Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization
Abstract
1. Introduction
2. Results
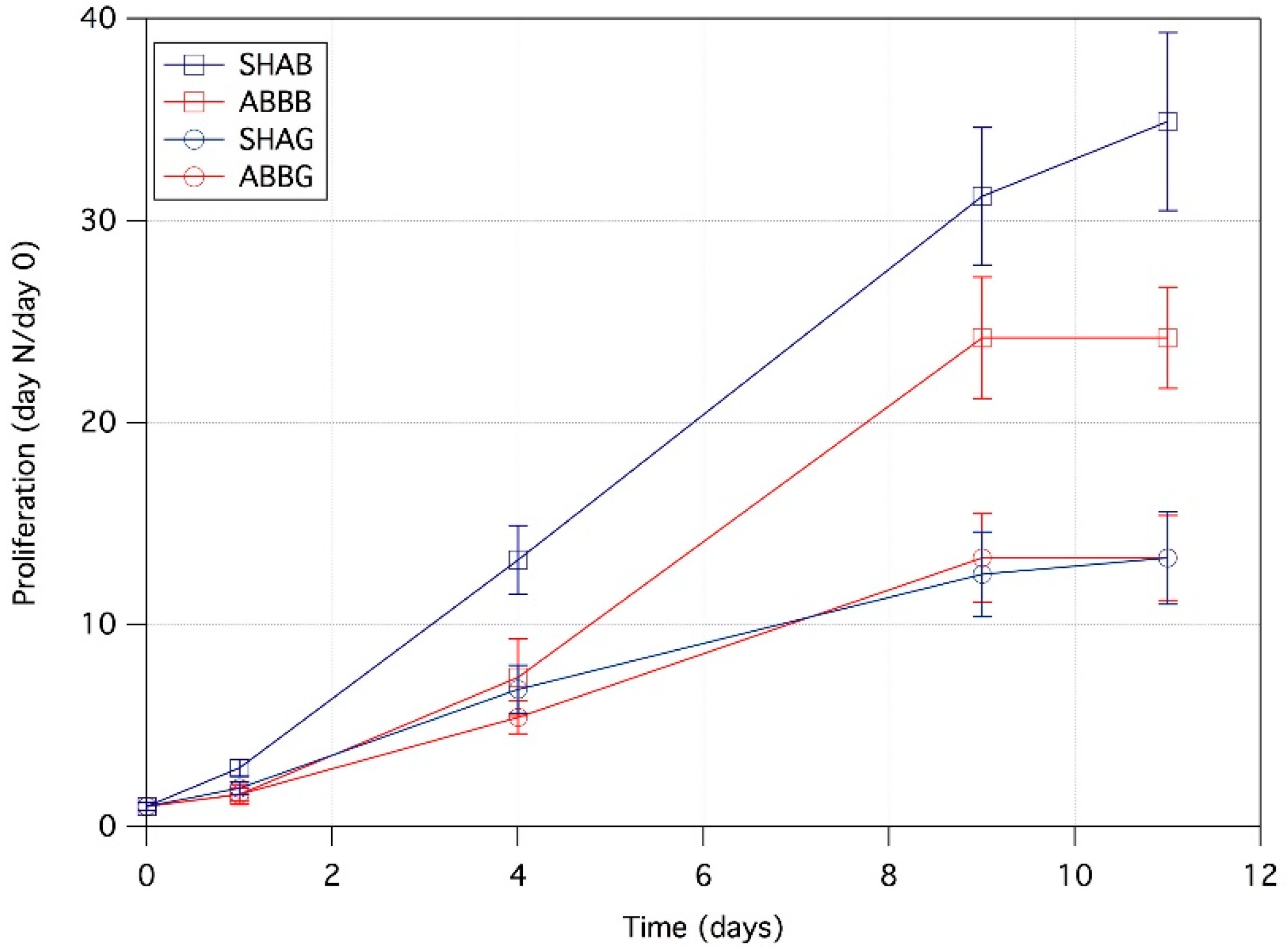
2.1. Cell Proliferation
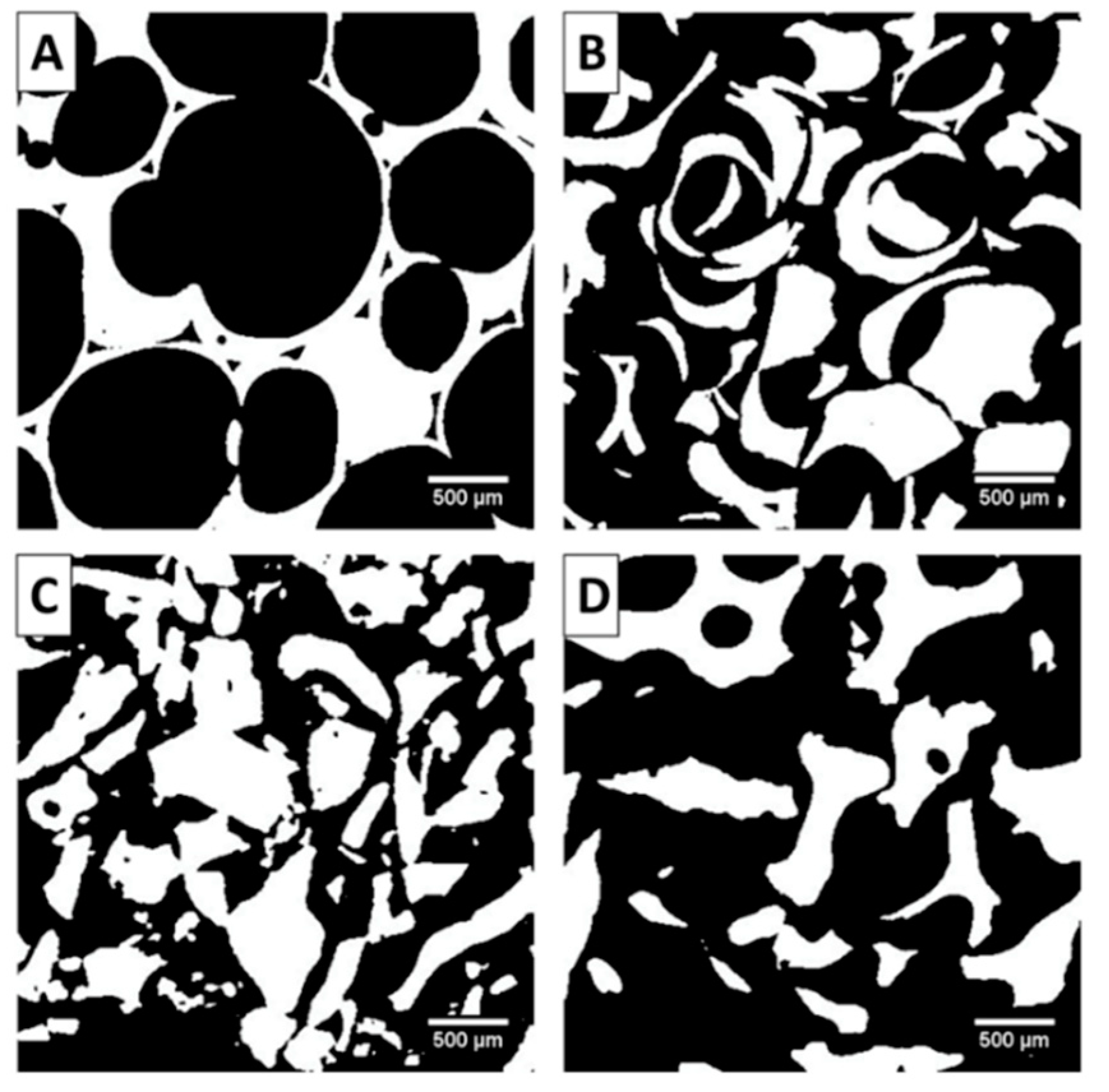
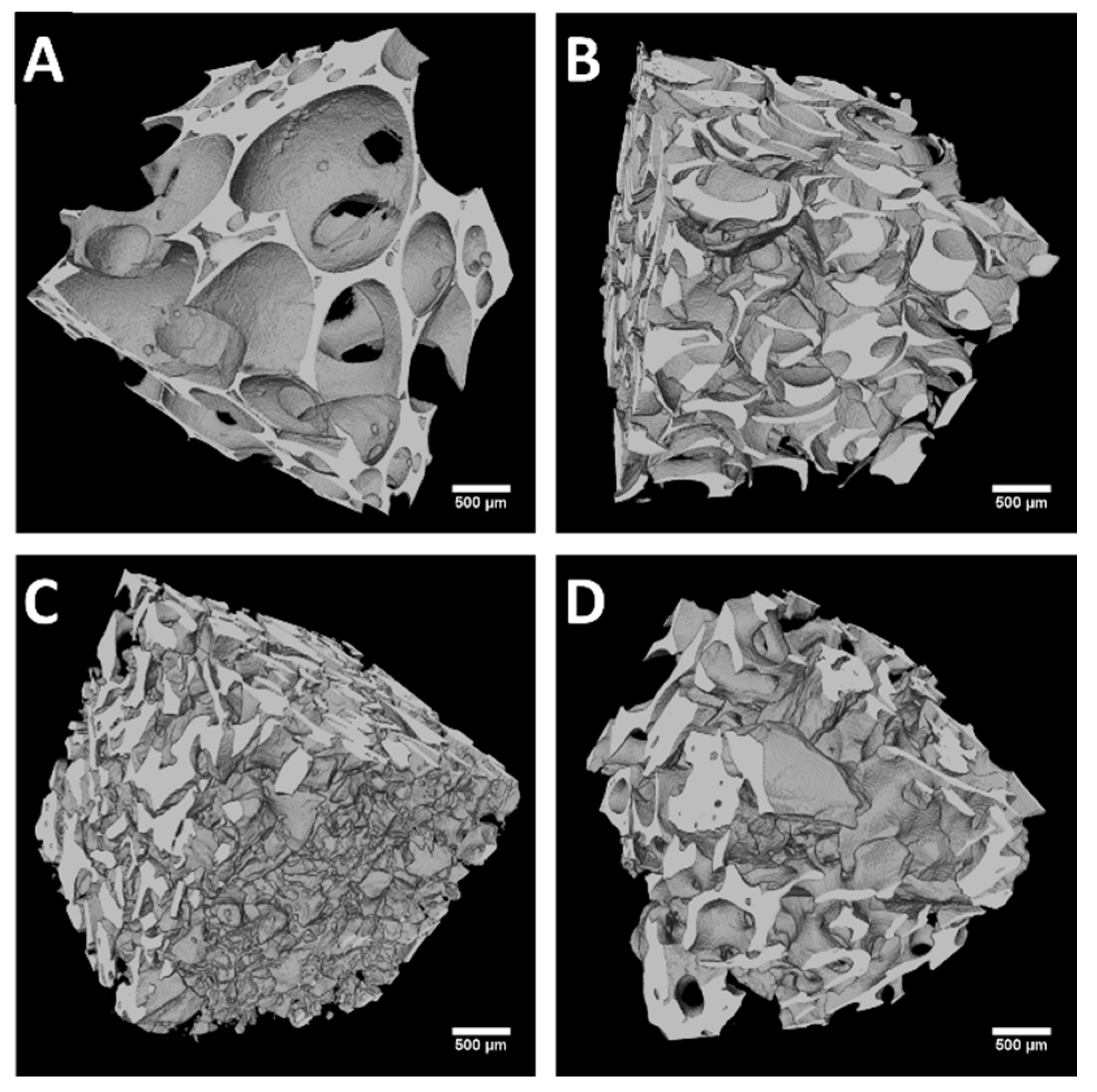
2.2. Structural Analysis: µ-CT Results
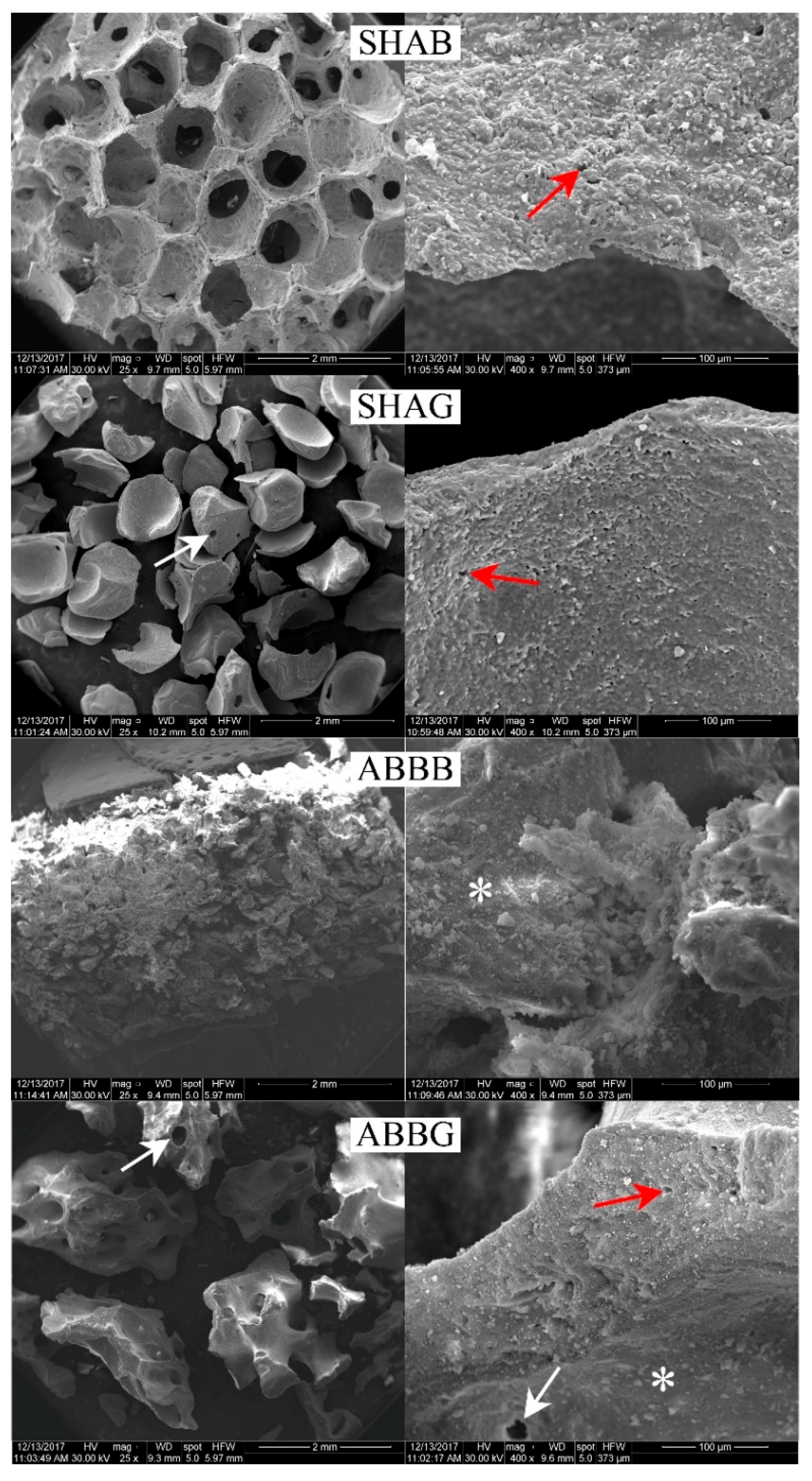
2.3. Structural Analysis: SEM Results
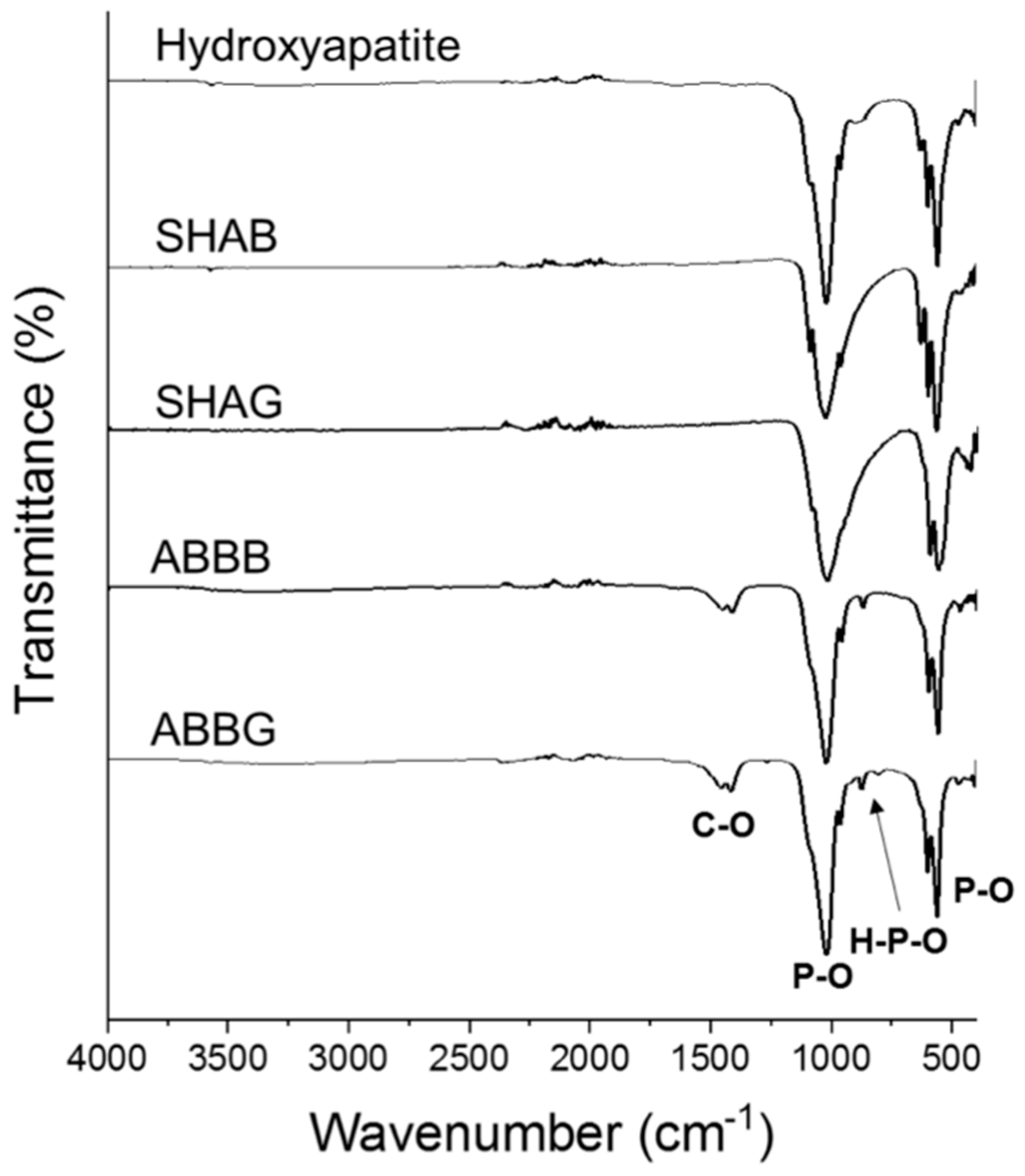
2.4. Fourier-Transformed Infrared Spectroscopy
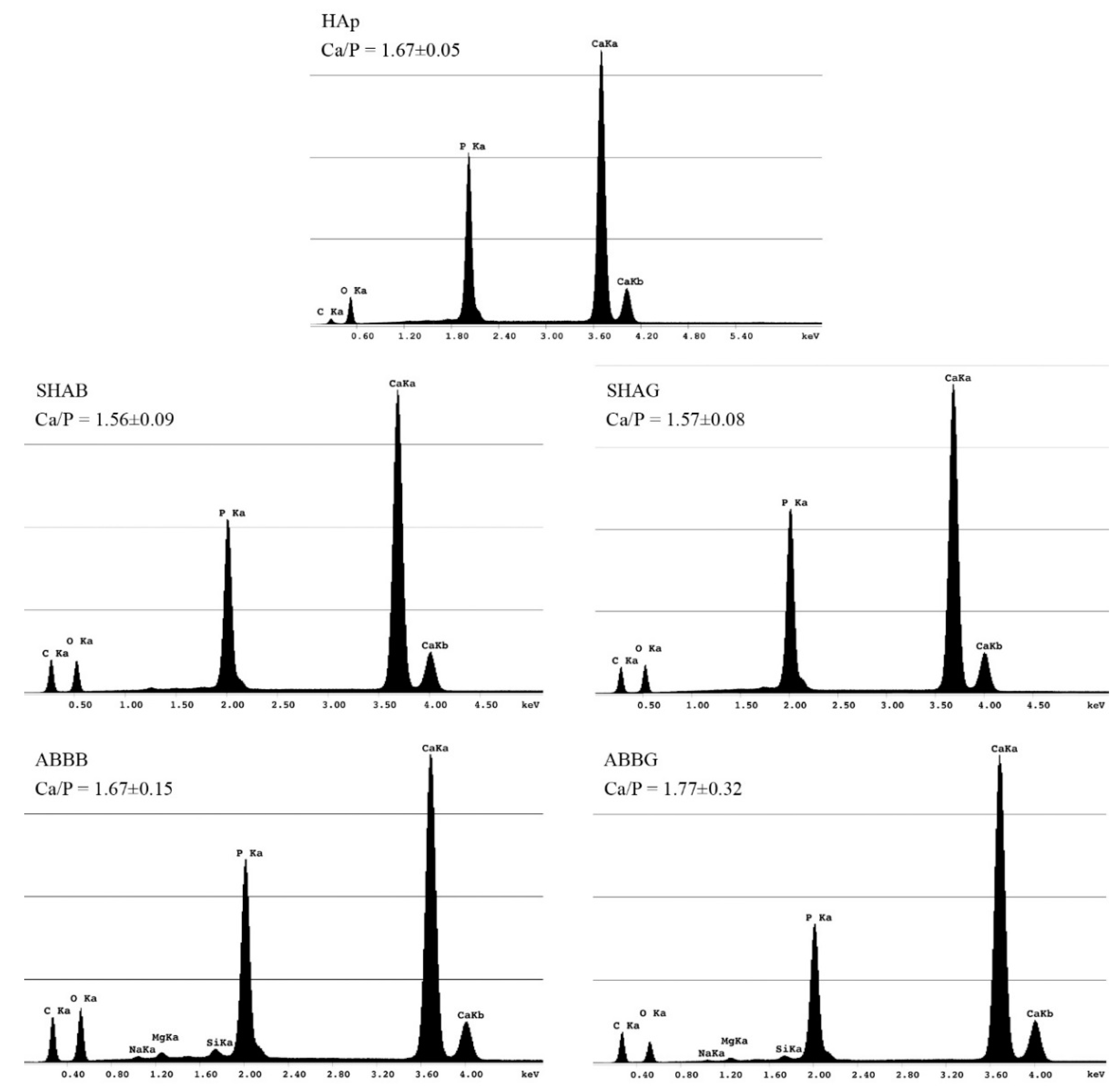
2.5. Energy Dispersive X-Ray Spectroscopy (EDS)
3. Discussion
4. Materials and Methods
4.1. Preparation of Biomaterials
4.2. Cell Culture and Seeding
4.3. Cells Proliferation and Viability
4.4. X-Ray Microcomputed Tomography
4.5. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy
4.6. Fourier-Transformed Infrared Spectroscopy
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABBB | anorganic bovine bone in blocks |
| ABBG | anorganic bovine bone in granules |
| EDS | energy dispersive X-ray spectroscopy |
| FTIR | Fourier transformed infrared spectroscopy |
| HAp | hydroxyapatite |
| µ-CT | micro-computed tomography |
| SEM | scanning electron microscopy |
| SHAB | synthetic hydroxyapatite in blocks |
| SHAG | synthetic hydroxyapatite in granules |
| VOI | volume of interest |
References
