.; Asher, M.A.; Hassanein, R.S. Iliac crest bone-graft harvest donor site morbidity—A statistical evaluation. Spine 1995, 20, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium Phophate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, J.D.; Shankar, K.; Yuan, H.; Habibovic, P. Osteoinduction and its evaluation. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 199–219. [Google Scholar]
- Nakamura, T.; Takemoto, M. Osteoconduction and its evaluation. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 183–198. [Google Scholar]
- Klawitter, J.J.; Hulbert, S.F. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J. Biomed. Mater. Res. 1971, 5, 161–229. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Cooke, F.W.; Klawitter, J.J.; Leonard, R.B.; Sauer, B.W.; Moyle, D.D.; Skinner, H.B. Attachment of prostheses to musculoskeletal system by tissue ingrowth and mechanical interlocking. J. Biomed. Mater. Res. 1973, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Best, S.M.; Tanner, K.E.; Bonfield, W.; Revell, P.A. Quantification of bone ingrowth within bone-derived porous hydroxyapatite implants of varying density. J. Mater. Sci.-Mater. Med. 1999, 10, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Tamai, N.; Myoui, A.; Tomita, T.; Nakase, T.; Tanaka, J.; Ochi, T.; Yoshikawa, H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biomed. Mater. Res. 2002, 59, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Saeed, S.; Annaz, B.; Buckland, T.; Revell, P.A. Microporosity affects bioactivity of macroporous hydroxyapatite bone graft substitutes. Key Eng. Mater. 2004, 254–256, 273–276. [Google Scholar] [CrossRef]
- Hing, K.A. Bioceramic bone graft substitutes: Influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar] [CrossRef]
- Okamoto, M.; Dohi, Y.; Ohgushi, H.; Shimaoka, H.; Ikeuchi, M.; Matsushima, A.; Yonemasu, K.; Hosoi, H. Influence of the porosity of hydroxyapatite ceramics on in vitro and in vivo bone formation by cultured rat bone marrow stromal cells. J. Mater. Sci. Mater. Med. 2006, 17, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Epple, M. Biomaterialien und Biomineralisation—Eine Einführung für Naturwissenschaftler, Mediziner und Ingenieure; Teubner: Wiesbaden, Germany, 2003; 162p. [Google Scholar]
