Direct-Contact Cytotoxicity Evaluation of CoCrFeNi-Based Multi-Principal Element Alloys
Abstract
:1. Introduction
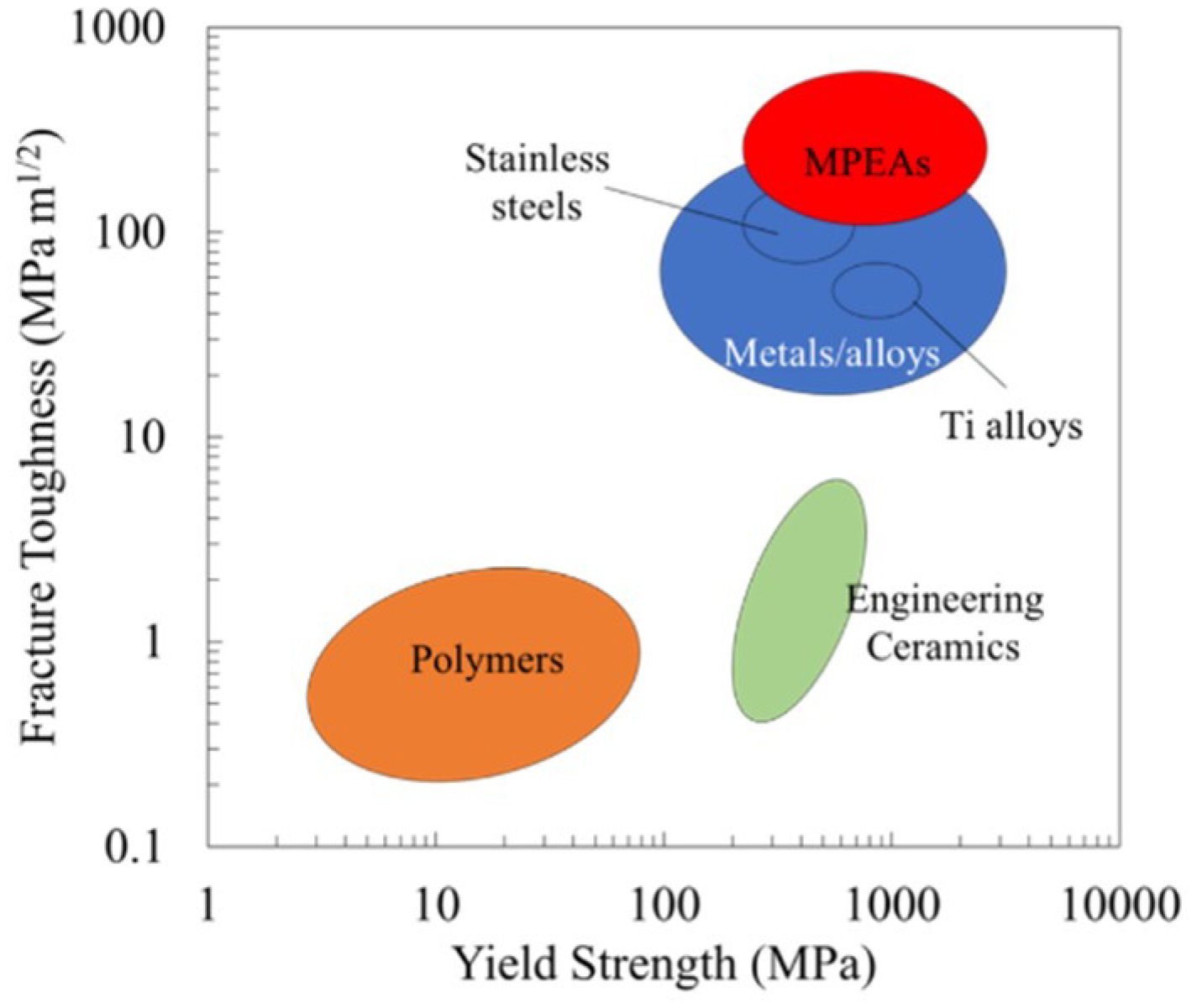
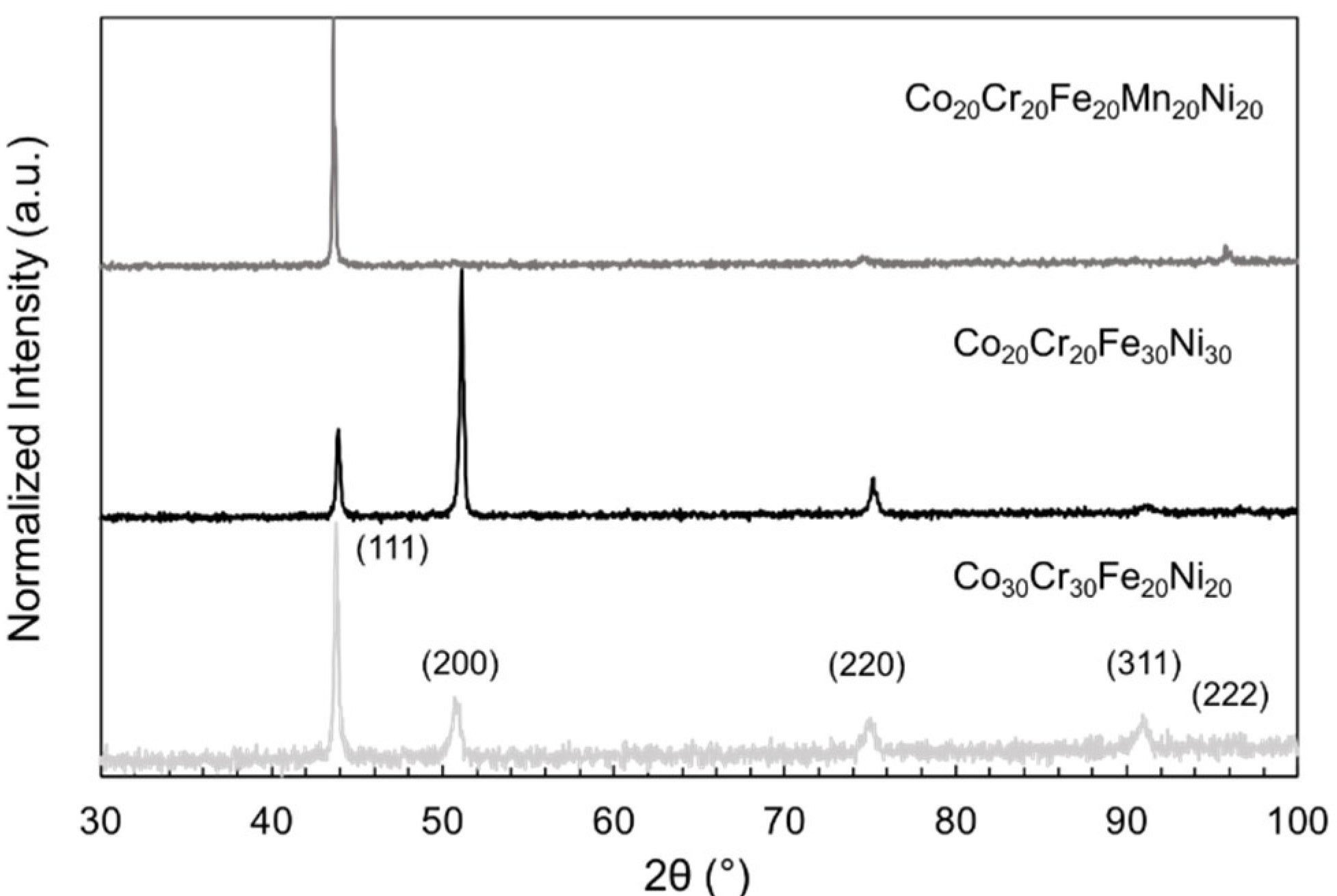
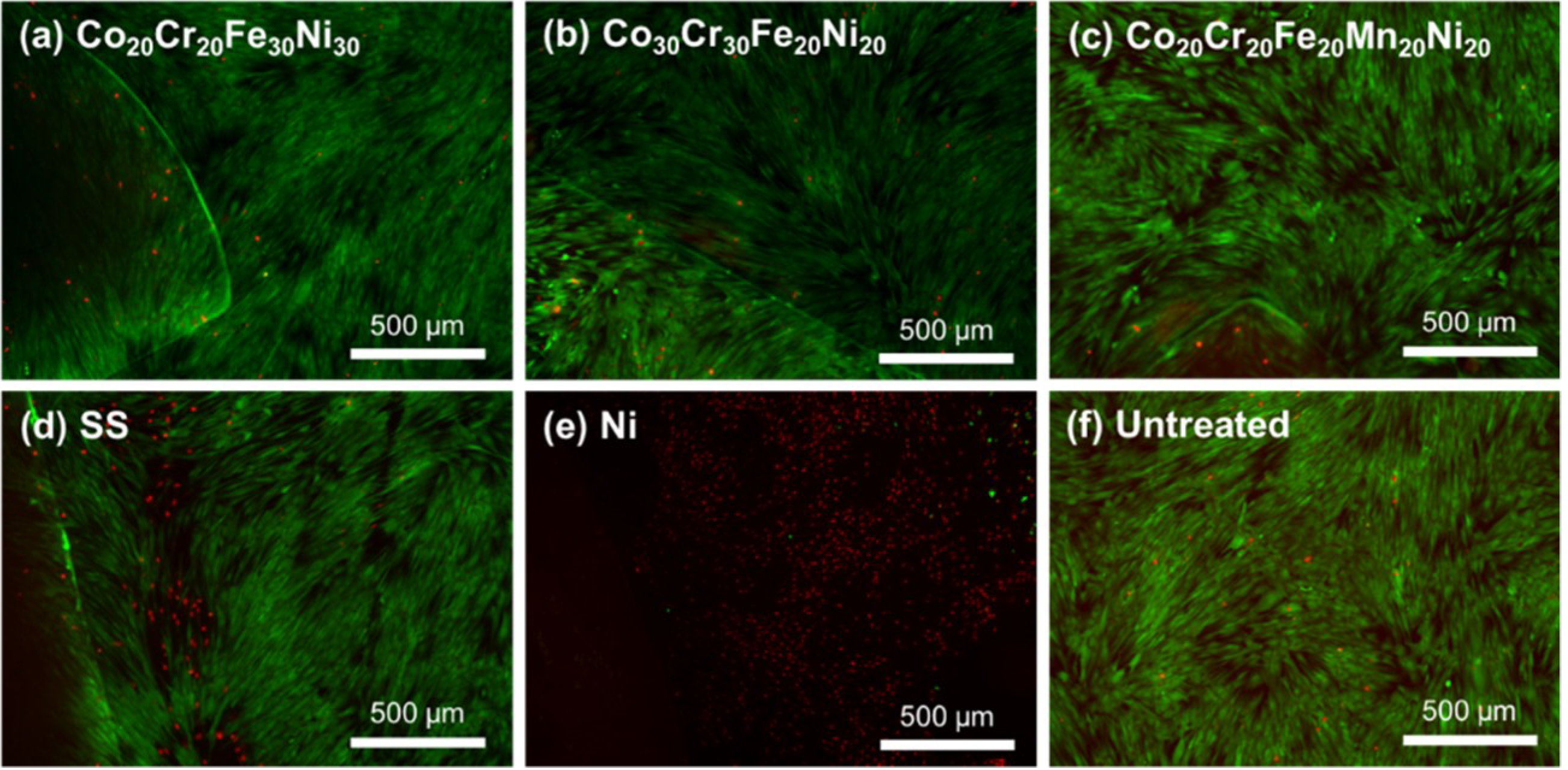
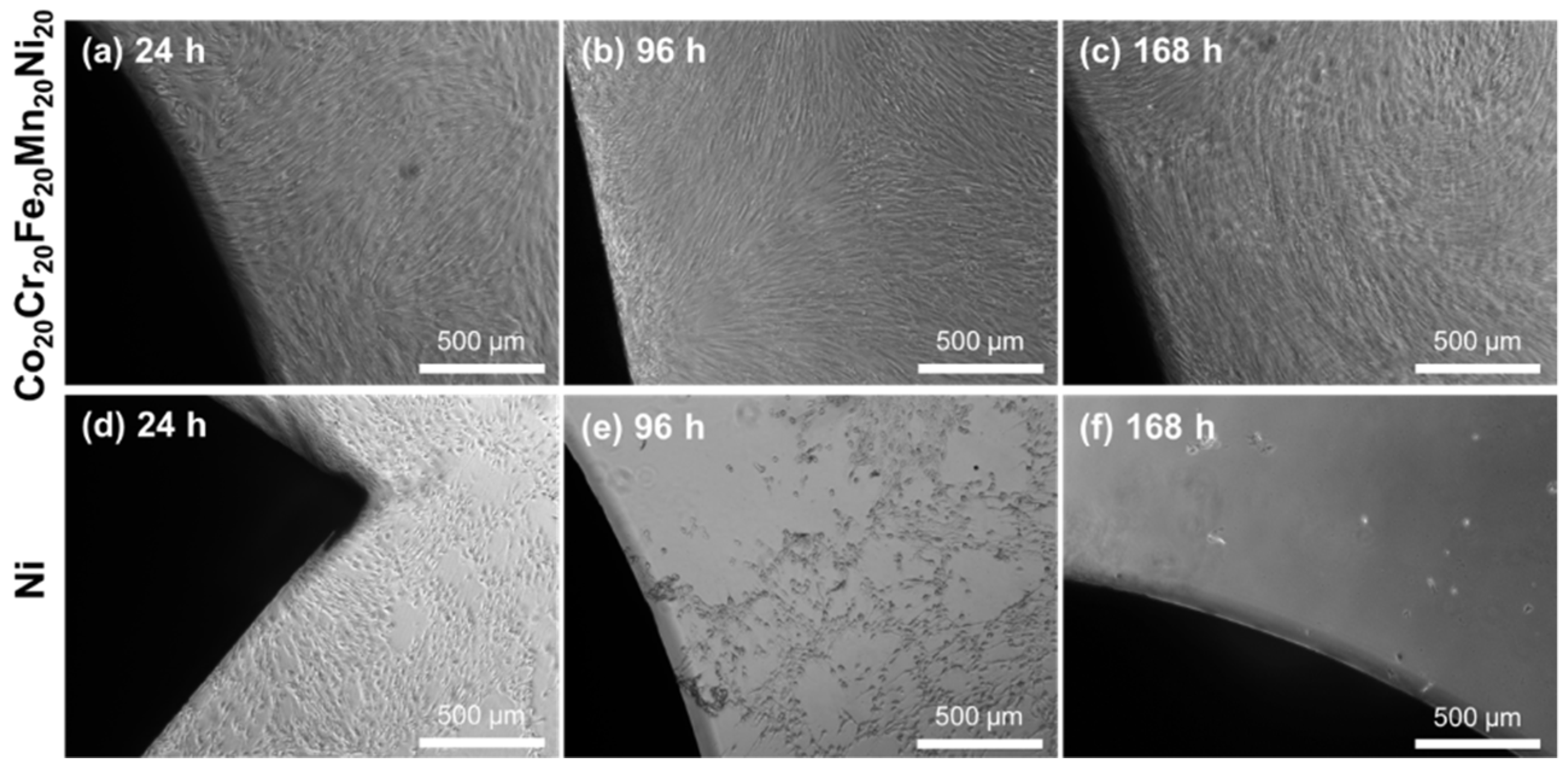
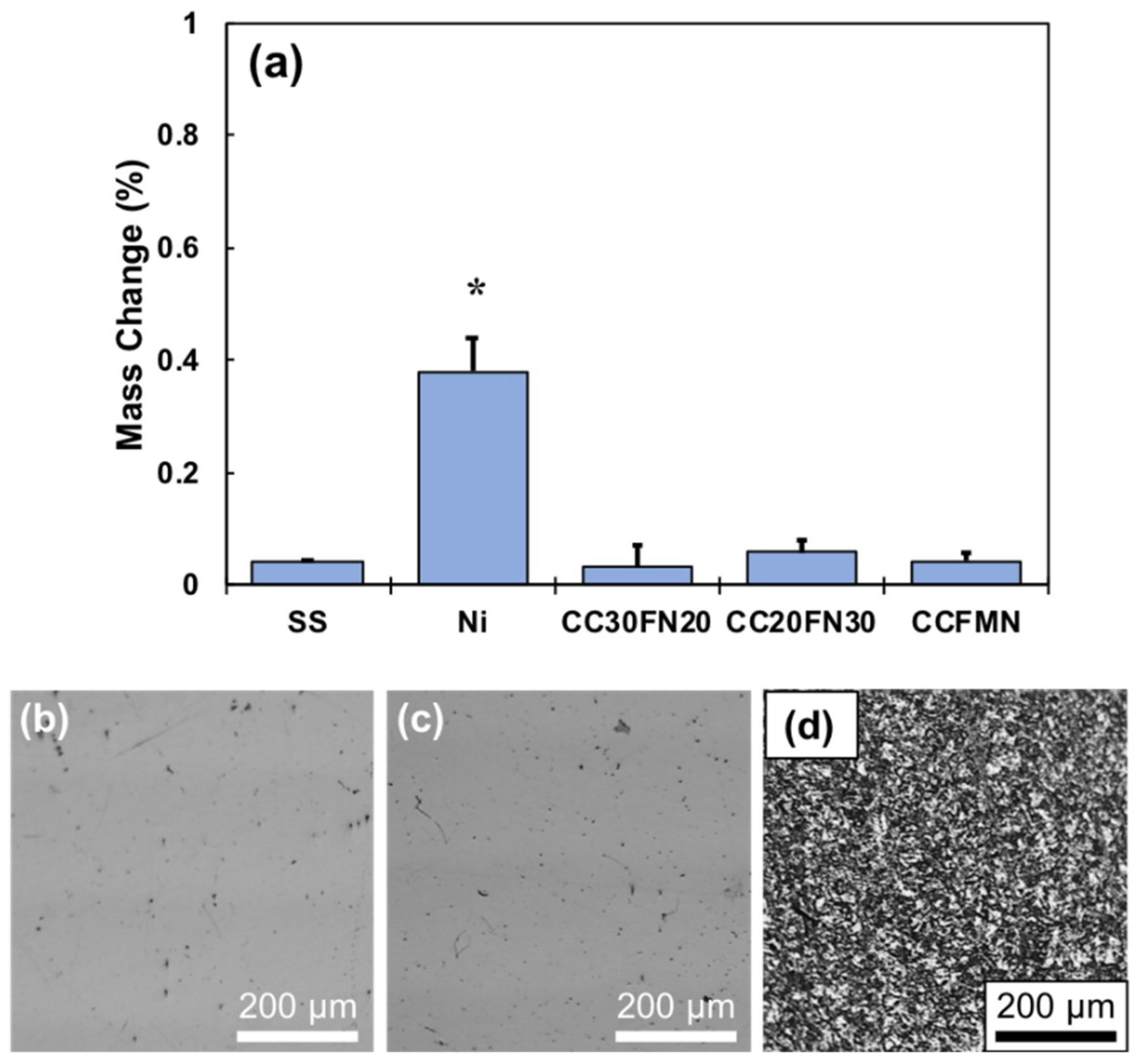
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Fabrication
4.2. Metal Multi-Principal Element Alloy Characterization
4.3. Cell Expansion
4.4. Direct Contact Cytotoxicity Evaluation
4.5. Cell Number
4.6. Viability Assessment
4.7. Mass Loss
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gioe, T.J. American Joint Replacement Registry 2016 Annual Report; American Joint Replacement Registry: Rosemont, IL, USA, 2016. [Google Scholar]
- Gludovatz, B.; Hohenwarter, A.; Thurston, K.V.S.; Bei, H.; Wu, Z.; George, E.P.; Ritchie, R.O. Exceptional damage-tolerance of a medium-entropy alloy crconi at cryogenic temperatures. Nat. Commun. 2016, 7, 10602. [Google Scholar] [CrossRef] [PubMed]
- Langton, D.; Jameson, S.; Joyce, T.; Hallab, N.; Natu, S.; Nargol, A. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J. Bone Jt. Surg. Br. 2010, 92, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, M.; Wegner, R.; Baur, X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J. Arthroplast. 