- Moura, L.B.; Carvalho, P.H.; Xavier, C.B.; Post, L.K.; Torriani, M.A.; Santagata, M.; Chagas Júnior, O.L. Autogenous non-vascularized bone graft in segmental mandibular reconstruction: A systematic review. Int. J. Oral Maxillofac. Surg. 2016, 45, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Kent, J.N. Sinus augmentation for dental implants: The use of autogenous bone. J. Oral Maxillofac. Surg. 1997, 55, 1281–1286. [Google Scholar] [CrossRef]
- Ahmed, W.; Asim, M.A.; Ehsan, A.; Abbas, Q. Non-vascularized autogenous bone grafts for reconstruction of maxillofacial osseous defects. J. Coll. Phys. Surg. Pak. 2018, 28, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-T.; Wang, H.-L. How to select replacement grafts for various periodontal and implant indications. Clin. Adv. Periodontics 2013, 3, 167–179. [Google Scholar] [CrossRef]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N.; American Academy of Orthopaedic Surgeons. The Committee on Biological, I. Bone-graft substitutes: Facts, fictions, and applications. J. Bone Joint Surg. Am. 2001, 83, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Orsini, G.; Di Iorio, D.; Breschi, L.; Di Lenarda, R. Clinical, histologic, and histomorphometric analyses of regenerated bone in maxillary sinus augmentation using fresh frozen human bone allografts. J. Periodontol. 2008, 79, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Stacchi, C.; Visintini, E.; Di Iorio, D.; Putignano, A.; Breschi, L.; Di Lenarda, R. Clinical and histologic evaluation of fresh frozen human bone grafts for horizontal reconstruction of maxillary alveolar ridges. Int. J. Periodontics Restor. Dent. 2011, 31, 535–544. [Google