Tailoring the Interface of Biomaterials to Design Effective Scaffolds
Abstract
1. Introduction
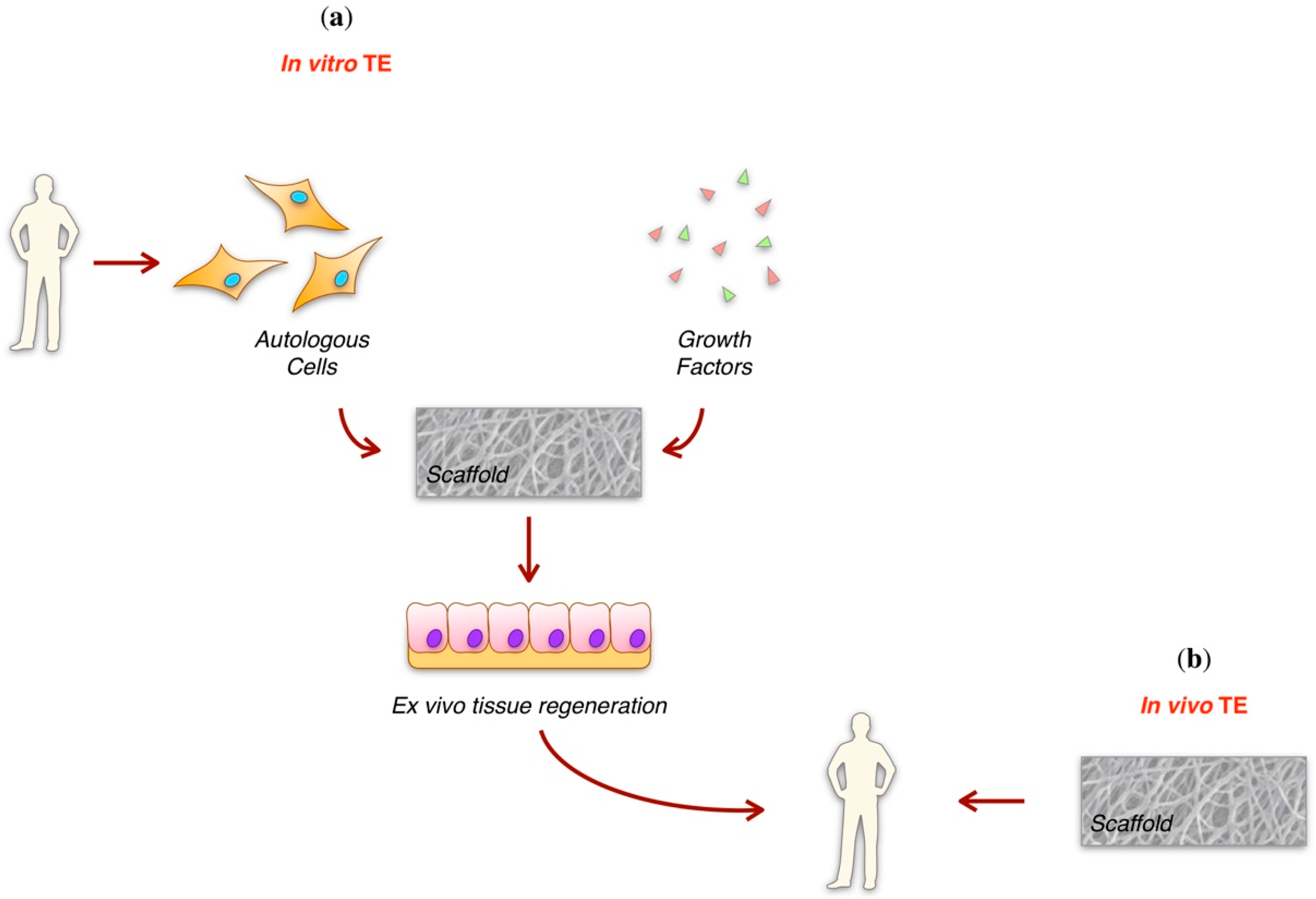
2. Tissue Engineering
3. Scaffold Requirements
3.1. Biodegradability Requirements
3.1.1. Synthetic Biodegradable Polymers
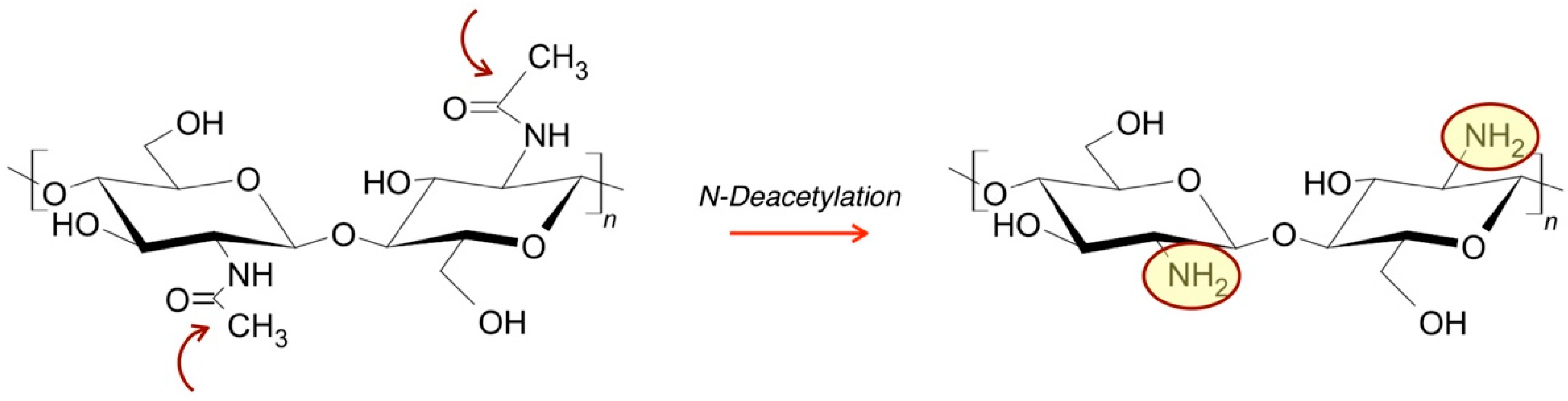
3.1.2. Natural Biodegradable Polymers
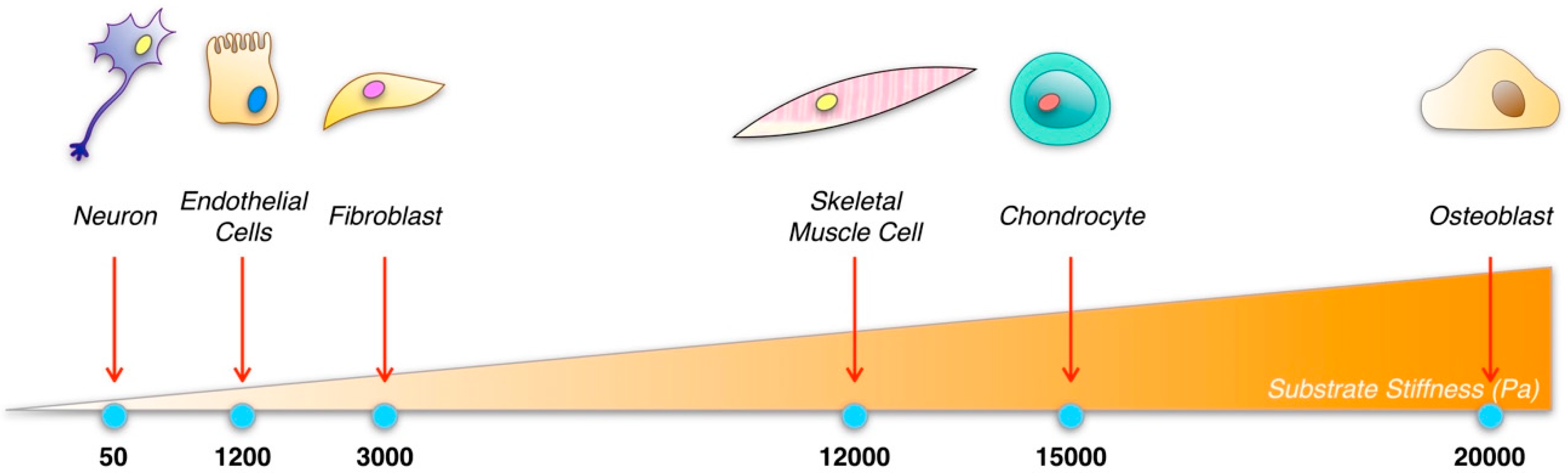
3.2. Mechanical Requirements
3.2.1. Porous Scaffolds
3.2.2. Hydrogels
3.2.3. Fibrous Scaffolds
3.3. Porosity Requirements
3.3.1. Top-Down Approaches
3.3.2. Bottom-Up Approaches
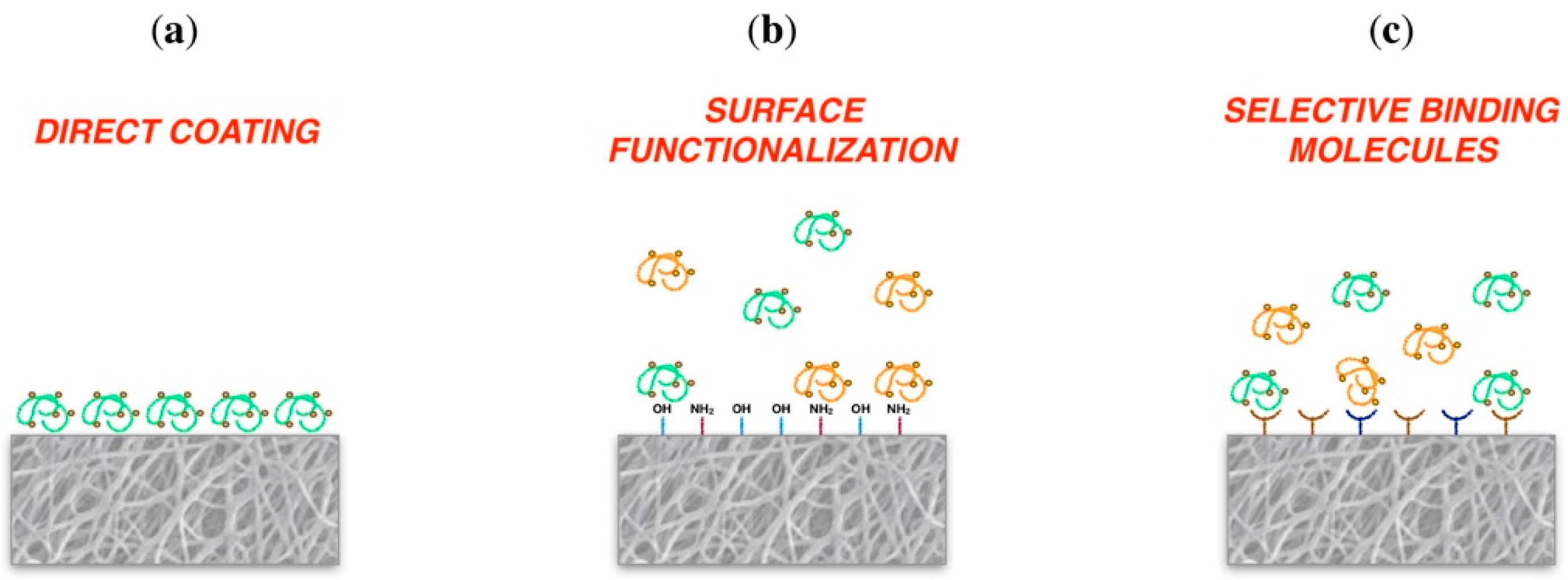
3.4. Bioactivity Requirements
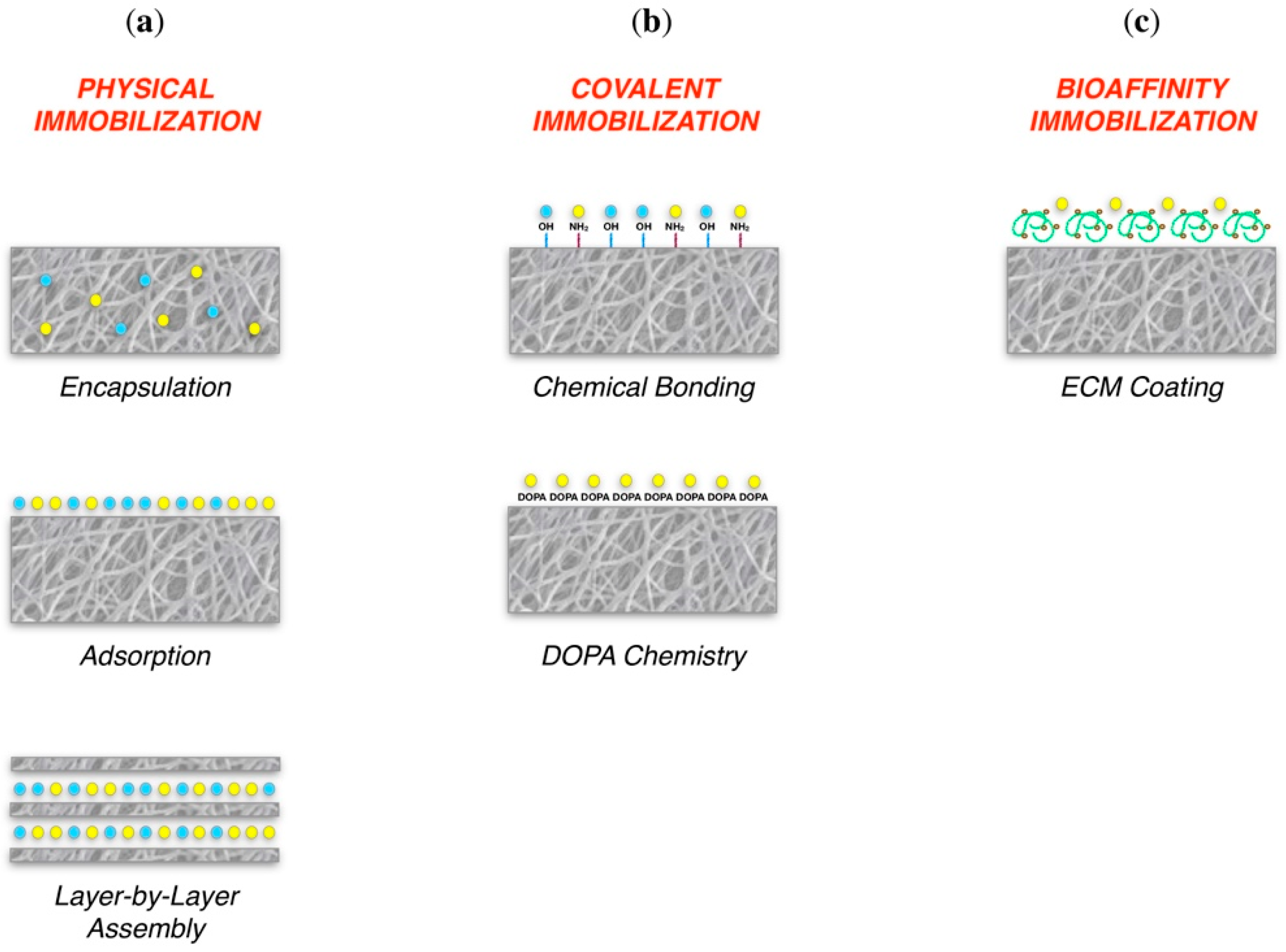
3.4.1. Control of Cell Adhesion
3.4.2. Control of Cell Fate and Function
3.5. Through a Functionally Graded Scaffold
4. A Case Study on Chitosan
4.1. Chitosan in Tissue Engineering
4.1.1. Bone
4.1.2. Skin
4.1.3. Cartilage
4.1.4. Cornea
5. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, D. The Williams Dictionary of Biomaterials; Liverpool University Press: Liverpool, UK, 1999. [Google Scholar]
- William, D. The inert-bioactivity conundrum. In The Implant Tissue Interface; Ellingsen, J., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 407–430. [Google Scholar]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Wejde, G.; Kugelberg, M.; Zetterstrom, C. Posterior capsule opacification: Comparison of 3 intraocular lenses of different materials and design. J. Cataract Refract. Surg. 2003, 29, 1556–1559. [Google Scholar] [CrossRef]
- Van Zele, D.; Heymans, O. Breast implants—A review. Acta Chir. Belg. 2004, 104, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Schoen, F.J.; Levy, R.J. Calcification of tissue heart valve substitutes: Progress toward understanding and prevention. Ann. Thorac. Surg. 2005, 79, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.M.; Doherty, P.J.; Williams, D.F. Mechanisms of polymer degradation in implantable devices. 2. Poly(dl-lactic acid). J. Biomed. Mater. Res. 1993, 27, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.M.; Zhong, S.P.; Doherty, P.J.; Williams, D.F. Mechanisms of polymer degradation in implantable devices. 1. poly(caprolactone). Biomaterials 1993, 14, 648–656. [Google Scholar] [CrossRef]
- Bostman, O.; Pihlajamaki, H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: A review. Biomaterials 2000, 21, 2615–2621. [Google Scholar] [CrossRef]
- Van Rooden, C.J.; Tesselaar, M.E.T.; Osanto, S.; Rosendaal, F.R.; Huisman, M.V. Deep vein thrombosis associated with central venous catheters—A review. J. Thromb. Haemost. 2005, 3, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I. Tissue and Organ Regeneration in Adults; Springer: New York, NY, USA, 2001. [Google Scholar]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Peters, M.C.; Mooney, D.J. Synthetic extracellular matrices for cell transplantation. Porous Mater. Tissue Eng. 1997, 250, 43–52. [Google Scholar]
- Hutmacher, D.W.; Goh, J.C.H.; Teoh, S.H. An introduction to biodegradable materials for tissue engineering applications. Ann. Acad. Med. Singap. 2001, 30, 183–191. [Google Scholar] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Tian, H.Y.; Tang, Z.H.; Zhuang, X.L.; Chen, X.S.; Jing, X.B. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Ma, Z.W.; Gao, C.Y.; Gong, Y.H.; Shen, J.C. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials 2005, 26, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotechnol. 2018, 60, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Bernick, S.; Cheung, D.T.; Ertl, D.C.; Nishimoto, S.K.; Paule, W.J.; Salka, C.; Strates, B.S. Biochemical differences between dystrophic calcification of cross-linked collagen implants and mineralization during bone induction. Calcif. Tissue Int. 1988, 42, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Goto, T.; Young, R.G.; Mansour, J.M.; Goldberg, V.M.; Caplan, A.I. Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng. 1998, 4, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Buttafoco, L.; Kolkman, N.G.; Engbers-Buijtenhuijs, P.; Poot, A.A.; Dijkstra, P.J.; Vermes, I.; Feijen, J. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials 2006, 27, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, B.C. Biodegradation of Silk Biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Galassi, G.; Brun, P.; Radice, M.; Cortivo, R.; Zanon, G.F.; Genovese, P.; Abatangelo, G. In vitro reconstructed dermis implanted in human wounds: Degradation studies of the HA-based supporting scaffold. Biomaterials 2000, 21, 2183–2191. [Google Scholar] [CrossRef]