- Chappard, D.; Zhioua, A.; Grizon, F.; Basle, M.F.; Rebel, A. Biomaterials for bone filling: Comparisons between autograft, hydroxyapatite and one highly purified bovine xenograft. Bull. Assoc. Anat. 1993, 77, 59–65. [Google Scholar]
- Begley, C.T.; Doherty, M.J.; Mollan, R.A.B.; Wilson, D.J. Comparative study of the osteoinductive properties of bioceramic, coral and processed bone-graft substitutes. Biomaterials 1995, 16, 1181–1185. [Google Scholar] [CrossRef]
- Emery, S.E.; Fuller, D.A.; Stevenson, S. Ceramic anterior spinal fusion—Biologic and biomechanical comparison in a canine model. Spine 1996, 21, 2713–2719. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.D.; Frierson, K.E.; Keller, T.S.; Cook, C.; Scheinberg, R.; Zerwekh, J.; Meyers, L.; Sciadini, M.F. Porous ceramics as bone graft substitutes in long bone defects: A biomechanical, histological, and radiographic analysis. J. Orthop. Res. 1996, 14, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Rueger, J.M.; Linhard, W.; Sommerfeldt, D. Biological reactions to calcium phosphate ceramic implants—Results of animal experiments. Orthopäde 1998, 27, 89–95. [Google Scholar] [PubMed]
- Klein, C.; Driessen, A.A.; Degroot, K.; Vandenhooff, A. Biodegradation behavior of various calcium-phosphate materials in bone tissue. J. Biomed. Mater. Res. 1993, 17, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M. Calcium-phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Rel. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Le Huec, J.C.; Lesprit, E.; Delavigne, C.; Clement, D.; Chauveaux, D.; Le Rebeller, A. Tri-calcium phosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis as bone substitutes for spinal fusion in idiopathic scoliosis: Comparative clinical results at four years. Acta Orthop. Belg. 1997, 63, 202–211. [Google Scholar] [PubMed]
- Daculsi, G.; LeGeros, R.Z. Tricalcium phosphate/hydroxyapatite biphasic ceramics. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 395–423. [Google Scholar]