Scholar]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral Implants Res. 2014, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Laino, L.; Cicciu, M.; Lo Russo, L. Combination of bone graft and resorbable membrane for alveolar ridge preservation: A systematic review, meta-analysis and trial sequential analysis. J. Periodontol. 2018, 89, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; de Buitrago, J.G.; Padial-Molina, M.; Fernandez-Barbero, J.E.; Ata-Ali, J.; O’Valle, F. Histopathological comparison of healing after maxillary sinus augmentation using xenograft mixed with autogenous bone versus allograft mixed with autogenous bone. Clin. Oral Implants Res. 2018, 29, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of prion disease transmission through bovine-derived bone substitutes: A systematic review. Clin. Implant Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Bannister, S.R.; Powell, C.A. Foreign body reaction to anorganic bovine bone and autogenous bone with platelet-rich plasma in guided bone regeneration. J. Periodontol. 2008, 79, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Yla-Soininmaki, A.; Moritz, N.; Turco, G.; Paoletti, S.; Aro, H.T. Quantitative characterization of porous commercial and experimental bone graft substitutes with microcomputed tomography. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/Hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Piattelli, A.; Caputi, S.; Degidi, M.; Mangano, C.; Scarano, A.; Perrotti, V.; Iezzi, G. Regeneration of human bone using different bone substitute biomaterials. Clin. Implant Dent. Relat. Res. 2015, 17, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lombardi, T.; Oreglia, F.; Alberghini Maltoni, A.; Traini, T. Histologic and histomorphometric comparison between sintered nanohydroxyapatite and anorganic bovine xenograft in maxillary sinus grafting: A split-mouth randomized controlled clinical trial. Biomed. Res. Int. 2017, 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Rehman, R.; Rehman, I.U. Advances of proteomic sciences in dentistry. Int. J. Mol. Sci. 2016, 17, 728. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Pelillo, C.; Donati, I.; Marsich, E.; Benincasa, M.; Scarpa, T.; Semeraro, S.; Turco, G.; Gennaro, R.; Paoletti, S. Non-cytotoxic silver nanoparticle-polysaccharide nanocomposites with antimicrobial activity. Biomacromolecules 2009, 10, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Bystrov, V.S.; Coutinho, J.; Bystrova, A.V.; Dekhtyar, Y.D.; Pullar, R.C.; Poronin, A.; Palcevskis, E.; Dindune, A.; Alkan, B.; Durucan, C.; et al. Computational study of hydroxyapatite structures, properties and defects. J. Phys. D Appl. Phys. 2015, 48, 195302. [Google Scholar] [CrossRef]