- Robinson, D.; Halperin, N.; Nevo, Z. Regenerating hyaline cartilage in articular defects of old chickens using implants of embryonal chick chondrocytes embedded in a new natural delivery substance. Calcif. Tissue Int. 1990, 46, 246–253. [Google Scholar] [CrossRef] [PubMed]
- DeVos, P.; DeHaan, B.J.; Wolters, G.H.J.; Strubbe, J.H.; VanSchilfgaarde, R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia 1997, 40, 262–270. [Google Scholar]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Kreke, M.R.; Huckle, W.R.; Goldstein, A.S. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone 2005, 36, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, P. Bone Mechanics Handbook; CRC Press LLC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Goulet, R.W.; Goldstein, S.A.; Ciarelli, M.J.; Kuhn, J.L.; Brown, M.B.; Feldkamp, L.A. The relationship between the structural and orthogonal compressive properties of trabecular bone. J. Biomech. 1994, 27, 375–389. [Google Scholar] [CrossRef]
- Wu, J.Z.; Cutlip, R.G.; Andrew, M.E.; Dong, R.G. Simultaneous determination of the nonlinear-elastic properties of skin and subcutaneous tissue in unconfined compression tests. Skin Res. Technol. 2007, 13, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Xiao, Y.; Liu, C.S. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R.; Sakamaki, T.; Pioquinto, R.; Leonard, T.O.; Goldberg, S.F.; Hon, Q.; Erikson, R.L.; Rieber, M.; Rieber, M.S.; Hicks, D.J.; et al. Transfection of constitutively active mitogen-activated protein/extracellular signal-regulated kinase kinase confers tumorigenic and metastatic potentials to NIH3T3 cells. Cancer Res. 2000, 60, 1552–1556. [Google Scholar] [PubMed]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Mammoto, T.; Ingber, D.E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 2012, 125, 3061–3073. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Sweeney, L.; Sen, S.; Engler, A. Matrix elasticity directs stem cell lineage specification. Biophys. J. 2007. [Google Scholar] [CrossRef]
- Li, Q.; Mu, L.; Zhang, F.; Sun, Y.; Chen, Q.; Xie, C.; Wang, H. A novel fish collagen scaffold as dural substitute. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.F.; Avci-Adali, M.; Alarcin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.X.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Heilshorn, S.C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Zhao, L.A.; Weir, M.D.; Xu, H.H.K. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive Silicate Nanoplatelets for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Bae, H.; Cha, J.M.; Mun, J.Y.; Chen, Y.C.; Tekin, H.; Shin, H.; Farshchi, S.; Dokmeci, M.R.; Tang, S.; et al. Carbon Nanotube Reinforced Hybrid Microgels as Scaffold Materials for Cell Encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive Nanoengineered Hydrogels for Bone Tissue Engineering: A Growth-Factor-Free Approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, S.; Nachlas, A.L.Y.; Brown, M.E.; Pete, T.; Johnson, C.T.; García, A.J.; Davis, M.E. Evaluation of Hydrogels Presenting Extracellular Matrix-Derived Adhesion Peptides and Encapsulating Cardiac Progenitor Cells for Cardiac Repair. ACS Biomater. Sci. Eng. 2018, 4, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agarwal, P.; Xiao, Y.; Peng, H.; Zhao, S.; Liu, X.; Zhou, S.; Li, J.; Liu, Z.; He, X. A Nano-In-Micro System for Enhanced Stem Cell Therapy of Ischemic Diseases. ACS Cent. Sci. 2017, 3, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Chang, F.; Xu, W.; Ding, J. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater. Sci. Eng. 2018, 88, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2018, 138, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Khoroushi, M.; Foroughi, M.; Karbasi, S.; Hashemibeni, B.; Khademi, A. Effect of polyhydroxybutyrate/chitosan/bioglass nanofiber scaffold on proliferation and differentiation of stem cells from human exfoliated deciduous teeth into odontoblast-like cells. Mater. Sci. Eng. C 2018, 89, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Chen, S.; Morsi, Y.; El-Hamshary, H.; El-Newehy, M.; Fan, C.Y.; Mo, X.M. Superabsorbent 3D Scaffold Based on Electrospun Nanofibers for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 24415–24425. [Google Scholar] [CrossRef] [PubMed]