2009, 24, 825.e815–e825. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.; Swann, M. Metal wear and tissue response in failed titanium alloy total hip replacements. J. Bone Jt. Surg. Br. 1991, 73, 559–563. [Google Scholar] [CrossRef]
- Lombardi, J.A.; Mallory, T.; Vaughn, B.; Drouillard, P. Aseptic loosening in total hip arthroplasty secondary to osteolysis induced by wear debris from titanium-alloy modular femoral heads. J. Bone Jt. Surg. Am. 1989, 71, 1337–1342. [Google Scholar] [CrossRef]
- Huber, M.; Reinisch, G.; Trettenhahn, G.; Zweymüller, K.; Lintner, F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009, 5, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Maloney, W.J.; Smith, R.L. Periprosthetic osteolysis in total hip arthroplasty: The role of particulate wear debris. J. Bone Jt. Surg. 1995, 77, 1448–1461. [Google Scholar] [CrossRef]
- Park, Y.-S.; Moon, Y.-W.; Lim, S.-J.; Yang, J.-M.; Ahn, G.; Choi, Y.-L. Early osteolysis following second-generation metal-on-metal hip replacement. J. Bone Jt. Surg. Am. 2005, 87, 1515–1521. [Google Scholar]
- Huo, M.H.; Salvati, E.A.; Lieberman, J.R.; Betts, F.; Bansal, M. Metallic debris in femoral endosteolysis in failed cemented total hip arthroplasties. Clin. Orthop. Relat. Res. 1992, 157–168. [Google Scholar] [CrossRef]