- Roy, D.M.; Linnehan, S.K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature 1974, 247, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, D.; Yang, L.; Tang, H. Hydrothermal conversion of coral into hydroxyapatite. Mater. Charact. 2001, 47, 83–87. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of sea urchin spines to Mg-substituted tricalcium phosphate for bone implants. Acta Biomater. 2007, 3, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, M.; Fröls, S.; Hauf, U.; Sethmann, I.; Schultheiss, S.; Pfeifer, F.; Kleebe, H.-J. Combined hydrothermal conversion and vapor transport sintering of Ag-modified calcium phosphate scaffolds. J. Am. Ceram. Soc. 2013, 96, 412–419. [Google Scholar] [CrossRef]
- Shors, E.C. Coralline bone graft substitutes. Orthop. Clin. N. Am. 1999, 30, 599–613. [Google Scholar] [CrossRef]
- Pountos, I.; Giannoudis, P.V. Is there a role of coral bone substitutes in bone repair? Inj. Int. J. Care Inj. 2016, 47, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Posner, A.S. Effect of magnesium on lipid-induced calcification: An in vitro model for bone mineralization. Calcif. Tissue Int. 1980, 32, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating WNT/catenin signalling. Stem Cells 2001, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Optimizing bone matabolism in osteoporosis: Insight into the pharmacologic profile of strontium ranelate. Osteoporos. Int. 2003, 14, S9–S12. [Google Scholar] [CrossRef] [PubMed]
- Barbara, A.; Delennoy, D.P.; Denis, B.G.; Marie, P.J. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism 2004, 53, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Feng, Q.L.; Kim, J.O.; Wu, J.; Wang, H.; Chen, G.Q.; Cui, F.Z. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci.-Mater. Med. 1998, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Yang, X.H.; Tian, X.G. Preparation of silver/hydroxyapatite nanocomposite spheres. Powder Technol. 2008, 184, 21–24. [Google Scholar] [CrossRef]