- Zambuzzi, W.F.; Fernandes, G.V.; Iano, F.G.; Fernandes Mda, S.; Granjeiro, J.M.; Oliveira, R.C. Exploring anorganic bovine bone granules as osteoblast carriers for bone bioengineering: A study in rat critical-size calvarial defects. Braz. Dent. J. 2012, 23, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Epple, M. Synthetic Hydroxyapatite as a Biomimetic Oral Care Agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.S.; Jadhav, G.R.; Gathani, K.M.; Kotadia, P. Bioceramics in endodontics—A review. J. Istanb. Univ. Fac. Dent. 2017, 51, S128–S137. [Google Scholar] [CrossRef] [PubMed]
- Porrelli, D.; Travan, A.; Turco, G.; Marsich, E.; Borgogna, M.; Paoletti, S.; Donati, I. Alginate-hydroxyapatite bone scaffolds with isotropic or anisotropic pore structure: Material properties and biological behavior. Macromol. Mater. Eng. 2015, 300, 989–1000. [Google Scholar] [CrossRef]
- Marsich, E.; Bellomo, F.; Turco, G.; Travan, A.; Donati, I.; Paoletti, S. Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: Preparation, characterization and biological properties. J. Mater. Sci. Mater. Med. 2013, 24, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Marsich, E.; Travan, A.; Donati, I.; Turco, G.; Kulkova, J.; Moritz, N.; Aro, H.T.; Crosera, M.; Paoletti, S. Biological responses of silver-coated thermosets: An in vitro and in vivo study. Acta Biomater. 2013, 9, 5088–5099. [Google Scholar] [CrossRef] [PubMed]
- Rustom, L.E.; Boudou, T.; Lou, S.; Pignot-Paintrand, I.; Nemke, B.W.; Lu, Y.; Markel, M.D.; Picart, C.; Wagoner Johnson, A.J. Micropore-induced capillarity enhances bone distribution in vivo in biphasic calcium phosphate scaffolds. Acta Biomater. 