- Pauly, H.M.; Kelly, D.J.; Popat, K.C.; Trujillo, N.A.; Dunne, N.J.; McCarthy, H.O.; Donahue, T.L.H. Mechanical properties and cellular response of novel electrospun nanofibers for ligament tissue engineering: Effects of orientation and geometry. J. Mech. Behav. Biomed. Mater. 2016, 61, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Zahari, N.K.; Idrus, R.B.H.; Chowdhury, S.R. Laminin-Coated Poly(Methyl Methacrylate) (PMMA) Nanofiber Scaffold Facilitates the Enrichment of Skeletal Muscle Myoblast Population. Int. J. Mol. Sci. 2017, 18, 2242. [Google Scholar] [CrossRef] [PubMed]
- Shefa, A.; Amirian, J.; Kang, H.; Bae, S.; Jung, H.; Choi, H.; Lee, S.; Lee, B. In vitro and in vivo evaluation of effectiveness of a novel TEMPO-oxidized cellulose nanofiber-silk fibroin scaffold in wound healing. Carbohydr. Polym. 2017, 177, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Darling, A.; Starly, B.; Nam, J. Computer-aided tissue engineering: Overview, scope and challenges. Biotechnol. Appl. Biochem. 2004, 39, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.; Liao, E.; Moffitt, E.; Jeong, C.; Kemppainen, J. Defining design targets for tissue engineering scaffolds. In Fundamentals of Tissue Engineering and Regenerative Medicine; Springer: Berlin, Germany, 2009. [Google Scholar]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Vagaska, B.; Bacakova, L.; Filova, E.; Balik, K. Osteogenic Cells on Bio-Inspired Materials for Bone Tissue Engineering. Physiol. Res. 2010, 59, 309–322. [Google Scholar] [PubMed]
- Galarneau, L.; Loranger, A.; Gilbert, S.; Marceau, N. Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp. Cell Res. 2007, 313, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Leong, K.F.; Du, Z.H.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part 1. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Oota, Y.; Ono, K.; Miyazima, S. 3D modeling for sagittal suture. Phys. A Statist. Mech. Appl. 2006, 359, 538–546. [Google Scholar] [CrossRef]
- Groeber, F.; Holeitera, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin Tissue Engineering-In Vivo and In Vitro Applications CPS (vol. 39, pg 1, 2012). Clin. Plast. Surg. 2012, 39, XI. [Google Scholar]
- Wimpenny, I.; Ashammakhi, N.; Yang, Y. Chondrogenic potential of electrospun nanofibres for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2012, 6, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Korossis, S.; Bolland, F.; Southgate, J.; Ingham, E.; Fisher, J. Regional biomechanical and histological characterisation of the passive porcine urinary bladder: Implications for augmentation and tissue engineering strategies. Biomaterials 2009, 30, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Li, Y.H.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Simonet, M.; Pandit, A.; Neuenschwander, P. Characterization of a slowly degrading biodegradable polyesterurethane for tissue engineering scaffolds. J. Biomed. Mater. Res. Part A 2007, 82A, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Zonari, A.; Cerqueira, M.T.; Novikoff, S.; Goes, A.M.; Marques, A.P.; Correlo, V.M.; Reis, R.L. Poly(hydroxybutyrate-co-hydroxyvalerate) Bilayer Skin Tissue Engineering Constructs with Improved Epidermal Rearrangement. Macromol. Biosci. 2014, 14, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly(dl-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Plikk, P.; Malberg, S.; Albertsson, A.C. Design of Resorbable Porous Tubular Copolyester Scaffolds for Use in Nerve Regeneration. Biomacromolecules 2009, 10, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Ebrahimi-Barough, S.; Azami, M.; Vahdat, S.; Baharvand, H. Preparation of a porous conductive scaffold from aniline pentamer-modified polyurethane/PCL blend for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Czernuszka, J. Making tissue engineering scaffolds work. Review on the application of solid free from fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 29–40. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Hatami, J.; Lehner, A.N.; da Silva, C.L.; Ferreira, F.C.; Antunes, A.P.M. Gelatin porous scaffolds fabricated using a modified gas foaming technique: Characterisation and cytotoxicity assessment. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Minton, J.; Janney, C.; Akbarzadeh, R.; Focke, C.; Subramanian, A.; Smith, T.; McKinney, J.; Liu, J.Y.; Schmitz, J.; James, P.F.; et al. Solvent-free polymer/bioceramic scaffolds for bone tissue engineering: Fabrication, analysis, and cell growth. J. Biomater. Sci. Polym. Ed. 2014, 25, 1856–1874. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Kundu, S.C. Non-bioengineered silk gland fibroin protein: Characterization and evaluation of matrices for potential tissue engineering applications. Biotechnol. Bioeng. 