- Diaz, M.; Barba, F.; Miranda, M.; Guitian, F.; Torrecillas, R.; Moya, J.S. Synthesis and antimicrobial activity of a silver-hydroxyapatite nanocomposite. J. Nanomater. 2009, 2009, 498505. [Google Scholar] [CrossRef]
- Buckley, J.J.; Lee, A.F.; Olivi, L.; Wilson, K. Hydroxyapatite supported antibacterial Ag3PO4 nanoparticles. J. Mater. Chem. 2010, 20, 8056–8063. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M.; Azadegan, M.; Liu, G.Q. Bioactive delivery systems for the slow-release of antibiotics: Incorporation of Ag+ ions into micro-porous hydroxyapatite coatings. Mater. Lett. 1995, 24, 7–12. [Google Scholar] [CrossRef]
- Feng, Q.L.; Kim, T.N.; Wu, J.; Park, E.S.; Kim, J.O.; Lim, D.Y.; Cui, F.Z. Antibacterial effects of Ag-HAp thin films on alumina substrates. Thin Solid Films 1998, 335, 214–219. [Google Scholar] [CrossRef]
- Chen, Y.K.; Zheng, X.B.; Xie, Y.T.; Ding, C.X.; Ruan, H.J.; Fan, C.Y. Anti-bacterial and cytotoxic properties of plasma sprayed silver-containing HA coatings. J. Mater. Sci. Mater. Med. 2008, 19, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Miyamoto, H.; Noda, I.; Sakurai, N.; Akiyama, T.; Yonekura, Y.; Shimazaki, T.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. Calcium phosphate coating containing silver shows high antibacterial activity and low cytotoxicity and inhibits bacterial adhesion. Mater. Sci. Eng. C 2010, 30, 175–180. [Google Scholar] [CrossRef]
- Bai, X.A.; More, K.; Rouleau, C.M.; Rabiei, A. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 2010, 6, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Rameshbabu, N.; Kumar, T.S.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.; Rao, K.P. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. Part A 2007, 80A, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Kalmodia, S.; Basu, B. Densification, phase stability and in vitro biocompatibility property of hydroxyapatite-10 wt% silver composites. J. Mater. Sci. Mater. Med. 2010, 21, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, Q.-S.; Zhang, Y.; Xia, H.; Ai, F.-Z.; Jiao, Y.-P.; Chen, X.