- Willert, H.; Brobäck, L.; Buchhorn, G.; Jensen, P.; Köster, G.; Lang, I.; Ochsner, P.; Schenk, R. Crevice corrosion of cemented titanium alloy stems in total hip replacements. Clin. Orthop. Relat. Res. 1996, 51–75. [Google Scholar] [CrossRef]
- Urban, R.M.; Jacobs, J.J.; Gilbert, J.L.; Galante, J.O. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J. Bone Jt. Surg. Am. 1994, 76, 1345–1359. [Google Scholar] [CrossRef]
- Mistry, J.B.; Chughtai, M.; Elmallah, R.K.; Diedrich, A.; Le, S.; Thomas, M.; Mont, M.A. Trunnionosis in total hip arthroplasty: A review. J. Orthop. Traumatol. 2016, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sumner, D.R.; Galante, J.O. Determinants of stress shielding. Clin. Orthop. Relat. Res. 1991, 274, 202–212. [Google Scholar] [CrossRef]
- Bobyn, J.D.; Mortimer, E.S.; Glassman, A.H.; Engh, C.A.; Miller, J.E.; Brooks, C.E. Producing and avoiding stress shielding. Laboratory and clinical observations of noncemented total hip arthroplasty. Clin. Orthop. Relat. Res. 1992, 274, 79–96. [Google Scholar]
- Senthi, S.; Munro, J.T.; Pitto, R.P. Infection in total hip replacement: Meta-analysis. Int. Orthop. 