2016, 44, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes–comparison with human bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatia, Y.D.; Ayukawa, Y.; Jinno, Y.; Furuhashi, A.; Koyano, K. Micro-computed tomography analysis of early stage bone healing using micro-porous titanium mesh for guided bone regeneration: Preliminary experiment in a canine model. Odontology 2017, 105, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Mate Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Gomez-Moreno, G.; Perez-Albacete Martinez, C.; Mazon, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity, and crystallinity on tissue reaction in vivo. Part A: Synthesis, characterization of the materials, and SEM analysis. Clin. Oral Implants Res. 2016, 27, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Prieto, E.M.; Talley, A.D.; Gould, N.R.; Zienkiewicz, K.J.; Drapeau, S.J.; Kalpakci, K.N.; Guelcher, S.A. Effects of particle size and porosity on in vivo remodeling of settable allograft bone/polymer composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Wake, M.C.; Patrick, C.W.; Mikos, A.G. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994, 3, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Whang, K.; Healy, K.E.; Elenz, D.R.; Nam, E.K.; Tsai, D.C.; Thomas, C.H.; Nuber, G.W.; Glorieux, F.H.; Travers, R.; Sprague, S.M. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999, 5, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, A.C.; Pochapski, M.T.; Daher, J.B.; da Silva, J.C.; Pilatti, G.L. Physico-chemical characterization and biocompatibility evaluation of hydroxyapatites. J. Oral Sci. 2006, 48, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Annaz, B.; Hing, K.A.; Kayser, M.; Buckland, T.; Di Silvio, L. An ultrastructural study of cellular response to variation in porosity in phase-pure hydroxyapatite. J. Microsc. 2004, 216, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Annaz, B.; Saeed, S.; Revell, P.A.; Buckland, T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J. Mater. Sci. Mater. Med. 2005, 16, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S.; Panduranga Rao, K. Nanoporous hydroxy-carbonate apatite scaffold made of natural bone. Mater. Lett. 2006, 60, 2844–2847. [Google Scholar] [CrossRef]
- Polak, S.J.; Rustom, L.E.; Genin, G.M.; Talcott, M.; Wagoner Johnson, A.J. A mechanism for effective cell-seeding in rigid, microporous substrates. Acta Biomater. 2013, 9, 7977–7986. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2017, 7. [Google Scholar] [CrossRef]
- Berberi, A.; Samarani, A.; Nader, N.; Noujeim, Z.; Dagher, M.; Kanj, W.; Mearawi, R.; Salemeh, Z.; Badran, B. Physicochemical characteristics of bone substitutes used in oral surgery in comparison to autogenous bone. Biomed. Res. Int. 2014, 2014, 320790. [Google Scholar] [CrossRef] [PubMed]
- TomoLab. Available online: https://www.elettra.trieste.it/it/lightsources/labs-and-services/tomolab/tomolab.html (accessed on 4 January 2018).