-Q. Determination of antibacterial properties and cytocompatibility of silver-loaded coral hydroxyapatite. J. Mater. Sci. Mater. Med. 2010, 21, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Sato, K.; Yoshida, K.; Hashimoto, K.; Toda, Y. Preparation and characterization of beta-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater. 2009, 5, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.C.; Mohn, D.; Fortunato, G.; Astasov-Frauenhoffer, M.; Imfeld, T.; Waltimo, T.; Zehnder, M.; Stark, W.J. Incorporation of reactive silver-tricalcium phosphate nanoparticles into polyamide 6 allows preparation of self-disinfecting fibers. Polym. Eng. Sci. 2011, 51, 71–77. [Google Scholar] [CrossRef]
- Manjubala, I.; Sivakumar, M.; Kumar, T.S.S.; Rao, K.P. Synthesis and characterization of functional gradient materials using Indian corals. J. Mater. Sci. Mater. Med. 2000, 11, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Manjubala, I.; Kumar, T.S.S. Effect of TiO2-Ag2O additives on the formation of calcium phosphate based functionally graded bioceramics. Biomaterials 2000, 21, 1995–2002. [Google Scholar] [CrossRef]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Jones, J.R.; Ehrenfried, L.M.; Saravanavan, P.; Hench, L.L. Controlling ion release from bioactive glass foam scaffolds with antibacterial properties. J. Mater. Sci. Mater. Med. 2006, 17, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.L.; Modak, S.M. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob. Agents Chemother. 1974, 5, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z.Q. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Sethmann, I.; Luft, C.; Kleebe, H.-J. Development of phosphatized calcium carbonate biominerals as bioactive bone graft substitute materials, part I: Incorporation of magnesium and strontium ions. J. Funct. Biomater. 2018. submitted. [Google Scholar]
- Bigi, A.; Foresti, E.; Gandolfi, M.; Gazzano, M.; Roveri, N. Isomorphous substitutions in β-tricalcium phosphate: The different effects of zinc and strontium. J. Inorg. Biochem. 1997, 66, 259–265. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ, version 3.1; KTH, SEED: Stockholm, Sweden, 2016. [Google Scholar]