2008, 100, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Electrospinning: A whipping fluid jet generates submicron polymer fibers. Appl. Phys. Lett. 2001, 78, 1149–1151. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Moazzez-Lalaklo, N.; Zarghami, N. An update on clinical applications of electrospun nanofibers for skin bioengineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Nune, M.; Kumaraswamy, P.; Krishnan, U.M.; Sethuraman, S. Self-Assembling Peptide Nanofibrous Scaffolds for Tissue Engineering: Novel Approaches and Strategies for Effective Functional Regeneration. Curr. Protein Pept. Sci. 2013, 14, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Recha-Sancho, L.; Semino, C.E. Heparin-based self-assembling peptide scaffold reestablish chondrogenic phenotype of expanded de-differentiated human chondrocytes. J. Biomed. Mater. Res. Part A 2016, 104, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Borland, S.; Richardson, S.M.; Merry, C.L.R.; Saiani, A.; Gough, J.E. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016, 46, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Bikas, H.; Stavropoulos, P.; Chryssolouris, G. Additive manufacturing methods and modelling approaches: A critical review. Int. J. Adv. Manuf. Technol. 2016, 83, 389–405. [Google Scholar] [CrossRef]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Bon, K.; Dong, D.; Sang, J.; Min, S.; Chang, M.; Chun-Ho, K. 3-dimensional bioprinting for tissue engineering applications. Biomater. Res. 2016, 20, 12. [Google Scholar]
- Bettahalli, N.M.S.; Vicente, J.; Moroni, L.; Higuera, G.A.; van Blitterswijk, C.A.; Wessling, M.; Stamatialis, D.F. Integration of hollow fiber membranes improves nutrient supply in three-dimensional tissue constructs. Acta Biomater. 2011, 7, 3312–3324. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.Z.; Wu, A.; Keefe, M.; Qin, X.H.; Yu, J.Y.; Suhr, J.; Byun, J.H.; Kim, B.S.; Chou, T.W. Additive manufacturing of multidirectional preforms for composites: Opportunities and challenges. Mater. Today 2015, 18, 503–512. [Google Scholar] [CrossRef]
- Chartrain, N.; Williams, C.; Whittington, A. A review on fabricating tissue scaffolds using vat photo polymerization. Acta Biomater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, L.G.; Messina, M.; Winston, S.; Kuo, C.Y.; Lerman, M.; Fisher, J.P. 3D Printed Pericardium Hydrogels To Promote Wound Healing in Vascular Applications. Biomacromolecules 2017, 18, 3802–3811. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, O.; Geven, M.A.; Sprecher, C.M.; Stadelmann, V.A.; Grijpma, D.W.; Tang, T.T.; Qin, L.; Lai, Y.; Alini, M.; de Bruijn, J.D.; et al. Surface-enrichment with hydroxyapatite nanoparticles in stereolithography-fabricated composite polymer scaffolds promotes bone repair. Acta Biomater. 2017, 54, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Yingying, D.; Haoming, L.; Qin, Y.; Shuai, W.; Jianglin, W.; Ma, J.; Insup, N.; Antonios, G.; Shengmin, Z. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar]
- Yin, J.; Yan, M.L.; Wang, Y.C.; Fu, J.Z.; Suo, H.R. 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-linking Strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Z.; Wu, J.B.; Liu, M.; Wang, H.; Li, C.M.; Rodriguez, M.J.; Li, G.; Wang, X.Q.; Kaplan, D.L. 3D Bioprinting of Self-Standing Silk-Based Bioink. Adv. Healthc. Mater. 2018, 7, 1701026. [Google Scholar] [CrossRef] [PubMed]
- Hemmrich, K.; von Heimburg, D.; Rendchen, R.; Di Bartolo, C.; Milella, E.; Pallua, N. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials 2005, 26, 7025–7037. [Google Scholar] [CrossRef] [PubMed]
- Gerecht-Nir, S.; Cohen, S.; Ziskind, A.; Itskovitz-Eldor, J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol. Bioeng. 2004, 88, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Thomas, C.H.; Branger, A.B.; Waters, C.M.; Healy, K.E. The detachment strength and morphology of bone cells contacting materials modified with a peptide sequence found within bone sialoprotein. J. Biomed. Mater. Res. 1997, 37, 9–19. [Google Scholar] [CrossRef]
- Dee, K.C.; Andersen, T.T.; Bizios, R. Osteoblast population migration characteristics on substrates modified with immobilized adhesive peptides. Biomaterials 1999, 20, 221–227. [Google Scholar] [CrossRef]