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelieres, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Avila, G.; Wang, H.L.; Galindo-Moreno, P.; Misch, C.E.; Bagramian, R.A.; Rudek, I.; Benavides, E.; Moreno-Riestra, I.; Braun, T.; Neiva, R. The influence of the bucco-palatal distance on sinus augmentation outcomes. J. Periodontol. 2010, 81, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lombardi, T.; Ottonelli, R.; Berton, F.; Perinetti, G.; Traini, T. New bone formation after transcrestal sinus floor elevation was influenced by sinus cavity dimensions: A prospective histologic and histomorphometric study. Clin. Oral Implants Res. 2018, 29, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Laino, L.; Iezzi, G.; Piattelli, A.; Lo Muzio, L.; Cicciu, M. Vertical ridge augmentation of the atrophic posterior mandible with sandwich technique: Bone block from the chin area versus corticocancellous bone block allograft–clinical and histological prospective randomized controlled study. Biomed. Res. Int. 2014, 2014, 982104. [Google Scholar] [CrossRef] [PubMed]
| Biomaterials | Advantages | Disadvantages |
|---|---|---|
| Autologous Bone | Osteoconduction; osteoinduction; no risks of cross infection or immune response; cost-effective | High morbidity; limited availability; limited dimensional stability over time |
| Allogeneic Bone | Osteoconduction; osteoinduction; low morbidity; unlimited availability | Theoretical risk of cross infection or immune response; limited dimensional stability over time; high costs |
| Xenogeneic Bone | Osteoconduction; low morbidity; unlimited availability; high dimensional stability over time | No osteoinductive properties; theoretical risk of cross infection or immune response; high costs |
| Alloplastic Materials | Osteoconduction; low morbidity; unlimited availability; variable dimensional stability over time; no risk of cross infection | No osteoinductive properties; theoretical risk of immune response; high costs |
| Structural Parameters | SHAB | SHAG | ABBB | ABBG |
|---|---|---|---|---|
| Porosity (%) | 75 ± 2 a | 56 ± 2 c | 54 ± 1 c | 65 ± 1 b |
| Tb.Th (µm) | 194 ± 100 a | 207 ± 127 a | 150 ± 59 b | 193 ± 75 a |
| Tb.Sp (µm) | 992 ± 323 a | 223 ± 106 b | 156 ± 71 b | 397 ± 182 b |
| BS/TV (mm−1) | 3.4 ± 0.4 d | 7.6 ± 0.3 b | 9.0 ± 0.1 a | 5.4 ± 0.3 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turco, G.; Porrelli, D.; Marsich, E.; Vecchies, F.; Lombardi, T.; Stacchi, C.; Di Lenarda, R. Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization. J. Funct. Biomater. 2018, 9, 62. https://doi.org/10.3390/jfb9040062
Turco G, Porrelli D, Marsich E, Vecchies F, Lombardi T, Stacchi C, Di Lenarda R. Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization. Journal of Functional Biomaterials. 2018; 9(4):62. https://doi.org/10.3390/jfb9040062
Chicago/Turabian StyleTurco, Gianluca, Davide Porrelli, Eleonora Marsich, Federica Vecchies, Teresa Lombardi, Claudio Stacchi, and Roberto Di Lenarda. 2018. "Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization" Journal of Functional Biomaterials 9, no. 4: 62. https://doi.org/10.3390/jfb9040062
APA StyleTurco, G., Porrelli, D., Marsich, E., Vecchies, F., Lombardi, T., Stacchi, C., & Di Lenarda, R. (2018). Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization. Journal of Functional Biomaterials, 9(4), 62. https://doi.org/10.3390/jfb9040062