- Tsipouras, N.; Rix, C.J.; Brady, P.H. Solubility of silver sulfadiazine in physiological media and relevance to treatment of thermal burns with silver sulfadiazine cream. Clin. Chem. 1995, 41, 87–91. [Google Scholar] [PubMed]
- Nikaido, H.; Nakae, T. The outer membrane of Gram-negative bacteria. Adv. Microb. Physiol. 1980, 20, 163–250. [Google Scholar]

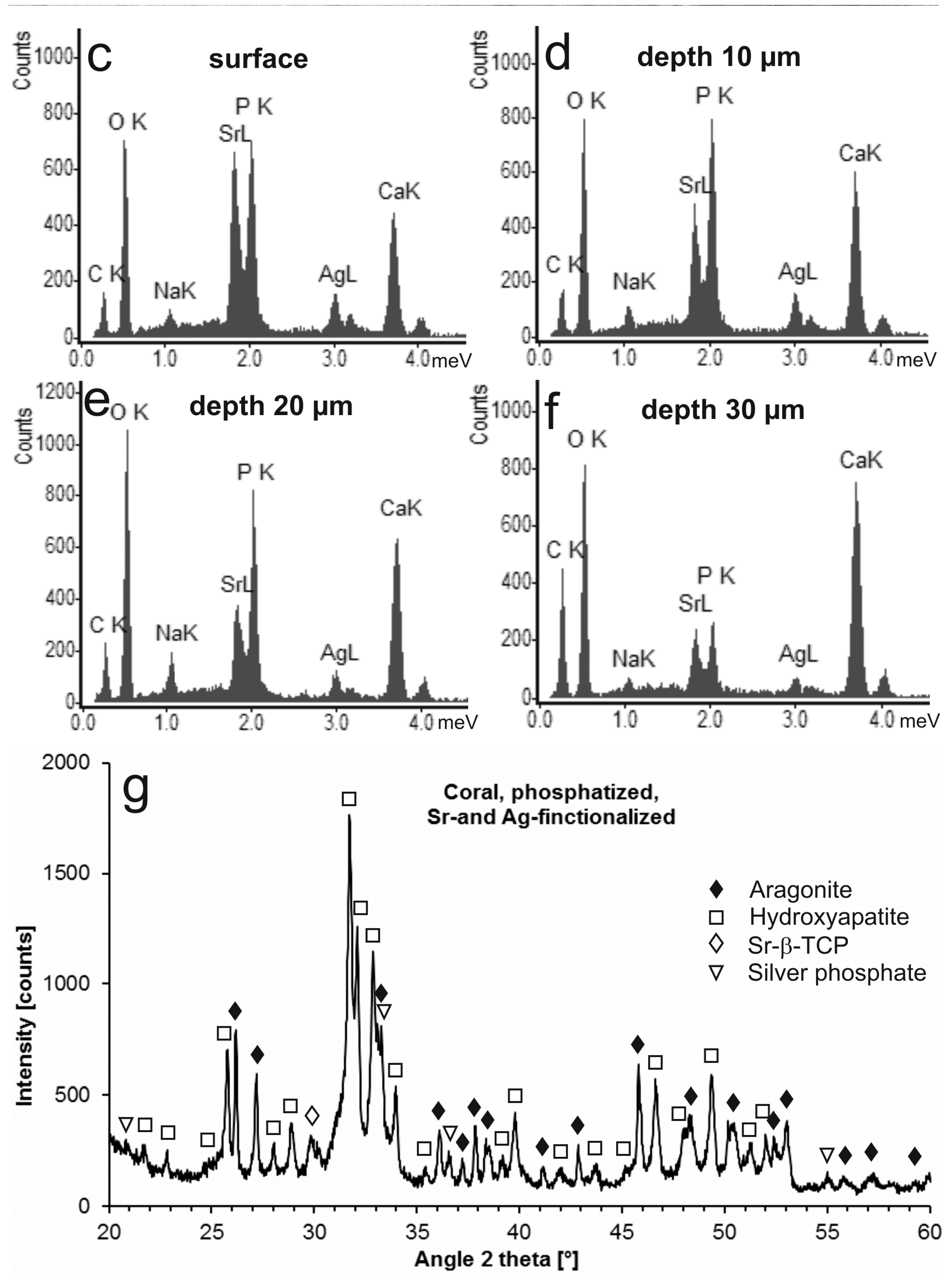
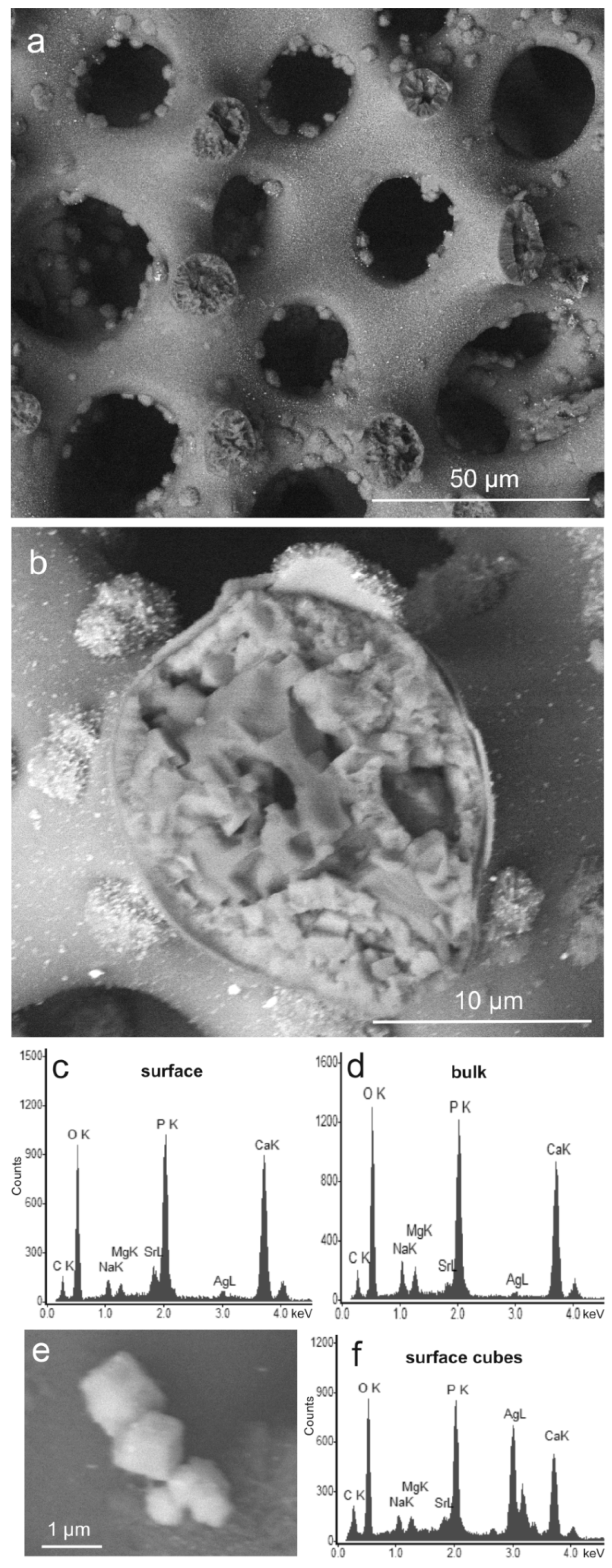
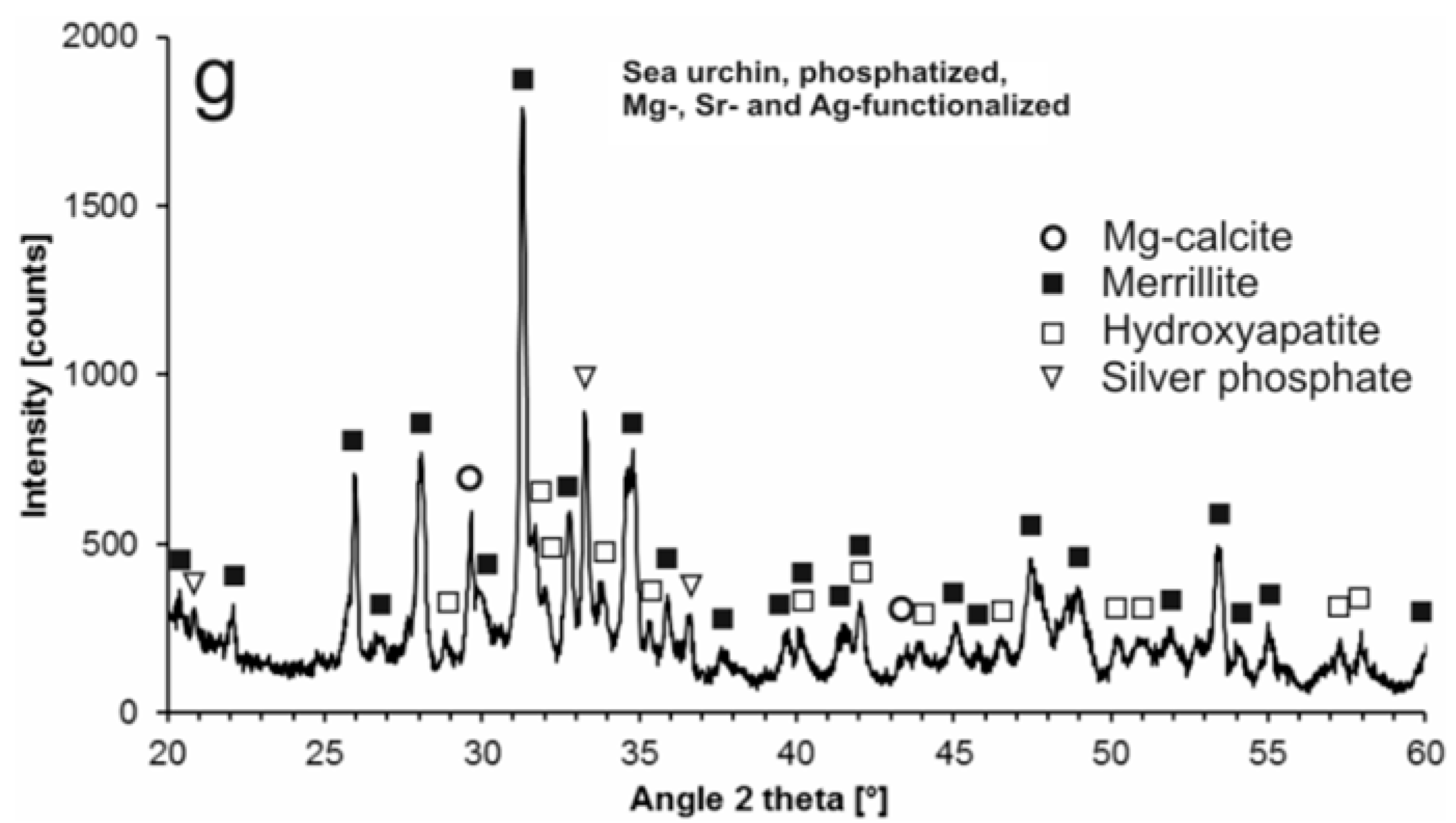
| Sample | Reaction Time | Ag+ (mg/L) |
|---|---|---|
| C Ag 100 | 24 h | 0.45 |
| C Ag 100 | 72 h | 0.51 |
| SU Ag 100 | 24 h | 0.51 |
| SU Ag 100 | 72 h | 0.47 |
| Ag3PO4 | 24 h | 0.49 |
| Ag3PO4 | 72 h | 0.59 |
| AgCl | 24 h | 0.46 |
| AgCl | 72 h | 0.46 |
| Ringer | -------- | 0.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethmann, I.; Völkel, S.; Pfeifer, F.; Kleebe, H.-J. Development of Phosphatized Calcium Carbonate Biominerals as Bioactive Bone Graft Substitute Materials, Part II: Functionalization with Antibacterial Silver Ions. J. Funct. Biomater. 2018, 9, 67. https://doi.org/10.3390/jfb9040067
Sethmann I, Völkel S, Pfeifer F, Kleebe H-J. Development of Phosphatized Calcium Carbonate Biominerals as Bioactive Bone Graft Substitute Materials, Part II: Functionalization with Antibacterial Silver Ions. Journal of Functional Biomaterials. 2018; 9(4):67. https://doi.org/10.3390/jfb9040067
Chicago/Turabian StyleSethmann, Ingo, Sabrina Völkel, Felicitas Pfeifer, and Hans-Joachim Kleebe. 2018. "Development of Phosphatized Calcium Carbonate Biominerals as Bioactive Bone Graft Substitute Materials, Part II: Functionalization with Antibacterial Silver Ions" Journal of Functional Biomaterials 9, no. 4: 67. https://doi.org/10.3390/jfb9040067
APA StyleSethmann, I., Völkel, S., Pfeifer, F., & Kleebe, H.-J. (2018). Development of Phosphatized Calcium Carbonate Biominerals as Bioactive Bone Graft Substitute Materials, Part II: Functionalization with Antibacterial Silver Ions. Journal of Functional Biomaterials, 9(4), 67. https://doi.org/10.3390/jfb9040067