2011, 35, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bai, C.; Xie, L.; Zhang, Y.; Wang, K. Inflammatory response to orthopedic biomaterials after total hip replacement. J. Orthop. Sci. 2012, 17, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Miracle, D.; Senkov, O. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Boston, MA, USA, 2004. [Google Scholar]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Lee, C.; Chang, C.; Chen, Y.; Yeh, J.; Shih, H. Effect of the aluminium content of alxcrfe1.5mnni0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Lee, C.; Chen, Y.; Hsu, C.; Yeh, J.; Shih, H. Enhancing pitting corrosion resistance of alxcrfe1.5mnni0.5 high-entropy alloys by anodic treatment in sulfuric acid. Thin Solid Films 2008, 517, 1301–1305. [Google Scholar] [CrossRef]
- Chen, Y.; Duval, T.; Hung, U.; Yeh, J.; Shih, H. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of alxco1.5crfeni1.5tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Wu, J.-M.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. Adhesive wear behavior of alxcocrcufeni high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Brif, Y.; Thomas, M.; Todd, I. The use of high-entropy alloys in additive manufacturing. Scr. Mater. 2015, 99, 93–96. [Google Scholar] [CrossRef]
- Joseph, J.; Jarvis, T.; Wu, X.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Comparative study of the microstructures and mechanical properties of direct laser fabricated and arc-melted alxcocrfeni high entropy alloys. Mater. Sci. Eng. A 2015, 633, 184–193. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Structure and hydrogen storage properties of a high entropy zrtivcrfeni alloy synthesized using laser engineered net shaping (lens). Int. J. Hydrog. Energy 2013, 38, 12180–12189. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. First demonstration of promising selective electron beam melting method for utilizing high-entropy alloys as engineering materials. Mater. Lett. 2015, 159, 12–15. [Google Scholar] [CrossRef]
- Ocelik, V.; Janssen, N.; Smith, S.; De Hosson, J.T.M. Additive manufacturing of high-entropy alloys by laser processing. JOM 2016, 68, 1810–1818. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, K.; Fortier, A.; Mishra, R.; Kumar, N. In Investigation of thermo-mechanical processing and mechanical properties of cocrfenimn high entropy alloy for peripheral vascular stent application. In Proceedings of the ASME 2016 11th International Manufacturing Science and Engineering Conference, Blacksburg, VA, USA, 27 June–1 July 2016. [Google Scholar]