- Zreiqat, H.; Akin, F.A.; Howlett, C.R.; Markovic, B.; Haynes, D.; Lateef, S.; Hanley, L. Differentiation of human bone-derived cells grown on GRGDSP-peptide bound titanium surfaces. J. Biomed. Mater. Res. Part A 2003, 64A, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.L.; Schneider, I.C. Directed cell migration in multi-cue environments. Integr. Biol. 2013, 5, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, C.R.; Kamm, R.D. 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials 2013, 34, 5995–6007. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Abdeen, A.A.; Zhang, D.; Kilian, K.A. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 2013, 34, 8140–8148. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B. Integrins. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Neff, J.A.; Caldwell, K.D.; Tresco, P.A. A novel method for surface modification to promote cell attachment to hydrophobic substrates. J. Biomed. Mater. Res. 1998, 40, 511–519. [Google Scholar] [CrossRef]
- Wang, D.A.; Williams, C.G.; Yang, F.; Elisseeff, J.H. Enhancing the tissue-biomaterial interface: Tissue-initiated integration of biomaterials. Adv. Funct. Mater. 2004, 14, 1152–1159. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Volpato, F.Z.; Cagol, N.; Siciliano, M.; Migliaresi, C.; Motta, A.; Sala, R. An innovative protocol for schwann cells extracellular matrix proteins extraction. J. Biomed. Mater. Res. Part A 2016, 104, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, B.; Meyer, J.; Jonczyk, A.; Kessler, H.; Adamietz, P.; Meenen, N.M.; Kantlehner, M.; Goepfert, C.; Nies, B. RGD-peptides for tissue engineering of articular cartilage. Biomaterials 2002, 23, 3455–3463. [Google Scholar] [CrossRef]
- Boxus, T.; Touillaux, R.; Dive, G.; Marchand-Brynaert, J. Synthesis and evaluation of RGD peptidomimetics aimed at surface bioderivatization of polymer substrates. Bioorg. Med. Chem. 1998, 6, 1577–1595. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Ann. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Shanmugam, K.; Katti, D.S. Improved biomaterials for tissue engineering applications: Surface modification of polymers. Curr. Top. Med. Chem. 2008, 8, 341–353. [Google Scholar] [PubMed]
- Ikada, Y. Surface modification of polymers for medical applications. Biomaterials 1994, 15, 725–736. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Parisi, L.; Elviri, L.; Bianchera, A.; Smerieri, A.; Lagonegro, P.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G.M. Chitosan scaffold modified with D-(+) raffinose and enriched with thiol-modified gelatin for improved osteoblast adhesion. Biomed. Mater. 2016, 11, 015004. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Firouzi, M.; Hassannejad, Z.; Haririan, I.; Zahedi, P. Fabrication and characterization of electrospun laminin-functionalized silk fibroin/poly(ethylene oxide) nanofibrous scaffolds for peripheral nerve regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.K.; Du, P.; Kim, I.G.; Ko, J.; Kim, S.W.; Park, K. Polymer mesh scaffold combined with cell-derived ECM for osteogenesis of human mesenchymal stem cells. Biomater. Res. 2016, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.K.; Luyten, F.P.; Patterson, J. RGD-functionalized polyethylene glycol hydrogels support proliferation and in vitro chondrogenesis of human periosteum-derived cells. J. Biomed. Mater. Res. Part A 2018, 106, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tegoulia, V.A.; Rao, W.S.; Kalambur, A.T.; Rabolt, J.R.; Cooper, S.L. Surface properties, fibrinogen adsorption, and cellular interactions of a novel phosphorylcholine-containing self-assembled monolayer on gold. Langmuir 2001, 17, 4396–4404. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Ratner, B.D.; Barbosa, M.A. Protein adsorption on mixtures of hydroxyl- and methylterminated alkanethiols self-assembled monolavers. J. Biomed. Mater. Res. Part A 2003, 67A, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Agashe, M.; Raut, V.; Stuart, S.J.; Latour, R.A. Molecular simulation to characterize the adsorption behavior of a fibrinogen gamma-chain fragment. Langmuir 2005, 21, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Means, G.E.; Feeney, R.E. Chemical Modifications of Proteins: History and Applications. Bioconjug. Chem. 1990, 1, 2–12. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Park, G.E.; Pattison, M.A.; Park, K.; Webster, T.J. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials 2005, 26, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovenko, S.; Pawlowski, L.; Smurov, I.; Veiko, V. Pulsed laser modification of plasma-sprayed coatings: Experimental processing of hydroxyapatite and numerical simulation. Surface Coat. Technol. 2006, 201, 2248–2255. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Wu, J.; He, Q.Y.; Zhou, X.Z.; Lu, G.; Shang, L.; Boey, F.; Venkatraman, S.S.; Zhang, H. Surface Modification of Smooth Poly(l-lactic acid) Films for Gelatin Immobilization. ACS Appl. Mater. Interfaces 2012, 4, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Parisi, L.; Piergianni, M.; Smerieri, A.; Passeri, G.; Guizzardi, S.; Costa, F.; Lumetti, S.; Manfredi, E.; Macaluso, G.M. Improved scaffold biocompatibility through anti-Fibronectin aptamer functionalization. Acta Biomater. 2016, 42, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Galli, C.; Bianchera, A.; Lagonegro, P.; Elviri, L.; Smerieri, A.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G.M. Anti-fibronectin aptamers improve the colonization of chitosan films modified with D-(+) Raffinose by murine osteoblastic cells. J. Mater. Sci. Mater. Med. 2017, 28, 136. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.R.; Gronowicz, G.; Hurley, M.M. Fibroblast Growth Factor-2, Bone Homeostasis and Fracture Repair. Curr. Pharm. Des. 2013, 19, 3354–3363. [Google Scholar] [CrossRef] [PubMed]
- Seeherman, H.; Wozney, J.M. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Jt. Surg. Am. Vol. 2008, 90, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Wang, Z.F.; Lu, W.W.; Zhen, W.X.; Yang, D.Z.; Peng, S.L. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Cai, Y.; Tong, S.; Zhang, R.; Zhu, T.; Wang, X.K. In vitro evaluation of a bone morphogenetic protein-2 nanometer hydroxyapatite collagen scaffold for bone regeneration. Mol. Med. Rep. 2018, 17, 5830–5836. [Google Scholar] [CrossRef] [PubMed]
- Ghiacci, G.; Graiani, G.; Cacchioli, A.; Galli, C.; Lumetti, S.; Ravanetti, F.; Elviri, L.; Manfredi, E.; Macaluso, G.M.; Sala, R. Stanozolol-soaked grafts enhance new bone formation in rat calvarial critical-size defects. Biomed. Mater. 2017, 12, 045016. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.B.; Jin, Q.M.; Giannobile, W.V.; Ma, P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials 2007, 28, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Angelatos, A.S.; Caruso, F. Template synthesis of nanostructured materials via layer-by-layer assembly. Chem. Mater. 2008, 20, 848–858. [Google Scholar] [CrossRef]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.L.; Samuel, R.E.; Shah, N.J.; Padera, R.F.; Beben, Y.M.; Hammond, P.T. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials 2011, 32, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Qin, X.X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Font Tellado, S.; Chiera, S.; Bonani, W.; Poh, S.; Migliaresi, C.; Motta, A.; Balmayor, E. Heparin functionalization increases retention of TGF-β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018, 72, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, M.; Martino, M.M.; Ventura, M.; Hubbell, J.A.; Hilborn, J.; Ossipov, D.A. Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials 2013, 34, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.; Miyamoto, Y.; Nogata, F. Functionally Graded Materials: Design, Processing and Applications; Kluwer Academic Publisher: Boston, MA, USA, 1999; pp. 7–28. [Google Scholar]
- Leong, K.F.; Chua, C.K.; Sudarmadji, N.; Yeong, W.Y. Engineering functionally graded tissue engineering scaffolds. J. Mech. Behav. Biomed. Mater. 2008, 1, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Sharma, B.; Williams, C.G.; Ruffner, M.A.; Malik, A.; McFarland, E.G.; Elisseeff, J.H. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage 2003, 11, 653–664. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jerome, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Jiang, T.; Deng, M.; James, R.; Nair, L.S.; Laurencin, C.T. Micro- and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014, 10, 1632–1645. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vazquez, M.; Vega-Ruiz, B.; Ramos-Zuniga, R.; Saldana-Koppel, D.A.; Quinones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N. Chitosan in Nanostructured Thin Films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.D.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Francesko, A.; Tzanov, T. Chitin, Chitosan and Derivatives for Wound Healing and Tissue Engineering. In Biofunctionalization of Polymers and Their Applications; Springer: Berlin/Heidelberg, Germany, 2011; Volume 125, pp. 1–27. [Google Scholar]