- Alagarsamy, K.; Fortier, A.; Komarasamy, M.; Kumar, N.; Mohammad, A.; Banerjee, S.; Han, H.-C.; Mishra, R.S. Mechanical properties of high entropy alloy al 0.1 cocrfeni for peripheral vascular stent application. Cardiovasc. Eng. Technol. 2016, 7, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.; Bao, X.; Huang, Y.; Qu, Y.; Lu, H.; Lu, Z. Mechanisms of cytotoxicity of nickel ions based on gene expression profiles. Biomaterials 2009, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Skreb, Y. Cytotoxicity of manganese for mammalian cells in vitro—Comparisons with lead, mercury and cadmium. Zentralbl Bakteriol. Mikrobiol. Hyg. B 1980, 171, 525–537. [Google Scholar] [PubMed]
- Castle, J.E.; Clayton, C.R. The use of in the x-ray photo-electron spectroscopy analyses of passive layers on stainless steel. Corrosi. Sci. 1977, 17, 7–26. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]

| Material | ASTM Designation | Yield Strength (MPa) | Elastic Modulus (GPa) | Endurance Limit (MPa) |
|---|---|---|---|---|
| Cortical Bone | - | 70–114 | 15–40 | 30–45 |
| CoCr | F75 | 448–841 | 280 | 207–950 |
| Ti-6Al-4V | F136 | 897–1034 | 116 | 620–689 |
| SS316L | F138 | 331–792 | 210 | 310–820 |
| TM MPEAs | - | 220–1500 | 55–230 | 400–950 |
| MPEA Sample | Co Content (at.%) | Cr Content (at.%) | Fe Content (at.%) | Mn Content (at.%) | Ni Content (at.%) |
|---|---|---|---|---|---|