- Silva, S.S.; Luna, S.M.; Gomes, M.E.; Benesch, J.; Pashkuleva, I.; Mano, J.F.; Reis, R.L. Plasma surface modification of chitosan membranes: Characterization and preliminary cell response studies. Macromol. Biosci. 2008, 8, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Rodrigues, M.T.; Motta, A.; Gomes, M.E.; Mano, J.F.; Migliaresi, C.; Reis, R.L. Novel genipin cross-linked chitosan-silk based sponges for the regeneration and repair of cartilage using a tissue engineering approach. Tissue Eng. Part A 2008, 14, 763. [Google Scholar]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.H.; Hwa, H.D. Effect of molecular weight of chitosan with the same degree of deacetylation on the thermal, mechanical, and permeability properties of the prepared membrane. Carbohydr. Polym. 1996, 29, 353–358. [Google Scholar]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Nishikawa, H.; Ueno, A.; Nishikawa, S.; Kido, J.; Ohishi, M.; Inoue, H.; Nagata, T. Sulfated glycosaminoglycan synthesis and its regulation by transforming growth factor-beta in rat clonal dental pulp cells. J. Endod. 2000, 26, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jia, Z.; Xiong, P.; Yan, J.; Li, M.; Cheng, Y.; Zheng, Y. Novel pH-responsive tobramycin-embedded micelles in nanostructured multilayer-coatings of chitosan/heparin with efficient and sustained antibacterial properties. Mater. Sci. Eng. C 2018, 90, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic modification of polysaccharides: Mechanisins, properties, and potential applications: A review. Enzyme Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Vazquez-Duhalt, R.; Wu, C.F.; Bentley, W.E.; Payne, G.F. Combinatorial screening for enzyme-mediated coupling. Tyrosinase-catalyzed coupling to create protein-chitosan conjugates. Biomacromolecules 2001, 2, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Rocasalbas, G.; Francesko, A.; Tourino, S.; Fernandez-Francos, X.; Guebitz, G.M.; Tzanov, T. Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr. Polym. 2013, 92, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.; Shahrezaee, M.; Shekarchi, B.; Oryan, A.; Azma, K. Three-Dimensional Porous Gelapin-Simvastatin Scaffolds Promoted Bone Defect Healing in Rabbits. Calcif. Tissue Int. 2015, 96, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Giannoudis, P.V. Enhancement of fracture healing with the diamond concept: The role of the biological chamber. Inj.-Int. J. Care Inj. 2011, 42, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [PubMed]
- Nandi, S.K.; Kundu, B.; Basu, D. Protein growth factors loaded highly porous chitosan scaffold: A comparison of bone healing properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials 2005, 26, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Liu, M.; Xu, T.; Wang, K.; Tan, H.; LU, X. Chitosan/biphasic calcium phosphate scaffolds functionalized with BMP-2-encapsulated nanoparticles and RGD for bone regeneration. J. Biomed. Mater. Res. Part A 2018. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W.J. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.H.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.M.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Belmonte, M.M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, Y.; Xiao, L.; Li, M.; Liu, H.; Li, S.; Liao, X.; Chen, Y.; Li, J.; Zhang, Y. Experimental study on effects of adipose-derived stem cell-seeded silk fibroin chitosan film on wound healing of a diabetic rat model. Transplant. Surg. Res. 2018, 80, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Dozin, B.; Giannoni, P.; Quarto, R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Tuli, R.; Li, W.J.; Tuan, R.S. Current state of cartilage tissue engineering. Arthritis Res. Ther. 2003, 5, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Kandel, R.A. In vitro cartilage tissue formation by co-culture of primary and passaged chondrocytes. Tissue Eng. 2007, 13, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Sekiya, I.; Miyazaki, K.; Ichinose, S.; Hata, Y.; Muneta, T. In vitro cartilage formation of composites of synovium-derived mesenchymal stem cells with collagen gel. Cell Tissue Res. 2005, 322, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Sakai, T.; Hiraiwa, H.; Nakashima, M.; Ono, Y.; Mitsuyama, H.; Ishiguro, N. Surface markers and gene expression to characterize the differentiation of monolayer expanded human articular chondrocytes. Nagoya J. Med. Sci. 2013, 75, 101–111. [Google Scholar] [PubMed]