| Co20Cr20Fe30Ni30 | 20.9 ± 0.5 | 18.9 ± 0.2 | 29.8 ± 0.2 | - | 30.4 ± 0.6 |
| Co30Cr30Fe20Ni20 | 29.9 ± 0.2 | 30.0 ± 0.2 | 20.5 ± 0.2 | - | 19.6 ± 0.2 |
| Co20Cr20Fe20Mn20Ni20 | 19.2 ± 0.2 | 20.6 ± 0.2 | 20.1 ± 0.2 | 20.9 ± 0.2 | 19.2 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newell, R.; Wang, Z.; Arias, I.; Mehta, A.; Sohn, Y.; Florczyk, S. Direct-Contact Cytotoxicity Evaluation of CoCrFeNi-Based Multi-Principal Element Alloys. J. Funct. Biomater. 2018, 9, 59. https://doi.org/10.3390/jfb9040059
Newell R, Wang Z, Arias I, Mehta A, Sohn Y, Florczyk S. Direct-Contact Cytotoxicity Evaluation of CoCrFeNi-Based Multi-Principal Element Alloys. Journal of Functional Biomaterials. 2018; 9(4):59. https://doi.org/10.3390/jfb9040059
Chicago/Turabian StyleNewell, Ryan, Zi Wang, Isabel Arias, Abhishek Mehta, Yongho Sohn, and Stephen Florczyk. 2018. "Direct-Contact Cytotoxicity Evaluation of CoCrFeNi-Based Multi-Principal Element Alloys" Journal of Functional Biomaterials 9, no. 4: 59. https://doi.org/10.3390/jfb9040059
APA StyleNewell, R., Wang, Z., Arias, I., Mehta, A., Sohn, Y., & Florczyk, S. (2018). Direct-Contact Cytotoxicity Evaluation of CoCrFeNi-Based Multi-Principal Element Alloys. Journal of Functional Biomaterials, 9(4), 59. https://doi.org/10.3390/jfb9040059