- Filardo, G.; Kon, E.; Roffi, A.; Di Martino, A.; Marcacci, M. Scaffold-Based Repair for Cartilage Healing: A Systematic Review and Technical Note. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Chen, C.H.; Hsiao, C.Y.; Chen, J.P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Kudva, A.K.; Guckert, N.L.; Linse, K.D.; Roy, K. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials 2011, 32, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; She, R.F.; Huang, W.L.; Dong, Z.J.; Mo, G.; Liu, B. A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. J. Mater. Sci. Mater. Med. 2013, 24, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Palchesko, R.; Carrasquilla, S.; Feinberg, A. Natural biomaterials for corneal tissue engineering, repair, and regeneration. Adv. Healthc. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.S.; Schottman, T.; Gulati, M. Turning the tide of corneal blindness. Indian J. Ophthalmol. 2012, 60, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.H.; Dart, J.K.G.; Holland, E.J.; Kinoshita, S. Ophthalmology 3 Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; DeLuca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Liu, Y.; Wang, L.; Jiang, Z.; Li, T.; Zhang, W.; Liang, Y. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr. Polym. 2018, 192, 240–250. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Ge, H.Y.; Tang, X.L.; Su, S.; Tian, P.; Xiao, N.; Zhang, H.; Zhang, L.; Liu, P. Use of a Silk Fibroin-Chitosan Scaffold to Construct a Tissue-Engineered Corneal Stroma. Cells Tissues Organs 2013, 198, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, K.; Bednarz, J.; Valtink, M. Prospects for endothelial transplantation. Exp. Eye Res. 2004, 78, 573–578. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.S.; Han, B.Q.; Yang, C.Z.; Ma, Q.; Zhao, W.W.; Rong, M.; Li, H. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J. Mater. Sci. Mater. Med. 2011, 22, 175–183. [Google Scholar] [CrossRef] [PubMed]
| Scaffold Requirement | Biological Significance | How to Control Scaffold Requirement | Example | Reference |
|---|---|---|---|---|
| Biodegradability | Once placed, the scaffold should be reabsorbed in order to:
| Synthetic polymers | Bone TE Cartilage TE Periodontal TE Muscle TE Skin TE Neural TE | Cai et al., 2018 [141] Ma et al., 2005 [19] Galli et al., 2016 [133] Zahari et al., 2017 [57] Sadeghi et al., 2016 [130] Rajabi et al., 2018 [121] |
| Natural polymers | Bone TE Ligament TE Periodontal TE Skin TE Neural TE | Kudva et al., 2018 [123] Font Tellado et al., 2018 [149] Parisi et al., 2017 [134] Shefa et al., 2017 [58] Li et al., 2017 [41] | ||
| Mechanical properties | The scaffold should be mechanistically similar to the tissue to regenerate in order to:
| Porous scaffolds | Neural TE | Li et al., 2017 [41] |
| Hydrogels | Cartilage TE Neural TE | Zhang et al., 2018 [51] Chedly et al., 2018 [52] | ||
| Fibrous scaffolds | Bone TE Cartilage TE Ligament TE Muscle TE Skin TE | Khorouschi et al., 2018 [54] Chen et al., 2016 [55] Pauly et al., 2016 [56] Zahari et al., 2017 [57] Shefa et al., 2017 [58] | ||
| Porosity | The scaffold should be porous in order to:
| Top-down approaches | Cartilage TE Skin TE Bladder TE Cardiac TE | Wimpenny et al., 2012 [69] Zonari et al., 2014 [73] Korossis et al., 2009 [70] Baheriraei et al., 2015 [76] |
| Bottom-up approaches | Cartilage TE | Yingying et al., 2017 [96] | ||
| Bioactivity | The scaffold should be bioactive in order to:
| Direct coating | Bone TE Neural TE | Noh et al., 2016 [122] Kudva et al., 2018 [123] Rajabi et al., 2018 [121] |
| Surface functionalization | Cartilage TE Skin TE | Ma et al., 2005 [19] Sadeghi et al., 2016 [130] | ||
| Selective binding molecules | Periodontal TE | Galli et al., 2016 [133] Parisi et al., 2017 [134] | ||
Physical immobilization:
| Bone TE | Cai et al., 2018 [141] Ghiacci et al., 2017 [142] Wei et al., 2007 [143] Macdonald et al., 2011 [147] | ||
Covalent immobilization:
| Bone TE | Lee et al., 2016 [149] | ||
| Bioaffinity immobilization | Bone TE Ligament TE | Kisiel et al., 2013 [151] Font Tellado et al., 2018 [150] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, L.; Toffoli, A.; Ghiacci, G.; Macaluso, G.M. Tailoring the Interface of Biomaterials to Design Effective Scaffolds. J. Funct. Biomater. 2018, 9, 50. https://doi.org/10.3390/jfb9030050
Parisi L, Toffoli A, Ghiacci G, Macaluso GM. Tailoring the Interface of Biomaterials to Design Effective Scaffolds. Journal of Functional Biomaterials. 2018; 9(3):50. https://doi.org/10.3390/jfb9030050
Chicago/Turabian StyleParisi, Ludovica, Andrea Toffoli, Giulia Ghiacci, and Guido M. Macaluso. 2018. "Tailoring the Interface of Biomaterials to Design Effective Scaffolds" Journal of Functional Biomaterials 9, no. 3: 50. https://doi.org/10.3390/jfb9030050
APA StyleParisi, L., Toffoli, A., Ghiacci, G., & Macaluso, G. M. (2018). Tailoring the Interface of Biomaterials to Design Effective Scaffolds. Journal of Functional Biomaterials, 9(3), 50. https://doi.org/10.3390/jfb9030050







